Phase I Radiation Dose-Escalation Study to Investigate the Dose-Limiting Toxicity of Concurrent Intra-Arterial Chemotherapy for Unresectable Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Toxicities
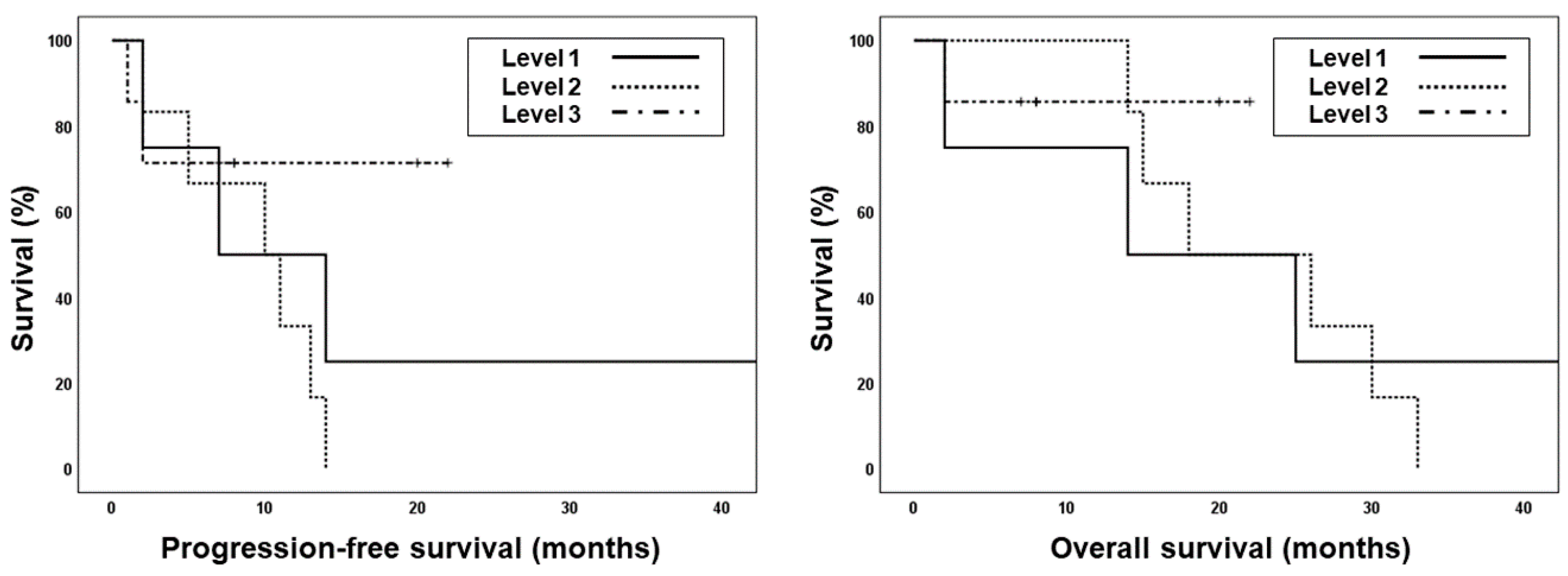
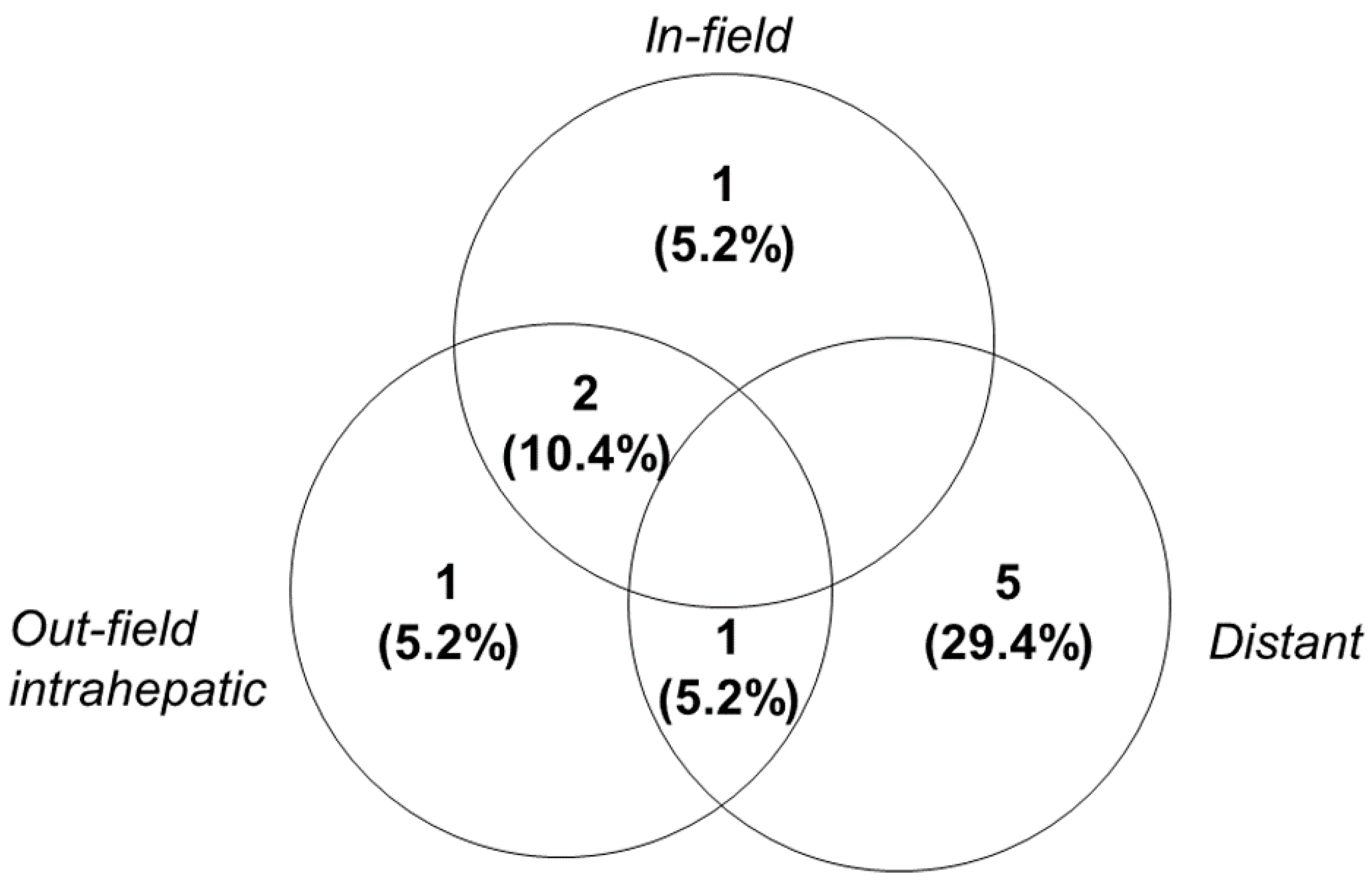
2.3. Treatment Outcomes
3. Discussion
4. Materials and Methods
4.1. Patient Eligibility
4.2. Simulation and Radiotherapy Planning
4.3. Intra-Arterial Chemotherapy
4.4. Dose-Limiting Toxicity
4.5. Follow-Up and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| AFP | α-fetoprotein |
| ALP | Alkaline phosphatase |
| BCLC | Barcelona clinic liver cancer |
| CR | Complete response |
| CT | Computed tomography |
| CTCAE | Common Toxicity Criteria for Adverse Events |
| CTV | Clinical target volume |
| DLT | Dose-limiting toxicity |
| ECOG | Eastern Cooperative Oncology Group |
| HCC | Hepatocellular carcinoma |
| IGRT | Image-guided radiotherapy |
| IMRT | Intensity-modulated radiotherapy |
| INR | International normalized ratio |
| IRB | Institutional Review Board |
| ITV | Internal target volume |
| OAR | Organs at risk |
| OS | Overall survival |
| PD | Progressive disease |
| PFS | Progression-free survival |
| PR | Partial response |
| PTV | Planning target volume |
| RILD | Radiation-induced liver disease |
| SBRT | Stereotactic body radiotherapy |
| SD | Stable disease |
| TACE | Transarterial chemoembolization |
References
- Sherman, M. Epidemiology of hepatocellular carcinoma. Oncology 2010, 78 (Suppl. 1), 7–10. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M. Updated treatment approach to hepatocellular carcinoma. J. Gastroenterol. 2005, 40, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Beaugrand, M. Hepatocellular carcinoma: Present status and future prospects. J. Hepatol. 2003, 38 (Suppl. 1), S136–S149. [Google Scholar] [CrossRef]
- Decadt, B.; Siriwardena, A.K. Radiofrequency ablation of liver tumours: Systematic review. Lancet Oncol. 2004, 5, 550–560. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montana, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Sola, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: Jsh-lcsgj criteria 2014 update. Oncology 2014, 87 (Suppl. 1), 22–31. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, W.S.; Koom, W.S.; Rim, C.H. Role of local treatment including radiotherapy in barcelona clinic of liver cancer stage c patients: A nationwide cohort analysis in south korea. Cancer Manag. Res. 2019, 11, 1373–1382. [Google Scholar] [CrossRef]
- Yoon, H.I.; Jung, I.; Han, K.H.; Seong, J. The effect of radiotherapy in liver-confined but non-resectable barcelona clinic liver cancer stage c large hepatocellular carcinoma. Oncotarget 2016, 7, 62715–62725. [Google Scholar] [CrossRef][Green Version]
- Do Seon Song, S.H.B. Treatments other than sorafenib for patients with advanced hepatocellular carcinoma. J. Liver Cancer 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic toxicity resulting from cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1237–1248. [Google Scholar] [CrossRef]
- Hawkins, M.A.; Dawson, L.A. Radiation therapy for hepatocellular carcinoma: From palliation to cure. Cancer 2006, 106, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Ben-Josef, E.; Normolle, D.; Ensminger, W.D.; Walker, S.; Tatro, D.; Ten Haken, R.K.; Knol, J.; Dawson, L.A.; Pan, C.; Lawrence, T.S. Phase ii trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2005, 23, 8739–8747. [Google Scholar] [CrossRef]
- Dawson, L.A.; McGinn, C.J.; Normolle, D.; Ten Haken, R.K.; Walker, S.; Ensminger, W.; Lawrence, T.S. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2000, 18, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Park, H.C.; Han, K.H.; Lee, D.Y.; Lee, J.T.; Chon, C.Y.; Moon, Y.M.; Suh, C.O. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1331–1335. [Google Scholar] [CrossRef]
- Song, J.E.; Jung, K.S.; Kim, D.Y.; Song, K.; Won, J.Y.; Lee, H.W.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; et al. Transarterial radioembolization versus concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma: A propensity score matching analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 396–406. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Chon, C.Y.; Seong, J. Combination treatment of localized concurrent chemoradiation therapy and transarterial chemoembolization in locally advanced hepatocellular carcinoma with intrahepatic metastasis. Cancer Chemother. Pharmacol. 2013, 71, 165–173. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association; National Cancer Center. 2018 korean liver cancer association-national cancer center korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver 2019, 13, 227–299. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, H.J.; Im, Y.R.; Kim, D.Y.; Han, K.H.; Seong, J. Dose escalation in radiotherapy for incomplete transarterial chemoembolization of hepatocellular carcinoma. Strahlenther. Onkol. 2019. [Google Scholar] [CrossRef]
- Choi, C.; Koom, W.S.; Kim, T.H.; Yoon, S.M.; Kim, J.H.; Lee, H.S.; Nam, T.K.; Seong, J. A prospective phase 2 multicenter study for the efficacy of radiation therapy following incomplete transarterial chemoembolization in unresectable hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1051–1060. [Google Scholar] [CrossRef]
- Koo, J.E.; Kim, J.H.; Lim, Y.S.; Park, S.J.; Won, H.J.; Sung, K.B.; Suh, D.J. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 180–187. [Google Scholar] [CrossRef]
- Oh, D.; Lim, D.H.; Park, H.C.; Paik, S.W.; Koh, K.C.; Lee, J.H.; Choi, M.S.; Yoo, B.C.; Lim, H.K.; Lee, W.J.; et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: A prospective evaluation of efficacy and toxicity. Am. J. Clin. Oncol. 2010, 33, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Oh, D.; Park, H.C.; Lim, D.H.; Shin, S.W.; Cho, S.K.; Gwak, G.Y.; Choi, M.S.; Paik, Y.H.; Paik, S.W. Transcatheter arterial chemoembolization and radiation therapy for treatment-naive patients with locally advanced hepatocellular carcinoma. Radiat. Oncol. J. 2014, 32, 14–22. [Google Scholar] [CrossRef]
- Yoon, S.M.; Ryoo, B.Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.H.; Lee, H.C.; Lim, Y.S. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: A randomized clinical trial. JAMA Oncol. 2018, 4, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Keum, K.C.; Han, K.H.; Lee, D.Y.; Lee, J.T.; Chon, C.Y.; Moon, Y.M.; Suh, C.O.; Kim, G.E. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 393–397. [Google Scholar] [CrossRef]
- Yamada, K.; Izaki, K.; Sugimoto, K.; Mayahara, H.; Morita, Y.; Yoden, E.; Matsumoto, S.; Soejima, T.; Sugimura, K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 113–119. [Google Scholar] [CrossRef]
- Yoon, H.I.; Lee, I.J.; Han, K.H.; Seong, J. Improved oncologic outcomes with image-guided intensity-modulated radiation therapy using helical tomotherapy in locally advanced hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2014, 140, 1595–1605. [Google Scholar] [CrossRef]
- Han, K.H.; Seong, J.; Kim, J.K.; Ahn, S.H.; Lee, D.Y.; Chon, C.Y. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer 2008, 113, 995–1003. [Google Scholar] [CrossRef]
- Park, H.C.; Seong, J.; Han, K.H.; Chon, C.Y.; Moon, Y.M.; Suh, C.O. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 150–155. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, H.J.; Im, Y.R.; Kim, D.Y.; Han, K.H.; Seong, J. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced bclc stage c hepatocellular carcinoma. Radiother. Oncol. 2019, 133, 1–8. [Google Scholar] [CrossRef]
- Tse, R.V.; Hawkins, M.; Lockwood, G.; Kim, J.J.; Cummings, B.; Knox, J.; Sherman, M.; Dawson, L.A. Phase i study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2008, 26, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Seong, J.; Lee, I.J.; Woo, J.Y.; Han, K.H. Phase i dose escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget 2016, 7, 40756–40766. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, D.Y.; Han, K.H.; Seong, J. Phase i/ii trial of helical imrt-based stereotactic body radiotherapy for hepatocellular carcinoma. Dig. Liver Dis. 2019, 51, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Park, H.C.; Yoon, W.S.; Yoon, S.M.; Jung, I.H.; Lee, I.J.; Kim, J.W.; Seong, J.; Kim, T.H.; Nam, T.K.; et al. Treatment outcome after fractionated conformal radiotherapy for hepatocellular carcinoma in patients with child-pugh classification b in korea (krog 16-05). Cancer Res. Treat. 2019, 51, 1589–1599. [Google Scholar] [CrossRef]
- Milano, M.T.; Constine, L.S.; Okunieff, P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat. Oncol. 2008, 3, 36. [Google Scholar] [CrossRef]
- Guha, C.; Kavanagh, B.D. Hepatic radiation toxicity: Avoidance and amelioration. Semin. Radiat. Oncol. 2011, 21, 256–263. [Google Scholar] [CrossRef]
- Toyoda, H.; Lai, P.B.; O’Beirne, J.; Chong, C.C.; Berhane, S.; Reeves, H.; Manas, D.; Fox, R.P.; Yeo, W.; Mo, F.; et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: Application of the albi grade. Br. J. Cancer 2016, 114, 744–750. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the albi grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Su, T.S.; Yang, H.M.; Zhou, Y.; Huang, Y.; Liang, P.; Cheng, T.; Chen, L.; Li, L.Q.; Liang, S.X. Albumin—Bilirubin (albi) versus child-turcotte-pugh (ctp) in prognosis of hcc after stereotactic body radiation therapy. Radiat. Oncol. 2019, 14, 50. [Google Scholar] [CrossRef]
- Ronald, J.; Wang, Q.; Choi, S.S.; Suhocki, P.V.; Hall, M.D.; Smith, T.P.; Kim, C.Y. Albumin-bilirubin grade versus meld score for predicting survival after transjugular intrahepatic portosystemic shunt (tips) creation. Diagn. Interv. Imaging 2018, 99, 163–168. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhong, J.H.; Su, Z.Y.; Huang, J.F.; Lu, S.D.; Xiang, B.D.; Ma, L.; Qi, L.N.; Ou, B.N.; Li, L.Q. Albumin-bilirubin versus child-pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br. J. Surg. 2016, 103, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; George, K.; Engineer, R.; Rajamanickam, K.; Nojin, S.; Joshi, K.; Swamidas, J.; Shetty, N.; Patkar, S.; Patil, P.; et al. Stereotactic body radio therapy for inoperable large hepatocellular cancers: Results from a clinical audit. Br. J. Radiol. 2019, 92, 20181053. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, M.S.; Chang, J.S.; Han, K.H.; Kim, D.Y.; Seong, J. Therapeutic benefit of radiotherapy in huge (>/=10 cm) unresectable hepatocellular carcinoma. Liver Int. 2014, 34, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Fukumitsu, N.; Kamizawa, S.; Numajiri, H.; Nemoto Murofushi, K.; Ohnishi, K.; Aihara, T.; Ishikawa, H.; Okumura, T.; Tsuboi, K.; et al. A validated proton beam therapy patch-field protocol for effective treatment of large hepatocellular carcinoma. J. Radiat. Res. 2018, 59, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, S.; Oshiro, Y.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Matsuzaki, Y.; Thono, E.; Tokita, M.; et al. Proton beam therapy for large hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Yu, J.I.; Cheng, J.C.; Zeng, Z.C.; Hong, J.H.; Wang, M.L.; Kim, M.S.; Chi, K.H.; Liang, P.C.; Lee, R.C.; et al. Consensus for radiotherapy in hepatocellular carcinoma from the 5th asia-pacific primary liver cancer expert meeting (apple 2014): Current practice and future clinical trials. Liver Cancer 2016, 5, 162–174. [Google Scholar] [CrossRef]
- Kim, J.W.; Seong, J.; Park, M.S.; Kim, K.S.; Park, Y.N.; Han, K.H.; Keum, K.C.; Lee, I.J. Radiological-pathological correlation study of hepatocellular carcinoma undergoing local chemoradiotherapy and surgery. J. Gastroenterol. Hepatol. 2016, 31, 1619–1627. [Google Scholar] [CrossRef]
- Lee, I.J.; Seong, J.; Koom, W.S.; Kim, Y.B.; Jeon, B.C.; Kim, J.H.; Han, K.H. Selection of the optimal radiotherapy technique for locally advanced hepatocellular carcinoma. Jpn. J. Clin. Oncol. 2011, 41, 882–889. [Google Scholar] [CrossRef]

| Characteristics | Median | (Range) | No. of Patients (n = 17) | (%) |
|---|---|---|---|---|
| Age | 63 | (33–80) | ||
| Sex | ||||
| Male | 15 | (88.2) | ||
| Female | 2 | (11.8) | ||
| ECOG PS | ||||
| 0 | 8 | (47.1) | ||
| 1 | 9 | (52.9) | ||
| Underlying liver disease | ||||
| HBV | 12 | (70.6) | ||
| HCV | 1 | (5.9) | ||
| Without viral infections | 4 | (23.5) | ||
| Underlying liver cirrhosis | ||||
| No | 4 | 23.5 | ||
| Yes | 13 | 76.5 | ||
| AFP (ng/mL) | 45 | (2.2–38,300) | ||
| >9 ng/mL | 12 | 70.6 | ||
| PIVKA-II (mIU/mL) | 381.4 | (23–185,072) | ||
| >35 mIU/mL | 13 | 76.5 | ||
| Child-Pugh class | ||||
| A5 | 14 | 82.4 | ||
| A6 | 3 | 17.6 | ||
| Platelet count | 163 k | (55–408 k) | 5 | 29.4 |
| Mild thrombocytopenia (75–150 k/uL) | 3 | 15.8 | ||
| Moderate thrombocytopenia (50–75 k/μL) | 2 | 11.8 | ||
| UICC stage | ||||
| T2 | 2 | 11.8 | ||
| T3 | 8 | 47.1 | ||
| T4 | 7 | 41.2 | ||
| N0 | 16 | 94.1 | ||
| N1 | 1 | 5.9 | ||
| Primary tumor size (cm) | 8 | (2.6–16) | ||
| Number of tumor(s) | ||||
| 1 | 8 | 47.1 | ||
| 2–4 | 7 | 41.2 | ||
| ≥5 | 2 | 11.8 | ||
| Involved site | ||||
| Right Lobe | 11 | 64.7 | ||
| Left Lobe | 2 | 11.8 | ||
| Both Lobes | 4 | 23.5 | ||
| Vascular invasion | ||||
| No | 3 | 17.6 | ||
| Yes | 14 | 82.4 | ||
| Previous treatment | ||||
| None | 13 | 76.5 | ||
| TACE | 4 | 23.5 | ||
| TACI | 1 | 5.9 | ||
| RFA | 1 | 5.9 |
| Level 1 | Level 2 | Level 3 | Total | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 4) | (n = 6) | (n = 7) | (n = 17) | |||||
| Parameters | Median | (Range) | Median | (Range) | Median | (Range) | Median | (Range) |
| PTV1 (cc) | 398 | (277–467) | 490 | (69–2086) | 355 | (260–909) | 398 | (69–2086) |
| PTV2 (cc) | 819 | (561–2066) | 717 | (209–2814) | 758 | (525–1634) | 784 | (209–2814) |
| Uninvolved liver volume (cc) | 1018 | (876–1643) | 1138 | (814–1393) | 1176 | (855–1511) | 1122 | (814–1643) |
| Mean dose of whole liver (Gy) | 30.4 | (20.5–42.1) | 28.1 | (18.8–38.2) | 30.1 | (17.7–39.6) | 30.4 | (18.8–42.1) |
| Mean dose of uninvolved liver (Gy) | 21.3 | (15.35–27.4) | 21.6 | (19.2–25.7) | 18.2 | (11.5–24.4) | 20.4 | (11.5–27.4) |
| Maximum dose of stomach (Gy) | 42.6 | (20.3–55.4) | 27.9 | (15.1–54.0) | 51.2 | (26.8–56.3) | 40.9 | (15.1–56.3) |
| Maximum dose of duodenum (Gy) | 40.0 | (21.4–52.2) | 37.8 | (2.1–54.6) | 48.5 | (15.1–54.1) | 40 | (2.1–54.6) |
| Maximum dose of spinal cord (Gy) | 29.8 | (26.8–35.2) | 25.7 | (18.7–37.4) | 36.8 | (24.7–44.0) | 30.5 | (18.7–44.0) |
| Mean dose of right kidney (Gy) | 5.6 | (2.5–19.4) | 6.4 | (1.1–20.4) | 2.8 | (1.3–17.5) | 6.4 | (1.1–20.4) |
| Mean dose of left kidney (Gy) | 4.6 | (0.8–16.9) | 3.2 | (0.6–6.9) | 2.4 | (0.6–7.5) | 3.2 | (0.6–16.9) |
| Level 1 (n = 4) | Level 2 (n = 6) | Level 3 (n = 7) | Total (n = 17) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||||||||
| Toxicities | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| GI toxicity | Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 1 (5.9) | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pain | 1 (25) | 0 | 0 | 1 (16.7) | 1 (16.7) | 0 | 1 (14.3) | 0 | 0 | 3 (17.6) | 1 (5.9) | 0 | |
| Liver function | AST | 2 (50) | 1 (25) | 0 | 5 (83.3) | 0 | 0 | 6 (85.7) | 0 | 0 | 13 (76.5) | 1 (5.9) | 0 |
| ALT | 2 (50) | 0 | 0 | 0 | 0 | 0 | 4 (57.1) | 0 | 0 | 6 (35.3) | 0 | 0 | |
| Albumin | 3 (75) | 1 (25) | 0 | 4 (66.7) | 1 (16.7) | 0 | 4 (57.1) | 1 (14.3) | 0 | 11 (64.7) | 3 (17.6) | 0 | |
| Bilirubin | 0 | 0 | 1 (25) | 0 | 2 (33.3) | 0 | 0 | 0 | 1 (14.3) | 0 | 2 (11.8) | 2 (11.8) | |
| INR | 4 (100) | 0 | 0 | 6 (100) | 0 | 0 | 5 (71.4) | 0 | 0 | 15 (88.2) | 0 | 0 | |
| ALP | 2 (50) | 1 (25) | 0 | 5 (83.3) | 0 | 0 | 4 (57.1) | 3 (42.9) | 0 | 11 (64.7) | 4 (23.5) | 0 | |
| Hematologic | Hemoglobin | 2 (50) | 2 (50) | 0 | 2 (33.3) | 0 | 0 | 3 (42.9) | 0 | 1 (14.3) | 7 (41.2) | 2 (11.8) | 1 (5.9) |
| WBC | 1 (25) | 2 (50) | 0 | 2 (33.3) | 0 | 0 | 2 (28.6) | 3 (42.9) | 0 | 5 (29.4) | 5 (29.4) | 0 | |
| ANC | 2 (50) | 0 | 1 (25) | 1 (16.7) | 1 (16.7) | 0 | 0 | 4 (57.1) | 1 (14.3) | 3 (17.6) | 5 (29.4) | 2 (11.8) | |
| Platelet | 2 (50) | 1 (25) | 1 (25) | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (28.6) | 0 | 2 (11.8) | 4 (23.5) | 2 (11.8) | |
| Other | General weakness | 0 | 1 (25) | 0 | 3 (50) | 0 | 0 | 0 | 1 (14.3) | 0 | 3 (17.6) | 2 (11.8) | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Kim, J.W.; Kim, J.K.; Lee, K.S.; Lee, J.I.; Lee, H.W.; Lee, K.-H.; Joo, S.-M.; Lim, J.H.; Lee, I.J. Phase I Radiation Dose-Escalation Study to Investigate the Dose-Limiting Toxicity of Concurrent Intra-Arterial Chemotherapy for Unresectable Hepatocellular Carcinoma. Cancers 2020, 12, 1612. https://doi.org/10.3390/cancers12061612
Cho Y, Kim JW, Kim JK, Lee KS, Lee JI, Lee HW, Lee K-H, Joo S-M, Lim JH, Lee IJ. Phase I Radiation Dose-Escalation Study to Investigate the Dose-Limiting Toxicity of Concurrent Intra-Arterial Chemotherapy for Unresectable Hepatocellular Carcinoma. Cancers. 2020; 12(6):1612. https://doi.org/10.3390/cancers12061612
Chicago/Turabian StyleCho, Yeona, Jun Won Kim, Ja Kyung Kim, Kwan Sik Lee, Jung Il Lee, Hyun Woong Lee, Kwang-Hun Lee, Seung-Moon Joo, Jin Hong Lim, and Ik Jae Lee. 2020. "Phase I Radiation Dose-Escalation Study to Investigate the Dose-Limiting Toxicity of Concurrent Intra-Arterial Chemotherapy for Unresectable Hepatocellular Carcinoma" Cancers 12, no. 6: 1612. https://doi.org/10.3390/cancers12061612
APA StyleCho, Y., Kim, J. W., Kim, J. K., Lee, K. S., Lee, J. I., Lee, H. W., Lee, K.-H., Joo, S.-M., Lim, J. H., & Lee, I. J. (2020). Phase I Radiation Dose-Escalation Study to Investigate the Dose-Limiting Toxicity of Concurrent Intra-Arterial Chemotherapy for Unresectable Hepatocellular Carcinoma. Cancers, 12(6), 1612. https://doi.org/10.3390/cancers12061612





