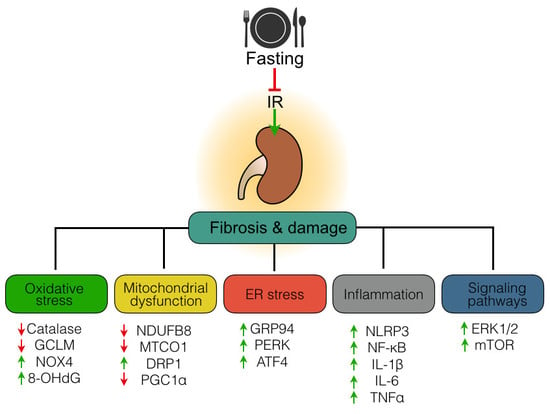
Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Materials, and Antibodies
2.2. Animals, Experimental Design, and Study Approval
2.3. Unilateral Ischemia-Reperfusion Injury
2.4. Plasma Biochemistry
2.5. Histology and Transmission Electron Microscopy
2.6. Immunohistochemistry and Immunofluorescence
2.7. Subcellular Fractionation
2.8. Western Blot
2.9. Enzyme-Linked Immunosorbent Assays
2.10. Statistical Analysis
3. Results
3.1. Fasting Attenuates Tubular Injury and Fibrosis Induced by Ischemia-Reperfusion Injury
3.2. Fasting Prevents Oxidative Stress Long after Ischemia-Reperfusion Injury
3.3. Fasting Protects Mitochondria Long After Ischemia-Reperfusion Injury
3.4. Fasting Protects Against Ischemia-Reperfusion Induced Endoplasmatic Reticulum Stress
3.5. Fasting Suppresses Inflammation Long After Ischemia-Reperfusion Injury
3.6. Effect of Fasting on ERK and mTOR Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duffield, J.S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Investig. 2014, 124, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Group AXW. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front Physiol. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. [Google Scholar] [CrossRef]
- Eltzsching, H.K.; Eckle, T. Ischemia and reperfusion—From mechanisms to translation. Nat. Medicine 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Huynh, P.; Chai, Z. Transforming growth factor β (TGFβ) and related molecules in chronic kidney disease (CKD). Clin. Sci. (London) 2019, 133, 287–313. [Google Scholar] [CrossRef]
- Andrikopoulos, P.; Kieswich, J.; Pacheco, S.; Nadarajah, L.; Harwood, S.M.; O’Riordan, C.E.; Thiemermann, C.; Yaqoob, M.M. The MEK inhibitor trametinib ameliorates kidney fibrosis by suppressing ERK1/2 and mTORC1 signaling. J. Am. Soc. Nephrol. 2019, 30, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, L.; Mao, J.; Li, J.; Fang, L.; Zhou, Y.; Liu, W.; He, W.; Zhao, A.Z.; Yang, J.; et al. Rheb/mTORC1 signaling promotes kidney fibroblast activation and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 1114–1126. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.T.; Mitchell, J.R. Benefits of short-term dietary restriction in mammals. Exp. Gerontol. 2013, 48, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Harputlugil, E.; Mitchell, J.R.; Longo, V.D. Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Res. Rev. 2017, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Morales, P.; León-Contreras, J.C.; Aparicio-Trejo, O.E.; Reyes-Ocampo, J.G.; Medina-Campos, O.N.; Jiménez-Osorio, A.S.; González-Reyes, S.; Marquina-Castillo, B.; Hernández-Pando, R.; Barrera-Oviedo, D.; et al. Fasting reduces oxidative stress, mitochondrial dysfunction and fibrosis induced by renal ischemia-reperfusion injury. Free Radic. Biol. Med. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Dong, Z. Rodent models of AKI-CKD transition. Am. J. Physiol. Renal Physiol. 2018, 315, F1098–F1106. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, G.S.; Dusting, G.J.; Chan, E.C. NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses. Redox Biol. 2014, 2, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Müller, C.; de Jong, M.; et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food restriction ameliorates the development of polycystic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 1437–1447. [Google Scholar] [CrossRef]
- Jongbloed, F.; Saat, T.C.; Verweij, M.; Payan-Gomez, C.; Hoeijmakers, J.H.; van den Engel, S.; van Oostrom, C.T.; Ambagtsheer, G.; Imholz, S.; Pennings, J.L.; et al. A signature of renal stress resistance induced by short-term dietary restriction, fasting, and protein restriction. Sci. Rep. 2017, 7, 40901. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Meyer, T.W.; Rennke, H.G.; Brenner, B.M. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int. 1986, 30, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Cai, G.Y.; Chen, X.M. Energy restriction in renal protection. Br. J. Nutr. 2018, 120, 1149–1158. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Morales, P.; Tapia, E.; León-Contreras, J.C.; González-Reyes, S.; Jiménez-Osorio, A.S.; Trujillo, J.; Pavón, N.; Granados-Pineda, J.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; et al. Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury. Biomolecules 2019, 9, 404. https://doi.org/10.3390/biom9090404
Rojas-Morales P, Tapia E, León-Contreras JC, González-Reyes S, Jiménez-Osorio AS, Trujillo J, Pavón N, Granados-Pineda J, Hernández-Pando R, Sánchez-Lozada LG, et al. Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury. Biomolecules. 2019; 9(9):404. https://doi.org/10.3390/biom9090404
Chicago/Turabian StyleRojas-Morales, Pedro, Edilia Tapia, Juan Carlos León-Contreras, Susana González-Reyes, Angélica Saraí Jiménez-Osorio, Joyce Trujillo, Natalia Pavón, Jessica Granados-Pineda, Rogelio Hernández-Pando, Laura Gabriela Sánchez-Lozada, and et al. 2019. "Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury" Biomolecules 9, no. 9: 404. https://doi.org/10.3390/biom9090404
APA StyleRojas-Morales, P., Tapia, E., León-Contreras, J. C., González-Reyes, S., Jiménez-Osorio, A. S., Trujillo, J., Pavón, N., Granados-Pineda, J., Hernández-Pando, R., Sánchez-Lozada, L. G., Osorio-Alonso, H., & Pedraza-Chaverri, J. (2019). Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury. Biomolecules, 9(9), 404. https://doi.org/10.3390/biom9090404