Optimal Time Interval between Neoadjuvant Platinum-Based Chemotherapy and Interval Debulking Surgery in High-Grade Serous Ovarian Cancer
Abstract
Simple Summary
Highlights
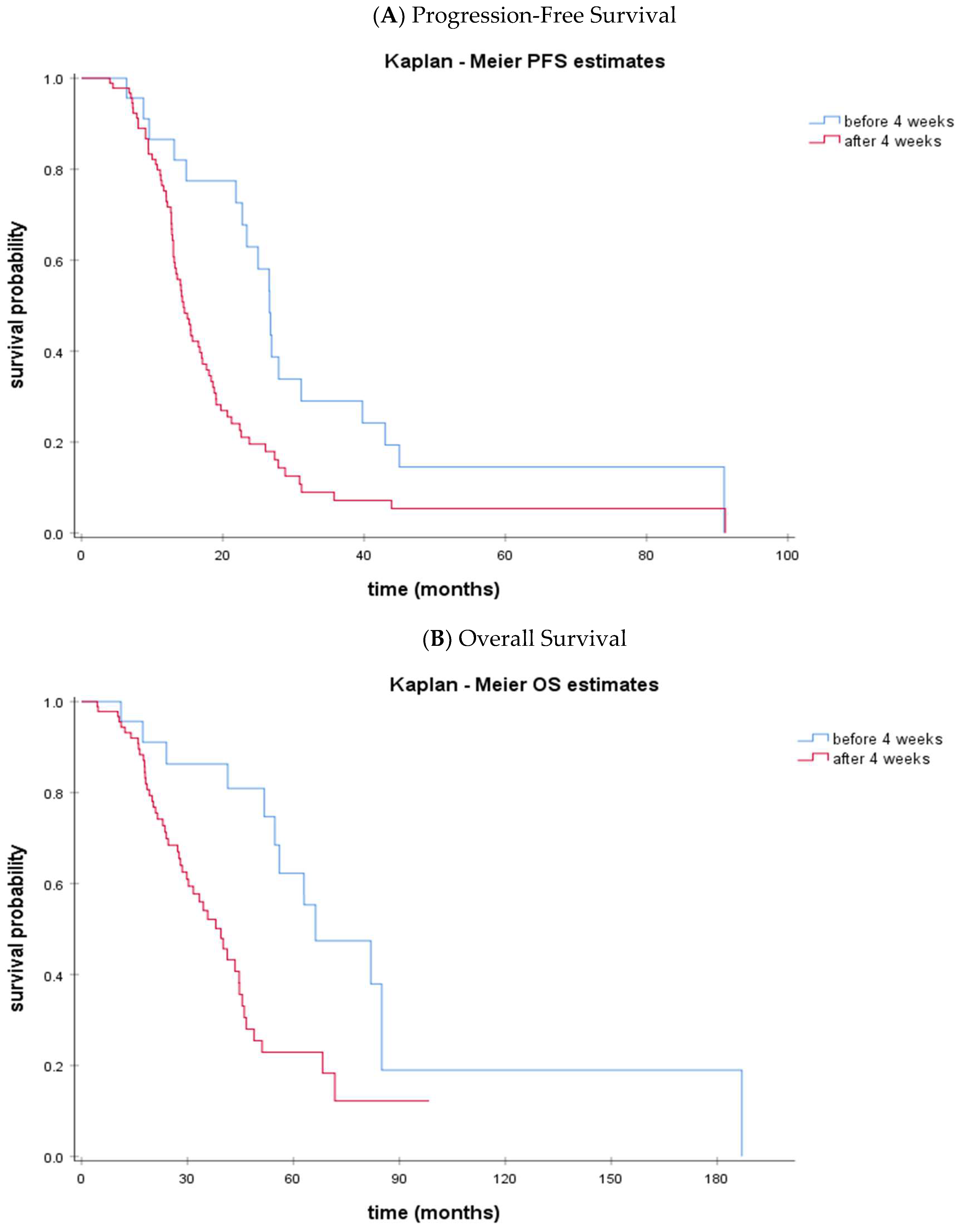
- The time interval NACT to IDS < 4 weeks was significantly associated with a prolonged PFS (p = 0.004) and OS (p = 0.002).
- Median OS was 66.3 months (95% CI: 39.1–93.4) vs. 39.4 months (95% CI: 31.8–47.0) in the <4 week vs. ≥4 week time interval NACT to IDS groups (p = 0.002)
- On multivariate analysis, the performance of IDS within 4 weeks after NACT and optimal debulking were independent factors for both PFS and OS
- Performing IDS early after NACT proved to be a good prognostic factor among ovarian cancer patients
- Multidisciplinary coordination is required so as to avoid any unnecessary delays
Abstract
1. Background
2. Methods
Statistical Analysis
3. Results
3.1. Study Population
3.2. Subgroup Analysis
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/online-analysis-multi-bars?v=2018&mode=cancer&mode_population=countries&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&type_multiple=%257B%2522inc%2522%253Atrue%252C%2522mort%2522%253Afalse%252C%2522prev%2522%253Afalse%257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522bottom%2522%253Afalse%257D&population_group_globocan_id= (accessed on 16 November 2020).
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Gueli Alletti, S.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Onda, T.; Satoh, T.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Nakamura, K.; Wakabayashi, M.; Takehara, K.; Saito, M.; Ushijima, K.; et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur. J. Cancer 2016, 64, 22–31. [Google Scholar] [CrossRef]
- Rouzier, R.; Gouy, S.; Selle, F.; Lambaudie, E.; Floquet, A.; Fourchotte, V.; Pomel, C.; Colombo, P.E.; Kalbacher, E.; Martin-Francoise, S.; et al. Efficacy and safety of bevacizumab-containing neoadjuvant therapy followed by interval debulking surgery in advanced ovarian cancer: Results from the ANTHALYA trial. Eur. J. Cancer 2017, 70, 133–142. [Google Scholar] [CrossRef]
- Garcia, Y.G.; De Juan Ferré, A.; Mendiola, C.; Barretina-Ginesta, M.P.; Gaba Garcia, L.; Santaballa Bertrán, A.; Bover Barcelo, I.; Gil-Martin, M.; Manzano, A.; Pérez, M.J.R.; et al. Efficacy and safety results from GEICO 1205, a randomized phase II trial of neoadjuvant chemotherapy with or without bevacizumab for advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 1050–1056. [Google Scholar] [CrossRef]
- Timmermans, M.; van der Aa, M.A.; Lalisang, R.I.; Witteveen, P.O.; Van de Vijver, K.K.; Kruitwagen, R.F.; Sonke, G.S. Interval between debulking surgery and adjuvant chemotherapy is associated with overall survival in patients with advanced ovarian cancer. Gynecol. Oncol. 2018, 150, 446–450. [Google Scholar] [CrossRef]
- Mahner, S.; Eulenburg, C.; Staehle, A.; Wegscheider, K.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J.; Du Bois, A. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: Analysis of prospective randomised phase III trials. Eur. J. Cancer 2013, 49, 142–149. [Google Scholar] [CrossRef]
- Alencar, V.; Pirolli, R.; Estati, F.L.; Ribeiro, A.R.G.; Guimaraes, A.P.; Baiocchi, G.; Costa, A.A.B.A. Da Association of time interval from neoadjuvant chemotherapy to interval cytoreduction and interval cytoreduction to adjuvant chemotherapy with survival outcomes and risk of platinum resistance. J. Clin. Oncol. 2020, 38, e18043. [Google Scholar] [CrossRef]
- Wang, D.F.; Zhang, G.N.; Peng, C.R.; Shi, Y.; Shi, X.W. Analysis of factors related to the prognostic benefit of neoadjuvant chemotherapy followed by interval debulking surgery in patients with advanced ovarian cancer. Zhonghua Fu Chan Ke Za Zhi 2021, 56, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhu, J.; Kim, J.W.; Liu, J.; Kato, K.; Kim, H.S.; Zhang, Y.; Zhang, P.; Zhu, T.; Aoki, D.; et al. Study of upfront surgery versus neoadjuvant chemotherapy followed by interval debulking surgery for patients with stage IIIC and IV ovarian cancer, SGOG SUNNY (SOC-2) trial concept. J. Gynecol. Oncol. 2020, 31, e86. [Google Scholar] [CrossRef] [PubMed]
- Clamp, A.R.; James, E.C.; McNeish, I.A.; Dean, A.; Kim, J.W.; O’Donnell, D.M.; Hook, J.; Coyle, C.; Blagden, S.; Brenton, J.D.; et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019, 394, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Z.; Xu, M.; Liu, D.; Liu, T.; He, M.; Yao, S. Impact of the Time Interval from Neoadjuvant Chemotherapy to Surgery in Primary Ovarian, Tubal, and Peritoneal Cancer Patients. J. Cancer 2018, 9, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Chung, Y.S.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Impact of the time interval from completion of neoadjuvant chemotherapy to initiation of postoperative adjuvant chemotherapy on the survival of patients with advanced ovarian cancer. Gynecol. Oncol. 2018, 148, 62–67. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Peng, C.; Shi, Y.; Shi, X. Choosing the right timing for interval debulking surgery and perioperative chemotherapy may improve the prognosis of advanced epithelial ovarian cancer: A retrospective study. J. Ovarian Res. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Clark, M.; Lee, Y.; Xu, W.; Brown, T.; May, T. Does the time interval off neoadjuvant chemotherapy before and after interval debulking surgery affect the overall survival of women with advanced-epithelial ovarian cancer? Gynecol. Oncol. 2018, 149, 92. [Google Scholar] [CrossRef]
- Omarini, C.; Guaitoli, G.; Noventa, S.; Andreotti, A.; Gambini, A.; Palma, E.; Papi, S.; Tazzioli, G.; Balduzzi, S.; Dominici, M.; et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur. J. Surg. Oncol. 2017, 43, 613–618. [Google Scholar] [CrossRef]
- Sanford, R.A.; Lei, X.; Barcenas, C.H.; Mittendorf, E.A.; Caudle, A.S.; Valero, V.; Tripathy, D.; Giordano, S.H.; Chavez-MacGregor, M. Impact of Time from Completion of Neoadjuvant Chemotherapy to Surgery on Survival Outcomes in Breast Cancer Patients. Ann. Surg. Oncol. 2016, 23, 1515–1521. [Google Scholar] [CrossRef]
- Yu, M.; Wang, D.C.; Li, S.; Huang, L.Y.; Wei, J. Does a long interval between neoadjuvant chemoradiotherapy and surgery benefit the clinical outcomes of locally advanced rectal cancer? A systematic review and meta analyses. Int. J. Colorectal Dis. 2022, 37, 855–868. [Google Scholar] [CrossRef]
- Gao, S.J.; Corso, C.D.; Wang, E.H.; Blasberg, J.D.; Detterbeck, F.C.; Boffa, D.J.; Decker, R.H.; Kim, A.W. Timing of Surgery after Neoadjuvant Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 314–322. [Google Scholar] [CrossRef]
- Liontos, M.; Sotiropoulou, M.; Kaparelou, M.; Tzannis, K.; Tsironis, G.; Kyriazoglou, A.; Tsiara, A.; Zakopoulou, R.; Koutsoukos, K.; Zagouri, F.; et al. Lymphocytic infiltration and Chemotherapy Response Score as prognostic markers in ovarian cancer patients treated with Neoadjuvant chemotherapy. Gynecol. Oncol. 2020, 157, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Faruqi, A.; Said, I.; Lockley, M.; Brockbank, E.; Jeyarajah, A.; Fitzpatrick, A.; Ennis, D.; Dowe, T.; Santos, J.L.; et al. Chemotherapy response score: Development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J. Clin. Oncol. 2015, 33, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.A.K.; Tripsiannis, G.; Markakidis, S.K.; Karanikiotis, C.N.; Tzegas, G.; Georgiadis, G.; Avgidou, K. Peritoneal Cancer Index: A Prognostic Indicator of Survival in Advanced Ovarian Cancer. Eur. J. Surg. Oncol. 2003, 29, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Ercoli, A.; Lorusso, D.; Rossi, M.; Scambia, G. A Laparoscopy-Based Score to Predict Surgical Outcome in Patients with Advanced Ovarian Carcinoma: A Pilot Study. Ann. Surg. Oncol. 2006, 13, 1156–1161. [Google Scholar] [CrossRef]
- Brun, J.L.; Rouzier, R.; Uzan, S.; Daraï, E. External Validation of a Laparoscopic-Based Score to Evaluate Resectability of Advanced Ovarian Cancers: Clues for a Simplified Score. Gynecol. Oncol. 2008, 110, 354–359. [Google Scholar] [CrossRef]

| Characteristic | Total N (%) |
|---|---|
| Age at diagnosis, median (IQR: 25–75), years | 62.7 (14.0; 58.1–71.8) |
| Initial stage (FIGO) | |
| IIIC | 88 (76.5%) |
| IV | 27 (23.5%) |
| Debulking status | |
| Optimal | 69 (60%) |
| Suboptimal | 32 (27.8%) |
| Unknown | 14 (12.2%) |
| ECOG performance status | |
| 0/1 | 97 (84.3%) |
| 2/3 | 14 (12.2%) |
| Unknown | 4 (3.5%) |
| Time interval NACT to IDS, median (IQR: 25–75), weeks | 5.6 (2.9; 4.1–7.0) |
| Time interval NACT to IDS, weeks | |
| <4 | 23 (20%) |
| ≥4 to <5 | 20 (17.4%) |
| ≥5 to <6 | 22 (19.1%) |
| ≥6 | 50 (43.5%) |
| BRCA1/2 somatic mutation | |
| YES | 18 (15.7%) |
| NO | 61 (53%) |
| Unknown | 36 (31.3%) |
| PFS, median (range), months | 15.7 (13.0–18.5) |
| OS, median (range), months | 44.7 (38.8–50.5) |
| Characteristic | <4 Weeks N (%) | ≥4 Weeks N (%) |
|---|---|---|
| Age at diagnosis, median (IQR: 25–75), years | 61.6 (15.0; 57.0—71.8) | 64.0 (14.0; 58.2–71.7) |
| Initial stage (FIGO) | ||
| IIIC | 18 (78.3%) | 70 (76.1%) |
| IV | 5 (21.7%) | 22 (23.9%) |
| Debulking status | ||
| Optimal | 14 (60.9%) | 55 (59.8%) |
| Suboptimal | 8 (34.8%) | 24 (26.1%) |
| Unknown | 1 (4.3%) | 13 (14.1%) |
| ECOG performance status | ||
| 0/1 | 19 (82.6%) | 78 (84.8%) |
| 2/3 | 3 (13%) | 11 (12%) |
| Unknown | 1 (4.3%) | 3 (3.3%) |
| BRCA1/2 somatic mutation | ||
| Yes | 3 (13%) | 15 (16.3%) |
| No | 14 (60.9%) | 47 (51.1%) |
| Unknown | 6 (26.1%) | 30 (32.6%) |
| Interval NACT to IDS, median (IQR: 25–75), weeks | 3.1 (0.7; 3.0–3.7) | 6.0 (2.2; 5.0–7.3) |
| PFS, median (95% CI), months | 26.6 (24.0–29.2) | 14.4 (12.6–16.3) |
| OS, median (95% CI), months | 66.3 (39.1–93.4) | 39.4 (31.8–47.0) |
| Characteristic | <4 Weeks Group A N (%) | ≥4 to <5 Weeks Group B N (%) | ≥5 to <6 Weeks Group C N (%) | ≥6 Weeks Group D N (%) |
|---|---|---|---|---|
| Age at diagnosis, median (IQR: 25–75), years | 61.6 (15.0; 57.0–71.8) | 60.8 (10.0; 56.1–65.8) | 61.6 (19.0; 51.8–71.3) | 67.0 (17.0; 59.1–76.1) |
| Initial stage (FIGO) | ||||
| IIIC | 18 (78.3%) | 14 (70.0%) | 17 (77.3%) | 39 (78.0%) |
| IV | 5 (21.7%) | 6 (30.0%) | 5 (22.7%) | 11 (22.0%) |
| Debulking status | ||||
| Optimal | 14 (60.9%) | 11 (55.0%) | 16 (72.7%) | 28 (56.0%) |
| Suboptimal | 8 (34.8%) | 5 (25.0%) | 5 (22.7%) | 14 (28.0%) |
| Unknown | 1 (4.3%) | 4 (20.0%) | 1 (4.5%) | 8 (16.0%) |
| ECOG performance status | ||||
| 0/1 | 19 (82.6%) | 19 (95.0%) | 17 (77.3%) | 42 (84.0%) |
| 2/3 | 3 (13.0%) | 1 (5.0%) | 3 (13.6%) | 7 (14.0%) |
| Unknown | 1 (4.3%) | 0 (0%) | 2 (9.1%) | 1 (2.0%) |
| BRCA1/2 somatic mutation | ||||
| Yes | 3 (13.0%) | 4 (20.0%) | 3 (13.6%) | 8 (16.0%) |
| No | 14 (60.9%) | 9 (45.0%) | 10 (45.5%) | 28 (56.0%) |
| Unknown | 6 (26.1%) | 7 (35.0%) | 9 (40.9%) | 14 (28.0%) |
| Interval NACT to IDS, median (IQR: 25–75), weeks | 3.1 (0.7; 3.0–3.7) | 4.4 (0.6; 4.1–4.7) | 5.4 (0.6; 5.1–5.7) | 7.1 (1.5; 6.4–7.9) |
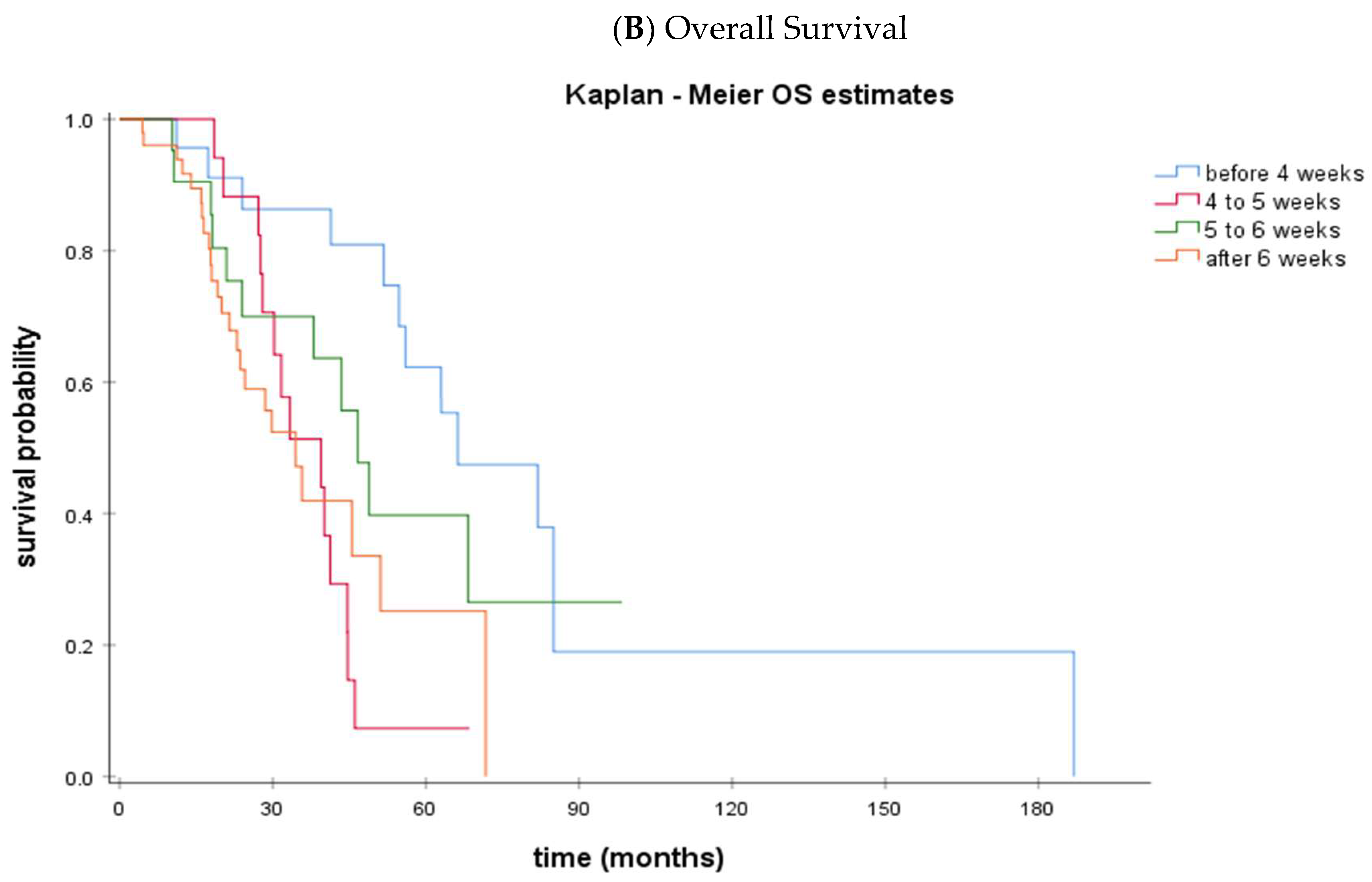
| PFS, median (95% CI), months | 26.6 (24.0–29.2) | 12.8 (12.2–13.3) | 14.6 (12.7–16.4) | 16.6 (13.9–19.2) |
| OS, median (95% CI), months | 66.3 (39.1–93.4) | 39.4 (25.4–53.5) | 46.6 (38.1–55.2) | 34.5 (25.3–43.7) |
| Variables | Category | PFS | OS | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Disease stage | IIIC vs. IV | 0.97 (0.85–1.11) | 0.689 | 0.89 (0.75–1.05) | 0.175 |
| Debulking | Optimal vs. Suboptimal | 1.96 (1.19–3.23) | 0.008 | 3.09 (1.63–5.87) | 0.001 |
| Performance status | 0/1 vs. 2/3 | 1.41 (0.67–2.95) | 0.370 | 2.35 (0.99–5.60) | 0.053 |
| Time interval NACT to IDS | <4 weeks vs. ≥4 weeks | 2.33 (1.31–4.17) | 0.004 | 3.23 (1.48–7.05) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrikopoulou, A.; Theofanakis, C.; Markellos, C.; Kaparelou, M.; Koutsoukos, K.; Apostolidou, K.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; Dimopoulos, M.-A.; et al. Optimal Time Interval between Neoadjuvant Platinum-Based Chemotherapy and Interval Debulking Surgery in High-Grade Serous Ovarian Cancer. Cancers 2023, 15, 3519. https://doi.org/10.3390/cancers15133519
Andrikopoulou A, Theofanakis C, Markellos C, Kaparelou M, Koutsoukos K, Apostolidou K, Thomakos N, Haidopoulos D, Rodolakis A, Dimopoulos M-A, et al. Optimal Time Interval between Neoadjuvant Platinum-Based Chemotherapy and Interval Debulking Surgery in High-Grade Serous Ovarian Cancer. Cancers. 2023; 15(13):3519. https://doi.org/10.3390/cancers15133519
Chicago/Turabian StyleAndrikopoulou, Angeliki, Charalampos Theofanakis, Christos Markellos, Maria Kaparelou, Konstantinos Koutsoukos, Kleoniki Apostolidou, Nikolaos Thomakos, Dimitrios Haidopoulos, Alexandros Rodolakis, Meletios-Athanasios Dimopoulos, and et al. 2023. "Optimal Time Interval between Neoadjuvant Platinum-Based Chemotherapy and Interval Debulking Surgery in High-Grade Serous Ovarian Cancer" Cancers 15, no. 13: 3519. https://doi.org/10.3390/cancers15133519
APA StyleAndrikopoulou, A., Theofanakis, C., Markellos, C., Kaparelou, M., Koutsoukos, K., Apostolidou, K., Thomakos, N., Haidopoulos, D., Rodolakis, A., Dimopoulos, M.-A., Zagouri, F., & Liontos, M. (2023). Optimal Time Interval between Neoadjuvant Platinum-Based Chemotherapy and Interval Debulking Surgery in High-Grade Serous Ovarian Cancer. Cancers, 15(13), 3519. https://doi.org/10.3390/cancers15133519






