Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins
Abstract
1. Introduction
2. Materials and Methods
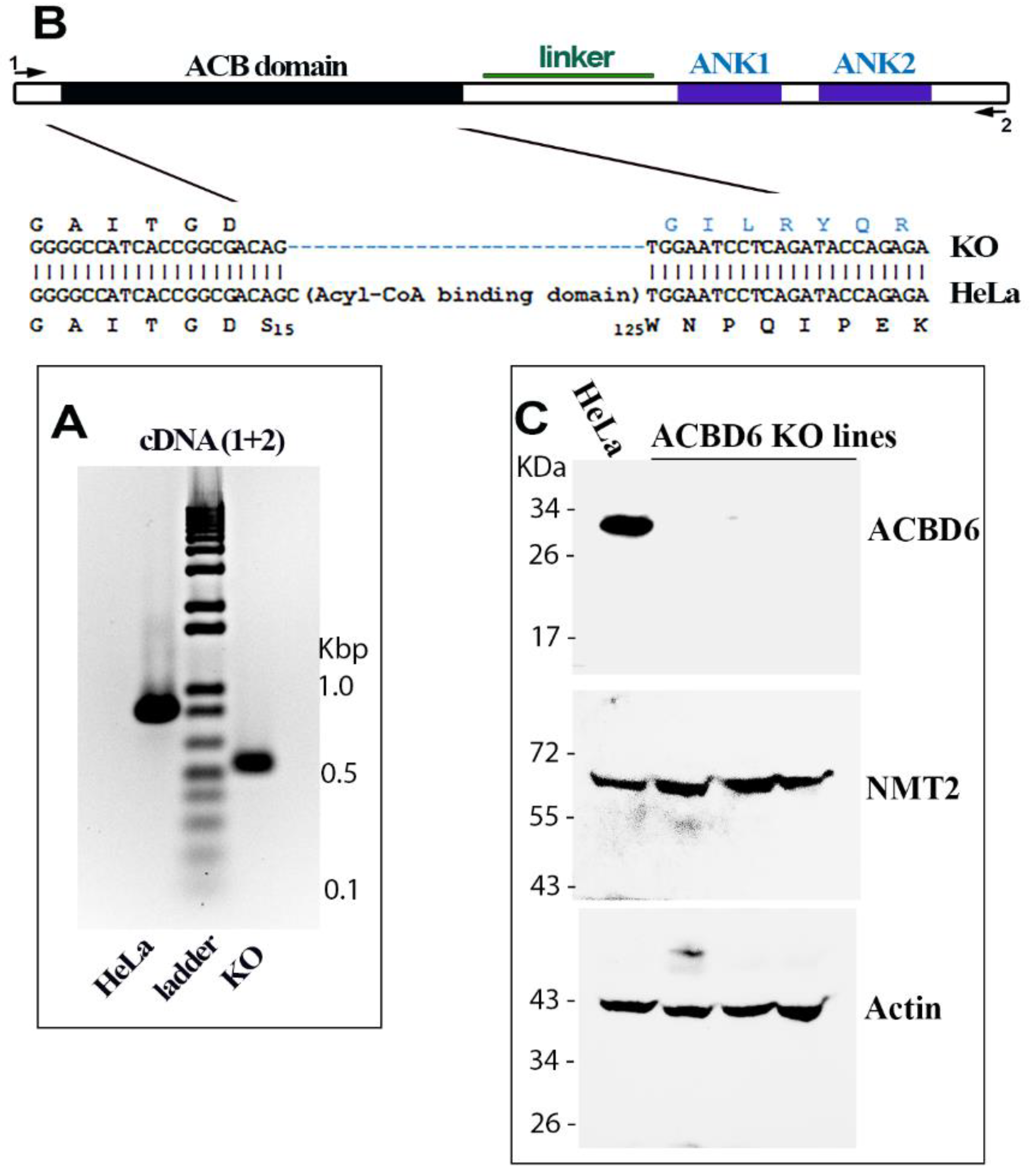
2.1. Construction and Characterization of the ACBD6.KO Cells
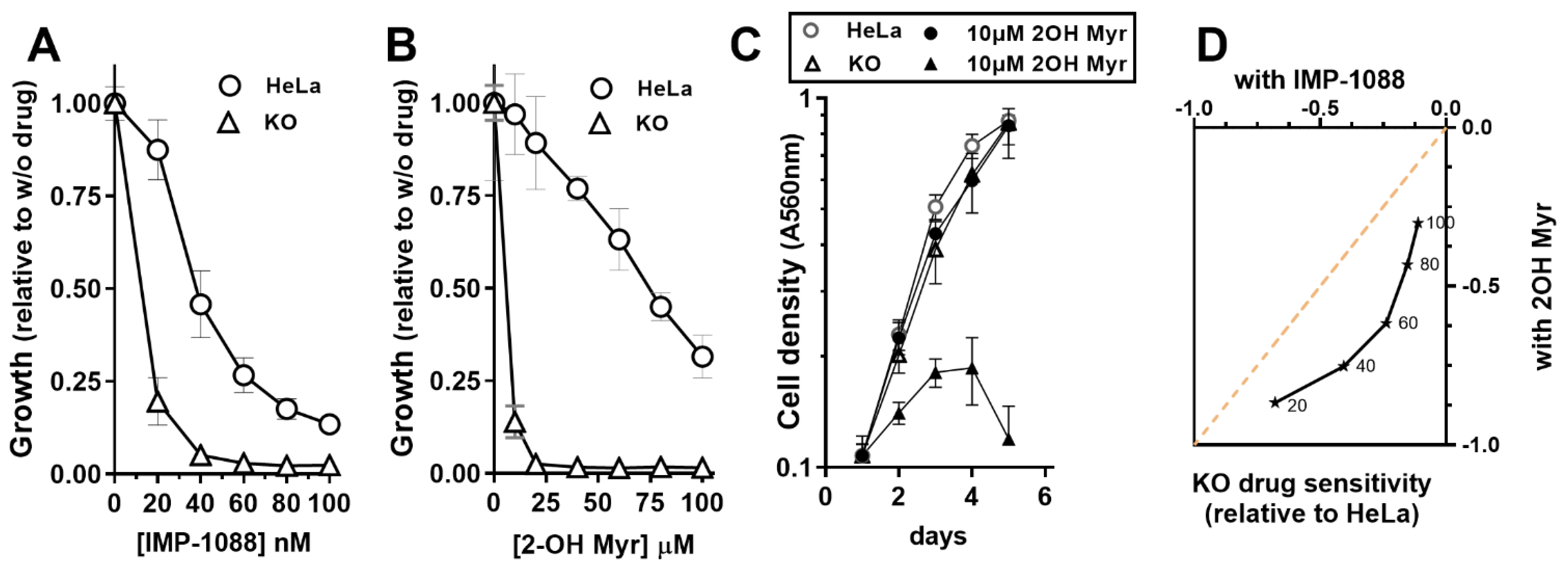
2.2. Cell Culture and Growth Experiments
2.3. Lipid Droplet Quantification and Isolation
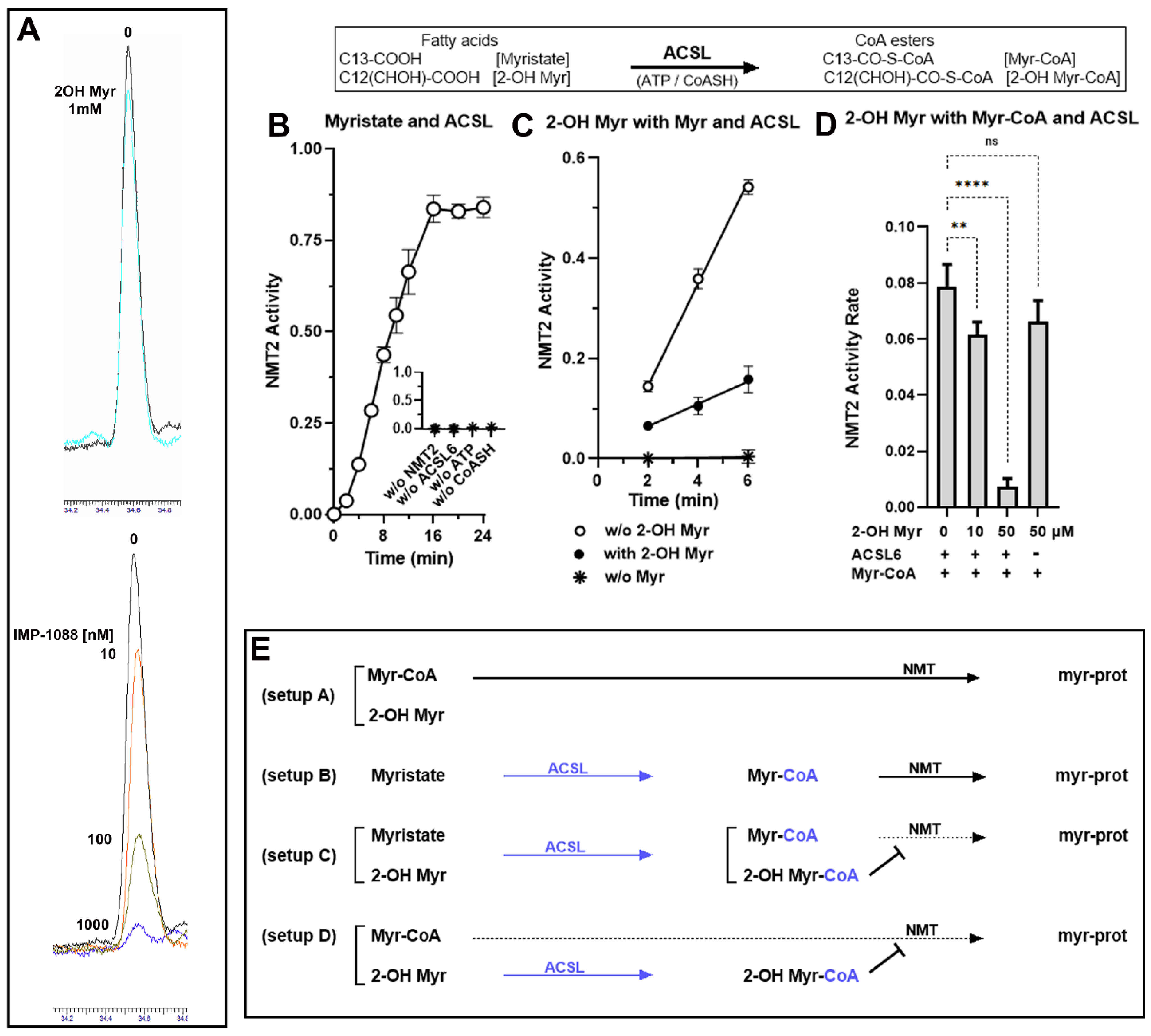
2.4. N-myristoyltransferase Activity Measurements
2.5. Protein Palmitoylation Quantification
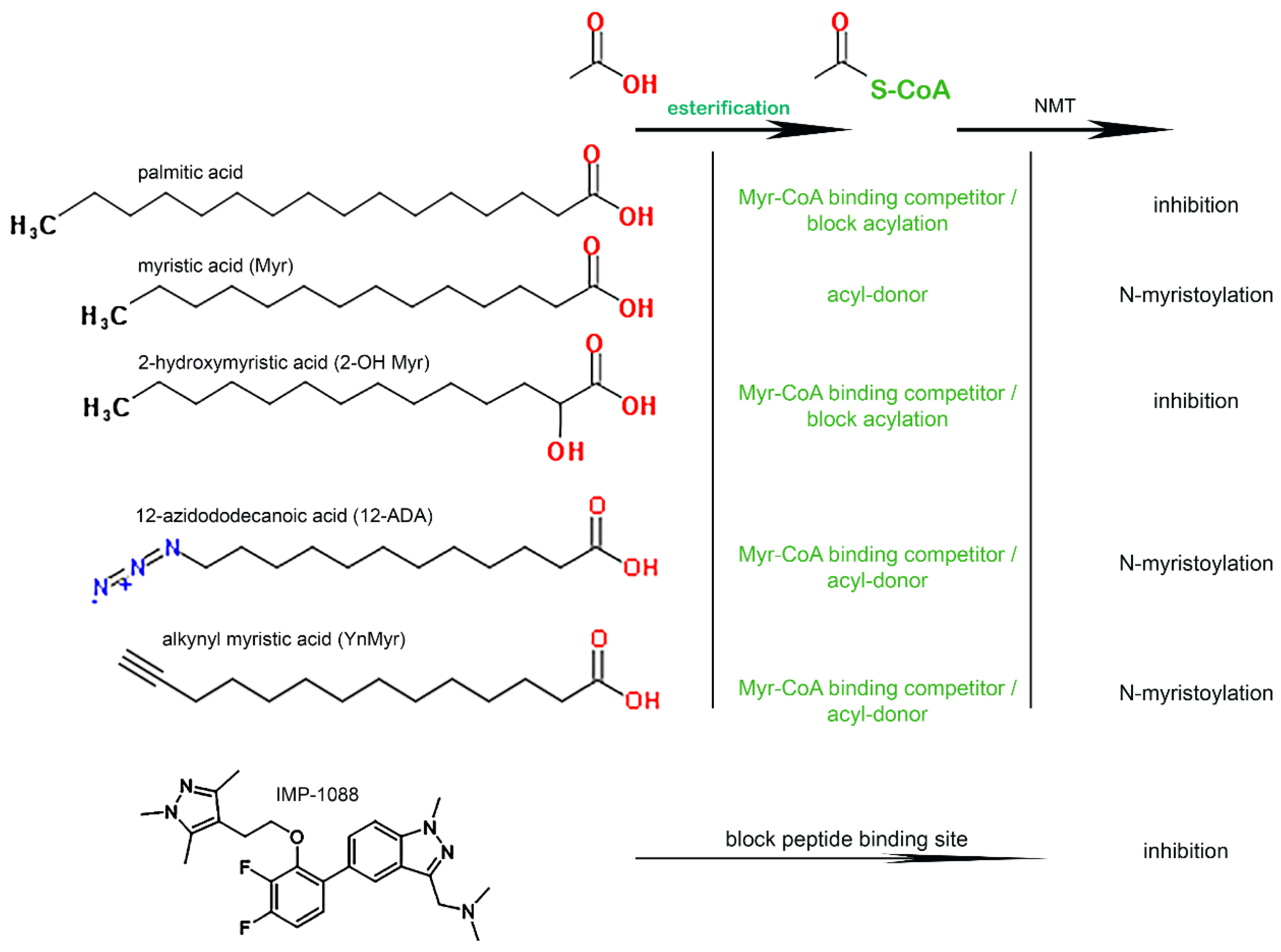
2.6. Fatty Acid Incorporation
2.7. Acyl-CoA Synthetase and lysoPL Acyltransferase Assays
3. Results
3.1. Role of the Acyl-CoA Synthetase Enzymes in the Myristoylation Reaction
3.2. Disruption of ACBD6 in Human Cells
3.3. NMT Activity Deficiency in the Absence of ACBD6
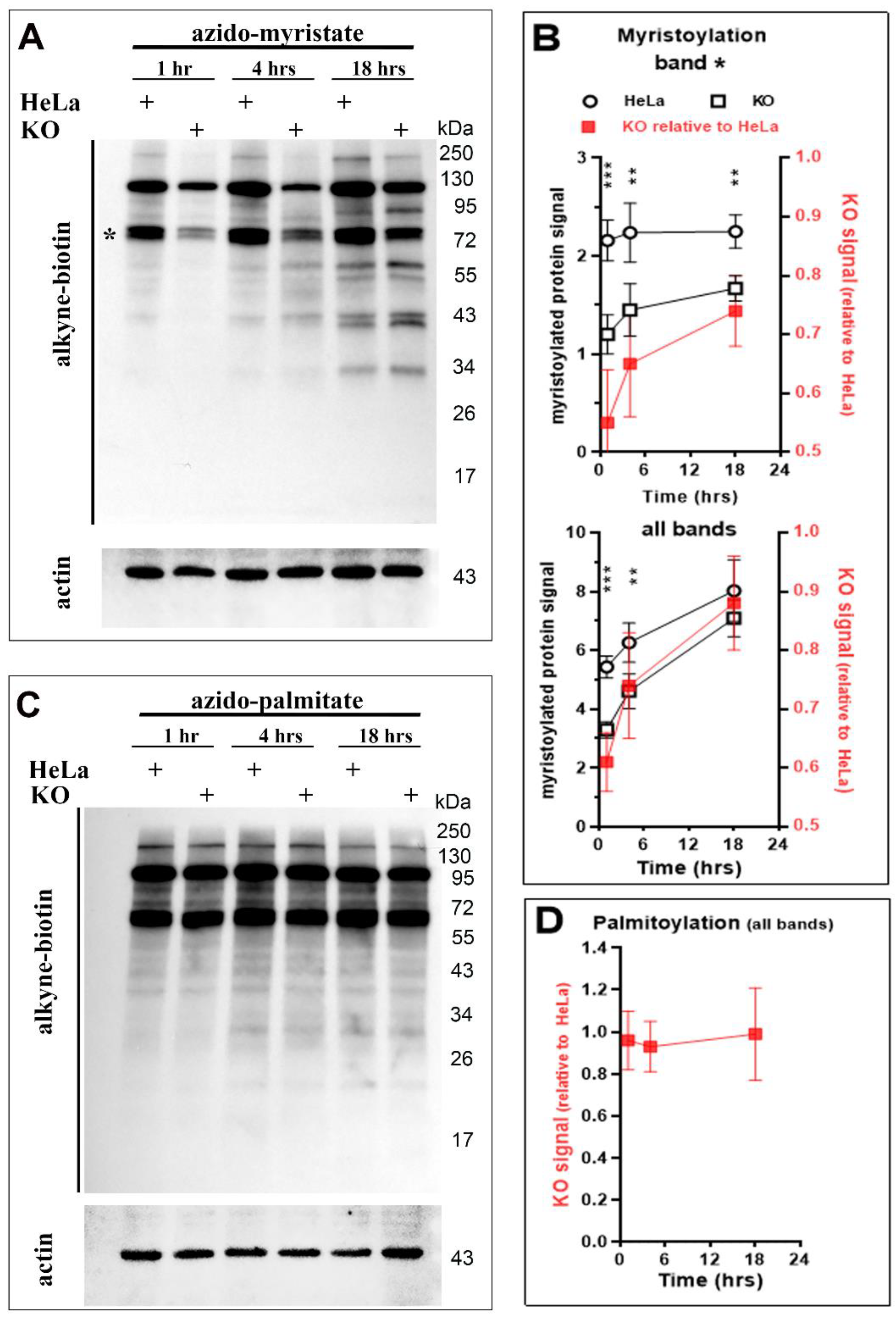
3.4. Protein N-Myristoylation Deficiency in the Absence of ACBD6
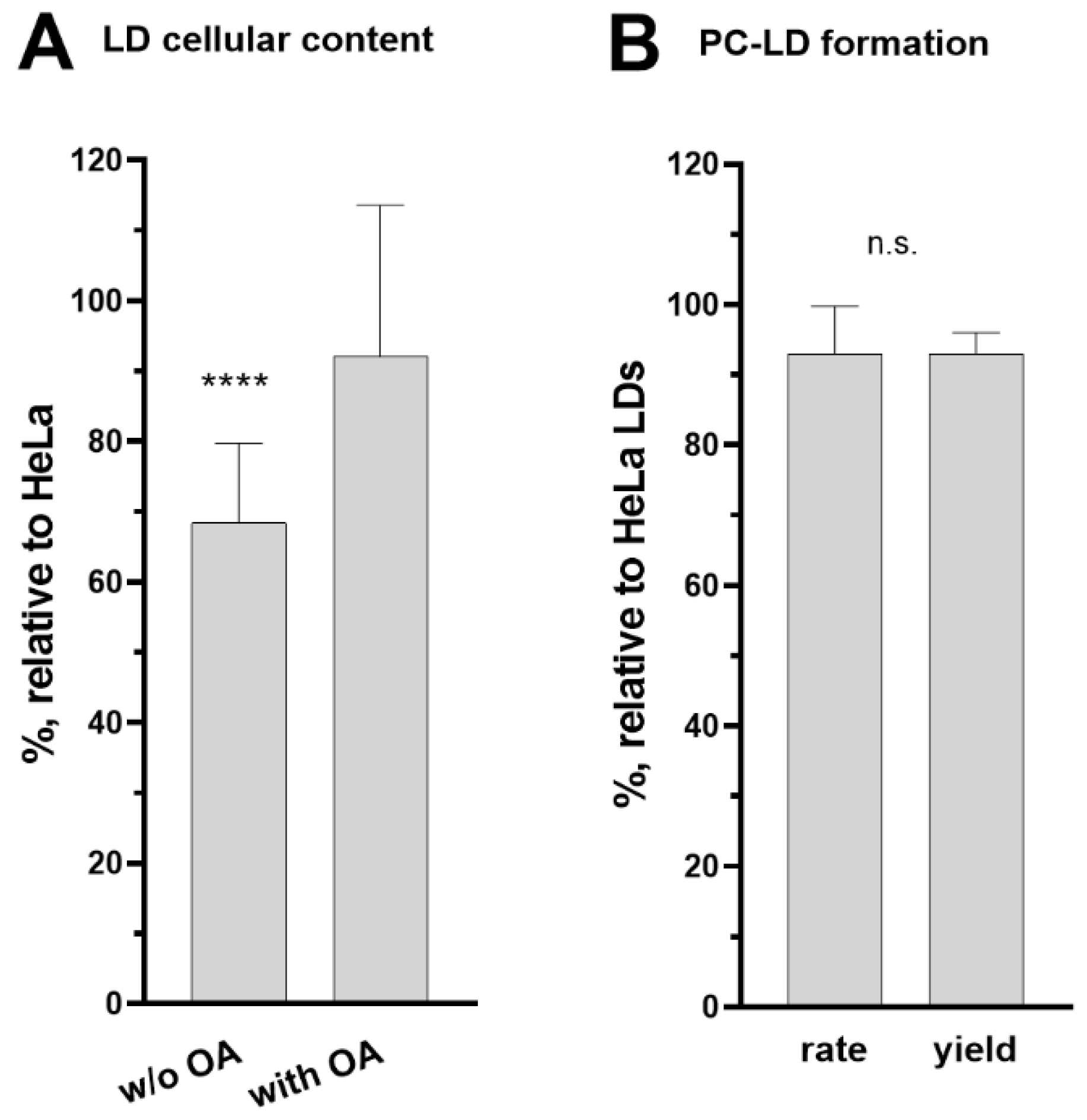
3.5. Role of ACBD6 in the Acyl-CoA Dependent Acylation of Lipids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Soupene, E.; Kao, J.; Cheng, D.H.; Wang, D.; Greninger, A.L.; Knudsen, G.M.; DeRisi, J.L.; Kuypers, F.A. Association of NMT2 with the acyl-CoA carrier ACBD6 protects the N-myristoyltransferase reaction from palmitoyl-CoA. J. Lipid Res. 2016, 57, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Kuypers, F.A. ACBD6 protein controls acyl chain availability and specificity of the N-myristoylation modification of proteins. J. Lipid Res. 2019, 60, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Schatz, U.A.; Rudnik-Schoneborn, S.; Kuypers, F.A. Requirement of the acyl-CoA carrier ACBD6 in myristoylation of proteins: Activation by ligand binding and protein interaction. PLoS ONE 2020, 15, e0229718. [Google Scholar] [CrossRef] [PubMed]
- Castrec, B.; Dian, C.; Ciccone, S.; Ebert, C.L.; Bienvenut, W.V.; Le Caer, J.P.; Steyaert, J.M.; Giglione, C.; Meinnel, T. Structural and genomic decoding of human and plant myristoylomes reveals a definitive recognition pattern. Nat. Chem. Biol. 2018, 14, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Giglione, C.; Meinnel, T. Mapping the myristoylome through a complete understanding of protein myristoylation biochemistry. Prog. Lipid Res. 2022, 85, 101139. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Chen, X.; Aramsangtienchai, P.; Tong, Z.; Lin, H. Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem. Rev. 2018, 118, 919–988. [Google Scholar] [CrossRef]
- Kallemeijn, W.W.; Lanyon-Hogg, T.; Panyain, N.; Goya Grocin, A.; Ciepla, P.; Morales-Sanfrutos, J.; Tate, E.W. Proteome-wide analysis of protein lipidation using chemical probes: In-gel fluorescence visualization, identification and quantification of N-myristoylation, N- and S-acylation, O-cholesterylation, S-farnesylation and S-geranylgeranylation. Nat. Protoc. 2021, 16, 5083–5122. [Google Scholar] [CrossRef]
- Resh, M.D. Fatty acylation of proteins: The long and the short of it. Prog. Lipid Res. 2016, 63, 120–131. [Google Scholar] [CrossRef]
- Udenwobele, D.I.; Su, R.C.; Good, S.V.; Ball, T.B.; Varma Shrivastav, S.; Shrivastav, A. Myristoylation: An Important Protein Modification in the Immune Response. Front. Immunol. 2017, 8, 751. [Google Scholar] [CrossRef]
- Varland, S.; Osberg, C.; Arnesen, T. N-terminal modifications of cellular proteins: The enzymes involved, their substrate specificities and biological effects. Proteomics 2015, 15, 2385–2401. [Google Scholar] [CrossRef]
- Meinnel, T. Comment on “Binding Affinity Determines Substrate Specificity and Enables Discovery of Substrates for N-Myristoyltransferases”. ACS Catal. 2022, 12, 8195–8201. [Google Scholar] [CrossRef]
- Meinnel, T.; Dian, C.; Giglione, C. Myristoylation, an Ancient Protein Modification Mirroring Eukaryogenesis and Evolution. Trends Biochem. Sci. 2020, 45, 619–632. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Futterer, K.; Waksman, G.; Gordon, J.I. The structure of myristoyl-CoA:protein N-myristoyltransferase. Biochim. Biophys. Acta 1999, 1441, 162–172. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Schall, O.F.; Jackson-Machelski, E.; Sikorski, J.A.; Devadas, B.; Gokel, G.W.; Gordon, J.I. Titration calorimetric analysis of AcylCoA recognition by myristoylCoA:protein N-myristoyltransferase. Biochemistry 1997, 36, 6700–6708. [Google Scholar] [CrossRef]
- DeMar, J.C., Jr.; Anderson, R.E. Identification and quantitation of the fatty acids composing the CoA ester pool of bovine retina, heart, and liver. J. Biol. Chem. 1997, 272, 31362–31368. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Glaser, L.; Gordon, J.I. Heteroatom-substituted fatty acid analogs as substrates for N-myristoyltransferase: An approach for studying both the enzymology and function of protein acylation. Proc. Natl. Acad. Sci. USA 1988, 85, 8795–8799. [Google Scholar] [CrossRef]
- Kishore, N.S.; Wood, D.C.; Mehta, P.P.; Wade, A.C.; Lu, T.; Gokel, G.W.; Gordon, J.I. Comparison of the acyl chain specificities of human myristoyl-CoA synthetase and human myristoyl-CoA:protein N-myristoyltransferase. J. Biol. Chem. 1993, 268, 4889–4902. [Google Scholar] [CrossRef]
- Rudnick, D.A.; Lu, T.; Jackson-Machelski, E.; Hernandez, J.C.; Li, Q.; Gokel, G.W.; Gordon, J.I. Analogs of palmitoyl-CoA that are substrates for myristoyl-CoA:protein N-myristoyltransferase. Proc. Natl. Acad. Sci. USA 1992, 89, 10507–10511. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Jackson-Machelski, E.; McWherter, C.A.; Gordon, J.I. Isothermal titration calorimetric studies of Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase. Determinants of binding energy and catalytic discrimination among acyl-CoA and peptide ligands. J. Biol. Chem. 1994, 269, 11045–11053. [Google Scholar] [CrossRef]
- Kishore, N.S.; Lu, T.B.; Knoll, L.J.; Katoh, A.; Rudnick, D.A.; Mehta, P.P.; Devadas, B.; Huhn, M.; Atwood, J.L.; Adams, S.P.; et al. The substrate specificity of Saccharomyces cerevisiae myristoyl-CoA:protein N-myristoyltransferase. Analysis of myristic acid analogs containing oxygen, sulfur, double bonds, triple bonds, and/or an aromatic residue. J. Biol. Chem. 1991, 266, 8835–8855. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Hu, X.; Cassady, J.M.; Paige, L.A.; Geahlen, R.L. Synthesis of myristoyl CoA analogues and myristoyl peptides as inhibitors of myristoyl CoA:protein N-myristoyltransferase. J. Pharm. Sci. 1994, 83, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Paige, L.A.; Zheng, G.Q.; DeFrees, S.A.; Cassady, J.M.; Geahlen, R.L. Metabolic activation of 2-substituted derivatives of myristic acid to form potent inhibitors of myristoyl CoA:protein N-myristoyltransferase. Biochemistry 1990, 29, 10566–10573. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Eppinger, E.; Schuster, I.G.; Weigand, L.U.; Liang, X.; Kremmer, E.; Peschel, C.; Krackhardt, A.M. Formin-like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. J. Biol. Chem. 2009, 284, 33409–33417. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.R.; Gilbert, R.L.; Blunt, C.; McIlhinney, R.A. Inhibition of varicella-zoster virus replication by an inhibitor of protein myristoylation. J. Gen. Virol. 1993, 74 Pt 6, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Harrison, M.L.; Ashendel, C.L.; Cassady, J.M.; Geahlen, R.L. Treatment of T cells with 2-hydroxymyristic acid inhibits the myristoylation and alters the stability of p56lck. Biochemistry 1993, 32, 9250–9255. [Google Scholar] [CrossRef]
- Perez, M.; Greenwald, D.L.; de la Torre, J.C. Myristoylation of the RING finger Z protein is essential for arenavirus budding. J. Virol. 2004, 78, 11443–11448. [Google Scholar] [CrossRef]
- Renna, L.; Stefano, G.; Majeran, W.; Micalella, C.; Meinnel, T.; Giglione, C.; Brandizzi, F. Golgi traffic and integrity depend on N-myristoyl transferase-1 in Arabidopsis. Plant Cell 2013, 25, 1756–1773. [Google Scholar] [CrossRef]
- Urata, S.; Yasuda, J.; de la Torre, J.C. The z protein of the new world arenavirus tacaribe virus has bona fide budding activity that does not depend on known late domain motifs. J. Virol. 2009, 83, 12651–12655. [Google Scholar] [CrossRef]
- Vaandrager, A.B.; Ehlert, E.M.; Jarchau, T.; Lohmann, S.M.; de Jonge, H.R. N-terminal myristoylation is required for membrane localization of cGMP-dependent protein kinase type II. J. Biol. Chem. 1996, 271, 7025–7029. [Google Scholar] [CrossRef]
- Van’t Hof, W.; Resh, M.D. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J. Cell Biol. 1999, 145, 377–389. [Google Scholar] [CrossRef]
- Vilas, G.L.; Corvi, M.M.; Plummer, G.J.; Seime, A.M.; Lambkin, G.R.; Berthiaume, L.G. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc. Natl. Acad. Sci. USA 2006, 103, 6542–6547. [Google Scholar] [CrossRef]
- Galbiati, F.; Guzzi, F.; Magee, A.I.; Milligan, G.; Parenti, M. Chemical inhibition of myristoylation of the G-protein Gi1 alpha by 2-hydroxymyristate does not interfere with its palmitoylation or membrane association. Evidence that palmitoylation, but not myristoylation, regulates membrane attachment. Biochem. J. 1996, 313 Pt 3, 717–720. [Google Scholar] [CrossRef]
- Kallemeijn, W.W.; Lueg, G.A.; Faronato, M.; Hadavizadeh, K.; Goya Grocin, A.; Song, O.R.; Howell, M.; Calado, D.P.; Tate, E.W. Validation and Invalidation of Chemical Probes for the Human N-myristoyltransferases. Cell Chem. Biol. 2019, 26, 892–900.e894. [Google Scholar] [CrossRef]
- Charron, G.; Zhang, M.M.; Yount, J.S.; Wilson, J.; Raghavan, A.S.; Shamir, E.; Hang, H.C. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J. Am. Chem. Soc. 2009, 131, 4967–4975. [Google Scholar] [CrossRef]
- Ritzefeld, M.; Wright, M.H.; Tate, E.W. New developments in probing and targeting protein acylation in malaria, leishmaniasis and African sleeping sickness. Parasitology 2018, 145, 157–174. [Google Scholar] [CrossRef]
- Wright, M.H.; Clough, B.; Rackham, M.D.; Rangachari, K.; Brannigan, J.A.; Grainger, M.; Moss, D.K.; Bottrill, A.R.; Heal, W.P.; Broncel, M.; et al. Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach. Nat. Chem. 2014, 6, 112–121. [Google Scholar] [CrossRef]
- Broncel, M.; Dominicus, C.; Vigetti, L.; Nofal, S.D.; Bartlett, E.J.; Touquet, B.; Hunt, A.; Wallbank, B.A.; Federico, S.; Matthews, S.; et al. Profiling of myristoylation in Toxoplasma gondii reveals an N-myristoylated protein important for host cell penetration. Elife 2020, 9. [Google Scholar] [CrossRef]
- Thinon, E.; Serwa, R.A.; Broncel, M.; Brannigan, J.A.; Brassat, U.; Wright, M.H.; Heal, W.P.; Wilkinson, A.J.; Mann, D.J.; Tate, E.W. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat. Commun. 2014, 5, 4919. [Google Scholar] [CrossRef]
- Witten, A.J.; Ejendal, K.F.K.; Gengelbach, L.M.; Traore, M.A.; Wang, X.; Umulis, D.M.; Calve, S.; Kinzer-Ursem, T.L. Fluorescent imaging of protein myristoylation during cellular differentiation and development. J. Lipid Res. 2017, 58, 2061–2070. [Google Scholar] [CrossRef]
- Su, D.; Kosciuk, T.; Lin, H. Reply to Comment on “Binding Affinity Determines Substrate Specificity and Enables Discovery of substrates for N-Myristoyltransferases”. ACS Catal. 2022, 12, 8829–8832. [Google Scholar] [CrossRef]
- Islinger, M.; Costello, J.L.; Kors, S.; Soupene, E.; Levine, T.P.; Kuypers, F.A.; Schrader, M. The diversity of ACBD proteins—From lipid binding to protein modulators and organelle tethers. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118675. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Kuypers, F.A. Ligand binding to the ACBD6 protein regulates the acyl-CoA transferase reactions in membranes. J. Lipid Res. 2015, 56, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Wang, D.; Kuypers, F.A. Remodeling of host phosphatidylcholine by Chlamydia acyltransferase is regulated by acyl-CoA binding protein ACBD6 associated with lipid droplets. Microbiologyopen 2015, 4, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Kuypers, F.A. Phosphatidylserine decarboxylase CT699, lysophospholipid acyltransferase CT775, and acyl-ACP synthase CT776 provide membrane lipid diversity to Chlamydia trachomatis. Sci. Rep. 2017, 7, 15767. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, J.A.; Smith, B.A.; Yu, Z.; Brzozowski, A.M.; Hodgkinson, M.R.; Maroof, A.; Price, H.P.; Meier, F.; Leatherbarrow, R.J.; Tate, E.W.; et al. N-myristoyltransferase from Leishmania donovani: Structural and functional characterisation of a potential drug target for visceral leishmaniasis. J. Mol. Biol. 2010, 396, 985–999. [Google Scholar] [CrossRef]
- Dian, C.; Perez-Dorado, I.; Riviere, F.; Asensio, T.; Legrand, P.; Ritzefeld, M.; Shen, M.; Cota, E.; Meinnel, T.; Tate, E.W.; et al. High-resolution snapshots of human N-myristoyltransferase in action illuminate a mechanism promoting N-terminal Lys and Gly myristoylation. Nat. Commun. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Mousnier, A.; Bell, A.S.; Swieboda, D.P.; Morales-Sanfrutos, J.; Perez-Dorado, I.; Brannigan, J.A.; Newman, J.; Ritzefeld, M.; Hutton, J.A.; Guedan, A.; et al. Fragment-derived inhibitors of human N-myristoyltransferase block capsid assembly and replication of the common cold virus. Nat. Chem. 2018, 10, 599–606. [Google Scholar] [CrossRef]
- Boyle, J.J.; Ludwig, E.H. Analysis of fatty acids of continuously cultured mammalian cells by gas-liquid chromatography. Nature 1962, 196, 893–894. [Google Scholar] [CrossRef]
- Shindou, H.; Hishikawa, D.; Harayama, T.; Eto, M.; Shimizu, T. Generation of membrane diversity by lysophospholipid acyltransferases. J. Biochem. 2013, 154, 21–28. [Google Scholar] [CrossRef]
- Valentine, W.J.; Yanagida, K.; Kawana, H.; Kono, N.; Noda, N.N.; Aoki, J.; Shindou, H. Update and nomenclature proposal for mammalian lysophospholipid acyltransferases, which create membrane phospholipid diversity. J. Biol. Chem. 2022, 298, 101470. [Google Scholar] [CrossRef]
- Soupene, E.; Kuypers, F.A. Phosphatidylcholine formation by LPCAT1 is regulated by Ca(2+) and the redox status of the cell. BMC Biochem. 2012, 13, 8. [Google Scholar] [CrossRef]
- Lands, W.E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J. Biol. Chem. 1960, 235, 2233–2237. [Google Scholar] [CrossRef]
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339. [Google Scholar] [CrossRef]
- Beilstein, F.; Lemasson, M.; Pène, V.; Rainteau, D.; Demignot, S.; Rosenberg, A.R. Lysophosphatidylcholine acyltransferase 1 is downregulated by hepatitis C virus: Impact on production of lipo-viro-particles. Gut 2017, 66, 2160–2169. [Google Scholar] [CrossRef]
- Cotte, A.K.; Aires, V.; Fredon, M.; Limagne, E.; Derangère, V.; Thibaudin, M.; Humblin, E.; Scagliarini, A.; de Barros, J.P.; Hillon, P.; et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 2018, 9, 322. [Google Scholar] [CrossRef]
- Knudsen, J.; Mandrup, S.; Rasmussen, J.T.; Andreasen, P.H.; Poulsen, F.; Kristiansen, K. The function of acyl-CoA-binding protein (ACBP)/diazepam binding inhibitor (DBI). Mol. Cell. Biochem. 1993, 123, 129–138. [Google Scholar] [CrossRef]
- Rasmussen, J.T.; Rosendal, J.; Knudsen, J. Interaction of acyl-CoA binding protein (ACBP) on processes for which acyl-CoA is a substrate, product or inhibitor. Biochem. J. 1993, 292 Pt 3, 907–913. [Google Scholar] [CrossRef]
- Elle, I.C.; Simonsen, K.T.; Olsen, L.C.; Birck, P.K.; Ehmsen, S.; Tuck, S.; Le, T.T.; Faergeman, N.J. Tissue- and paralogue-specific functions of acyl-CoA-binding proteins in lipid metabolism in Caenorhabditis elegans. Biochem. J. 2011, 437, 231–241. [Google Scholar] [CrossRef]
- Heal, W.P.; Wickramasinghe, S.R.; Bowyer, P.W.; Holder, A.A.; Smith, D.F.; Leatherbarrow, R.J.; Tate, E.W. Site-specific N-terminal labelling of proteins in vitro and in vivo using N-myristoyl transferase and bioorthogonal ligation chemistry. Chem. Commun. 2008, 4, 480–482. [Google Scholar] [CrossRef]
- Heal, W.P.; Wickramasinghe, S.R.; Leatherbarrow, R.J.; Tate, E.W. N-Myristoyl transferase-mediated protein labelling in vivo. Org. Biomol. Chem. 2008, 6, 2308–2315. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soupene, E.; Kuypers, F.A. Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins. Biomolecules 2022, 12, 1726. https://doi.org/10.3390/biom12121726
Soupene E, Kuypers FA. Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins. Biomolecules. 2022; 12(12):1726. https://doi.org/10.3390/biom12121726
Chicago/Turabian StyleSoupene, Eric, and Frans A. Kuypers. 2022. "Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins" Biomolecules 12, no. 12: 1726. https://doi.org/10.3390/biom12121726
APA StyleSoupene, E., & Kuypers, F. A. (2022). Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins. Biomolecules, 12(12), 1726. https://doi.org/10.3390/biom12121726



