Foam-in-Vein: Characterisation of Blood Displacement Efficacy of Liquid Sclerosing Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physician Compounded Foam (PCF) Formulation
2.2. Measuring Foam Apparent Viscosity Using Pipe Viscometry
2.3. Selection of a Blood Surrogate Fluid
2.4. Displacement Flow of Sclerosing Foams and a Blood Surrogate
3. Results and Discussion
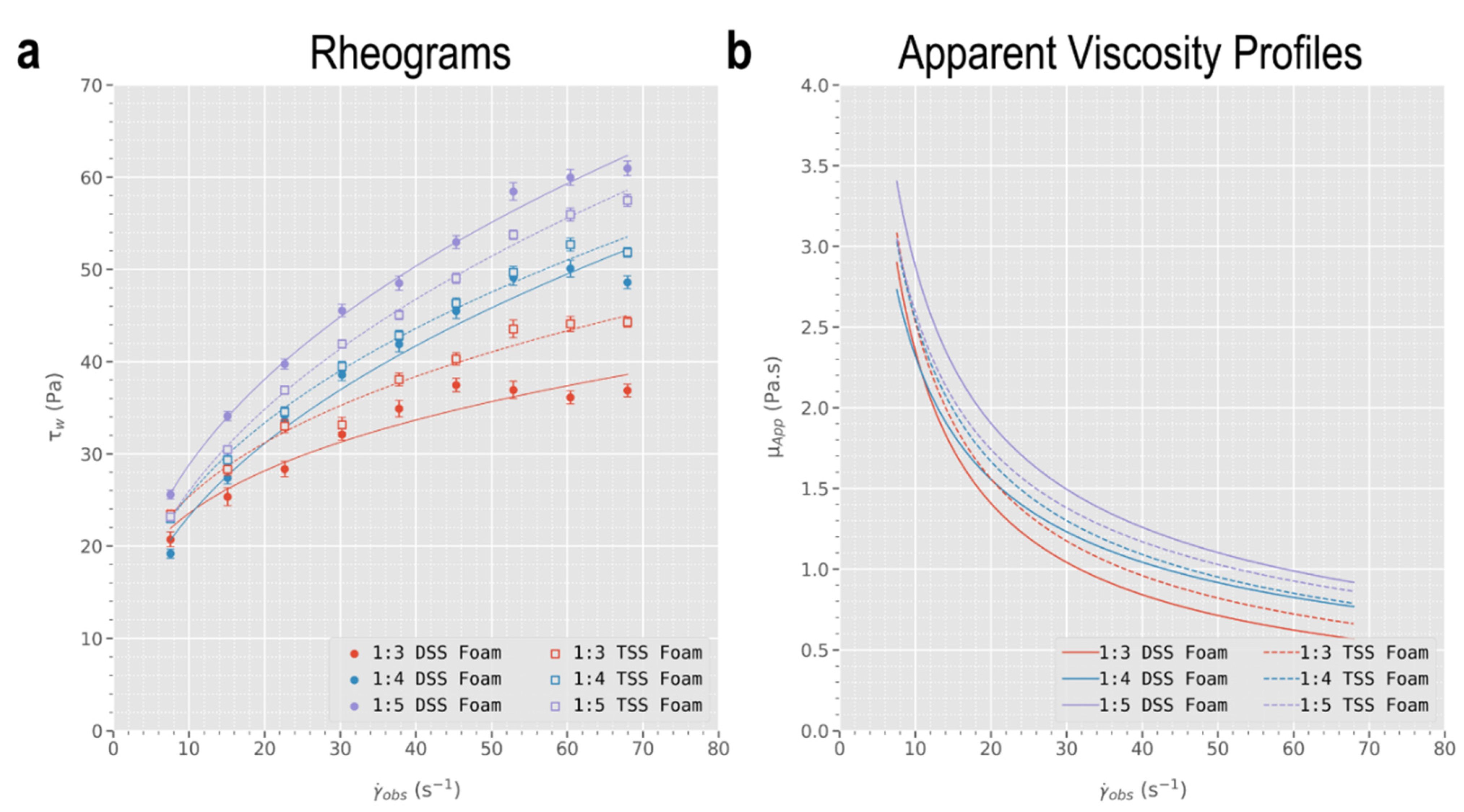
3.1. Characterisation of Foams’ Apparent Viscosity against Different Formulations
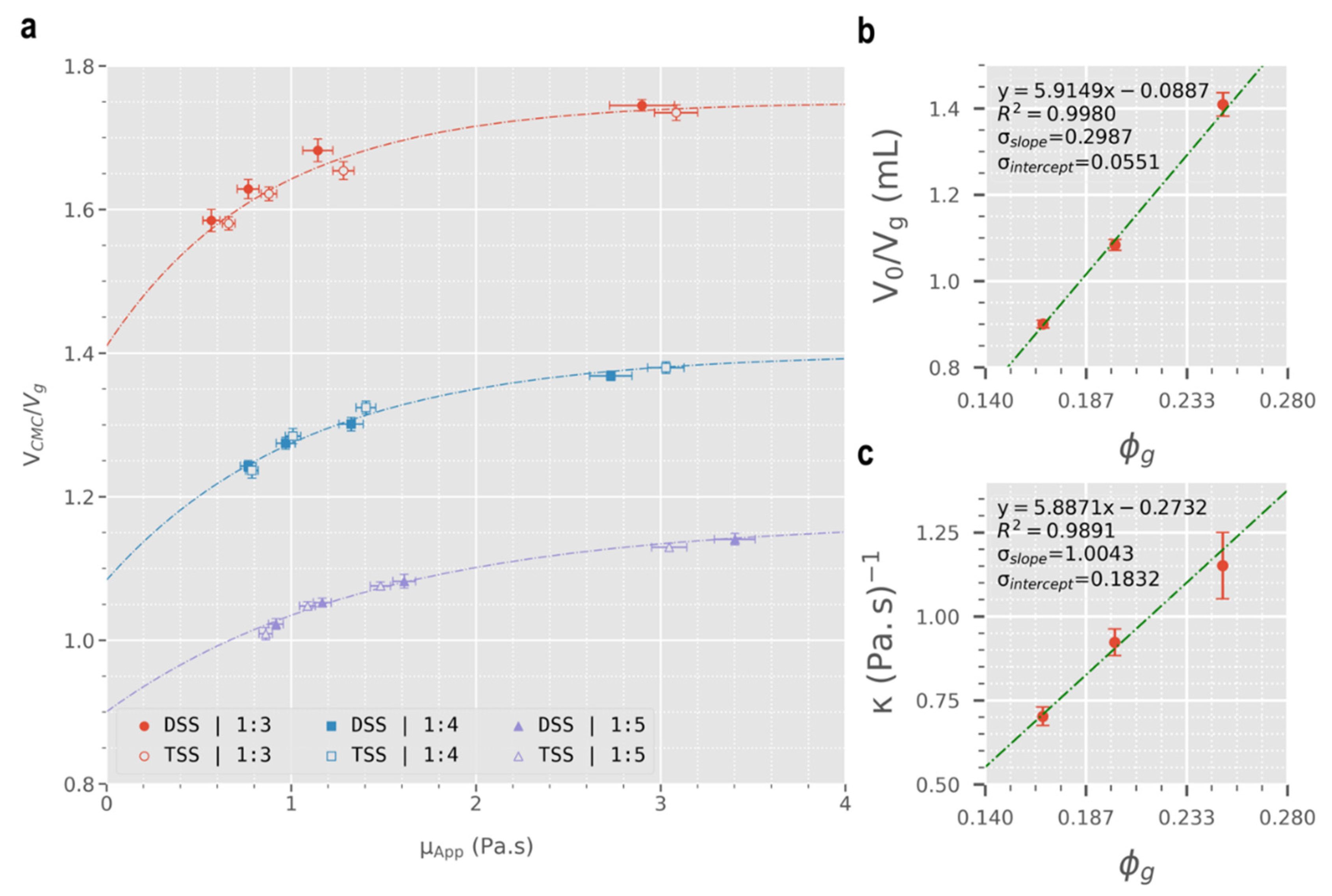
3.2. Quantification of Sclerosing Foams’ Efficacy at Displacing a Blood Surrogate
3.3. Prediction of Clinical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, M.-J.; Kim, Y.-K.; Kang, D.-M.; Kim, J.-E.; Ha, W.-C.; Jung, K.-Y.; Choi, H.-W. A Study on Prevalence and Risk Factors for Varicose Veins in Nurses at a University Hospital. Saf. Health Work. 2018, 9, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Mowatt, G.; Burr, J.M.; Cassar, K.; Cook, J.; Fraser, C. Systematic review of foam sclerotherapy for varicose veins. Br. J. Surg. 2007, 94, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Meghdadi, A.; Jones, S.A.; Patel, V.A.; Lewis, A.L.; Millar, T.M.; Carugo, D. Foam-in-vein: A review of rheological properties and characterization methods for optimization of sclerosing foams. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 109, 69–91. [Google Scholar] [CrossRef]
- Connor, D.E.; Cooley-Andrade, O.; Goh, W.X.; Ma, D.D.F.; Parsi, K. Detergent sclerosants are deactivated and consumed by circulating blood cells. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, M.R. Deactivation of sodium tetradecyl sulphate injection by blood proteins. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Parsi, K.; Exner, T.; Connor, D.E.; Herbert, A.; Ma, D.D.F.; Joseph, J.E. The Lytic Effects of Detergent Sclerosants on Erythrocytes, Platelets, Endothelial Cells and Microparticles are Attenuated by Albumin and other Plasma Components in Vitro. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Parsi, K. Interaction of detergent sclerosants with cell membranes. Phlebology 2015, 30, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Jiang, W.; Chen, Y.; Yan, F.; Xu, Z.; Fan, Y. Effect of Multiple Factors on Foam Stability in Foam Sclerotherapy. Sci. Rep. 2018, 8, 15683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottaro, E.; Paterson, J.; Zhang, X.; Hill, M.; Patel, V.A.; Jones, S.A.; Lewis, A.L.; Millar, T.M.; Carugo, D. Physical Vein Models to Quantify the Flow Performance of Sclerosing Foams. Front. Bioeng. Biotechnol. 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Chen, T.; Connor, D.E.; Behnia, M.; Parsi, K. Sclerosant foam structure and stability is strongly influenced by liquid air fraction. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Ankrett, D.N.; O’Byrne, V.; Wright, D.D.I.; Lewis, A.L.; Hill, M.; Zhang, X. The role of clinically-relevant parameters on the cohesiveness of sclerosing foams in a biomimetic vein model. J. Mater. Sci. Mater. Med. 2015, 26, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nastasa, V.; Samaras, K.; Ampatzidis, C.; Karapantsios, T.D.; Trelles, M.A.; Moreno-Moraga, J.; Smarandache, A.; Pascu, M.L. Properties of polidocanol foam in view of its use in sclerotherapy. Int. J. Pharm. 2015, 478, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Chen, T.; Connor, D.E.; Behnia, M.; Parsi, K. Basic physiochemical and rheological properties of detergent sclerosants. Phlebology 2015, 30, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Meghdadi, A.; Jones, S.A.; Patel, V.A.; Lewis, A.L.; Millar, T.M.; Carugo, D. Foam-in-vein: Rheological characterisation of liquid sclerosing foams using a pipe viscometer. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128916. [Google Scholar] [CrossRef]
- Bottaro, E.; Paterson, J.A.J.; Quercia, L.; Zhang, X.; Hill, M.; Patel, V.A.; Jones, S.A.; Lewis, A.L.; Millar, T.M.; Carugo, D. In vitro and ex vivo evaluation of the biological performance of sclerosing foams. Sci. Rep. 2019, 9, 9880. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Crooks, S.; Eckmann, D.M. Dose- and time-dependent liquid sclerosant effects on endothelial cell death. Dermatol. Surg. 2006, 32, 1444–1452. [Google Scholar]
- Mol, W.; Furukawa, H.; Sasaki, S.; Tomaru, U.; Hayashi, T.; Saito, A.; Nagao, M.; Saito, N.; Hata, S.; Yamamoto, Y. Evaluation of the sclerotherapeutic efficacy of ethanol, polidocanol, and OK-432 using an in vitro model. Dermatol. Surg. 2007, 33, 1452–1459. [Google Scholar] [CrossRef]
- Carugo, D.; Ankrett, D.N.; Zhao, X.; Zhang, X.; Hill, M.; O’Byrne, V.; Hoad, J.; Arif, M.; Wright, D.D.I.; Lewis, A.L. Benefits of polidocanol endovenous microfoam (Varithena®) compared with physician-compounded foams. Phlebology 2016, 31, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Uchida, B.; Horikawa, M.; Mimura, H.; Farsad, K. Effects of Different Mixing Agents on the Stability of Sodium Tetradecyl Sulfate (STS) Foam: An Experimental Study. CardioVascular Interv. Radiol. 2018, 41, 1952–1957. [Google Scholar] [CrossRef]
- Eslami, A.; Mollaabbasi, R.; Roustaei, A.; Taghavi, S.M. Pressure-driven displacement flows of yield stress fluids: Viscosity ratio effects. Can. J. Chem. Eng. 2019, 97, 2804–2817. [Google Scholar] [CrossRef]
- Etrati, A.; Alba, K.; Frigaard, I.A. Two-layer displacement flow of miscible fluids with viscosity ratio: Experiments. Phys. Fluids 2018, 30, 052103. [Google Scholar] [CrossRef]
- Etrati, A.; Frigaard, I.A. Viscosity effects in density-stable miscible displacement flows: Experiments and simulations. Phys. Fluids 2018, 30, 123104. [Google Scholar] [CrossRef]
- Taghavi, S.M.; Séon, T.; Martinez, D.M.; Frigaard, I.A. Influence of an imposed flow on the stability of a gravity current in a near horizontal duct. Phys. Fluids 2010, 22, 031702. [Google Scholar] [CrossRef]
- Alba, K.; Taghavi, S.M.; de Bruyn, J.R.; Frigaard, I.A. Incomplete fluid–fluid displacement of yield-stress fluids. Part 2: Highly inclined pipes. J. Non-Newton. Fluid Mech. 2013, 201, 80–93. [Google Scholar] [CrossRef]
- Taghavi, S.M.; Alba, K.; Moyers-Gonzalez, M.; Frigaard, I.A. Incomplete fluid–fluid displacement of yield stress fluids in near-horizontal pipes: Experiments and theory. J. Non-Newton. Fluid Mech. 2012, 167–168, 59–74. [Google Scholar] [CrossRef]
- Taghavi, S.M.; Alba, K.; Seon, T.; Wielage-Burchard, K.; Martinez, D.M.; Frigaard, I.A. Miscible displacement flows in near-horizontal ducts at low Atwood number. J. Fluid Mech. 2012, 696, 175–214. [Google Scholar] [CrossRef] [Green Version]
- Taghavi, S.M.; Seon, T.; Martinez, D.M.; Frigaard, I.A. Buoyancy-dominated displacement flows in near-horizontal channels: The viscous limit. J. Fluid Mech. 2009, 639, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Taghavi, S.M.; Séon, T.; Wielage-Burchard, K.; Martinez, D.M.; Frigaard, I.A. Stationary residual layers in buoyant Newtonian displacement flows. Phys. Fluids 2011, 23, 044105. [Google Scholar] [CrossRef]
- Rasmussen, H.K.; Hassager, O.; Saasen, A. Viscous flow with large fluid–fluid interface displacement. Int. J. Numer. Methods Fluids 1998, 28, 859–881. [Google Scholar] [CrossRef]
- Zhou, M.-Y.; Sheng, P. Dynamics of immiscible-fluid displacement in a capillary tube. Phys. Rev. Lett. 1990, 64, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Vitello, D.J.; Ripper, R.M.; Fettiplace, M.R.; Weinberg, G.L.; Vitello, J.M. Blood Density Is Nearly Equal to Water Density: A Validation Study of the Gravimetric Method of Measuring Intraoperative Blood Loss. J. Vet. Med. 2015, 2015, 152730. [Google Scholar] [CrossRef]
- Alba, K.; Taghavi, S.M.; Frigaard, I.A. Miscible density-stable displacement flows in inclined tube. Phys. Fluids 2012, 24, 123102. [Google Scholar] [CrossRef]
- Cavezzi, A.; Tessari, L. Foam sclerotherapy techniques: Different gases and methods of preparation, catheter versus direct injection. Phlebology 2009, 24, 247–251. [Google Scholar] [CrossRef]
- Hamel-Desnos, C.; Ouvry, P.; Benigni, J.P.; Boitelle, G.; Schadeck, M.; Desnos, P.; Allaert, F.A. Comparison of 1% and 3% Polidocanol Foam in Ultrasound Guided Sclerotherapy of the Great Saphenous Vein: A Randomised, Double-Blind Trial with 2 Year-Follow-up. “The 3/1 Study”. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaki, T.; Nozaki, M.; Sakurai, H.; Takeuchi, M.; Soejima, K.; Kono, T. Multiple Small-Dose Injections Can Reduce the Passage of Sclerosant Foam into Deep Veins During Foam Sclerotherapy for Varicose Veins. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessari, L.; Cavezzi, A.; Frullini, A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol. Surg. 2001, 27, 58–60. [Google Scholar] [PubMed]
- Musil, D.; Herman, J.; Mazuch, J. Width of the Great Saphenous Vein Lumen in the Groin and Occurrence of Significant Reflux in the Sapheno-Femoral Junction. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2008, 152, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urrutia, A.; Fernandez de Arroiabe, P.; Ramirez, M.; Martinez-Agirre, M.; Mounir Bou-Ali, M. Contact angle measurement for LiBr aqueous solutions on different surface materials used in absorption systems. Int. J. Refrig. 2018, 95, 182–188. [Google Scholar] [CrossRef]
- Boyce, J.F.; Wong, P.C.; Schurch, S.; Roach, M.R. Rabbit arterial endothelium and subendothelium. A change in interfacial free energy that may promote initial platelet adhesion. Circ. Res. 1983, 53, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Mallik, B.B.; Das, S.; Chatterjee, S.S.; Ghosh, S. A Study on Bingham Plastic Characteristics of blood flow through multiple overlapped stenosed arteries. Saudi J. Eng. Technol. 2017, 2, 349–357. [Google Scholar]
- Shibeshi, S.S.; Collins, W.E. The rheology of blood flow in a branced arterial system. Appl. Rheol. 2005, 15, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.A.; Deutsch, S.; Tarbell, J.M.; Geselowitz, D.B.; Rosenberg, G.; Pierce, W.S. An experimental study of newtonian and non-newtonian flow dynamics in a ventricular assist device. J. Biomech. Eng. 1987, 109, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mowlavi, S.; Engmann, J.; Burbidge, A.; Lloyd, R.; Hayoun, P.; Le Reverend, B.; Ramaioli, M. In vivo observations and in vitro experiments on the oral phase of swallowing of Newtonian and shear-thinning liquids. J. Biomech. 2016, 49, 3788–3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173–1180. [Google Scholar] [CrossRef]
- Benslimane, A.; Bekkour, K.; François, P.; Sadaoui, D.; Benchabane, A. Carboxymethyl Cellulose: Rheological and Pipe Flow Properties. Recent Adv. Petrochem. Sci. 2019, 5, 555675. [Google Scholar]
- Enzendorfer, C.; Harris, R.A.; Valkó, P.; Economides, M.J.; Fokker, P.A.; Davies, D.D. Pipe viscometry of foams. J. Rheol. 2002, 39, 345–358. [Google Scholar] [CrossRef]
- Gardiner, B.S.; Dlugogorski, B.Z.; Jameson, G.J. Rheology of fire-fighting foams. Fire Saf. J. 1998, 31, 61–75. [Google Scholar] [CrossRef]
- Larmignat, S.; Vanderpool, D.; Lai, H.K.; Pilon, L. Rheology of colloidal gas aphrons (microfoams). Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Osei-Bonsu, K.; Shokri, N.; Grassia, P. Fundamental investigation of foam flow in a liquid-filled Hele-Shaw cell. J. Colloid Interface Sci. 2016, 462, 288–296. [Google Scholar] [CrossRef]

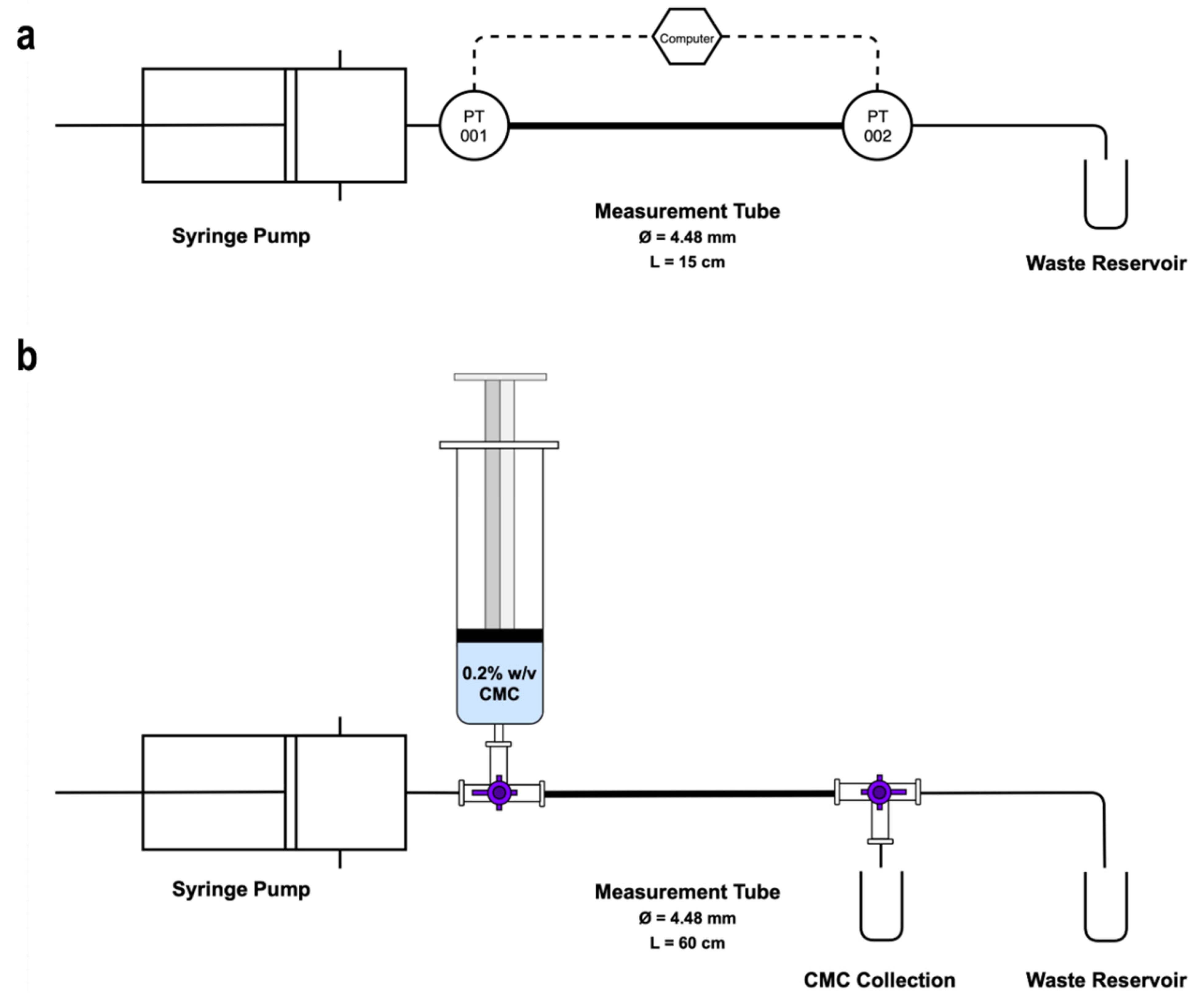

| Volume of Foam | PCF Formulations | Flowrates | Tube ID | Tube Length | Wall Shear Rates | Repeats | |
|---|---|---|---|---|---|---|---|
| Pipe Viscometry | 8 mL | DSS 1:3, 1:4, 1:5 TSS 1:3, 1:4, 1:5 | 4, 14, 24, 36 mL·min−1 | 4.48 mm | 15 cm | 7.55–67.97 s−1 | |
| Blood Displacement | 7 mL | 60 cm |
| Original Data | ||||||
|---|---|---|---|---|---|---|
| slope | y-intercept | p-value | ||||
| 2.4302 | 1.3895 | 4.9776 | 0.2661 | 0.8843 | 0.2210 | |
| 5.8871 | 1.0043 | −0.2733 | 0.1832 | 0.9891 | 0.0665 | |
| Normalised Data | ||||||
| slope | y-intercept | p-value | ||||
| 5.9149 | 0.2987 | −0.0887 | 0.0551 | 0.9980 | 0.0288 | |
| 5.8871 | 1.0043 | −0.2732 | 0.1832 | 0.9891 | 0.0665 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meghdadi, A.; Jones, S.A.; Patel, V.A.; Lewis, A.L.; Millar, T.M.; Carugo, D. Foam-in-Vein: Characterisation of Blood Displacement Efficacy of Liquid Sclerosing Foams. Biomolecules 2022, 12, 1725. https://doi.org/10.3390/biom12121725
Meghdadi A, Jones SA, Patel VA, Lewis AL, Millar TM, Carugo D. Foam-in-Vein: Characterisation of Blood Displacement Efficacy of Liquid Sclerosing Foams. Biomolecules. 2022; 12(12):1725. https://doi.org/10.3390/biom12121725
Chicago/Turabian StyleMeghdadi, Alireza, Stephen A. Jones, Venisha A. Patel, Andrew L. Lewis, Timothy M. Millar, and Dario Carugo. 2022. "Foam-in-Vein: Characterisation of Blood Displacement Efficacy of Liquid Sclerosing Foams" Biomolecules 12, no. 12: 1725. https://doi.org/10.3390/biom12121725
APA StyleMeghdadi, A., Jones, S. A., Patel, V. A., Lewis, A. L., Millar, T. M., & Carugo, D. (2022). Foam-in-Vein: Characterisation of Blood Displacement Efficacy of Liquid Sclerosing Foams. Biomolecules, 12(12), 1725. https://doi.org/10.3390/biom12121725







