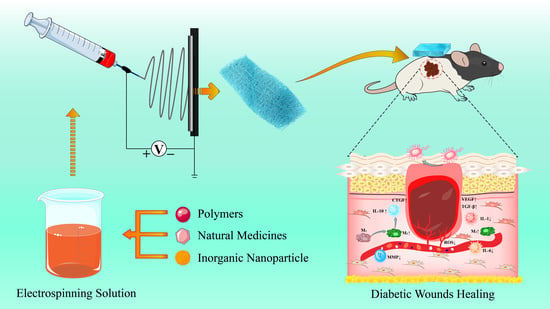
Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing
Abstract
1. Introduction
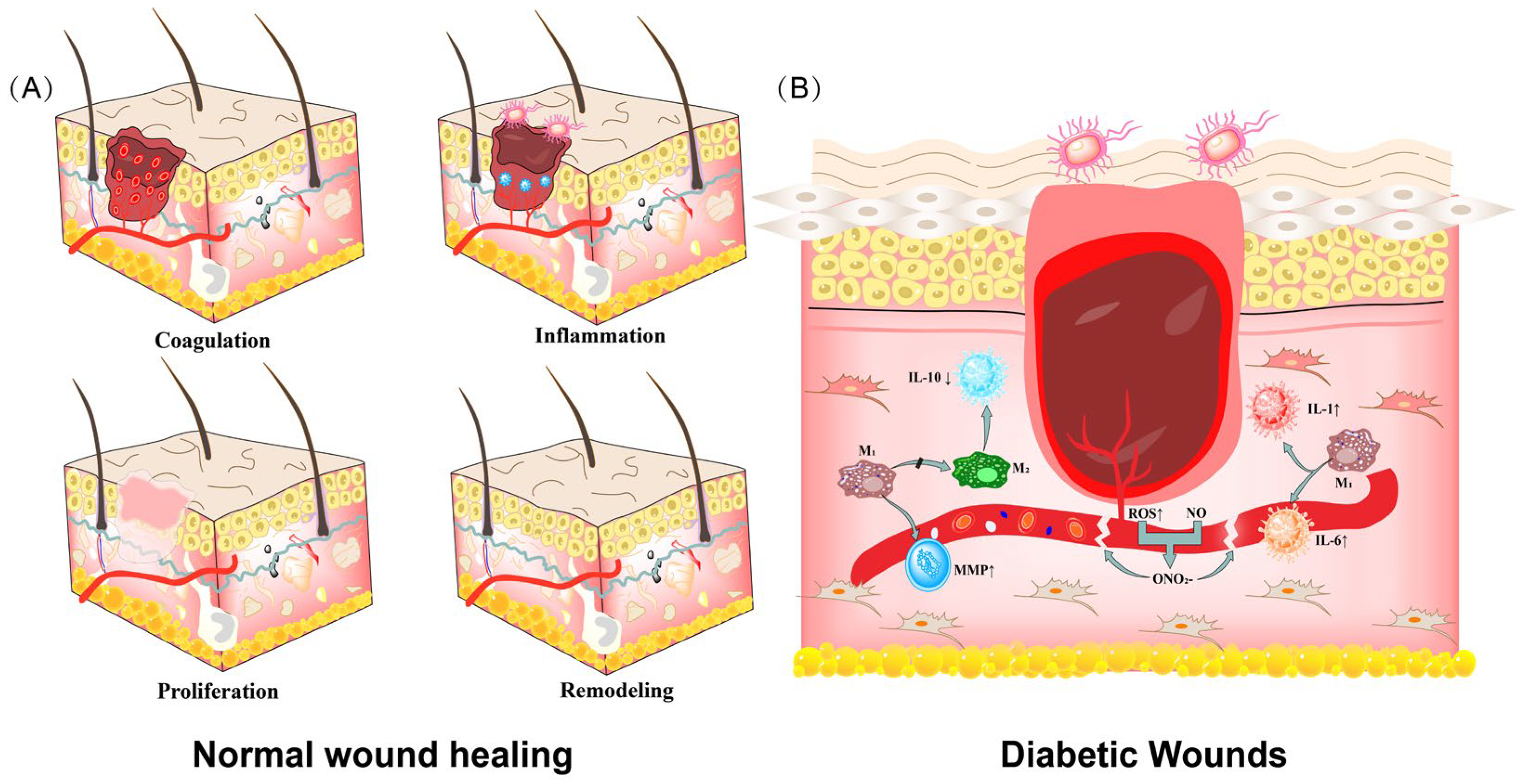
2. Diabetic Wounds and Diabetic Dressings
2.1. Diabetic Wounds
2.2. Diabetic Dressings
2.2.1. Basic Dressings
2.2.2. Antimicrobial Active Dressing
2.2.3. Chinese Medicine Poultice Dressing
2.2.4. Bioactive Dressings
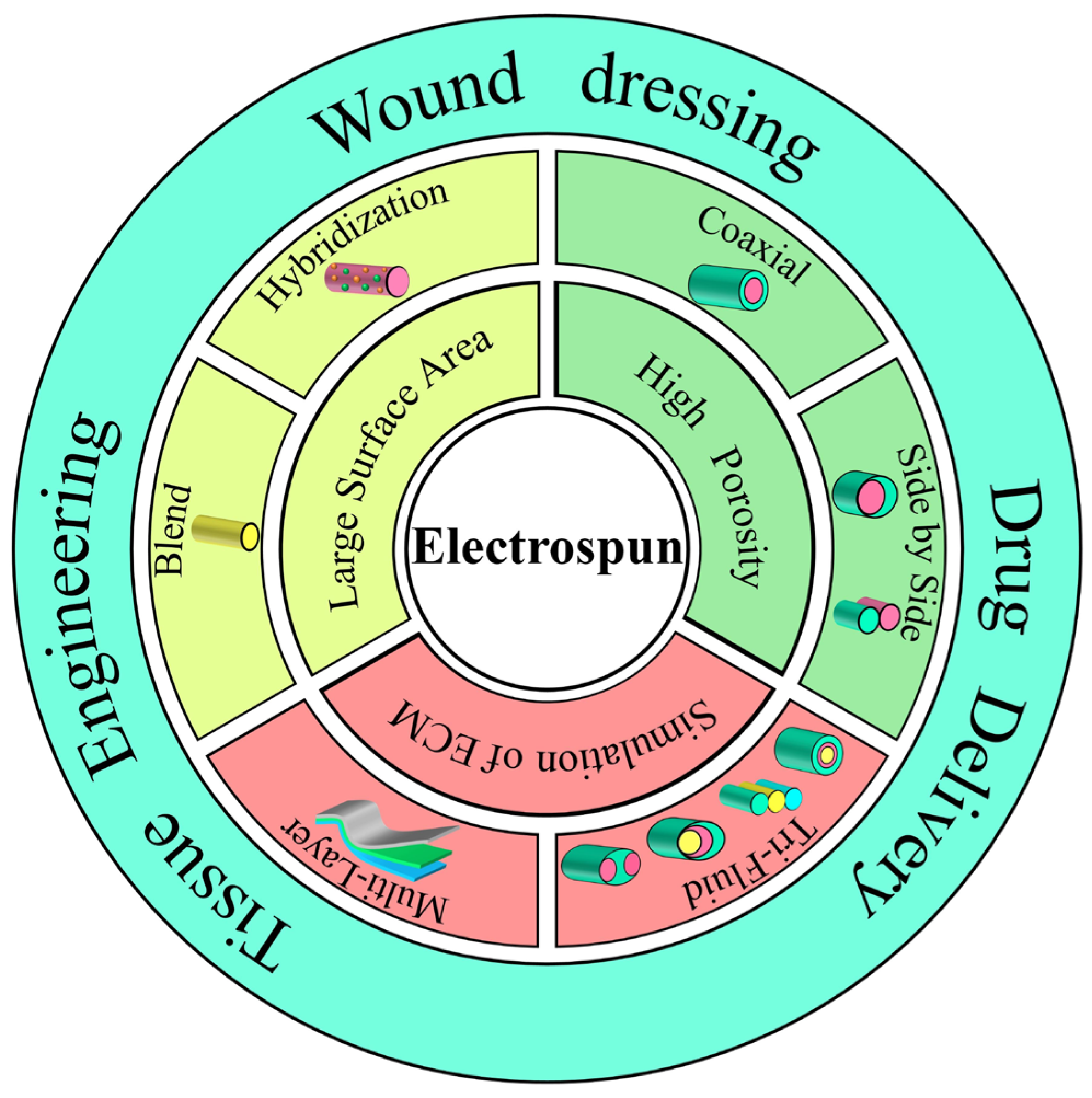
3. Electrospinning
3.1. Electrospinning Technology
3.2. Classification of Electrospinning Process
3.2.1. Single-Fluid Electrospinning
3.2.2. Multifluid Electrospinning
Coaxial Electrospinning
Side-by-Side Electrospinning
Multifluid Electrospinning
4. Electrospinning Nanofiber in Diabetic Wound Therapy
4.1. Natural Polymer Electrospinning Fiber for Diabetic Wounds
4.2. Synthetic Polymer Electrospinning Fiber for Diabetic Wounds
4.3. Natural/Synthetic Polymer Hybrid Electrospinning Fiber for Diabetic Wounds
4.4. Electrospinning Fiber Multi-Drug Combination for Diabetic Wounds
4.4.1. Inorganic Nanoparticle and Natural Drug Combination Promote Diabetic Wound Healing
4.4.2. Growth Factor-Loaded Electrospun Wound Dressing Promotes Diabetic Wound Healing
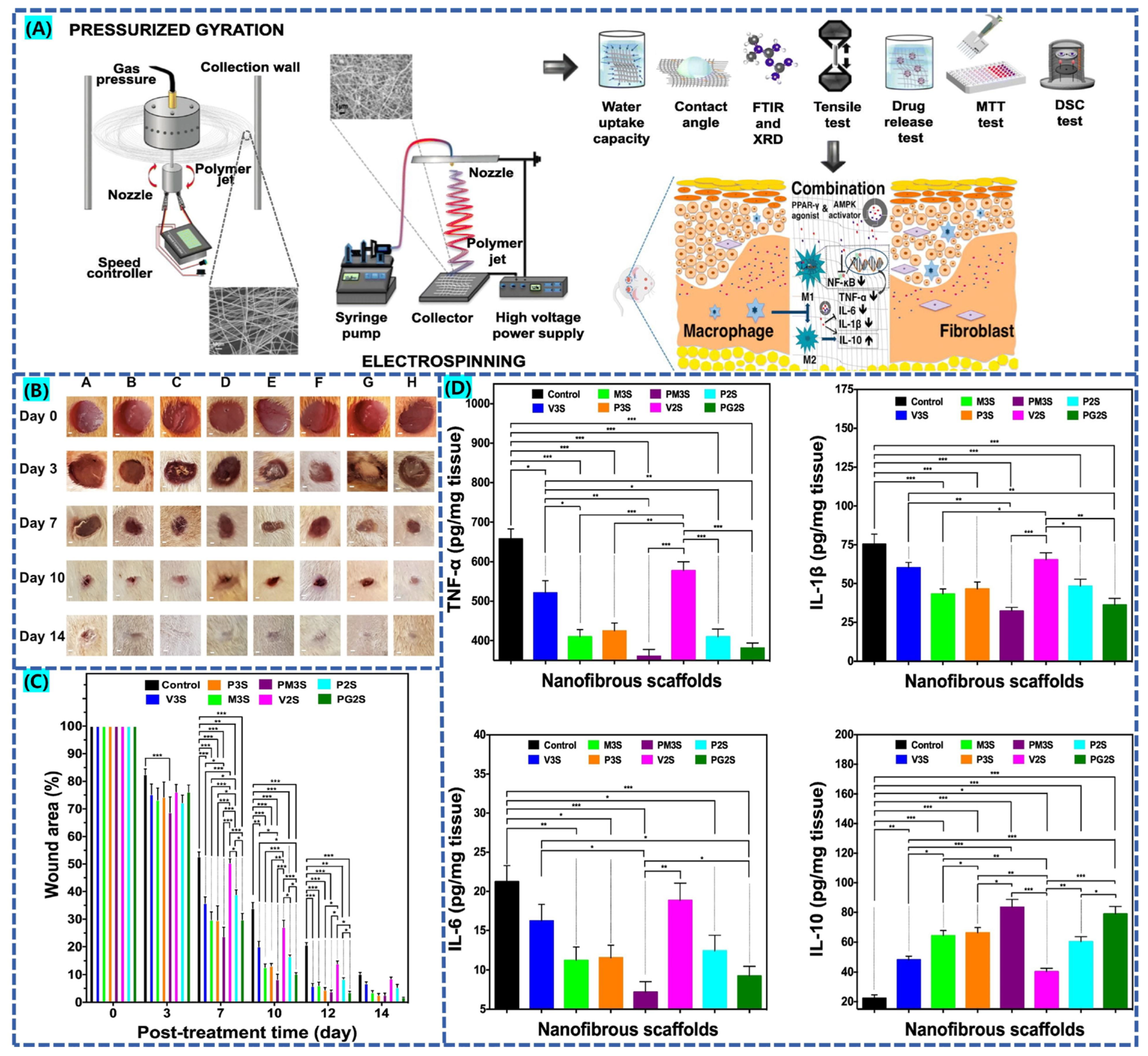
4.4.3. Combination of Glucose-Lowering Drugs to Promote Diabetic Wound Healing
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, J.; Wang, H.; Yang, K.; Wang, H.; Duan, C.; Ni, N.; An, L.; Luo, Y.; Zhao, P.; Gou, Y.; et al. Reversibly immortalized keratinocytes (ikera) facilitate re-epithelization and skin wound healing: Potential applications in cell-based skin tissue engineering. Bioact. Mater. 2022, 9, 523–540. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The role of extracellular matrix in skin wound healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef]
- Hao, R.; Cui, Z.; Zhang, X.; Tian, M.; Zhang, L.; Rao, F.; Xue, J. Rational design and preparation of functional hydrogels for skin wound healing. Front. Chem. 2022, 9, 839055. [Google Scholar] [CrossRef]
- Kimura, S.; Tsuji, T. Mechanical and immunological regulation in wound healing and skin reconstruction. Int. J. Mol. Sci. 2021, 22, 5474. [Google Scholar] [CrossRef]
- Xie, J.; Wu, X.; Zheng, S.; Lin, K.; Su, J. Aligned electrospun poly(l-lactide) nanofibers facilitate wound healing by inhibiting macrophage m1 polarization via the jak-stat and nf-κb pathways. J. Nanobiotechnol. 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Liu, J.; Xiao, J. Lymphatic regulation in tissue repair and regeneration: Recent advance and future perspective. Curr. Stem Cell Res. T. 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Wang, L.; Pan, W.; Chen, K. Polysaccharide electrospun nanofibers for wound healing applications. Int. J. Nanomed. 2022, 17, 3913–3931. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Keni, R.; Begum, F.; Gourishetti, K.; Viswanatha, G.L.; Nayak, P.G.; Nandakumar, K.; Shenoy, R.R. Diabetic wound healing approaches: An update. J. Basic. Clin. Physiol. Pharmacol. 2022, in press. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in diabetic wound healing, inflammation, and scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Matoori, S.; Veves, A.; Mooney, D.J. Advanced bandages for diabetic wound healing. Sci. Transl. Med. 2021, 13, eabe4839. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.; Avsar, P.; Wilson, P.; Mairghani, M.; O’Connor, T.; Nugent, L.; Moore, Z. Treatment of diabetic foot ulcers: Review of the literature with regard to the TIME clinical decision support tool. J. Wound Care 2022, 31, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, H.; Liang, Y.; Xuan, M.; Liu, G.; Xie, H. Hyaluronic acid-methacrylic anhydride/polyhexamethylene biguanide hybrid hydrogel with antibacterial and proangiogenic functions for diabetic wound repair. Chin. Chem. Lett. 2022, 33, 5030–5034. [Google Scholar] [CrossRef]
- Pamu, D.; Tallapaneni, V.; Karri, V.V.S.R.; Singh, S.K. Biomedical applications of electrospun nanofibers in the management of diabetic wounds. Drug Deliv. Transl. Res. 2022, 12, 158–166. [Google Scholar] [CrossRef]
- Priya, S.; Batra, U.; Samshritha, R.N.; Sharma, S.; Chaurasiya, A.; Singhvi, G. Polysaccharide-based nanofibers for pharmaceutical and biomedical applications: A review. Int. J. Biol. Macromol. 2022, 218, 209–224. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Bowers, D.T.; Liu, W.; Ma, M. Functional hydrogels for diabetic wound management. Apl. Bioeng. 2021, 5, 031503. [Google Scholar] [CrossRef]
- Mamidi, N.; García, R.G.; Martínez, J.D.H.; Briones, C.M.; Martínez Ramos, A.M.; Tamez, M.F.L.; Del Valle, B.G.; Segura, F.J.M. Recent advances in designing fibrous biomaterials for the domain of biomedical, clinical, and environmental applications. ACS Biomater. Sci. Eng. 2022, 8, 3690–3716. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wang, H.; Bai, J.; Miao, X.; Lin, Q.; Zheng, J.; Ding, S.; Li, X.; Tang, Y. Multidrug-loaded electrospun micro/nanofibrous membranes: Fabrication strategies, release behaviors and applications in regenerative medicine. J. Control. Release 2021, 330, 1264–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Bian, T.; Zhang, H.; Xing, H. Preparation and biological properties of collagen/nano-hydroxyapatite composite nanofibers based on ordered nano-hydroxyapatite ceramic fibers. Colloid. Surface. A 2020, 602, 124802. [Google Scholar] [CrossRef]
- Grasl, C.; Stoiber, M.; Rohrich, M.; Moscato, F.; Bergmeister, H.; Schima, H. Electrospinning of small diameter vascular grafts with preferential fiber directions and comparison of their mechanical behavior with native rat aortas. Mater. Sci. Eng. C 2021, 124, 112085. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloy. Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhao, P. The key elements for biomolecules to biomaterials and to bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (l-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharmaceut. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Costa, P.R.A.; Menezes, L.R.; Dias, M.L.; Silva, E.O. Advances in the use of electrospinning as a promising technique for obtaining nanofibers to guide epithelial wound healing in diabetics—Mini-review. Polym. Advan. Technol. 2022, 33, 1031–1046. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Liu, P.; Zhang, M.; Wang, C.; Zhang, B. Multifunctional chitosan/polycaprolactone nanofiber scaffolds with varied dual-drug release for wound-healing applications. ACS Biomater. Sci. Eng. 2020, 6, 4666–4676. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, M.; Wang, P.; Xu, X.; Liu, Y.; Yu, D.-G. Ingenious construction of Ni(DMG)2/TiO2-decorated porous nanofibers for the highly efficient photodegradation of pollutants in water. Colloid. Surface. A 2022, 650, 129561. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment-a review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-based wound dressing materials loaded with bioactive agents: Potential materials for the treatment of diabetic wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic diabetic wounds and their treatment with skin substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Gastaldi, G.; Pannier, F.; Roztocil, K.; Lugli, M.; Mansilha, A.; Haller, H.; Rabe, E.; Van Rijn, M.J. Chronic venous disease and diabetic microangiopathy center dot center dot pathophysiology and commonalities. Int. Angiol. 2021, 40, 457–469. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Wang, J.; Yao, M.; Peng, Y.; Liu, T.; Li, Z.; Luo, G.; Deng, J. Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing. Adv. Funct. Mater. 2021, 31, 2105749. [Google Scholar] [CrossRef]
- Chakraborty, R.; Borah, P.; Dutta, P.P.; Sen, S. Evolving spectrum of diabetic wound: Mechanistic insights and therapeutic targets. World J. Diabetes 2022, 13, 696–716. [Google Scholar] [CrossRef]
- Alexandrescu, V.A.; Van Overmeire, L.; Makrygiannis, G.; Azdad, K.; Popitiu, M.; Paquet, S.; Poppe, L.; Nodit, M. Clinical implications of diabetic peripheral neuropathy in primary infrapopliteal angioplasty approach for neuro-ischemic foot wounds. J. Endovasc. Ther. 2022, in press. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J.M. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M.; Kashif, M.U.R. Curcumin-laden hyaluronic acid-co-pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. Int. J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Yang, K.; Liu, P.; Wang, J. The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J. Ethnopharmacol. 2022, 282, 114662. [Google Scholar] [CrossRef]
- Lai, H.-J.; Kuan, C.-H.; Wu, H.-C.; Tsai, J.-C.; Chen, T.-M.; Hsieh, D.-J.; Wang, T.-W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta. Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Ji, S.; Liu, X.; Huang, J.; Bao, J.; Chen, Z.; Han, C.; Hao, D.; Hong, J.; Hu, D.; Jiang, Y.; et al. Consensus on the application of negative pressure wound therapy of diabetic foot wounds. Burns Trauma 2021, 9, tkab018. [Google Scholar] [CrossRef]
- Chen, J.; Zou, Q.; Hamblin, M.R.; Wen, X. A preliminary clinical trial comparing wet silver dressings versus wet-to-dry povidone-iodine dressings for wound healing in pemphigus vulgaris patients. Dermatol. Ther. 2021, 34, e14906. [Google Scholar] [CrossRef]
- Hoff, J.; Karl, B.; Gerstmeier, J.; Beekmann, U.; Schmölz, L.; Börner, F.; Kralisch, D.; Bauer, M.; Werz, O.; Fischer, D.; et al. Controlled release of the α-tocopherol-derived metabolite α-13′-carboxychromanol from bacterial nanocellulose wound cover improves wound healing. Nanomaterials 2021, 11, 1939. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel dressings for the treatment of burn wounds: An up-to-date overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Ajiteru, O.; Lee, O.J.; Kim, J.-H.; Lee, Y.J.; Lee, J.S.; Lee, H.; Sultan, M.T.; Park, C.H. Fabrication and characterization of a myrrh hydrocolloid dressing for dermal wound healing. Colloid. Interfac. Sci. 2022, 48, 100617. [Google Scholar] [CrossRef]
- De Francesco, F.; De Francesco, M.; Riccio, M. Hyaluronic acid/collagenase ointment in the treatment of chronic hard-to-heal wounds: An observational and retrospective study. J. Clin. Med. 2022, 11, 537. [Google Scholar] [CrossRef]
- Thomas, D.C.; Tsu, C.L.; Nain, R.A.; Arsat, N.; Fun, S.S.; Sahid Nik Lah, N.A. The role of debridement in wound bed preparation in chronic wound: A narrative review. Ann. Med. Surg. 2021, 71, 102876. [Google Scholar] [CrossRef]
- Barrigah-Benissan, K.; Ory, J.; Sotto, A.; Salipante, F.; Lavigne, J.-P.; Loubet, P. Antiseptic agents for chronic wounds: A systematic review. Antibiotics 2022, 11, 350. [Google Scholar] [CrossRef]
- Nagoba, B.; Gavkare, A.; Rayate, A.; Mumbre, S.; Rao, A.; Warad, B.; Nanaware, N.; Jamadar, N. Role of an acidic environment in the treatment of diabetic foot infections: A review. World J. Diabetes 2021, 12, 1539–1549. [Google Scholar] [CrossRef]
- Chen, Z.; Song, J.; Xia, Y.; Jiang, Y.; Murillo, L.L.; Tsigkou, O.; Wang, T.; Li, Y. High strength and strain alginate fibers by a novel wheel spinning technique for knitting stretchable and biocompatible wound-care materials. Mater. Sci. Eng. C 2021, 127, 112204. [Google Scholar] [CrossRef]
- Pahlevanneshan, Z.; Deypour, M.; Kefayat, A.; Rafienia, M.; Sajkiewicz, P.; Esmaeely Neisiany, R.; Enayati, M.S. Polyurethane-nanolignin composite foam coated with propolis as a platform for wound dressing: Synthesis and characterization. Polymers 2021, 13, 3191. [Google Scholar] [CrossRef]
- Shie Karizmeh, M.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Biomater. Adv. 2022, 135, 112667. [Google Scholar] [CrossRef]
- Tainara de Paula, d.L.L.; Passos, M.F. Skin wounds, the healing process, and hydrogel-based wound dressings: A short review. J. Biomat. Sci.-Polym. E. 2021, 32, 1910–1925. [Google Scholar] [CrossRef]
- Lin, H.; BoLatai, A.; Wu, N. Application progress of nano silver dressing in the treatment of diabetic foot. Diabetes Metab. Syndr. Obes. 2021, 14, 4145–4154. [Google Scholar] [CrossRef]
- Xu, D.; Chu, T.; Tao, G. Clinical study on the efficacy of silver ion dressing combined with prontosan gel dressing in the treatment of diabetic foot ulcers and the effect on serum inflammatory factors. Evid.-Based Complement Altern. Med. 2021, 2021, 2938625. [Google Scholar] [CrossRef]
- Winter, G.F. Medical-grade honey dressing use in developing countries. Adv. Skin Wound Care 2017, 30, 1–3. [Google Scholar] [CrossRef]
- Henry, N.; Jeffery, S.; Radotra, I. Properties and use of a honey dressing and gel in wound management. Br. J. Nurs. 2019, 28, S30–S35. [Google Scholar] [CrossRef]
- Salisbury, A.; Mullin, M.; Foulkes, L.; Chen, R.; Percival, S.L. Controlled-release iodine foam dressings demonstrate broad-spectrum biofilm management in several in vitro models. Int. Wound J. 2022, 19, 1717–1728. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Yang, S.; Li, C.; Jin, W.; Hou, W. Effects of the chinese herb medicine formula “she-xiang-yu-hong” ointment on wound healing promotion in diabetic mice. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Chandrika, A.M.; Selvi, V.S.K. Significance of interleukin-6 in diabetes mellitus and its complications. Int. J. Life Sci. Pharma Res. 2022, 12, L170–L174. [Google Scholar] [CrossRef]
- Huang, M.; Duan, J.; Yu, B.; Zheng, S.; Chen, Q.; Lin, F.; Zeng, N.; Ling, B. Clinical value of thalidomide on kk-rat model through TNF-a mediated inflammation approach. Food Sci. Technol. 2022, 42, e06821. [Google Scholar] [CrossRef]
- Fei, J.; Ling, Y.-M.; Zeng, M.-J.; Zhang, K.-W. Shixiang plaster, a traditional chinese medicine, promotes healing in a rat model of diabetic ulcer through the receptor for advanced glycation end products (rage)/nuclear factor kappa b (nf-kappa b) and vascular endothelial growth factor (vegf)/vascular cell adhesion molecule-1 (vcam-1)/endothelial nitric oxide synthase (enos) signaling pathways. Med. Sci. Monitor. 2019, 25, 9446–9457. [Google Scholar] [CrossRef]
- Nishad, R.; Tahaseen, V.; Kavvuri, R.; Motrapu, M.; Singh, A.K.; Peddi, K.; Pasupulati, A.K. Advanced-glycation end-products induce podocyte injury and contribute to proteinuria. Front. Med. 2021, 8, 685447. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Al-Oanzi, Z.H. Biotechnological applications of polymeric nanofiber platforms loaded with diverse bioactive materials. Polymers 2021, 13, 3734. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.-G.; Ge, R. Electrospun nanofiber-based glucose sensors for glucose detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef]
- Hajilou, H.; Farahpour, M.R.; Hamishehkar, H. Polycaprolactone nanofiber coated with chitosan and gamma oryzanol functionalized as a novel wound dressing for healing infected wounds. Int. J. Biol. Macromol. 2020, 164, 2358–2369. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, P.; Song, W.; Wang, M.; Yu, D.-G. Electrospun zein/polyoxyethylene core-sheath ultrathin fibers and their antibacterial food packaging applications. Biomolecules 2022, 12, 1110. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Yu, D.-G. Recent progresses of electrospun nanofibers and their applications in treating heavy metal wastewater. Front. Chem. Sci. Eng. 2022, 17, 1–27. [Google Scholar] [CrossRef]
- Mousavi, S.-M.; Nejad, Z.M.; Hashemi, S.A.; Salari, M.; Gholami, A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W. Bioactive agent-loaded electrospun nanofiber membranes for accelerating healing process: A review. Membranes 2021, 11, 702. [Google Scholar] [CrossRef]
- Madhukiran, D.R.; Jha, A.; Kumar, M.; Ajmal, G.; Bonde, G.V.; Mishra, B. Electrospun nanofiber-based drug delivery platform: Advances in diabetic foot ulcer management. Expert Opin. Drug Del. 2021, 18, 25–42. [Google Scholar] [CrossRef]
- Afsharian, Y.P.; Rahimnejad, M. Bioactive electrospun scaffolds for wound healing applications: A comprehensive review. Polym. Test 2021, 93, 106952. [Google Scholar] [CrossRef]
- Karimi Afshar, S.; Abdorashidi, M.; Dorkoosh, F.A.; Akbari Javar, H. Electrospun fibers: Versatile approaches for controlled release applications. Int. J. Polym. Sc. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun core (hpmc–acetaminophen)–shell (pvp–sucralose) nanohybrids for rapid drug delivery. Gels 2022, 8, 357. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun medicated nanofibers for wound healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Huang, J.; Luo, Q.; Zou, J.; Zeng, X.; Chen, B.; Wu, H.; Tang, Q. Electrostatic spun hierarchically porous carbon matrix with cose2/co heterostructure as bifunctional electrocatalysts for zinc-air batteries. J. Alloy. Compd. 2021, 875, 160056. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, R.G. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S. Silver Ionic Modification in Dual-Layer Hollow Fiber Membranes with Significant Enhancement in CO2/CH4 and O2/N2 Separation. J. Membrane. Sci. 2010, 350, 226–231. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Chen, Y.; Yuan, K.; He, W.; Yang, S.; Tang, T.; Yu, D.-G. Electrospraying Micro-Nano Structures on Chitosan Composite Coatings for Enhanced Antibacterial Effect. Prog. Org. Coat. 2023, 174, 107310. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun beads-on-the-string nanoproducts: Preparation and drug delivery application. Curr. Drug. Deliv. 2022, 19. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- Kazemzadeh, G.; Jirofti, N.; Mohebbi-Kalhori, D.; Sarhaddi, F.; Taheri, R. Pathological examination of blended and co-electrospinning hybrid polycaprolactone/polyurethane nanofibers for soft tissue engineering applications. J. Ind. Text. 2022, 51, 6816S–6837S. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Liu, P.; Zhang, Y.; Song, W.; Yu, D.-G.; Lu, X. Electrospun healthcare nanofibers from medicinal liquor of Phellinus igniarius. Adv. Compos. Hybrid Mater. 2022, 5, 3045–3056. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of electrospun polyvinylidene fluoride-based nanofibers, their piezoelectric properties, and several fantastic applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun porous nanofibers: Pore−forming mechanisms and applications for photocatalytic degradation of organic pollutants in wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef]
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.-G.; Shen, H. Sheath-separate-core nanocomposites fabricated using a trifluid electrospinning. Mater. Design. 2020, 192, 108782. [Google Scholar] [CrossRef]
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun functional nanofiber membrane for antibiotic removal in water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, K.; Yang, Y.; Liu, Y.; Yu, D.-G. Electrospun environment remediation nanofibers using unspinnable liquids as the sheath fluids: A review. Polymers 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chae, S.-H.; Kim, T.; Lee, D.; Kim, H. White-light-emitting triphasic fibers as a phosphor for light-emitting diodes. Nanoscale Adv. 2020, 2, 5403–5411. [Google Scholar] [CrossRef] [PubMed]
- Davani, F.; Alishahi, M.; Sabzi, M.; Khorram, M.; Arastehfar, A.; Zomorodian, K. Dual drug delivery of vancomycin and imipenem/cilastatin by coaxial nanofibers for treatment of diabetic foot ulcer infections. Mater. Sci. Eng. C 2021, 123, 111975. [Google Scholar] [CrossRef]
- Mostofizadeh, M.; Ghasemi-Mobarakeh, L.; Zamani, M. Dual drug release from Gelatin/PLGA core-shell fibers for diabetic neuropathic wound healing. Macromol. Mater. Eng. 2022, 307, 2100490. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.-G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos. Hybrid Mater. 2022, 5, 1017–1029. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.-G. Progresses on electrospun metal–organic frameworks nanofibers and their wastewater treatment applications. Mater. Today. Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Wang, M.; Yu, D.-G.; Williams, G.R.; Bligh, S.W.A. Co-loading of inorganic nanoparticles and natural oil in the electrospun janus nanofibers for a synergetic antibacterial effect. Pharmaceutics 2022, 14, 1208. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Liu, Y.; Yu, D.; Liu, P. Electrospun core-sheath nanofibers with variable shell thickness for modifying curcumin release to achieve a better antibacterial performance. Biomolecules 2022, 12, 1057. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, X.-Y.; Wang, X.; Yang, J.-H.; Bligh, S.W.A.; Williams, G.R. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.-G. Electrospun nanofibers for periodontal treatment: A recent progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Yan, C.; Liu, H.; Yu, D.-G. Advances in the application of electrospun drug-loaded nanofibers in the treatment of oral ulcers. Biomolecules 2022, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, Q.; Yao, Q.; Zhang, P. Application of electrospun nanofiber membrane in the treatment of diabetic wounds. Pharmaceutics 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Jeckson, T.A.; Neo, Y.P.; Sisinthy, S.P.; Gorain, B. Delivery of Therapeutics from Layer-by-Layer Electrospun Nanofiber Matrix for Wound Healing: An Update. J. Pharm. Sci. 2021, 110, 635–653. [Google Scholar] [CrossRef]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.-G.; Liu, Y. Recent progress in electrospun polyacrylonitrile nanofiber-based wound dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie Bligh, S.W. A review on electrospun poly(amino acid) nanofibers and their applications of hemostasis and wound healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef]
- Morsi, Y.; Zhu, T.; Ahmad, A.; Xie, X.; Yu, F.; Mo, X. Electrospinning: An emerging technology to construct polymer-based nanofibrous scaffolds for diabetic wound healing. Front. Mater. Sci. 2021, 15, 10–35. [Google Scholar] [CrossRef]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef]
- Abdel Khalek, M.A.; Abdel Gaber, S.A.; El-Domany, R.A.; El-Kemary, M.A. Photoactive electrospun cellulose acetate/polyethylene oxide/methylene blue and trilayered cellulose acetate/polyethylene oxide/silk fibroin/ciprofloxacin nanofibers for chronic wound healing. Int. J. Biol. Macromol. 2021, 193, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Khan, Z.U.; Saeed, A.; Shah, K.A.; Khan, N.U.; Menaa, B.; Iqbal, H.; Menaa, F. Development of cephradine-loaded gelatin/polyvinyl alcohol electrospun nanofibers for effective diabetic wound healing: In-vitro and in-vivo assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nanofibres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.S.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded PCL/PVA/SF based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef]
- Yang, M.; Yu, S.; Zhao, P.; Xie, L.; Lyu, G.; Yu, J. Fabrication of homogeneously-aligned nano-fillers encapsulated silk fibroin electrospun nanofibers for improved fibroblast attachment, epithelialization, and collagen depositions: In vitro and in vivo wound healing evaluation. J. Biomater. Sci. Polym. Ed. 2022, 33, 878–899. [Google Scholar] [CrossRef]
- Shin, Y.C.; Shin, D.-M.; Lee, E.J.; Lee, J.H.; Kim, J.E.; Song, S.H.; Hwang, D.-Y.; Lee, J.J.; Kim, B.; Lim, D.; et al. Hyaluronic acid/PLGA core/shell fiber matrices loaded with egcg beneficial to diabetic wound healing. Adv. Healthc. Mater. 2016, 5, 3035–3045. [Google Scholar] [CrossRef]
- Khan, A.U.R.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni.-Suef. Univ. J. Basic. Appl. Sci 2020, 9, 31. [Google Scholar] [CrossRef]
- Cam, M.E.; Ertas, B.; Alenezi, H.; Hazar-Yavuz, A.N.; Cesur, S.; Ozcan, G.S.; Ekentok, C.; Guler, E.; Katsakouli, C.; Demirbas, Z.; et al. Accelerated diabetic wound healing by topical application of combination oral antidiabetic agents-loaded nanofibrous scaffolds: An in vitro and in vivo evaluation study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiao, X.; Zhou, L.; Wang, J.; Wang, C.; Qin, Y.; Wen, Y. Nanofibrous composite aerogel with multi-bioactive and fluid gating characteristics for promoting diabetic wound healing. Biomaterials 2021, 276, 121040. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Nanditha, C.K.; Kumar, G.S.V. ECM-mimicking nanofibrous scaffold enriched with dual growth factor carrying nanoparticles for diabetic wound healing. Nanoscale Adv. 2021, 3, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Yu, B.; He, C.; Wang, W.; Ren, Y.; Yang, J.; Guo, S.; Zheng, Y.; Shi, X. Asymmetric wettable composite wound dressing prepared by electrospinning with bioinspired micropatterning enhances diabetic wound healing. ACS Appl. Biol. Mater. 2020, 3, 5383–5394. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Kumar, P.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Artificial, triple-layered, nanomembranous wound patch for potential diabetic foot ulcer intervention. Materials 2018, 11, 2128. [Google Scholar] [CrossRef]
- Derakhshan, M.A.; Nazeri, N.; Khoshnevisan, K.; Heshmat, R.; Omidfar, K. Three-layered PCL-collagen nanofibers containing melilotus officinalis extract for diabetic ulcer healing in a rat model. J. Diabetes Metab. Disord 2022, 21, 313–321. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Juang, J.-H.; Hsieh, I.-C.; Wen, M.-S.; Liu, S.-J. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomed. Nanotechnol. 2020, 24, 102123. [Google Scholar] [CrossRef]
- Venault, A.; Lin, K.-H.; Tang, S.-H.; Dizon, G.V.; Hsu, C.-H.; Maggay, I.V.B.; Chang, Y. Zwitterionic electrospun PVDF fibrous membranes with a well-controlled hydration for diabetic wound recovery. J. Membrane. Sci. 2020, 598, 117648. [Google Scholar] [CrossRef]
- Augustine, R.; Ur Rehman, S.R.; Joshy, K.S.; Hasan, A. Stromal cell-derived factor loaded co-electrospun hydrophilic/hydrophobic bicomponent membranes for wound protection and healing. RSC Adv. 2020, 11, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guan, N.; Miao, W.; Zhao, W.; Li, Q. An electrospun scaffold loaded with an enteromorpha polysaccharide for accelerated wound healing in diabetic mice. Marine Drugs 2022, 20, 95. [Google Scholar] [CrossRef]
- Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional biomimetic nanofibrous scaffold loaded with asiaticoside for rapid diabetic wound healing. Pharmaceutics 2022, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int. J. Nanomed. 2019, 14, 8573–8588. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Su, S.; Ulag, S.; Woźniak, A.; Grinholc, M.; Erdemir, G.; Erdem Kuruca, S.; Gunduz, O.; Muhammed, M.; El-Sherbiny, I.M.; et al. Development and in vitro evaluation of biocompatible pla-based trilayer nanofibrous membranes for the delivery of nanoceria: A novel approach for diabetic wound healing. Polymers 2021, 13, 3630. [Google Scholar] [CrossRef]
- Khazaeli, P.; Alaei, M.; Khaksarihadad, M.; Ranjbar, M. Preparation of PLA/chitosan nanoscaffolds containing cod liver oil and experimental diabetic wound healing in male rats study. J. Nanobiotechnol. 2020, 18, 176. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Gunduz, O.; Sahin, A.; Grinholc, M.; El-Sherbiny, I.M.; Megahed, M. Dual spinneret electrospun polyurethane/PVA-gelatin nanofibrous scaffolds containing cinnamon essential oil and nanoceria for chronic diabetic wound healing: Preparation, physicochemical characterization and in-vitro evaluation. Molecules 2022, 27, 2146. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, T.; Li, J.; Xu, Y.; Wang, R.; Ke, Q.; Shen, S.G.F.; Xu, H.; Lin, K. Hierarchical micro/nanofibrous scaffolds incorporated with curcumin and zinc ion eutectic metal organic frameworks for enhanced diabetic wound healing via anti-oxidant and anti-inflammatory activities. Chem. Eng. J. 2020, 402, 126273. [Google Scholar] [CrossRef]
- Campa-Siqueiros, P.I.; Madera-Santana, T.J.; Castillo-Ortega, M.M.; López-Cervantes, J.; Ayala-Zavala, J.F.; Ortiz-Vazquez, E.L. Electrospun and co-electrospun biopolymer nanofibers for skin wounds on diabetic patients: An overview. RSC Adv. 2021, 11, 15340–15350. [Google Scholar] [CrossRef]
- Puertas-Bartolome, M.; Mora-Boza, A.; Garcia-Fernandez, L. Emerging biofabrication techniques: A review on natural polymers for biomedical applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Antunes dos Santos, A.E.; dos Santos, F.V.; Freitas, K.M.; Santos Pimenta, L.P.; Andrade, L.d.O.; Marinho, T.A.; de Avelar, G.F.; da Silva, A.B.; Ferreira, R.V. Cellulose acetate nanofibers loaded with crude annatto extract: Preparation, characterization, and in vivo evaluation for potential wound healing applications. Mater. Sci. Eng. C 2021, 118, 111322. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Yuan, Z.; Xu, F.; Xie, X. Electrospun cellulose acetate wound dressings loaded with Pramipexole for diabetic wound healing: An in vitro and in vivo study. Cellulose 2022, 29, 3407–3422. [Google Scholar] [CrossRef]
- Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The development of hyaluronic acids used for skin tissue regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Yu, J.; Shao, N.; Lu, H.; Guo, J.; Qiu, X.; Zhou, D.; Huang, Y. Absorbable thioether grafted hyaluronic acid nanofibrous hydrogel for synergistic modulation of inflammation microenvironment to accelerate chronic diabetic wound healing. Adv. Healthc. Mater. 2020, 9, 2000198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Ren, D.-Y.; Feng, Z.-X.; Zhang, L.-Y.; Zhong, Y.-F.; Jin, M.-Y.; Xu, F.-W.; Feng, C.-Y.; Du, Y.-Z.; et al. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Mater. Today Biol. 2022, 15, 100320. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Sun, B.; Chu, D.; Yang, L.; Li, M.; Li, H.; Dai, Y.; Yu, Z.; Guo, J. Modulation of macrophages by a paeoniflorin-loaded hyaluronic acid-based hydrogel promotes diabetic wound healing. Mater. Today Biol. 2021, 12, 100139. [Google Scholar] [CrossRef] [PubMed]
- Sheir, M.M.; Nasra, M.M.A.; Abdallah, O.Y. Chitosan alginate nanoparticles as a platform for the treatment of diabetic and non-diabetic pressure ulcers: Formulation and in vitro/in vivo evaluation. Int. J. Pharm. 2021, 607, 120963. [Google Scholar] [CrossRef] [PubMed]
- Garcia Garcia, C.E.; Bossard, F.; Rinaudo, M. Electrospun biomaterials from chitosan blends applied as scaffold for tissue regeneration. Polymers 2021, 13, 1037. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, M.-L.; Fang, Q.-Q.; Zhao, W.-Y.; Lou, D.; Hu, Y.-Y.; Chen, J.; Wang, X.-Z.; Tan, W.-Q. Flexible electrical stimulation device with chitosan-vaseline (r) dressing accelerates wound healing in diabetes. Bioact. Mater. 2021, 6, 230–243. [Google Scholar] [CrossRef]
- Sharaf, S.S.; El-Shafei, A.M.; Refaie, R.; Gibriel, A.A.; Abdel-Sattar, R. Antibacterial and wound healing properties of cellulose acetate electrospun nanofibers loaded with bioactive glass nanoparticles; in-vivo study. Cellulose 2022, 29, 4565–4577. [Google Scholar] [CrossRef]
- Tombulturk, F.K.; Todurga-Seven, Z.G.; Huseyinbas, O.; Ozyazgan, S.; Ulutin, T.; Kanigur-Sultuybek, G. Topical application of metformin accelerates cutaneous wound healing in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2022, 49, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Almulathanon, A.A.Y.; Mohammad, J.A.; Fathi, F.H. Comparative effects of metformin and glibenclamide on the redox balance in type 2 diabetic patients. Pharmacia 2021, 68, 327–332. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Hong, H.; Mo, X.; Wang, H. Fabrication of scaffold based on gelatin and polycaprolactone (PCL) for wound dressing application. J. Drug. Deliv. Sci. Tec. 2021, 63, 102501. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohyd. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.O.; Permyakova, E.S.; Ershov, K.I.; Bakhareva, K.I.; Miroshnichenko, S.M.; Kiryukhantsev-Korneev, P.V.; Konopatsky, A.S.; Polčak, J.; Shtansky, D.V.; Manakhov, A.M. Plasma-coated PCL scaffolds with immobilized platelet-rich plasma enhance the wound healing in diabetics mice. Plasma. Process. Polym. 2022, 19, 2200032. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Gao, W.; Shi, M.; Tang, F.; Fu, X.; Chen, X. Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. J. Mat. Chem. B 2021, 9, 1395–1405. [Google Scholar] [CrossRef]
- Liu, M.; Wang, R.; Liu, J.; Zhang, W.; Liu, Z.; Lou, X.; Nie, H.; Wang, H.; Mo, X.; Abd-Elhamid, A.I.; et al. Incorporation of magnesium oxide nanoparticles into electrospun membranes improves pro-angiogenic activity and promotes diabetic wound healing. Biomater. Adv. 2022, 133, 112609. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Aijaz, T.; Candido, K.D.; Kovaieva, S.; Lissounov, A.; Knezevic, N. The effect of once-daily gabapentin extended release formulation in patients with postamputation pain. Front. Pharmacol. 2019, 10, 504. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, S.; Weng, T.; Yang, M.; Shao, J.; Zhang, M.; Wang, J.; Xu, P.; Wei, J.; Jin, R.; et al. Antibacterial coaxial hydro-membranes accelerate diabetic wound healing by tuning surface immunomodulatory functions. Mater. Today Biol. 2022, 16, 100395. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-containing wound dressing: Antimicrobial and healing effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Swielam, E.M.; Atwa, N.A.; Agwa, M.M. Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. Int. J. Biol. Macromol. 2022, 197, 121–130. [Google Scholar] [CrossRef]
- Saddik, M.S.; Elsayed, M.M.A.; El-Mokhtar, M.A.; Sedky, H.; Abdel-Aleem, J.A.; Abu-Dief, A.M.; Al-Hakkani, M.F.; Hussein, H.L.; Al-Shelkamy, S.A.; Meligy, F.Y.; et al. Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics 2022, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Ghiyasi, Y.; Salahi, E.; Esfahani, H. Synergy effect of Urtica dioica and ZnONPs on microstructure, antibacterial activity and cytotoxicity of electrospun PCL scaffold for wound dressing application. Mater. Today Commun. 2021, 26, 102163. [Google Scholar] [CrossRef]
- Kolanthai, E.; Fu, Y.; Kumar, U.; Babu, B.; Venkatesan, A.K.; Liechty, K.W.; Seal, S. Nanoparticle mediated RNA delivery for wound healing. Wires. Nanomed. Nanobi. 2022, 14, e1741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, J.; Zhang, Q.; Deng, T. Growth factors, as biological macromolecules in bioactivity enhancing of electrospun wound dressings for diabetic wound healing: A review. Int. J. Biol. Macromol. 2021, 193, 205–218. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, I.; Pandey, H.; Patil, S.; Mishra, S.B.; Pandey, A.C.; Zamboni, P.; Ramteke, P.W.; Singh, A.V. In viv diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J. Biomed. Mater. Res. 2018, 106, 641–651. [Google Scholar] [CrossRef]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.-C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef]
- McInnes, N.; Hall, S.; Sultan, F.; Aronson, R.; Hramiak, I.; Harris, S.; Sigal, R.J.; Woo, V.; Liu, Y.Y.; Gerstein, H.C. Remission of type 2 diabetes following a short-term intervention with insulin glargine, metformin, and dapagliflozin. J. Clin. Endocrinol. Metab. 2020, 105, 2532–2540. [Google Scholar] [CrossRef]
- Leutner, M.; Kaleta, M.; Bellach, L.; Kautzky, A.; Thurner, S.; Klimek, P.; Kautzky-Willer, A. Insulin as Monotherapy and in Combination with Other Glucose-Lowering Drugs Is Related to Increased Risk of Diagnosis of Pneumonia: A Longitudinal Assessment over Two Years. J. Pers. Med. 2021, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Deng, M.; Wang, J.; Fan, P.; Wang, Y.; Zhao, X.; He, Y.; Shi, B.; Sui, J. Efficacy and tolerability of sitagliptin and metformin compared with insulin as an initial therapy for newly diagnosed diabetic patients with severe hyperglycaemia. Exp. Ther. Med. 2021, 21, 217. [Google Scholar] [CrossRef] [PubMed]




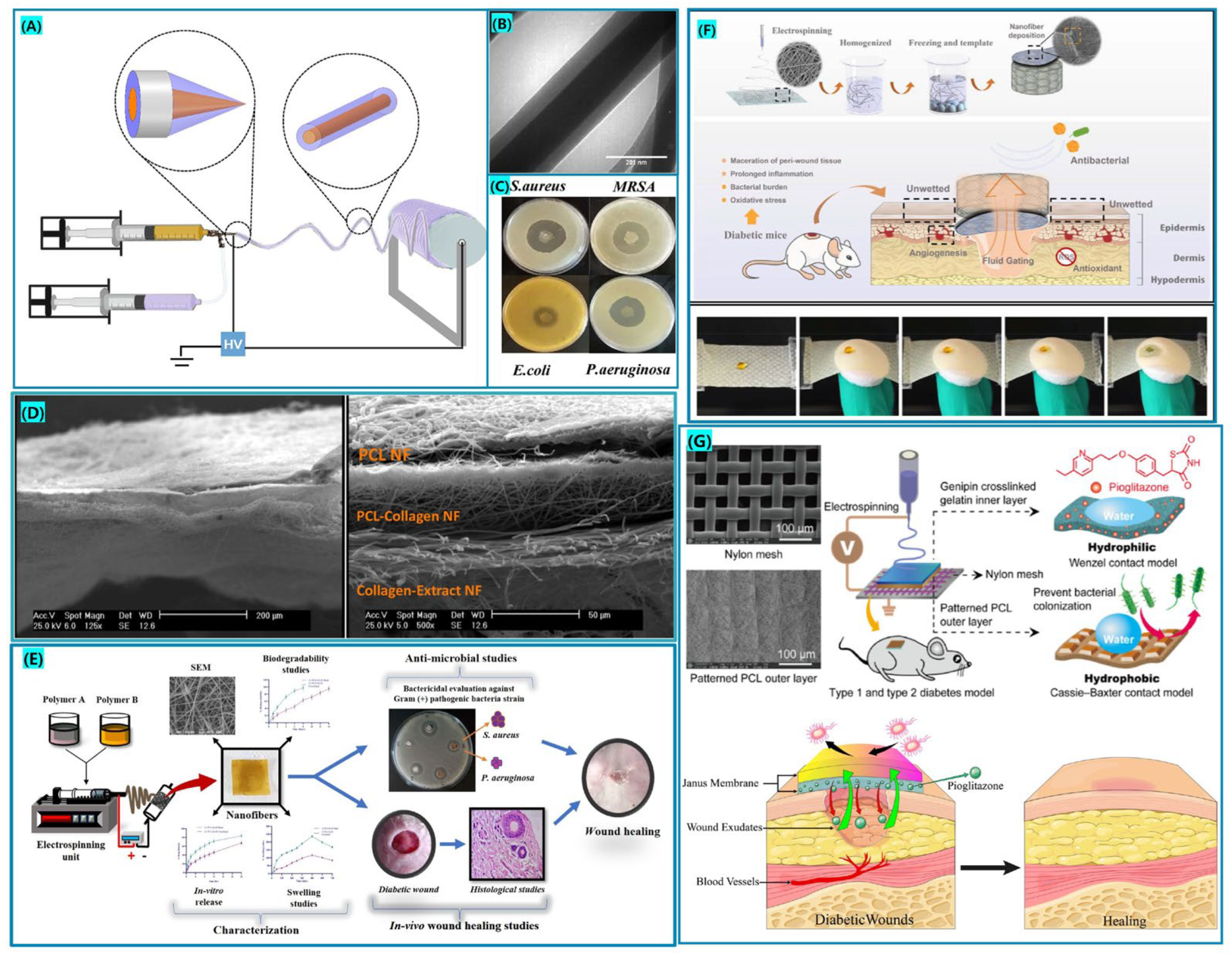

| Polymer | Additional Polymer | Solvent | Active Ingredients | Technique | Highlights | Ref. |
|---|---|---|---|---|---|---|
| CA | Gel | HFIP | Berberine | Blend | Antimicrobial research on wounds has shown that they effectively suppress the microbial growth. | [112] |
| Zein | Acetic acid, water | Sesamol | Blend | Synergistic effect of sesamol and nanofibrous membranes. | [113] | |
| PEO/SF | Acetone, Formic acid | MB, CIP | Blend | ROS generation during light-emitting diode irradiation, achieved combined PDT and antibiotic therapy. | [114] | |
| Gel | PVA | DMSO | Ceph | Blend | Stronger antibacterial activity and thermal stability, drug loaded fibrous membranes have a dual purpose. | [115] |
| PLGA | DMF Methanol TCM, DIW | GBP, CipHCl | Coaxial | Core-shell fibers for dual delivery of analgesic and antibiotic agents for the therapy of diabetic neuropathic ulcers. | [95] | |
| BC | Acetic acid, DMF, DIW | MET, GB | Blend | An “all-natural” drug-eluting wound dressing. Gel-BC-GB significantly reduces the expression of TNF-α. | [116] | |
| SF | PCL/PVA | DCM, Formic acid | Curcumin | Blend | Synthetic and natural polymer blends and active ingredients are encapsulated for electrospinning. | [117] |
| / | / | Hydroxyapatite, Curcumin | Blend | The mechanical characteristics and porosity of the fiber membrane improved when the CUR concentration was raised. | [118] | |
| HA | PLGA | HFIP, DIW | EGCG | Coaxial | The synergistic impact of HA and EGCG increased wound epithelialization and ECM rearrangement considerably. | [119] |
| PLCL | HFIP | ZnO, OEO | Coaxial | Controlled release of the volatile chemical OEO at the core of the fiber in the form of an oil-in-water emulsion. | [120] | |
| PLA | DCM | Valsartan | Blend | Composite fibers have a dynamic effect and promote re-epithelialization. | [121] | |
| CS | Gel/PCL/PVP | Acetic acid DIW, TCM Methanol | MET, PHR, GB | Three-layered | Multi-drug combination therapy accelerates diabetic wound healing in type 1 diabetic rats. | [122] |
| PVA/PCL | Acetic acid, DCM, DMF | Curcumin | Janus | Enables autonomous, rapid and unidirectional transfer of exudate, effectively preventing reverse penetration of liquids. | [123] | |
| Collagen/PLGA | HFIP, TFA | VEGF, bFGF | Blend | The collagen gene expression in composite growth factor fiber dressing is greater, and the dressing heals faster. | [124] | |
| PVA | Methanol, Acetic acid, DIW | ZnO | Blend | The composite nanofiber membrane has a 90% closure rate for diabetic wounds. | [125] | |
| PCL | Gel | HFIP | Pioglitazone | Blend | The scaffolds increased epidermal regeneration, angiogenesis, collagen deposition, and inflammatory responses in vivo. Synergistic improvement of diabetic wound healing efficiency | [126] |
| PAA/PVP | Ethanol, DCM | CIP | Three-layered | Excellent mechanical properties. | [127] | |
| Collagen | Acetic acid, Methanol, TFM | Melilotus | Three-layered | Each layer of the structure has unique properties that synergistically promote wound healing | [128] | |
| PLGA | / | HFIP | Insulin | Coaxial | The topical application of insulin reduces the amount of type I collagen in vitro and increases the amount of TGF-β in vivo, promoting the healing of diabetic wounds. | [129] |
| PVDF | ZP (S-r-4VP) | Acetone, DMF, DMAC | / | Blend | The amphoteric copolymer membrane possesses high hemocompatibility, non-adhesive characteristics, and resistance to contamination by plasma proteins. | [130] |
| PVA | PCL | Ultrapure water DMF, DCM | SDF1 | Blend | SDF1-loaded fiber membranes displayed a high level of cellular activity and enhanced cell proliferation considerably. | [131] |
| / | Acetic acid | EPP | Blend | Outstanding water absorption performance, inflammatory response suppression, and wound healing time reduction. | [132] | |
| SA/SF | Formic acid, Calcium chloride DIW | Asiaticoside | Blend | Low toxicity and considerable cell migration, restoring the skin’s natural capacity to recover. | [133] | |
| PLA | PVA | DIW DMF, DCM | CTGF | Coaxial | A core-shell membrane containing CTGF promotes cell proliferation, migration, and angiogenesis, allowing for faster wound healing. | [134] |
| PVA | DMF, DCM DIW | nCeO2 | Three-layered | The hierarchical structure may be used as a medication carrier system that also promotes cell migration and proliferation. | [135] | |
| CS | DMF, DIW | Cod liver oil | Blend | Provides the permeability and oxygen essential for tissue healing. | [136] | |
| PU | PVA/Gel | THF, DMF | nCeO2, CEO | Blend | CEO promotes antibacterial effectiveness and cell viability by increasing nCeO2 loading. | [137] |
| PLLA | / | Acetone DMF, DCM | Curcumin, Zn+2 | Blend | During the wound healing phase, the multilayered nanofiber scaffold releases curcumin and zinc ions as needed. | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, C.; Feng, Z.; Han, B.; Yu, D.-G.; Wang, K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules 2022, 12, 1727. https://doi.org/10.3390/biom12121727
Liu Y, Li C, Feng Z, Han B, Yu D-G, Wang K. Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules. 2022; 12(12):1727. https://doi.org/10.3390/biom12121727
Chicago/Turabian StyleLiu, Yukang, Chaofei Li, Zhangbin Feng, Biao Han, Deng-Guang Yu, and Ke Wang. 2022. "Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing" Biomolecules 12, no. 12: 1727. https://doi.org/10.3390/biom12121727
APA StyleLiu, Y., Li, C., Feng, Z., Han, B., Yu, D.-G., & Wang, K. (2022). Advances in the Preparation of Nanofiber Dressings by Electrospinning for Promoting Diabetic Wound Healing. Biomolecules, 12(12), 1727. https://doi.org/10.3390/biom12121727