Editorial (Preface) for the Special Issue on Advances in Minimally Invasive Liver Resection for Cancer Therapies
Conflicts of Interest
References
- Morise, Z.; Wakabayashi, G. First quarter century of laparoscopic liver resection. World J. Gastroenterol. 2017, 23, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [PubMed]
- Cherqui, D.; Wakabayashi, G.; Geller, D.A.; Buell, J.F.; Han, H.S.; Soubrane, O.; O’Rourke, N. International Laparoscopic Liver Society. The need for organization of laparoscopic liver resection. J. Hepatobiliary Pancreat. Sci. 2016, 23, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepatobiliary Pancreat. Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Wakabayashi, G.; Hasegawa, K.; Gotohda, N.; Mizuguchi, T.; Takahashi, Y.; Hirokawa, F.; Taniai, N.; Watanabe, M.; Katou, M.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: A multi-institutional Japanese study. J. Hepatobiliary Pancreat. Sci. 2015, 22, 711–720. [Google Scholar] [CrossRef] [PubMed]
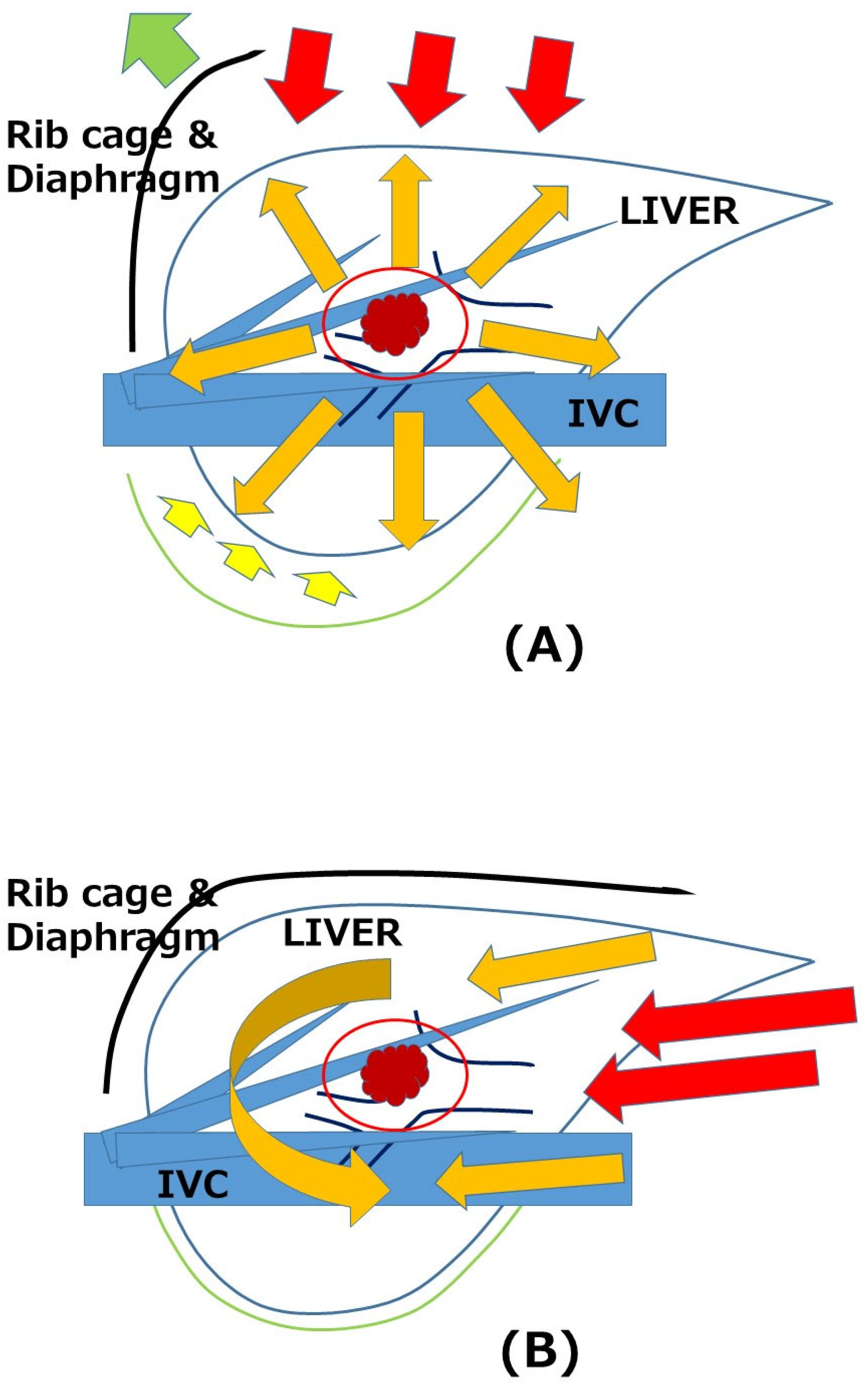
- Tomishige, H.; Morise, Z.; Kawabe, N.; Nagata, H.; Ohshima, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J. Gastrointest. Surg. 2013, 5, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; Morise, Z.; Sposito, C.; Igarashi, K.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.P.; Kubo, S.; Tanaka, S.; et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J. Hepatol. 2020, 72, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Belli, A.; Cherqui, D.; Tanabe, M.; Wakabayashi, G.; ILLS-Tokyo Collaborator group. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020, 107, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Katsuno, H.; Kikuchi, K.; Endo, T.; Matsuo, K.; Asano, Y.; Horiguchi, A. Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection. Cancers 2023, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z. Current status of minimally invasive liver surgery for cancers. World J. Gastroenterol. 2022, 28, 6090–6098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morise, Z. Editorial (Preface) for the Special Issue on Advances in Minimally Invasive Liver Resection for Cancer Therapies. Cancers 2023, 15, 3520. https://doi.org/10.3390/cancers15133520
Morise Z. Editorial (Preface) for the Special Issue on Advances in Minimally Invasive Liver Resection for Cancer Therapies. Cancers. 2023; 15(13):3520. https://doi.org/10.3390/cancers15133520
Chicago/Turabian StyleMorise, Zenichi. 2023. "Editorial (Preface) for the Special Issue on Advances in Minimally Invasive Liver Resection for Cancer Therapies" Cancers 15, no. 13: 3520. https://doi.org/10.3390/cancers15133520
APA StyleMorise, Z. (2023). Editorial (Preface) for the Special Issue on Advances in Minimally Invasive Liver Resection for Cancer Therapies. Cancers, 15(13), 3520. https://doi.org/10.3390/cancers15133520



