A Self-Referenced Diffraction-Based Optical Leaky Waveguide Biosensor Using Photofunctionalised Hydrogels
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Diffraction-Based LWs and Their Photopatterning
2.3. Preparation of Solutions for Characterisation of LWs
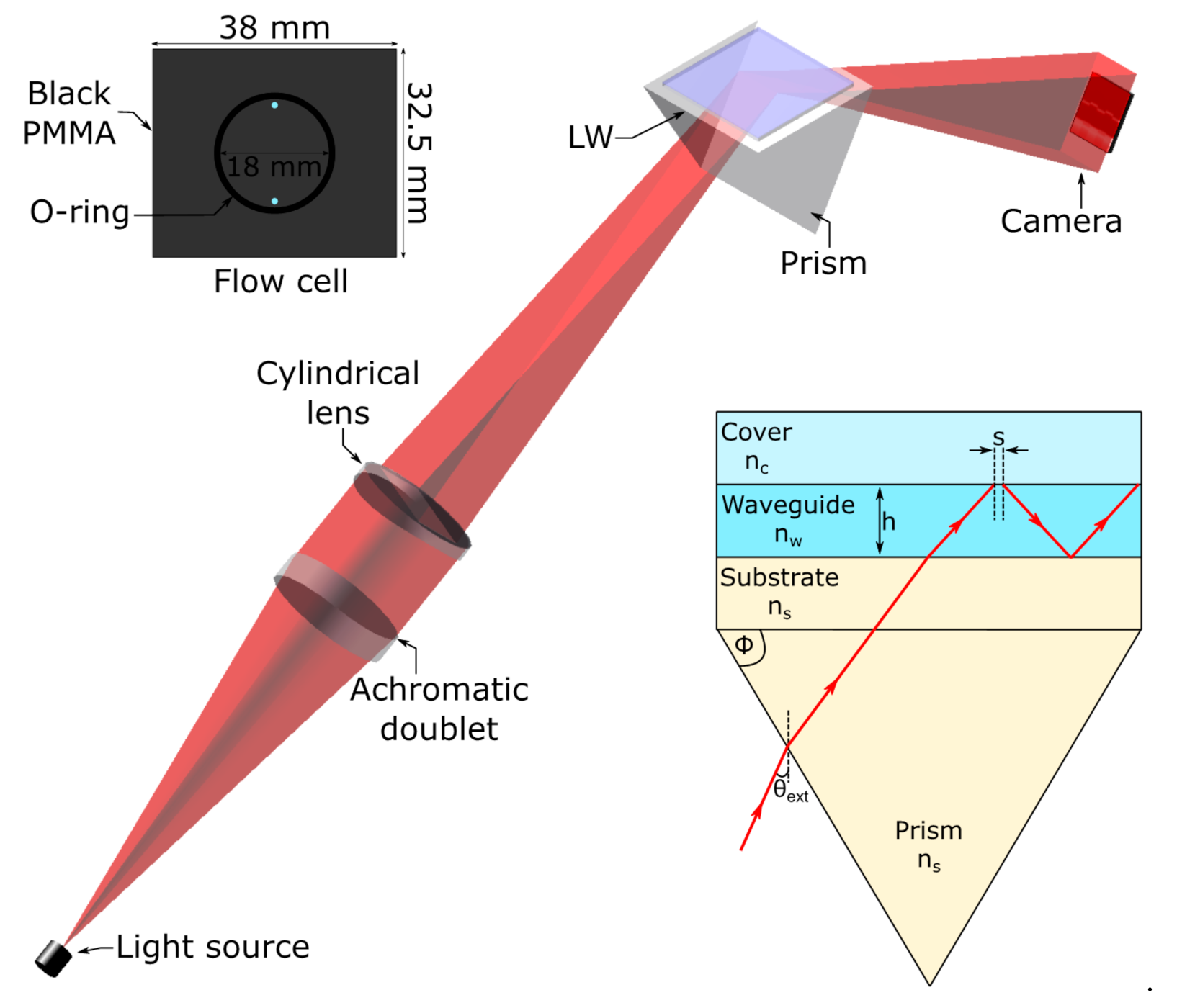
2.4. Instrumentation
3. Results and Discussion
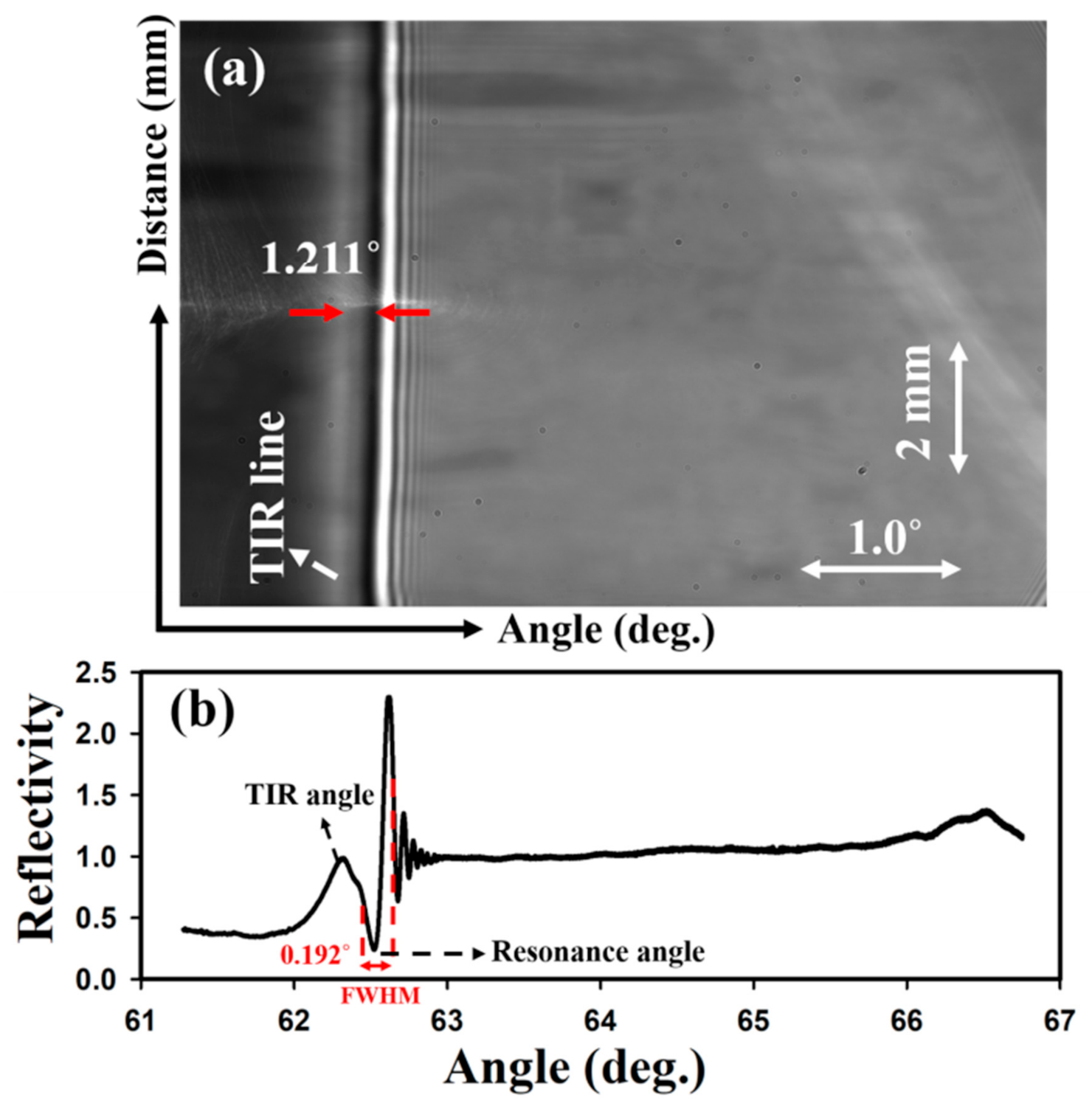
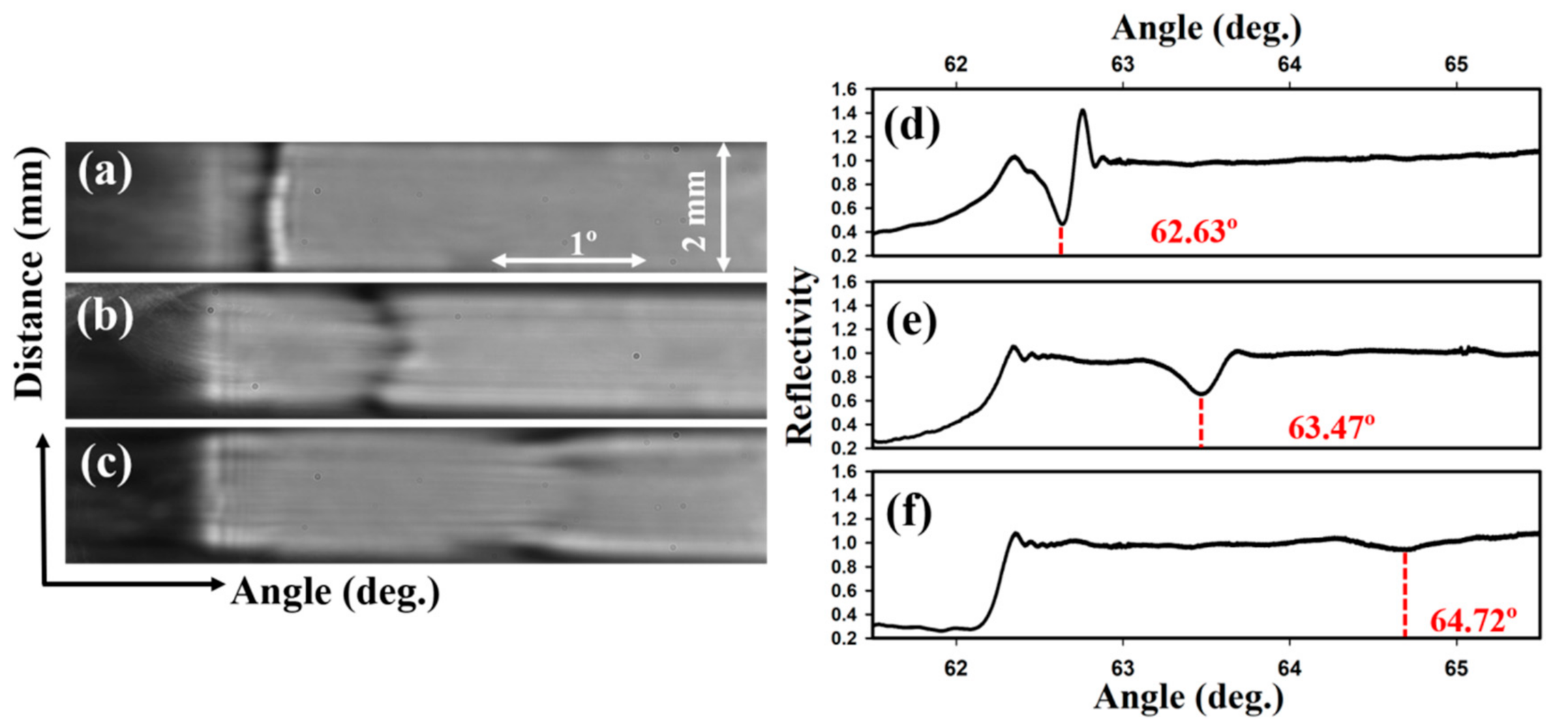
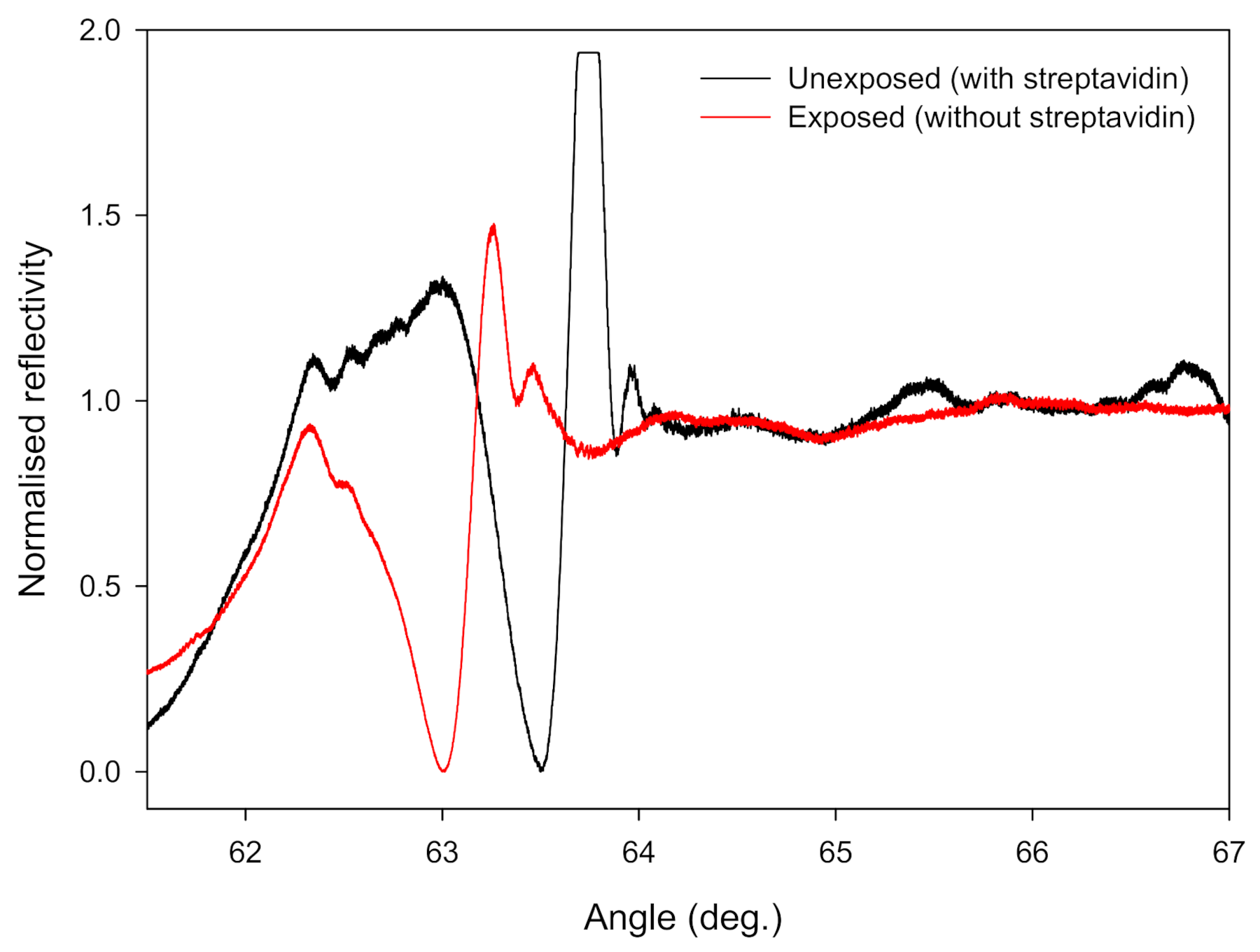
3.1. Optimisation of the Diffraction-Based LW
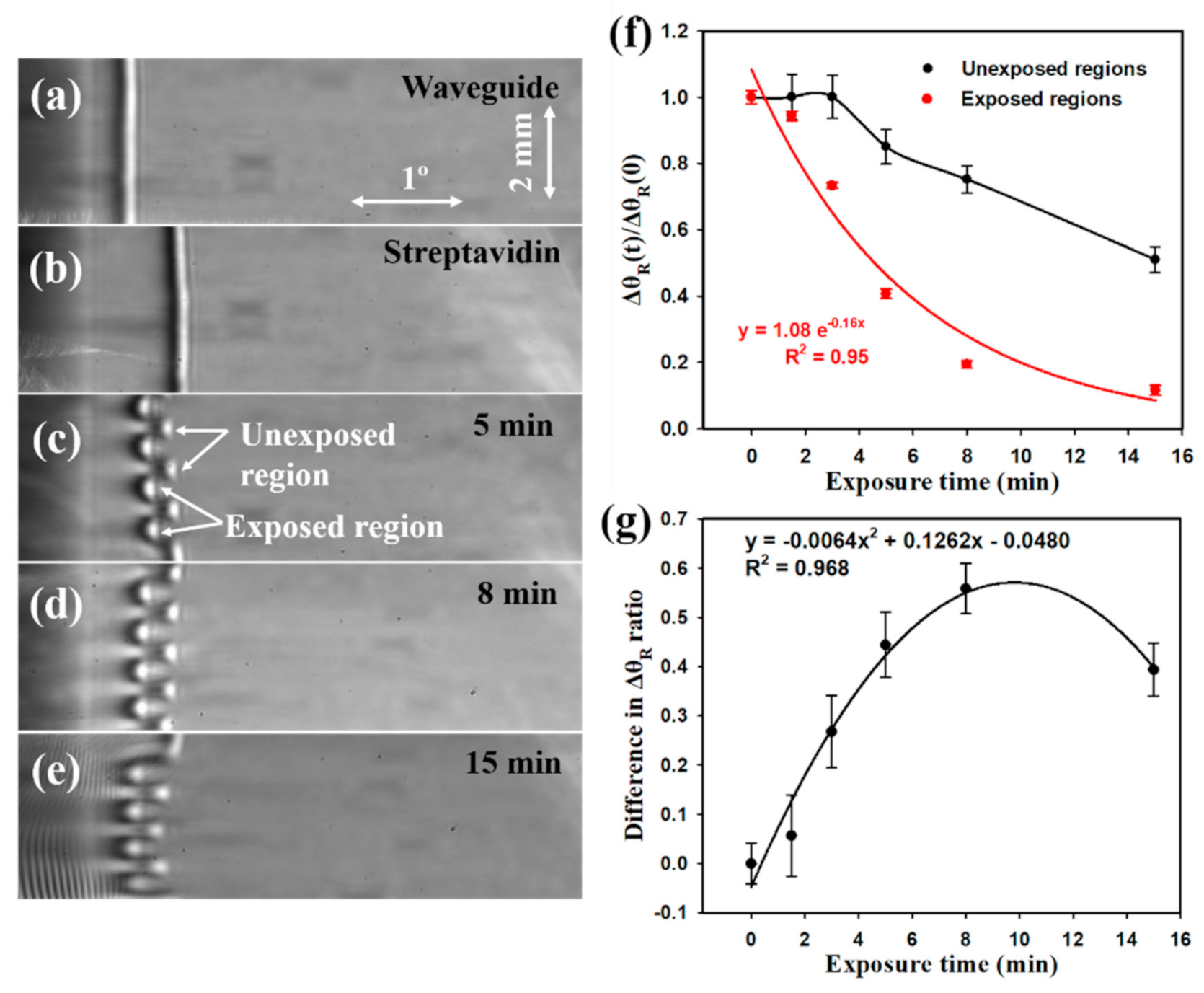
3.2. Self-Referenced Diffraction-Based LW Biosensor
3.2.1. Compensation for Changes in Temperature
3.2.2. Compensation for Changes in Sample Composition
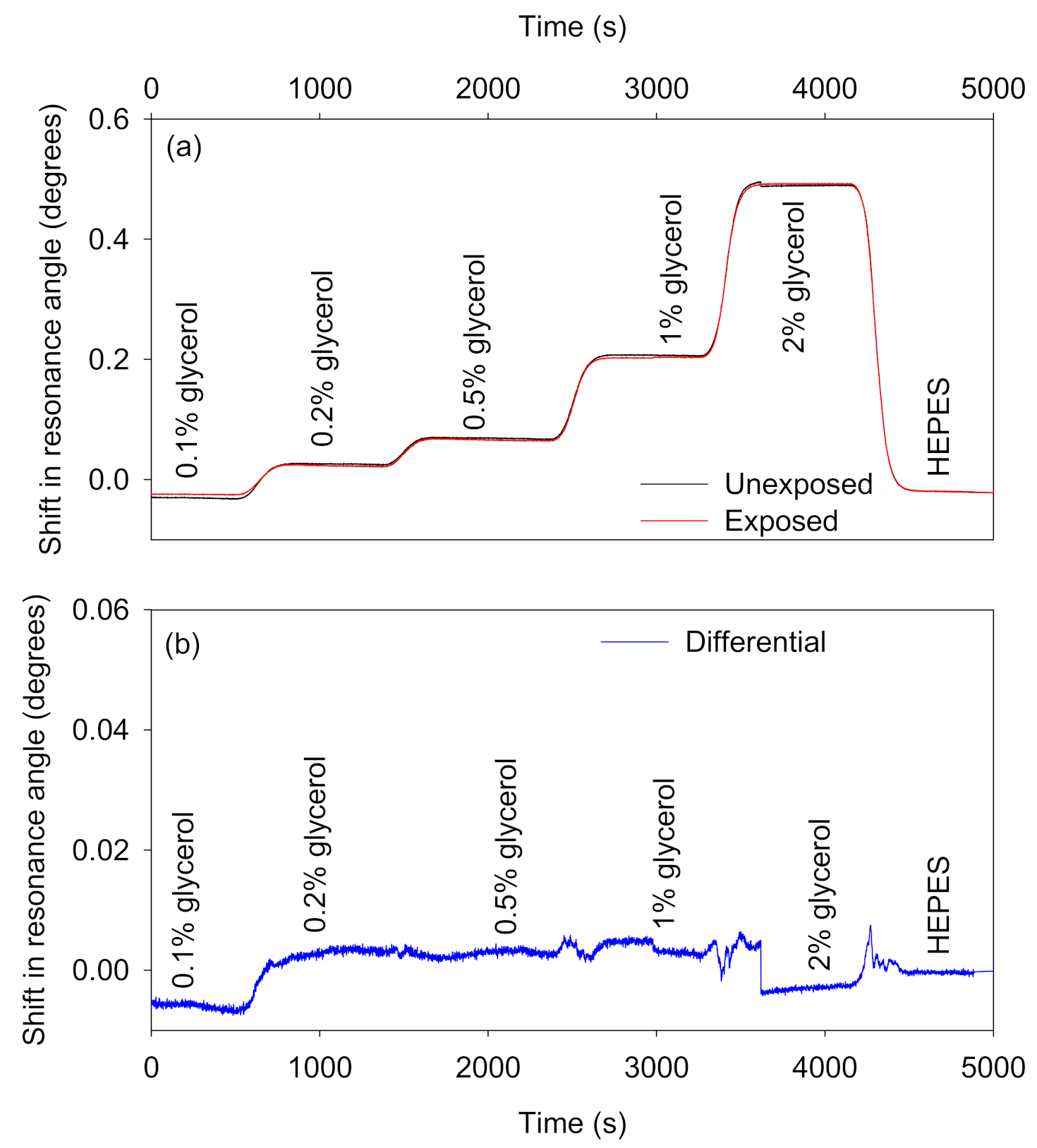
- Non-adsorbing interferents: Figure 7 shows the absolute and differential shifts in resonance angles as a result of changes in RI caused by different concentrations of glycerol solutions. The RI of glycerol solutions of different concentrations are provided in Table S1 in Supplementary Materials. On average, the differential response because of changes in bulk RI was ~99% lower than the absolute shifts in the resonance angles of the sensor and reference regions.
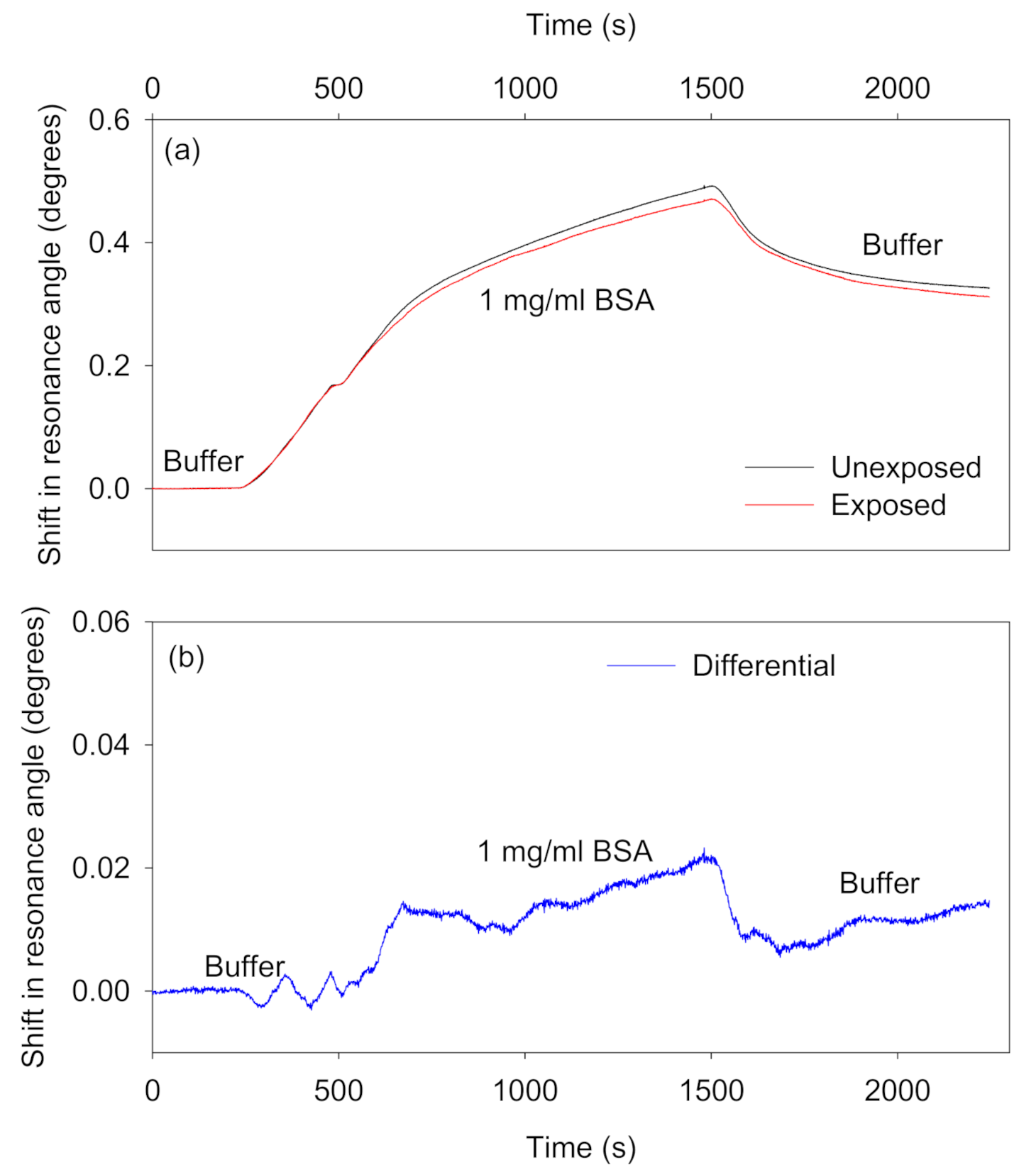
- Adsorbing interferents: Ideally, species in samples should not non-specifically adsorb to the waveguide materials. Alternatively, the non-specific adsorption of species to sensor and reference regions should at least be similar so that differential measurements can be performed to remove the contribution of non-specific adsorption from the overall output of the biosensor. We investigated the potential of the reported self-referenced LW to eliminate the effect of non-specific adsorption by using 1 mg/mL BSA solution prepared in 100 mM HEPES buffer, pH 7.4. As shown in Figure 8a, the resonance angle of sensor and reference regions increased as BSA solution was introduced on top of the patterned chitosan LW. The resonance angles did not return back to baseline after a buffer wash. This may be a result of electrostatic interactions between BSA and the chitosan films as well as hydrophobic interactions between BSA and the NVOC in unexposed regions of the film. The isoelectric point of BSA is 4.7–5.1 [30], whereas the pKa of the amines in chitosan is about 6.5 [31], which means that BSA will be negatively charged at the selected pH of the buffer, and hence can interact with positively charged primary amines in chitosan films [32]. Since the difference in response to BSA between the exposed and unexposed regions is quite small, it seems likely that electrostatic interactions are more significant. The shifts in resonance angles of sensor and reference regions because of non-specific adsorption of BSA were, however, similar. As a result, as shown in Figure 8b, the differential signal was up to 97% lower than the absolute shifts in the resonance angles of the sensor and reference regions. The remaining small difference between the exposed and unexposed regions can be explained by non-specific interaction of BSA with the higher concentration of streptavidin in the unexposed regions.
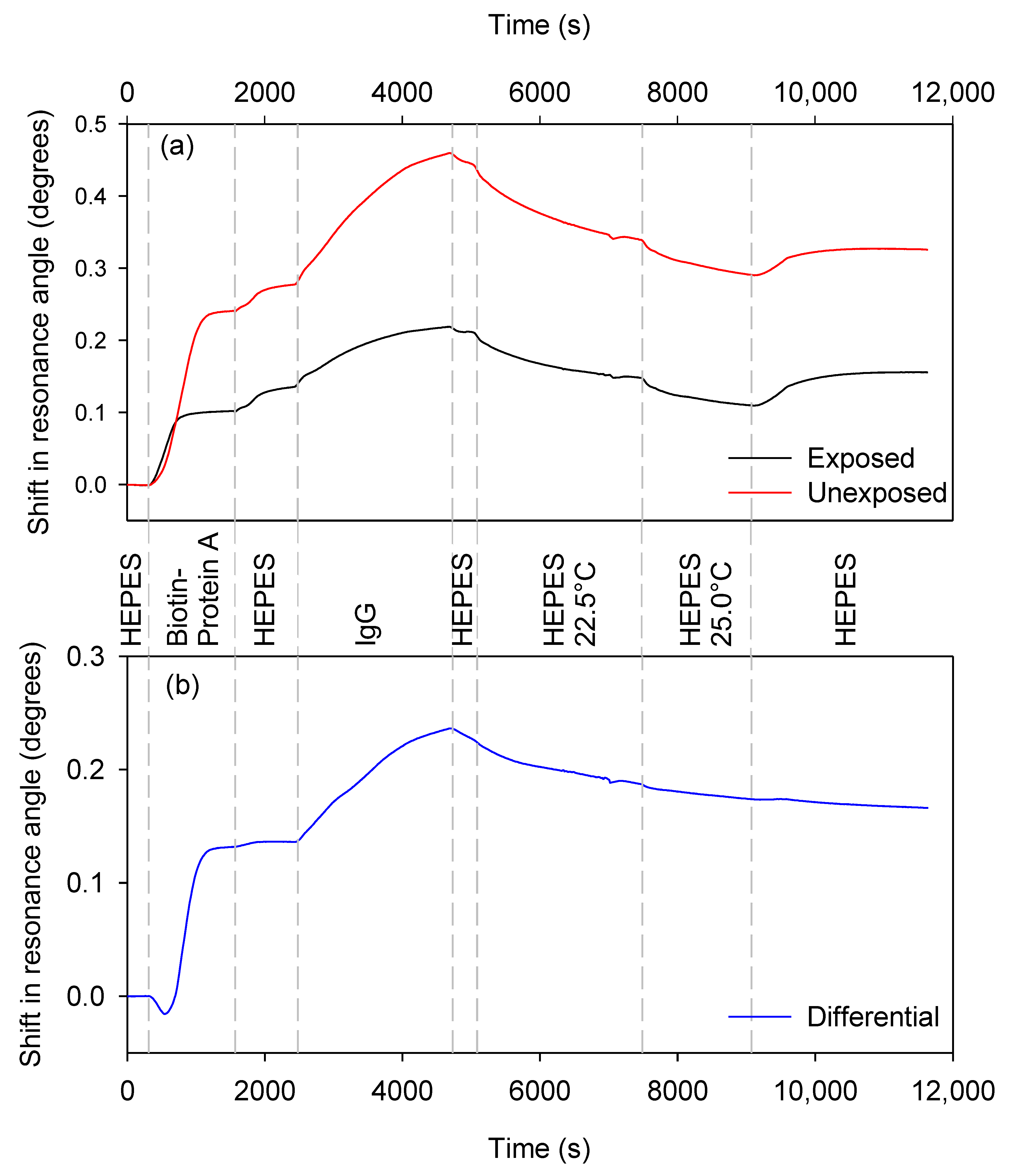
3.2.3. Kinetic Analysis of Analyte Binding
3.2.4. Temperature Compensation during Analyte Unbinding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zourob, M.; Goddard, N.J. Metal clad leaky waveguides for chemical and biosensing applications. Biosens. Bioelectron. 2005, 20, 1718. [Google Scholar] [CrossRef] [PubMed]
- Osterfeld, M.; Franke, H.; Feger, C. Optical gas-detection using metal-film enhanced leaky mode spectroscopy. Appl. Phys. Lett. 1993, 62, 2310. [Google Scholar] [CrossRef]
- Berz, F. On a quarter wave light condenser. Br. J. Appl. Phys. 1965, 16, 1733. [Google Scholar] [CrossRef]
- Podgorsek, R.P.; Franke, H. Selective optical detection of aromatic vapors. Appl. Opt. 2002, 41, 601. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Wang, Y.; Mateescu, A.; Sergelen, K.; Kibrom, A.; Jonas, U.; Wei, T.X.; Dostalek, J. Biosensor based on hydrogel optical waveguide spectroscopy for the detection of 17 beta-estradiol. Talanta 2013, 104, 149. [Google Scholar] [CrossRef]
- Zourob, M.; Simonian, A.; Wild, J.; Mohr, S.; Fan, X.D.; Abdulhalim, I.; Goddard, N.J. Optical leaky waveguide biosensors for the detection of organophosphorus pesticides. Analyst 2007, 132, 114. [Google Scholar] [CrossRef]
- Zourob, M.; Mohr, S.; Brown, B.J.T.; Fielden, P.R.; McDonnell, M.B.; Goddard, N.J. An integrated optical leaky waveguide sensor with electrically induced concentration system for the detection of bacteria. Lab Chip 2005, 5, 1360. [Google Scholar] [CrossRef]
- Alamrani, N.A.; Greenway, G.M.; Pamme, N.; Goddard, N.J.; Gupta, R. A feasibility study of a leaky waveguide aptasensor for thrombin. Analyst 2019, 144, 6048. [Google Scholar] [CrossRef]
- Im, W.J.; Kim, B.B.; Byun, J.Y.; Kim, H.M.; Kim, M.G.; Shin, Y.B. Immunosensing using a metal clad leaky waveguide biosensor for clinical diagnosis. Sens. Actuators B-Chem. 2012, 173, 288. [Google Scholar] [CrossRef]
- Kim, B.B.; Im, W.J.; Byun, J.Y.; Kim, H.M.; Kim, M.G.; Shin, Y.B. Label-free CRP detection using optical biosensor with one-step immobilization of antibody on nitrocellulose membrane. Sens. Actuators B-Chem. 2014, 190, 243. [Google Scholar] [CrossRef]
- Der, A.; Valkai, S.; Mathesz, A.; Ando, I.; Wolff, E.K.; Ormos, P. Protein-based all-optical sensor device. Sens. Actuators B-Chem. 2010, 151, 26. [Google Scholar] [CrossRef]
- Goddard, N.J.; Gupta, R. A novel manifestation at optical leaky waveguide modes for sensing applications. Sens. Actuators B-Chem. 2020, 309, 127776. [Google Scholar] [CrossRef]
- Gupta, R.; Goddard, N.J. Leaky waveguides (LWs) for chemical and biological sensing—A review and future perspective. Sens. Actuators B Chem. 2020, 322, 128628–128642. [Google Scholar] [CrossRef]
- Goddard, N.J.; Gupta, R. Speed and sensitivity—Integration of electrokinetic preconcentration with a leaky waveguide biosensor. Sens. Actuators B Chem. 2019, 301, 127063. [Google Scholar] [CrossRef]
- Thormahlen, I.; Straub, J.; Grigull, U. Refractive-index of water and its dependence on wavelength, temperature, and density. J. Phys. Chem. Ref. Data 1985, 14, 933. [Google Scholar] [CrossRef] [Green Version]
- Abbate, G.; Bernini, U.; Ragozzino, E.; Somma, F. The temperature dependence of the refractive index of water. J. Phys. D Appl. Phys. 1978, 11, 1167. [Google Scholar] [CrossRef]
- Manninen, P.; Orrevetelainen, P. On spectral and thermal behaviors of AlGaInP light-emitting diodes under pulse-width modulation. Appl. Phys. Lett. 2007, 91, 181121. [Google Scholar] [CrossRef] [Green Version]
- Yakes, B.J.; Buijs, J.; Elliott, C.T.; Campbell, K. Surface plasmon resonance biosensing: Approaches for screening and characterising antibodies for food diagnostics. Talanta 2016, 156, 55. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.B.; Homola, J.; Campbell, C.T.; Nenninger, G.G.; Yee, S.S.; Ratner, B.D. Protein contact printing for a surface plasmon resonance biosensor with on-chip referencing. Sens. Actuators B-Chem. 2001, 74, 91. [Google Scholar] [CrossRef]
- Yu, F.; Knoll, W. Immunosensor with self-referencing based on surface plasmon diffraction. Anal. Chem. 2004, 76, 1971. [Google Scholar] [CrossRef]
- Nizamov, S.; Scherbahn, V.; Mirsky, V.M. Self-referencing SPR-sensor based on integral measurements of light intensity reflected by arbitrarily distributed sensing and referencing spots. Sens. Actuators B-Chem. 2015, 207, 740. [Google Scholar] [CrossRef]
- Zou, Q.J.; Menegazzo, N.; Booksh, K.S. Development and investigation of a dual-pad in-channel referencing surface plasmon resonance sensor. Anal. Chem. 2012, 84, 7891. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.V.; Senturk, O.I.; Spatz, J.P. Photocleavable linker for the patterning of bioactive molecules. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.K.; Labella, E.; Goddard, N.J.; Gupta, R. Photofunctionalizable hydrogel for fabricating volume optical diffractive sensors. Macromol. Chem. Phys. 2019, 220, 1900228. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Alamrani, N.; Greenway, G.M.; Pamme, N.; Goddard, N.J. A method for determining average iron content of ferritin by measuring its optical dispersion. Anal. Chem. 2019, 91, 7366. [Google Scholar] [CrossRef] [Green Version]
- Löfås, S.; Johnsson, B. A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J. Chem. Soc. Chem. Commun. 1990, 1526–1528. [Google Scholar] [CrossRef]
- Cush, R.; Cronin, J.M.; Stewart, W.J.; Maule, C.H.; Molloy, J.; Goddard, N.J. The resonant mirror—A novel optical biosensor for direct sensing of biomolecular interactions. 1. Principle of operation and associated instrumentation. Biosens. Bioelectron. 1993, 8, 347. [Google Scholar] [CrossRef]
- Gupta, R.; Goddard, N.J.; Dixon, H.; Toole, N. 3D printed instrumentation for point-of-use leaky waveguide (LW) biochemical sensor. IEEE Trans. Instrum. Meas. 2020, 69, 6390. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.Y.; Brown, P.H.; Schuckt, P. On the distribution of protein refractive index increments. Biophys. J. 2011, 100, 2309. [Google Scholar] [CrossRef] [Green Version]
- Jachimska, B.; Pajor, A. Physico-chemical characterization of bovine serum albumin in solution and as deposited on surfaces. Bioelectrochemistry 2012, 87, 138. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.J.; Wang, X.H.; Luo, G.S.; Dai, Y.Y. Adsorption of bovin serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion system. Bioresour. Technol. 2008, 99, 3881–3884. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, M.J. Direct immunochemical sensing—Basic chemical principles and fundamental limitations. Biosensors 1987, 3, 1–15. [Google Scholar] [CrossRef]
- Gupta, R.; Goddard, N.J. A novel optical biosensor with internal referencing. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013; p. 1490. [Google Scholar]

| Device | FWHM (°) | Sensitivity (° nm−1) | DFOM (nm) |
|---|---|---|---|
| SPR (TM) | 4.3185 | 0.0657 | 65.8 |
| RM (TM) | 1.0212 | 8.62 × 10−3 | 118.5 |
| RM (TE) | 0.1941 | 0.0174 | 11.16 |
| Diffraction-based LW (TM) | 0.0518 | 3.62 × 10−4 | 143.1 |
| Diffraction-based LW (TE) | 0.0476 | 1.60 × 10−4 | 296.7 |
| Device | Compensation for Change in Temperature | Compensation for Changes in Sample Composition | Reduction in Sensitivity to Analyte Binding | |
|---|---|---|---|---|
| Non-Adsorbing Interferents | Adsorbing Interferents | |||
| Stacked MCLW [34] | 94% | 97% | Not tested | 78% |
| This work | 98% | 99% | 97% | 49% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, A.K.; Goddard, N.J.; Dixon, H.J.; Gupta, R. A Self-Referenced Diffraction-Based Optical Leaky Waveguide Biosensor Using Photofunctionalised Hydrogels. Biosensors 2020, 10, 134. https://doi.org/10.3390/bios10100134
Pal AK, Goddard NJ, Dixon HJ, Gupta R. A Self-Referenced Diffraction-Based Optical Leaky Waveguide Biosensor Using Photofunctionalised Hydrogels. Biosensors. 2020; 10(10):134. https://doi.org/10.3390/bios10100134
Chicago/Turabian StylePal, Anil K., Nicholas J. Goddard, Hazel J. Dixon, and Ruchi Gupta. 2020. "A Self-Referenced Diffraction-Based Optical Leaky Waveguide Biosensor Using Photofunctionalised Hydrogels" Biosensors 10, no. 10: 134. https://doi.org/10.3390/bios10100134
APA StylePal, A. K., Goddard, N. J., Dixon, H. J., & Gupta, R. (2020). A Self-Referenced Diffraction-Based Optical Leaky Waveguide Biosensor Using Photofunctionalised Hydrogels. Biosensors, 10(10), 134. https://doi.org/10.3390/bios10100134





