Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment
Abstract
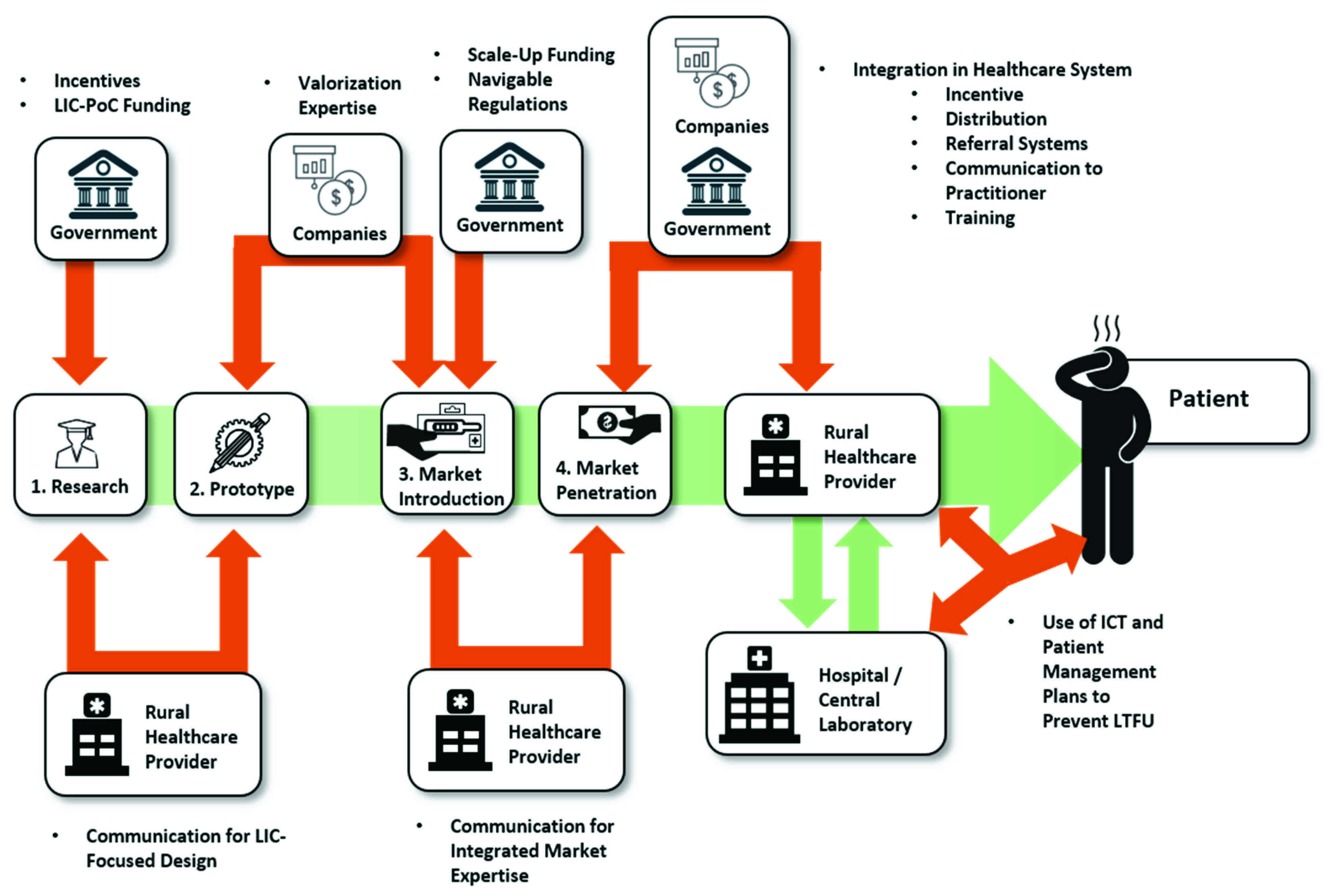
1. Introduction
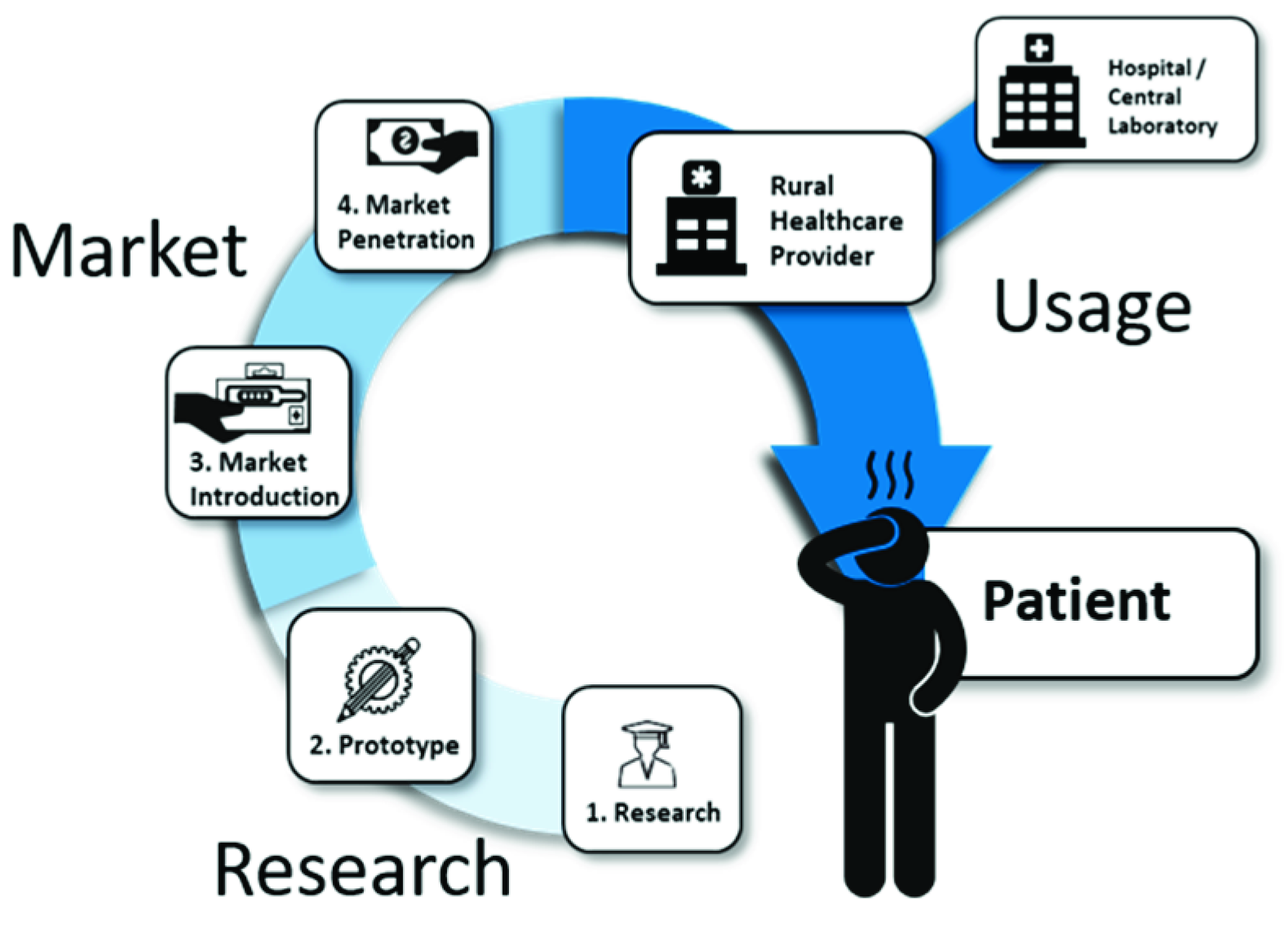
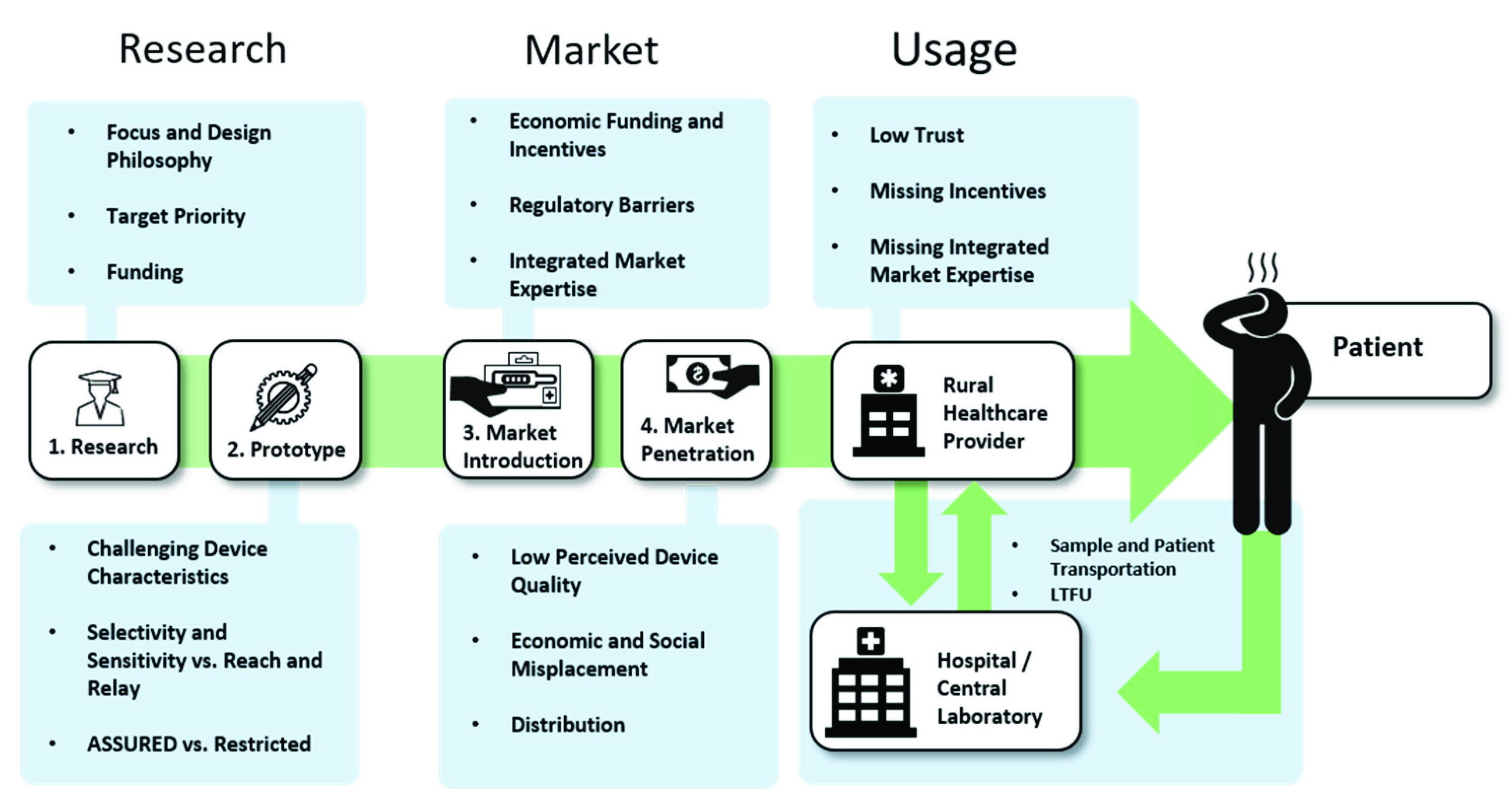
2. Research
2.1. Fundamental Research: Funding Availability and Focus
2.1.1. Choosing a Suitable Design Philosophy
2.1.2. Taking Aim: Proper Target Analytes
2.1.3. Funding in LICs
2.1.4. Incentives to Change Focus in HICs
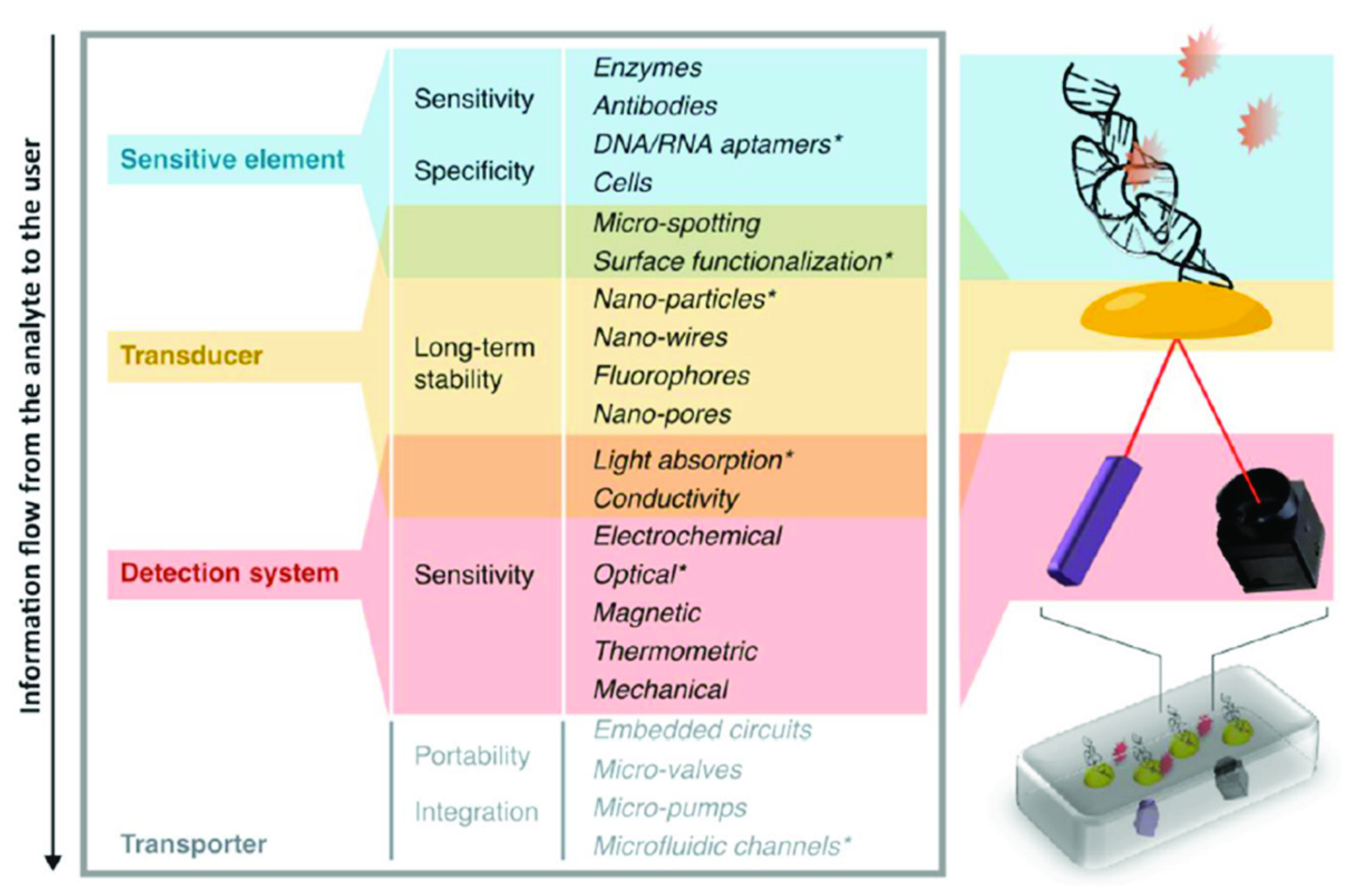
2.2. Proof-of-Concept and Prototypes: The Importance of Appropriate Device Characteristics.
2.2.1. Device Characteristics
2.2.2. Being ASSURED
2.2.3. How Necessary Is It to Be ASSURED?
2.2.4. Steps towards an Effectively Usable PoC Device
3. The Market
3.1. Market Introduction
3.1.1. Funding and IP
3.1.2. Regulations
3.1.3. Integrated Market Expertise
3.2. Market Penetration
3.2.1. Device Quality
3.2.2. Economic and Social Placement
3.2.3. Product Distribution
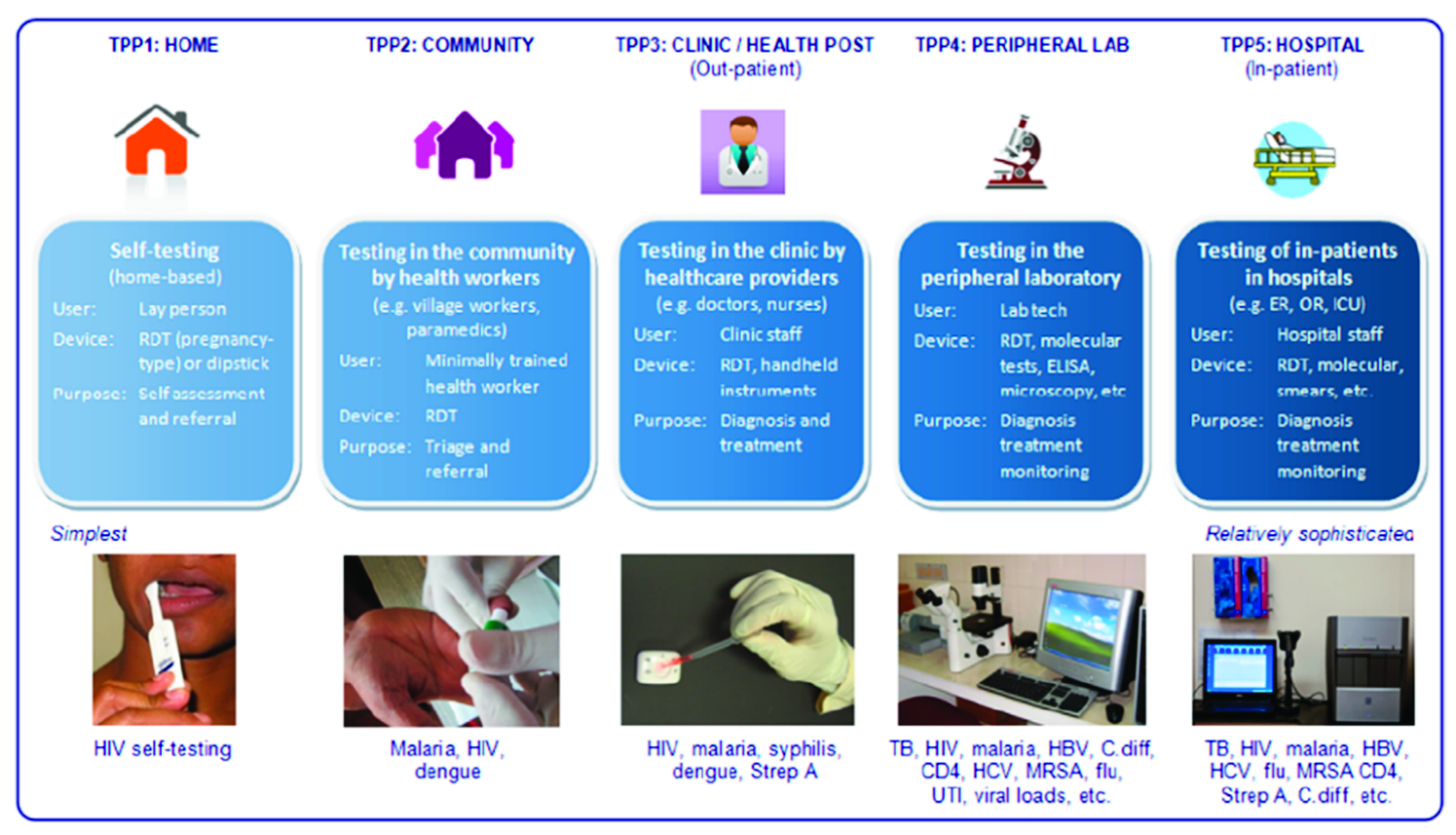
4. The Usage
4.1. Healthcare Management
4.2. Patient Management
4.3. Training
4.3.1. Use by Trained Doctors
4.3.2. View on PoC
5. Conclusions
5.1. Main Findings
5.2. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Boutayeb, A. The impact of infectious diseases on the development of Africa. In The Handbook of Disease Burdens and Quality of Life Measures; Preedy, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010; pp. 1171–1188. ISBN 978-0-387-78664-3. [Google Scholar]
- Kuupiel, D.; Bawontuo, V.; Drain, P.K.; Gwala, N.; Mashamba-Thompson, T.P. Supply chain management and accessibility to point-of-care testing in resource-limited settings: A systematic scoping review. BMC Heal. Serv. Res. 2019, 19, 519. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Graef, K.M.; Dent, J. Fostering innovative product development for neglected tropical diseases through partnerships. Pharm. Pat. Anal. 2016, 5, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Migliozzi, D.; Guibentif, T. Assessing the potential deployment of biosensors for point-of-care diagnostics in developing countries: Technological, economic and regulatory aspects. Biosensors 2018, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low-and middle-income countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef]
- Sia, S.K.; Kricka, L.J. Microfluidics and point-of-care testing. Lab Chip 2008, 8, 1982–1983. [Google Scholar] [CrossRef]
- Kozel, T.R.; Burnham-Marusich, A.R. Point-of-care testing for infectious diseases: Past, present, and future. J. Clin. Microbiol. 2017, 55, 2313–2320. [Google Scholar] [CrossRef]
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S.A. Comparison of optical, electrochemical, magnetic, and colorimetric point-of-care biosensors for infectious disease diagnosis. ACS Infect. Dis. 2018, 4, 1162–1178. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Eersels, K.; Rogosic, R.; Boonen, T.; Heidt, B.; Diliën, H.; Van Grinsven, B.; Cleij, T.J. Surface grafted molecularly imprinted polymeric receptor layers for thermal detection of the new psychoactive substance 2-methoxphenidine. Sens. Actuators 2019, 295, 586–595. [Google Scholar] [CrossRef]
- Vandenryt, T.; Van Grinsven, B.; Eersels, K.; Cornelis, P.; Kholwadia, S.; Cleij, T.J.; Thoelen, R.; De Ceuninck, W.; Peeters, M.; Wagner, P. Single-shot detection of neurotransmitters in whole-blood samples by means of the heat-transfer method in combination with synthetic receptors. Sensors 2017, 17, 2701. [Google Scholar] [CrossRef]
- Takemura, K.; Adegoke, O.; Takahashi, N.; Kato, T.; Li, T.-C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Versatility of a localized surface plasmon resonance-based gold nanoparticle-alloyed quantum dot nanobiosensor for immunofluorescence detection of viruses. Biosens. Bioelectron. 2017, 89, 998–1005. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Eersels, K.; Rogosic, R.; Heidt, B.; Diliën, H.; Redeker, E.S.; Peeters, M.; Van Grinsven, B.; Cleij, T.J. Substrate displacement colorimetry for the detection of diarylethylamines. Sens. Actuators 2019, 282, 137–144. [Google Scholar] [CrossRef]
- Xiong, L.-H.; Cui, R.; Zhang, Z.-L.; Yu, X.; Xie, Z.; Shi, Y.-B.; Pang, D.-W. Uniform fluorescent nanobioprobes for pathogen detection. ACS Nano 2014, 8, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Tang, X.; Bagarozzi, D.A.; Pan, D.; Locascio, L.; Walker, A.; Barron, F.E.; et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Molecular biosensors for electrochemical detection of infectious pathogens in liquid biopsies: Current trends and challenges. Sensors 2017, 17, 2533. [Google Scholar] [CrossRef]
- Cecchetto, J.; Fernandes, F.C.B.; Lopes, R.; Bueno, P.R. The capacitive sensing of NS1 Flavivirus biomarker. Biosens. Bioelectron. 2017, 87, 949–956. [Google Scholar] [CrossRef]
- Hsieh, K.; Ferguson, B.S.; Eisenstein, M.; Plaxco, K.W.; Soh, H.T. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc. Chem. Res. 2015, 48, 911–920. [Google Scholar] [CrossRef]
- Park, J.Y.; Kricka, L.J. Prospects for nano-and microtechnologies in clinical point-of-care testing. Lab Chip 2007, 7, 547–549. [Google Scholar] [CrossRef]
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the accessibility and efficiency of point-of-care diagnostics services in low-and middle-income countries: Lean and agile supply chain management. Diagnostics 2017, 7, 58. [Google Scholar] [CrossRef]
- Wang, S.; Lifson, M.A.; Inci, F.; Liang, L.-G.; Sheng, Y.-F.; Demirci, U. Advances in addressing technical challenges of point-of-care diagnostics in resource-limited settings. Expert Rev. Mol. Diagn. 2016, 16, 449–459. [Google Scholar] [CrossRef]
- Urdea, M.; Penny, L.A.; Olmsted, S.S.; Giovanni, M.Y.; Kaspar, P.; Shepherd, A.; Wilson, P.; Dahl, C.A.; Buchsbaum, S.; Moeller, G.; et al. Requirements for high impact diagnostics in the developing world. Nature 2006, 444 (Suppl. 1), 73–79. [Google Scholar] [CrossRef]
- Tayoun, A.N.A.; Ward, B.P.; Maltezos, G.; Scherer, A.; Tsongalis, G.J. Evaluating the thermostability of commercial fast real-time PCR master mixes. Exp. Mol. Pathol. 2012, 93, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y. Analysis of research intensity on infectious disease by disease burden reveals which infectious diseases are neglected by researchers. Proc. Natl. Acad. Sci. USA 2019, 116, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.M.; Luo, N.; Tembo, G.; Halwiindi, B.; Steenbergen, G.; Machiels, L.; Pobee, J.; Nunn, P.; Hayes, R.; McAdam, K.P. Impact of HIV on tuberculosis in Zambia: A cross sectional study. BMJ 1990, 301, 412–415. [Google Scholar] [CrossRef] [PubMed]
- McNerney, R. Diagnostics for developing countries. Diagnostics 2015, 5, 200–209. [Google Scholar] [CrossRef]
- Hecht, R.; Stover, J.; Bollinger, L.; Muhib, F.; Case, K.K.; De Ferranti, D. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009–2031. Lancet 2010, 376, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Kimani, F.W.; Mwangi, S.M.; Kwasa, B.; Kusow, A.M.; Ngugi, B.; Chen, J.; Liu, X.; Cademartiri, R.; Thuo, M.M. Rethinking the design of low-cost point-of-care diagnostic devices. Micromachines 2017, 8, 317. [Google Scholar] [CrossRef]
- Expedited Programs for Serious Conditions––Drugs and Biologics. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-serious-conditions-drugs-and-biologics (accessed on 9 July 2020).
- Ridley, D.B.; Régnier, S.A. The commercial market for priority review vouchers. Health Aff. 2016, 35, 776–783. [Google Scholar] [CrossRef]
- Slingsby, B.; Kurokawa, K. The Global Health Innovative Technology (GHIT) Fund: Financing medical innovations for neglected populations. Lancet Glob. Health 2013, 1, e184–e185. [Google Scholar] [CrossRef]
- Bessa, T.C.B.; De Aragão, E.S.; Guimarães, J.M.M.; Almeida, B.D.A. R&D in vaccines targeting neglected diseases: An exploratory case study considering funding for preventive tuberculosis vaccine development from 2007 to 2014. Biomed Res. Int. 2017, 2017, 4765719. [Google Scholar] [CrossRef]
- Mercer, M.A.; Thompson, S.M.; De Araujo, R.M. The role of international NGOs in health systems strengthening: The case of Timor-Leste. Int. J. Health Serv. 2014, 44, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Bhan, M.K.; Chopra, M.; Rudan, I.; Victora, C.G. Accelerating the health impact of the Gates Foundation. Lancet 2009, 373, 1584–1585. [Google Scholar] [CrossRef]
- Guha-Sapir, D. The Gates Foundation: Looking at the bigger picture. Lancet 2009, 374, 201–202. [Google Scholar] [CrossRef]
- McCoy, D.C.; Kembhavi, G.; Patel, J.; Luintel, A. The Bill & Melinda Gates Foundation’s grant-making programme for global health. Lancet 2009, 373, 1645–1653. [Google Scholar] [CrossRef]
- Rao, B.C. The science underlying frugal innovations should not be frugal. R. Soc. Open Sci. 2019, 6, 180421. [Google Scholar] [CrossRef]
- Prabhu, J. Frugal innovation: Doing more with less for more. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160372. [Google Scholar] [CrossRef]
- Tran, V.-T.; Ravaud, P. Frugal innovation in medicine for low resource settings. BMC Med. 2016, 14, 102. [Google Scholar] [CrossRef]
- Howitt, P.; Darzi, A.; Yang, G.-Z.; Ashrafian, H.; Atun, R.; Barlow, J.; Blakemore, A.; Bull, A.M.J.; Car, J.; Conteh, L.; et al. Technologies for global health. Lancet 2012, 380, 507–535. [Google Scholar] [CrossRef]
- Heidt, B.; Rogosic, R.; Bonni, S.; Jansen, J.P.; Dimech, D.; Lowdon, J.W.; Arreguin-Campos, R.; Redeker, E.S.; Eersels, K.; Diliën, H.; et al. The liberalization of microfluidics: Form 2 benchtop 3D printing as an affordable alternative to established manufacturing methods. Phys. Status Solidi (A) 2020. [Google Scholar] [CrossRef]
- Kleinman, Z. Coronavirus: 3D Printers Save Hospital with Valves. BBC News [Online], 16 March 2020. Available online: https://www.bbc.com/news/technology-51911070 (accessed on 12 July 2020).
- Tayoun, A.N.A.; Burchard, P.R.; Malik, I.; Scherer, A.; Tsongalis, G.J. Democratizing molecular diagnostics for the developing world. Am. J. Clin. Pathol. 2014, 141, 17–24. [Google Scholar] [CrossRef]
- Zachariah, R.; Reid, S.D.; Chaillet, P.; Massaquoi, M.; Schouten, E.J.; Harries, A.D. Viewpoint: Why do we need a point-of-care CD4 test for low-income countries? Trop. Med. Int. Health 2011, 16, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Mashamba-Thompson, T.P.; Jama, N.A.; Sartorius, B.; Drain, P.K.; Thompson, R.M. Implementation of point-of-care diagnostics in rural primary healthcare clinics in South Africa: Perspectives of key stakeholders. Diagnostics 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Toskin, I.; Murtagh, M.; Peeling, R.W.; Blondeel, K.; Cordero, J.P.; Kiarie, J. Advancing prevention of sexually transmitted infections through point-of-care testing: Target product profiles and landscape analysis. Sex. Transm. Infect. 2017, 93, S69–S80. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Holmes, K.K.; Mabey, D. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82, v1–v6. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D.; Herring, A.; Hook, E.W. Why do we need quality-assured diagnostic tests for sexually transmitted infections? Nat. Rev. Genet. 2006, 4, S7–S19. [Google Scholar] [CrossRef]
- Rasti, R.; Nanjebe, D.; Karlström, J.; Muchunguzi, C.; Mwanga-Amumpaire, J.; Gantelius, J.; Mårtensson, A.; Rivas, L.; Galban, F.; Reuterswärd, P.; et al. Health care workers’ perceptions of point-of-care testing in a low-income country-A qualitative study in Southwestern Uganda. PLoS ONE 2017, 12, e0182005. [Google Scholar] [CrossRef]
- Duchesne, L.; Hejblum, G.; Kane, N.C.T.; Njouom, R.; Toni, T.-D.; Moh, R.; Sylla, B.; Rouveau, N.; Attia, A.; Lacombe, K. Model-based cost-effectiveness estimates of testing strategies for diagnosing hepatitis C virus infection in people who use injecting drugs in Senegal. Int. J. Drug Policy 2020, 75, 102613. [Google Scholar] [CrossRef]
- Gift, T.L.; Pate, M.S.; Hook, E.W.; Kassler, W.J. The rapid test paradox: When fewer cases detected lead to more cases treated: A decision analysis of tests for Chlamydia trachomatis. Sex. Transm. Dis. 1999, 26, 232–240. [Google Scholar] [CrossRef]
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics based point-of-care diagnostics. Biotechnol. J. 2017, 13, 1700047. [Google Scholar] [CrossRef]
- Azhar, M.; Dendukuri, D. Microfluidic platforms for point of care (POC) medical diagnostics. In Medical Biosensors for Point of Care (POC) Applications; Narayan, R., Ed.; Woodhead Publishing is an Imprint of Elsevier: Duxford, UK, 2016; pp. 255–273. ISBN 9780081000724. [Google Scholar]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab. Chip. 2012, 12, 2118–2134. [Google Scholar] [CrossRef]
- Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O’Kennedy, R. Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, A.; Keefe, J.; Franco-Waite, L.; Konnaiyan, K.R.; Pyayt, A. Mobile phone based ELISA (MELISA). Biosens. Bioelectron. 2018, 103, 138–142. [Google Scholar] [CrossRef]
- Archibong, E.; Konnaiyan, K.R.; Kaplan, H.; Pyayt, A. A mobile phone-based approach to detection of hemolysis. Biosens. Bioelectron. 2017, 88, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, D.; Merkoçi, A. Mobile phone-based biosensing: An emerging “diagnostic and communication” technology. Biosens. Bioelectron. 2017, 92, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.C.; Yao, C.; Venkatesh, A.G.; Hall, D.A. An efficient power harvesting mobile phone-based electrochemical biosensor for point-of-care health monitoring. Sens. Actuators 2016, 235, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.L.; Hogan, C.F. Mobile phone based electrochemiluminescence detection in paper-based microfluidic sensors. Methods. Mol. Biol. 2015, 1256, 277–289. [Google Scholar] [CrossRef]
- Sarvestani, A.S.; Sienko, K. Medical device landscape for communicable and noncommunicable diseases in low-income countries. Glob. Health 2018, 14, 65. [Google Scholar] [CrossRef]
- Parvizi, N.; Woods, K. Regulation of medicines and medical devices: Contrasts and similarities. Clin. Med. 2014, 14, 6–12. [Google Scholar] [CrossRef]
- Basu, S.; Hassenplug, J.C. Patient access to medical devices—A comparison of US and European review processes. N. Engl. J. Med. 2012, 367, 485–488. [Google Scholar] [CrossRef]
- Ramamoorthi, R.; Graef, K.M.; Dent, J. WIPO Re:Search: Accelerating anthelmintic development through cross-sector partnerships. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 220–225. [Google Scholar] [CrossRef][Green Version]
- Manner, C.K.; Graef, K.M.; Dent, J. WIPO Re:Search: Catalyzing Public-Private Partnerships to Accelerate Tropical Disease Drug Discovery and Development. Trop. Med. Infect. Dis. 2019, 4, 53. [Google Scholar] [CrossRef]
- Peeling, R. Bringing diagnostics to developing countries: An interview with Rosanna Peeling. Expert Rev. Mol. Diagn. 2015, 15, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Jarosławski, S.; Pai, M. Why are inaccurate tuberculosis serological tests widely used in the Indian private healthcare sector? A root-cause analysis. J. Epidemiol. Glob. Health 2012, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Baloch, N.A.; Pai, M. Tuberculosis control: Business models for the private sector. Lancet Infect. Dis. 2012, 12, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Marseille, E.; Larson, B.A.; Kazi, D.S.; Kahn, J.G.; Rosen, S.B. Thresholds for the cost–effectiveness of interventions: Alternative approaches. Bull. World Health Organ. 2015, 93, 118–124. [Google Scholar] [CrossRef]
- Campos, N.G.; Tsu, V.D.; Jeronimo, J.; Mvundura, M.; Kim, J.J. Estimating the value of point-of-care HPV testing in three low-and middle-income countries: A modeling study. BMC Cancer 2017, 17, 791. [Google Scholar] [CrossRef]
- Simeon, K.; Sharma, M.; Dorward, J.; Naidoo, J.; Dlamini, N.; Moodley, P.; Samsunder, N.; Barnabas, R.V.; Garrett, N.; Drain, P.K. Comparative cost analysis of point-of-care versus laboratory-based testing to initiate and monitor HIV treatment in South Africa. PLoS ONE 2019, 14, e0223669. [Google Scholar] [CrossRef]
- Phillips, A.; Cambiano, V.; Nakagawa, F.; Ford, D.; Apollo, T.; Murungu, J.; Rousseau, C.; Garnett, G.; Ehrenkranz, P.; Bansi-Matharu, L.; et al. Point-of-care viral load testing for Sub-Saharan Africa: Informing a target product profile. Open Forum Infect. Dis. 2016, 3, ofw161. [Google Scholar] [CrossRef]
- Hyle, E.P.; Jani, I.; Lehe, J.; Su, A.E.; Wood, R.; Quevedo, J.; Losina, E.; Bassett, I.V.; Pei, P.P.; Paltiel, A.D.; et al. The clinical and economic impact of point-of-care CD4 testing in Mozambique and other resource-limited settings: A cost-effectiveness analysis. PLoS Med. 2014, 11, e1001725. [Google Scholar] [CrossRef]
- Estill, J.; Egger, M.; Blaser, N.; Salazar-Vizcaya, L.; Garone, D.; Wood, R.; Campbell, J.; Hallett, T.B.; Keiser, O.; Africa, I.S. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: Mathematical modelling study. AIDS 2013, 27, 1483–1492. [Google Scholar] [CrossRef]
- Nichols, B.E.; Girdwood, S.J.; Crompton, T.; Stewart-Isherwood, L.; Berrie, L.; Chimhamhiwa, D.; Moyo, C.; Kuehnle, J.; Stevens, W.; Rosen, S.; et al. Monitoring viral load for the last mile: What will it cost? J. Int. Aids Soc. 2019, 22, e25337. [Google Scholar] [CrossRef] [PubMed]
- Girdwood, S.J.; Nichols, B.E.; Moyo, C.; Crompton, T.; Chimhamhiwa, D.; Rosen, S. Optimizing viral load testing access for the last mile: Geospatial cost model for point of care instrument placement. PLoS ONE 2019, 14, e0221586. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Sabidó, M.; Camey, E.; Batres, A.; Casabona, J. Lessons learned from integrating simultaneous triple point-of-care screening for syphilis, hepatitis B, and HIV in prenatal services through rural outreach teams in Guatemala. Int. J. Gynecol. Obstet. 2015, 130, S70–S72. [Google Scholar] [CrossRef]
- Yao, K.; Wafula, W.; Bile, E.; Cheignsong, R.; Howard, S.; Demby, A.; Nkengasong, J. Ensuring the quality of HIV rapid testing in resource-poor countries using a systematic approach to training. Am. J. Clin. Pathol. 2010, 134, 568–572. [Google Scholar] [CrossRef]
- Engel, N.; Wachter, K.; Pai, M.; Gallarda, J.; Boehme, C.; Celentano, I.; Weintraub, R. Addressing the challenges of diagnostics demand and supply: Insights from an online global health discussion platform. BMJ Glob. Health 2016, 1, e000132. [Google Scholar] [CrossRef]
- Ackerman, E.; Strickland, E. Medical delivery drones take flight in East Africa. IEEE Spectr. 2018, 55, 34–35. [Google Scholar] [CrossRef]
- Ackerman, E.; Koziol, M. The blood is here: Zipline’s medical delivery drones are changing the game in Rwanda. IEEE Spectr. 2019, 56, 24–31. [Google Scholar] [CrossRef]
- Rustici, C. Drone Start-up Zipline is Helping Fight Covid-19 in Africa. MedicalExpo e-Magazine [Online], 13 June 2020. Available online: http://emag.medicalexpo.com/drone-start-up-zipline-is-helping-fight-covid-19-in-africa/ (accessed on 22 June 2020).
- Lewis, N. Zipline is Delivering COVID-19 Medical Supplies to Rural Ghana and Rwanda by Drone- Business Insider: A Tech Company Engineered Drones to Deliver Vital COVID-19 Medical Supplies to Rural Ghana and Rwanda in Minutes. Business Insider [Online], 12 May 2020. Available online: https://www.businessinsider.com/zipline-drone-coronavirus-supplies-africa-rwanda-ghana-2020-5?international=true&r=US&IR=T (accessed on 22 June 2020).
- Scott, J.E. Drone delivery models for healthcare. In Proceedings of the 50thHawaii International Conference on System Sciences 2017 (HICSS-50), Hilton Waikoloa Village, Hawaii, HI, USA, 4–7 January 2017; ISBN 9780998133102. [Google Scholar]
- Kaindjee-Tjituka, F.; Sawadogo, S.; Mutandi, G.; Maher, A.D.; Salomo, N.; Mbapaha, C.; Neo, M.; Beukes, A.; Gweshe, J.; Muadinohamba, A.; et al. Task-shifting point-of-care CD4+ testing to lay health workers in HIV care and treatment services in Namibia. Afr. J. Lab. Med. 2017, 6, 643. [Google Scholar] [CrossRef]
- Hsiao, N.-Y.; Stinson, K.; Myer, L. Linkage of HIV-infected infants from diagnosis to antiretroviral therapy services across the Western Cape, South Africa. PLoS ONE 2013, 8, e55308. [Google Scholar] [CrossRef]
- Ciaranello, A.L.; Park, J.-E.; Ramirez-Avila, L.; Freedberg, K.A.; Walensky, R.P.; Leroy, V. Early infant HIV-1 diagnosis programs in resource-limited settings: Opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011, 9, 59. [Google Scholar] [CrossRef]
- Dunning, L.; Hsiao, N.-Y.; Myer, L. Point-of-care HIV early infant diagnosis: Is test sensitivity everything? J. Int. Aids Soc. 2015, 18, 20235. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, Z.; Fajardo, E.; Mbofana, E.; Maparo, T.; Garone, D.; Metcalf, C.; Bygrave, H.; Kao, K.; Zinyowera, S. Multidisease testing for HIV and TB using the GeneXpert platform: A feasibility study in rural Zimbabwe. PLoS ONE 2018, 13, e0193577. [Google Scholar] [CrossRef] [PubMed]
- Zeh, C.; Rose, C.E.; Inzaule, S.; Desai, M.A.; Otieno, F.; Humwa, F.; Akoth, B.; Omolo, P.; Chen, R.T.; Kebede, Y.; et al. Laboratory-based performance evaluation of PIMA CD4+ T-lymphocyte count point-of-care by lay-counselors in Kenya. J. Immunol. Methods 2017, 448, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, N.-Y.; Dunning, L.; Kroon, M.; Myer, L. Laboratory evaluation of the alere q point-of-care system for early infant HIV diagnosis. PLoS ONE 2016, 11, e0152672. [Google Scholar] [CrossRef] [PubMed]
- Haenssgen, M.J.; Charoenboon, N.; Althaus, T.; Greer, R.; Intralawan, D.; Lubell, Y. The social role of C-reactive protein point-of-care testing to guide antibiotic prescription in Northern Thailand. Soc. Sci. Med. 2018, 202, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Johnson, F.A.; Frempong-Ainguah, F.; Nyarko, P.; Baschieri, A.; Aboagye, P.; Falkingham, J.; Matthews, Z.; Atkinson, P.M. Geographical access to care at birth in Ghana: A barrier to safe motherhood. BMC Public Health 2012, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Tayler-Smith, K.; Zachariah, R.; Massaquoi, M.; Manzi, M.; Pasulani, O.; Akker, T.V.D.; Bemelmans, M.; Bauernfeind, A.; Mwagomba, B.; Harries, A.D.D. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: Can we retain such patients within the general health system? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.; Fidler, S.J.; Cooke, G.S. Tracking the progress of HIV: The impact of point-of-care tests on antiretroviral therapy. Clin. Epidemiol. 2013, 5, 387–396. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Nene, B.M.; Shastri, S.S.; Jayant, K.; Muwonge, R.; Budukh, A.M.; Hingmire, S.; Malvi, S.G.; Thorat, R.; Kothari, A.; et al. HPV screening for cervical cancer in rural India. N. Engl. J. Med. 2009, 360, 1385–1394. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Muse, E.D.; Topol, E.J. The emerging field of mobile health. Sci. Transl. Med. 2015, 7, 283rv3. [Google Scholar] [CrossRef]
- Abaza, H.; Marschollek, M. mHealth application areas and technology combinations. A comparison of literature from high and low/middle income countries. Methods Inf. Med. 2017, 56, e105–e122. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Duclos, V.; Ye, M.; Kagoné, M.; Sanou, H.; Sawadogo, N.H.; Bibeau, G.; Sié, A. Situating mobile health: A qualitative study of mHealth expectations in the rural health district of Nouna, Burkina Faso. Health Res. Policy Syst. 2017, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Malkin, R. Effectiveness of medical equipment donations to improve health systems: How much medical equipment is broken in the developing world? Med. Biol. Eng. Comput. 2011, 49, 719–722. [Google Scholar] [CrossRef]
- Pham, M.D.; Agius, P.A.; Romero, L.; McGlynn, P.; Anderson, D.; Crowe, S.M.; Luchters, S. Acceptability and feasibility of point-of-care CD4 testing on HIV continuum of care in low and middle income countries: A systematic review. BMC Health Serv. Res. 2016, 16, 343. [Google Scholar] [CrossRef]
- Global Health Workforce Alliance; WHO. The Cost-Effectiveness of Close-to-Community Health Programmes: What do We Know and Where Are the Gaps? Available online: https://www.who.int/workforcealliance/knowledge/resources/cost_effectiveness_brief/en/ (accessed on 21 August 2020).
- WHO. Treat. Train. Retain-Task Shifting: Global Recommendations and Guidelines; WHO Document Production Services: Geneva, Switzerland, 2008; ISBN 978-92-4-159631-2. [Google Scholar]
- Scheffler, R.M.; Liu, J.X.; Kinfu, Y.; Poz, M.R.D. Forecasting the global shortage of physicians: An economic-and needs-based approach. Bull. World Health Organ. 2008, 86, 516–523. [Google Scholar] [CrossRef]
- Ritchie, L.M.P.; Van Lettow, M.; Makwakwa, A.; Chan, A.K.; Hamid, J.S.; Kawonga, H.; Martiniuk, A.; Schull, M.J.; Van Schoor, V.; Zwarenstein, M.; et al. The impact of a knowledge translation intervention employing educational outreach and a point-of-care reminder tool vs standard lay health worker training on tuberculosis treatment completion rates: Study protocol for a cluster randomized controlled trial. Trials 2016, 17, 439. [Google Scholar] [CrossRef]
- Ritchie, L.M.P.; Van Lettow, M.; Barnsley, J.; Chan, A.K.; Joshua, M.; Martiniuk, A.; Schull, M.J.; Zwarenstein, M. Evaluation of lay health workers’ needs to effectively support anti-tuberculosis treatment adherence in Malawi. Int. J. Tuberc. Lung Dis. 2012, 16, 1492–1497. [Google Scholar] [CrossRef]
- Schroeder, L.F.; Elbireer, A.; Jackson, J.B.; Amukele, T.K. Laboratory diagnostics market in East Africa: A survey of test types, test availability, and test prices in Kampala, Uganda. PLoS ONE 2015, 10, e0134578. [Google Scholar] [CrossRef]
- Mabey, D.; Sollis, K.A.; Kelly, H.A.; Benzaken, A.S.; Bitarakwate, E.; Changalucha, J.; Chen, X.-S.; Yin, Y.-P.; Garcia, P.J.; Strasser, S.; et al. Point-of-care tests to strengthen health systems and save newborn lives: The case of Syphilis. PloS Med. 2012, 9, e1001233. [Google Scholar] [CrossRef]
- Klatman, E.L.; Jenkins, A.J.; Ahmedani, M.Y.; Ogle, G.D. Blood glucose meters and test strips: Global market and challenges to access in low-resource settings. Lancet Diabetes Endocrinol. 2019, 7, 150–160. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidt, B.; Siqueira, W.F.; Eersels, K.; Diliën, H.; van Grinsven, B.; Fujiwara, R.T.; Cleij, T.J. Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment. Biosensors 2020, 10, 133. https://doi.org/10.3390/bios10100133
Heidt B, Siqueira WF, Eersels K, Diliën H, van Grinsven B, Fujiwara RT, Cleij TJ. Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment. Biosensors. 2020; 10(10):133. https://doi.org/10.3390/bios10100133
Chicago/Turabian StyleHeidt, Benjamin, Williane F. Siqueira, Kasper Eersels, Hanne Diliën, Bart van Grinsven, Ricardo T. Fujiwara, and Thomas J. Cleij. 2020. "Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment" Biosensors 10, no. 10: 133. https://doi.org/10.3390/bios10100133
APA StyleHeidt, B., Siqueira, W. F., Eersels, K., Diliën, H., van Grinsven, B., Fujiwara, R. T., & Cleij, T. J. (2020). Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment. Biosensors, 10(10), 133. https://doi.org/10.3390/bios10100133





