The Quality of Practice Guidelines for Melanoma: A Methodologic Appraisal with the AGREE II and AGREE-REX Instruments
Abstract
:1. Introduction
2. Results
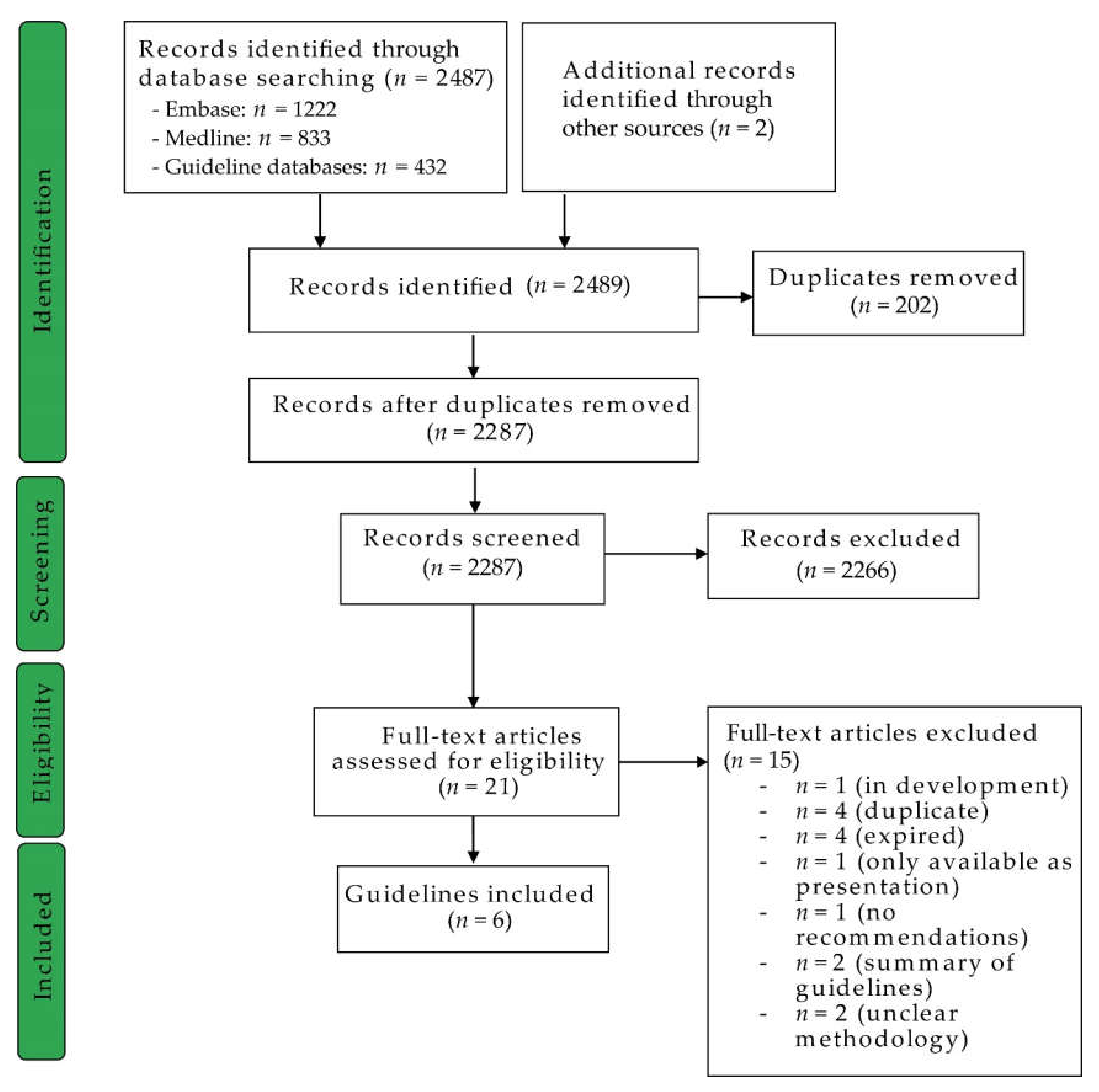
2.1. Guideline Identification
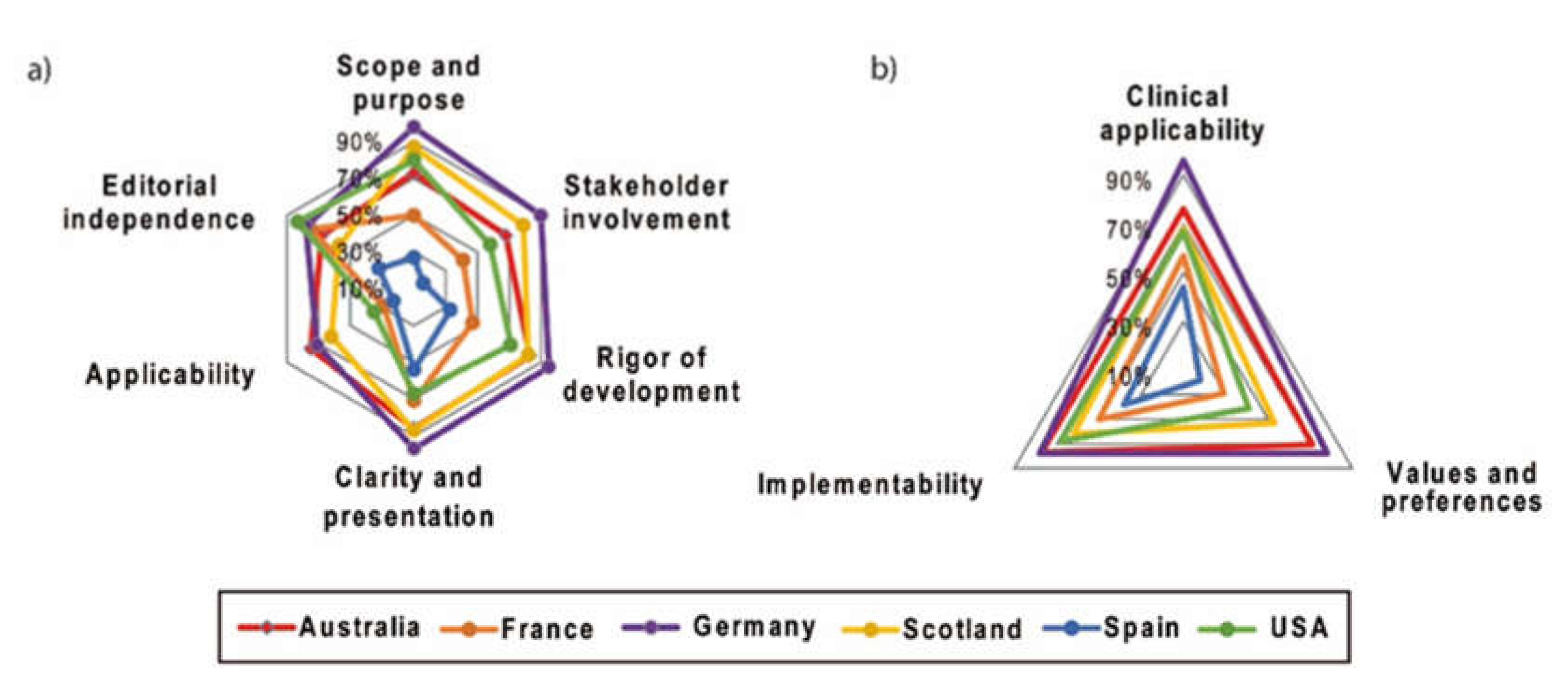
2.2. AGREE II
2.2.1. Scope and Purpose
2.2.2. Stakeholder Involvement
2.2.3. Rigor of Development
2.2.4. Clarity and Presentation
2.2.5. Application
2.2.6. Editorial Independence
2.2.7. Overall Assessment
2.3. AGREE-REX
2.3.1. Clinical Applicability
2.3.2. Values and Preferences
2.3.3. Implementability
2.4. Correlations of the AGREE II and AGREE-REX Domains
3. Discussion
4. Materials and Methods
4.1. Eligibility Criteria
4.2. Search Strategy and Guideline Selection
4.3. Data Extraction and Rating of the Guidelines
4.4. Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine; Board on Health Care Services; Committee on Standards for Developing Trustworthy Clinical Practice. Clinical Practice Guidelines We Can Trust; Graham, R., Mancher, M., Miller Wolman, D., Greenfield, S., Steinberg, E., Eds.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Wrobel, S.; Przybylo, M.; Stepien, E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019, 8, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico Iturrioz, R.; Gutierrez-Ibarluzea, I.; Asua Batarrita, J.; Navarro Puerto, M.A.; Reyes Dominguez, A.; Marin Leon, I.; Briones Perez de la Blanca, E. Assessment of clinical practice guidelines evaluation. Scales and criteria. Rev. Esp. Salud Publica 2004, 78, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, M.C.; Florez, I.D.; McNair, S.A.; Vella, E.T.; Yao, X. Clinical Practice Guidelines: Tools to Support High Quality Patient Care. Semin. Nucl. Med. 2019, 49, 145–152. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Vlayen, J.; Aertgeerts, B.; Hannes, K.; Sermeus, W.; Ramaekers, D. A systematic review of appraisal tools for clinical practice guidelines: Multiple similarities and one common deficit. Int. J. Qual. Health Care 2005, 17, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dans, A.L.; Dans, L.F. Appraising a tool for guideline appraisal (the AGREE II instrument). J. Clin. Epidemiol. 2010, 63, 1281–1282. [Google Scholar] [CrossRef]
- AGREE-REX Research Team. The Appraisal of Guidelines Research & Evaluation—Recommendation EXcellence (AGREE-REX). 2019. Available online: https://www.agreetrust.org/wp-content/uploads/2019/04/AGREE-REX-2019.pdf (accessed on 12 February 2020).
- National Institute for Health and Care Excellence (NICE). NICE Guideline: Melanoma: Assessment and Management. 2015. Available online: https://www.nice.org.uk/guidance/ng14 (accessed on 1 February 2020).
- Castro, L.G.M.; Duprat Neto, J.P.; Di Giacomo, T.H.B.; Messina, M.C.L.; Macarenco, R.S.S.; Gontijo, G.; Bakos, R.M.; Bittencourt, F.V.; Serpa, S.S.; Loureiro, W.R.; et al. Brazilian guidelines for diagnosis, treatment and follow-up of primary cutaneous melanoma-Part II. An. Bras. Dermatol. 2016, 91, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, L.G.M.; Messina, M.C.; Loureiro, W.; Macarenco, R.S.; Duprat Neto, J.P.; Di Giacomo, T.H.B.; Bittencourt, F.V.; Bakos, R.M.; Serpa, S.S.; Stolf, H.O.; et al. Guidelines of the Brazilian dermatology society for diagnosis, treatment and follow up of primary cutaneous melanoma-Part I. An. Bras. Dermatol. 2015, 90, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Qin, S.; Liang, J.; Lin, T.; Si, L.; Chen, X.; Chi, Z.; Cui, C.; Du, N.; Fan, Y.; et al. Chinese guidelines on the diagnosis and treatment of melanoma (2015 edition). Chin. Clin. Oncol. 2015, 3, 322. [Google Scholar] [CrossRef]
- American Society of Clinical Oncology. Guidelines in Development: Systemic Therapy for Melanoma. Available online: https://www.asco.org/research-guidelines/quality-guidelines/guidelines-tools-resources/guidelines-development (accessed on 17 February 2020).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Cuataneous Melanoma, Version 1.2020-December 19, 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed on 15 January 2020).
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment-Update 2019. Eur. J. Cancer 2020, 126, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics-Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrocal, A.; Espinosa, E.; Marin, S.; Malvehy, J.; Moreno, D.; Lozano, M.D.; Martin-Algarra, S.; Lopez, J.A.; Conill, C.; Rodriguez-Peralto, J.L. Spanish Multidisciplinary Melanoma Group (GEM) guidelines for the management of patients with advanced melanoma. Eur. J. Dermatol. 2015, 25, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline-Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hauschild, A.; Lindenblatt, N.; Pentheroudakis, G.; Keilholz, U.; Committee, E.G. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v126–v132. [Google Scholar] [CrossRef]
- Coit, D.G.; Thompson, J.A.; Albertini, M.R.; Barker, C.; Carson, W.E.; Contreras, C.; Daniels, G.A.; DiMaio, D.; Fields, R.C.; Fleming, M.D.; et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 367–402. [Google Scholar] [CrossRef] [Green Version]
- Barker, C.A.; Salama, A.K. New NCCN guidelines for uveal melanoma and treatment of recurrent or progressive distant metastatic melanoma. J. Natl. Compr. Cancer Netw. 2018, 16, 646–650. [Google Scholar] [CrossRef]
- Macbeth, F.; Newton-Bishop, J.; O’Connell, S.; Hawkins, J.E. Melanoma: Summary of NICE guidance. BMJ (Online) 2015, 35, h3708. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.R.S.; Fraser, S.J.; Quaba, O.; Simms, A.; Stein, A. Cutaneous melanoma: An updated SIGN guideline. J. R. Coll. Phys. Edinb. 2017, 47, 214–217. [Google Scholar] [CrossRef]
- Hoge, J.C.; Schadendorf, D. Update of the German S3 guideline on malignant melanoma: Current recommendations for advanced melanoma. Best Pract. Onkol. 2017, 12, 110–119. (In German) [Google Scholar] [CrossRef]
- Cancer Council Australia Melanoma Guidelines Working Party. Clinical Practice Guidelines for the Diagnosis and Management of Melanoma. Sydney: Cancer Council Australia. Available online: https://wiki.cancer.org.au/australiawiki/index.php?oldid=209426 (accessed on 12 February 2020).
- Guillot, B.; Dalac, S.; Denis, M.G.; Dupuy, A.; Emile, J.F.; De La Fouchardiere, A.; Hindie, E.; Jouary, T.; Lassau, N.; Mirabel, X.; et al. French updated recommendations in Stage I to III melanoma treatment and management. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 594–602. [Google Scholar] [CrossRef] [Green Version]
- Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF. Diagnostik, Therapie und Nachsorge des Melanoms. In Leitlinienprogramm Onkologie, Kurzversion 3.2; AWMF Registernummer: 032/024OL; AWMF: Berlin, Germany, 2019. [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN). Cutaneous Melanoma. SIGN Publication No. 146; SIGN: Edinburgh, UK, 2017. [Google Scholar]
- Berrocal, A.; Arance, A.; Castellon, V.E.; de la Cruz, L.; Espinosa, E.; Cao, M.G.; Larriba, J.L.G.; Marquez-Rodas, I.; Soria, A.; Algarra, S.M. SEOM clinical guideline for the management of malignant melanoma (2017). Clin. Transl. Oncol. 2018, 20, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Scottish Intercollegiate Guidelines Network (SIGN). SIGN 50: A Guideline Developer’s Handbook; SIGN Publication No. 50; SIGN: Edinburgh, UK, 2015. [Google Scholar]
- Heppt, M.V.; Steeb, T.; Berking, C.; Nast, A. Comparison of guidelines for the management of patients with high-risk and advanced cutaneous squamous cell carcinoma—A systematic review. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 25–32. [Google Scholar] [CrossRef] [Green Version]
- Hauschild, A.; Ascierto, P.A.; Schadendorf, D.; Grob, J.J.; Ribas, A.; Kiecker, F.; Dutriaux, C.; Demidov, L.V.; Lebbe, C.; Rutkowski, P.; et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib monotherapy: Analysis from phase 2 and 3 clinical trials. Eur. J. Cancer 2020, 125, 114–120. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Flaherty, K.T.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; et al. Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs. vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur. J. Cancer 2020, 126, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, K.; Miksch, S. Versioning computer-interpretable guidelines: Semi-automatic modeling of ‘Living Guidelines’ using an information extraction method. Artif. Intell. Med. 2009, 46, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Uhlig, K.; Berns, J.S.; Carville, S.; Chan, W.; Cheung, M.; Guyatt, G.H.; Hart, A.; Lewis, S.Z.; Tonelli, M.; Webster, A.C.; et al. Recommendations for kidney disease guideline updating: A report by the KDIGO Methods Committee. Kidney Int. 2016, 89, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Steeb, T.; Hayani, K.M.; Forster, P.; Liegl, R.; Toussaint, F.; Schlaak, M.; Berking, C.; Heppt, M.V. Guidelines for uveal melanoma: A critical appraisal of systematically identified guidelines using the AGREE II and AGREE-REX instrument. J. Cancer Res. Clin. Oncol. 2020, 146, 1079–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Title | Year | National Society and/or Authors |
|---|---|---|
| Clinical practice guidelines for the diagnosis and management of melanoma [25] | 2019 | Cancer Council Australia |
| French updated recommendations in Stage I to III melanoma treatment and management [26] | 2017 | Guillot et al., (France) |
| Diagnostik, Therapie und Nachsorge des Melanoms [27] | 2019 | AWMF, DKG, DKH (Germany) |
| Cutaneous melanoma [28] | 2017 | Scottish Intercollegiate Guidelines Network (SIGN) |
| SEOM clinical guideline for the management of malignant melanoma [29] | 2017 | Berrocal et al. (Spain) |
| Guidelines of care for the management of primary cutaneous melanoma [30] | 2019 | Swetter et al. (USA) |
| r < 0.29 | AGREE II | AGREE-REX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r ≥ 0.3–0.49 | |||||||||||
| r ≥ 0.5–0.69 | |||||||||||
| r ≥ 0.7–0.89 | |||||||||||
| r ≥ 0.9 | |||||||||||
| Domain | Scope and Purpose | Stakeholder Involvement | Rigor of Development | Clarity of Presentation | Applicability | Editorial Independence | Overall Assessment | Clinical Applicability | Values and Preferences | Implementability | |
| AGREE II | Scope and Purpose | 1.000 | 0.917 ** | 0.811 ** | 0.716 ** | 0.569 ** | 0.422 * | 0.845 ** | 0.770 ** | 0.555 ** | 0.638 ** |
| Stakeholder Involvement | 1.000 | 0.890 ** | 0.684 ** | 0.639 ** | 0.427 * | 0.839 ** | 0.845 ** | 0.584 ** | 0.589 ** | ||
| Rigor of Development | 1.000 | 0.536 ** | 0.680 ** | 0.326 | 0.798 ** | 0.858 ** | 0.660 ** | 0.571 ** | |||
| Clarity of Presentation | 1.000 | 0.418 * | 0.457 * | 0.739 ** | 0.624 ** | 0.299 | 0.505 ** | ||||
| Applicability | 1.000 | 0.021 | 0.587 ** | 0.785 ** | 0.735 ** | 0.585 ** | |||||
| Editorial Independence | 1.000 | 0.375 * | 0.276 | −0.050 | 0.127 | ||||||
| Overall Assessment | 1.000 | 0.768 ** | 0.581 ** | 0.617 ** | |||||||
| AGREE-REX | Clinical applicability | 1.000 | 0.730 ** | 0.686 ** | |||||||
| Values and preferences | 1.000 | 0.663 ** | |||||||||
| Implementability | 1.000 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steeb, T.; Wessely, A.; Drexler, K.; Salzmann, M.; Toussaint, F.; Heinzerling, L.; Reinholz, M.; Berking, C.; Heppt, M.V. The Quality of Practice Guidelines for Melanoma: A Methodologic Appraisal with the AGREE II and AGREE-REX Instruments. Cancers 2020, 12, 1613. https://doi.org/10.3390/cancers12061613
Steeb T, Wessely A, Drexler K, Salzmann M, Toussaint F, Heinzerling L, Reinholz M, Berking C, Heppt MV. The Quality of Practice Guidelines for Melanoma: A Methodologic Appraisal with the AGREE II and AGREE-REX Instruments. Cancers. 2020; 12(6):1613. https://doi.org/10.3390/cancers12061613
Chicago/Turabian StyleSteeb, Theresa, Anja Wessely, Konstantin Drexler, Martin Salzmann, Frédéric Toussaint, Lucie Heinzerling, Markus Reinholz, Carola Berking, and Markus V. Heppt. 2020. "The Quality of Practice Guidelines for Melanoma: A Methodologic Appraisal with the AGREE II and AGREE-REX Instruments" Cancers 12, no. 6: 1613. https://doi.org/10.3390/cancers12061613
APA StyleSteeb, T., Wessely, A., Drexler, K., Salzmann, M., Toussaint, F., Heinzerling, L., Reinholz, M., Berking, C., & Heppt, M. V. (2020). The Quality of Practice Guidelines for Melanoma: A Methodologic Appraisal with the AGREE II and AGREE-REX Instruments. Cancers, 12(6), 1613. https://doi.org/10.3390/cancers12061613





