Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides
Abstract
1. Introduction
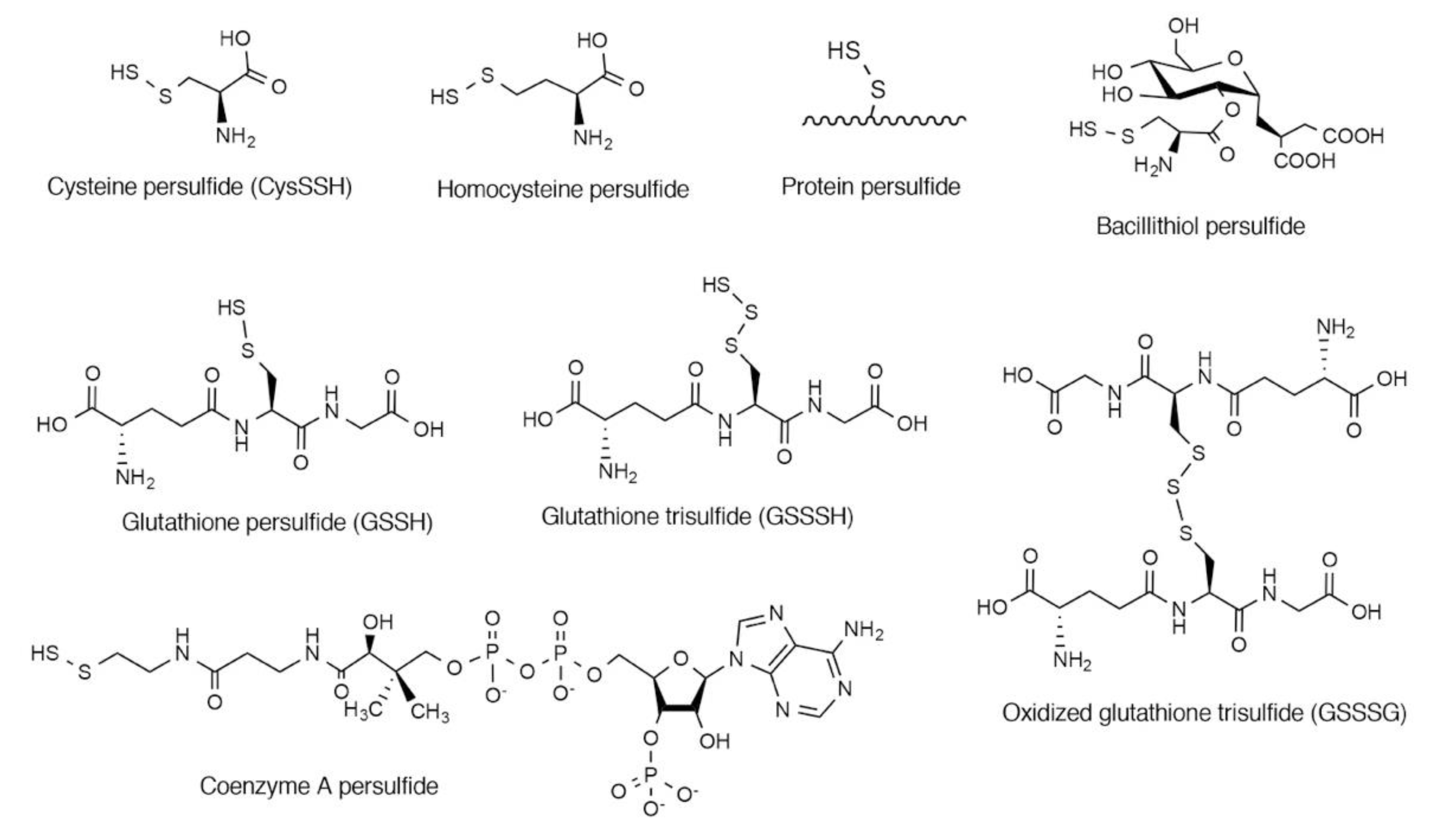
2. Endogenous Occurrence of CysSSH and Related Molecules
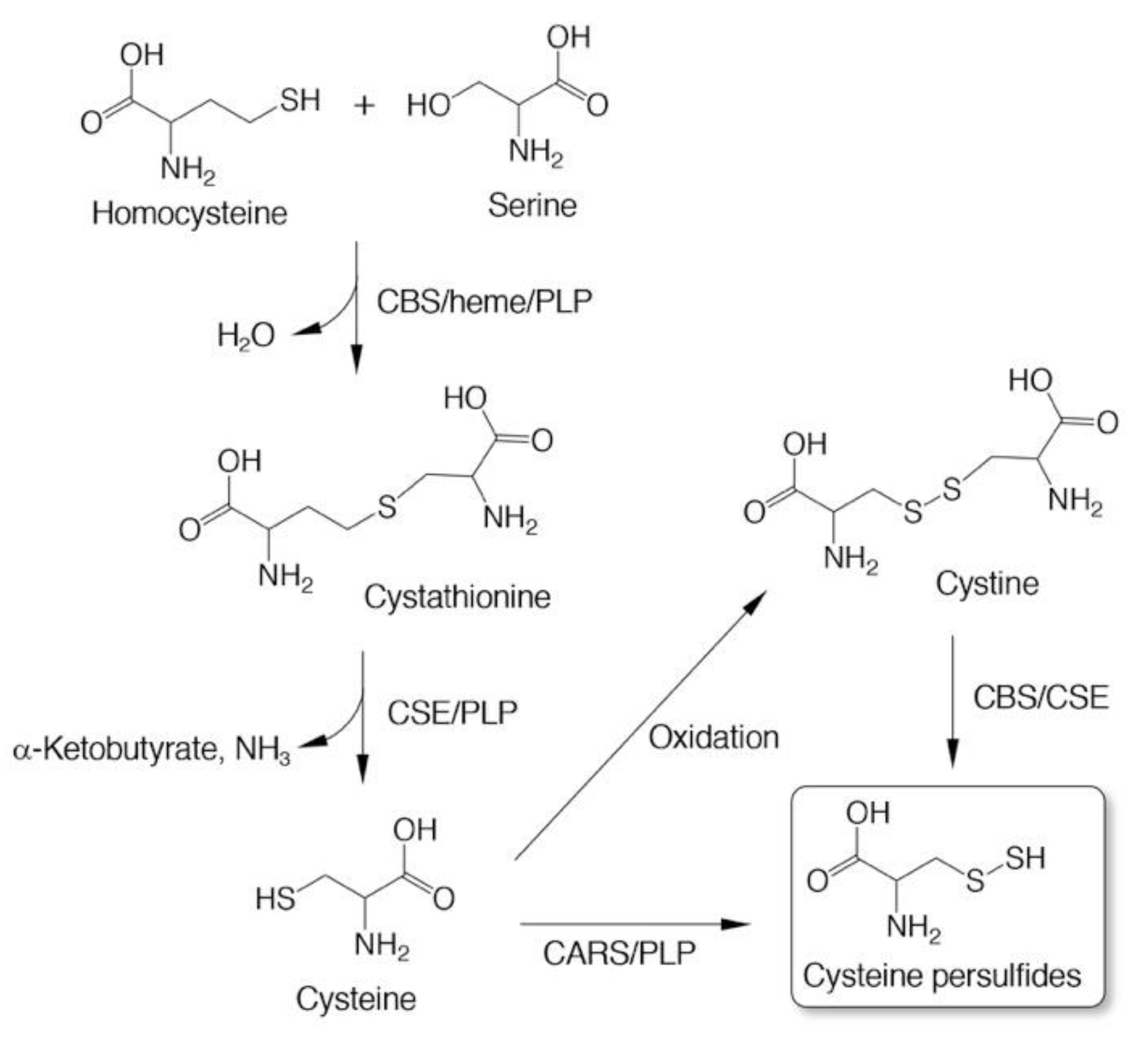
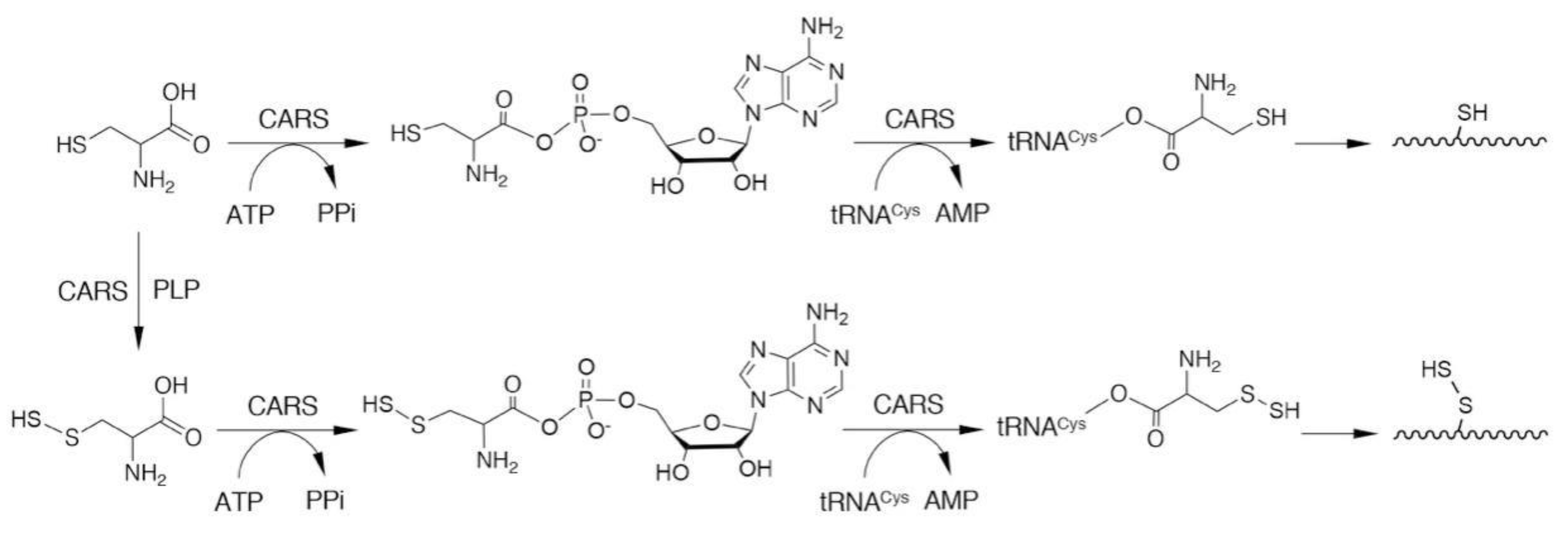
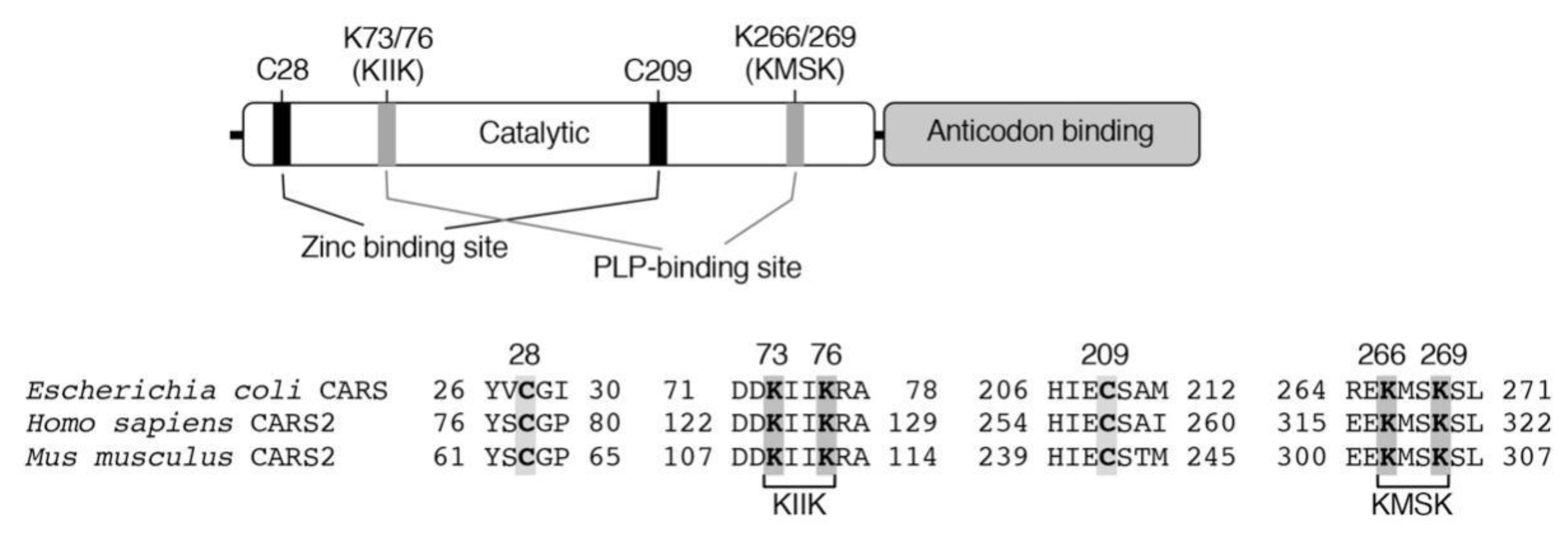
3. Biosynthesis of CysSSH and Related Molecules
4. Antioxidative and Nucleophilic Properties of Persulfides/Polysulfides
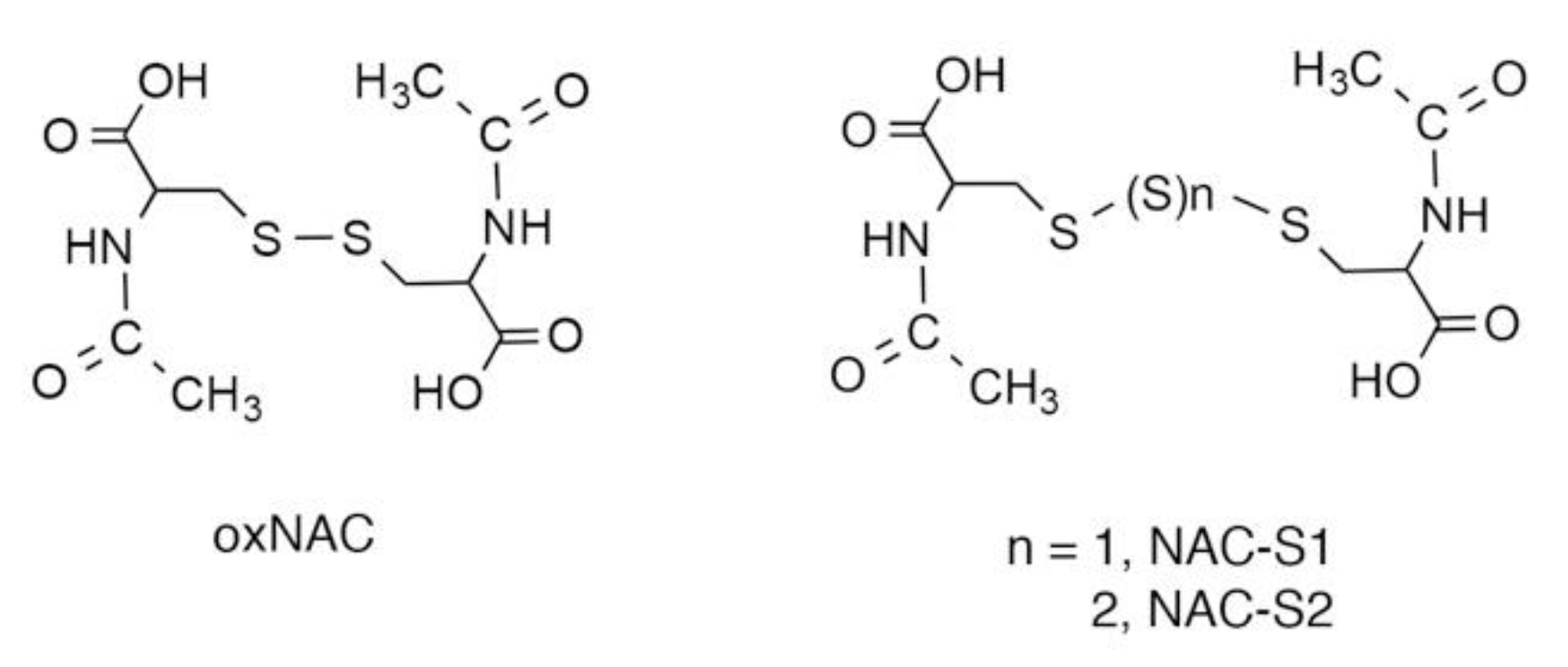
5. N-Acetyl-l-Cysteine (NAC) Polysulfides as Useful Chemical Tools to Investigate Biological Functions of Reactive Sulfur Species
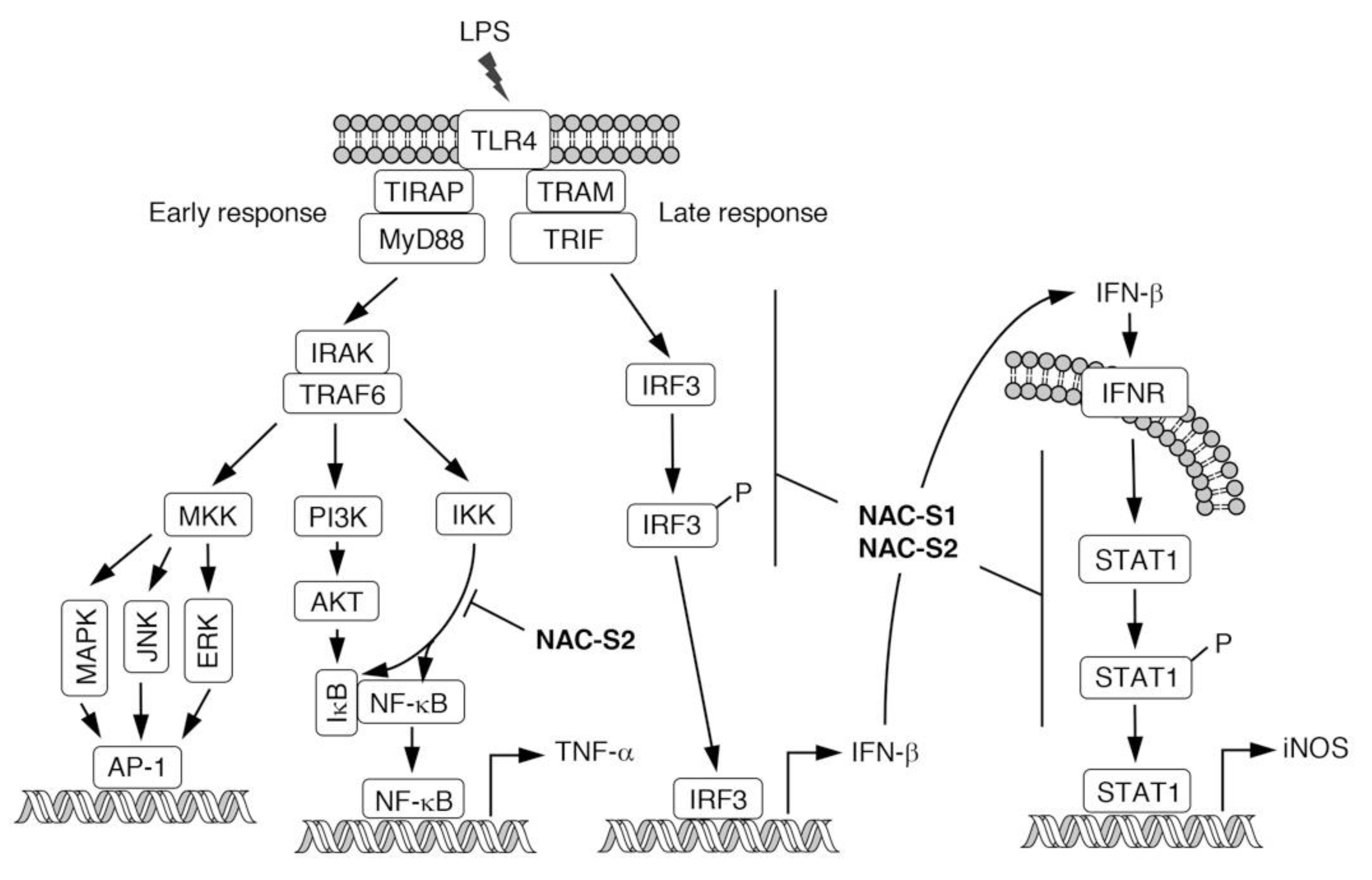
6. Anti-inflammatory Actions of NAC Polysulfides
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sawa, T.; Ono, K.; Tsutsuki, H.; Zhang, T.; Ida, T.; Nishida, M.; Akaike, T. Reactive cysteine persulphides: Occurrence, biosynthesis, antioxidant activity, methodologies, and bacterial persulphide signalling. Adv. Microb. Physiol. 2018, 72, 1–28. [Google Scholar]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Fujii, S.; Sawa, T.; Motohashi, H.; Akaike, T. Persulfide synthases that are functionally coupled with translation mediate sulfur respiration in mammalian cells. Br. J. Pharmacol. 2019, 176, 607–615. [Google Scholar] [CrossRef]
- Motohashi, H.; Akaike, T. Sulfur-utilizing cytoprotection and energy metabolism. Curr. Opin. Physiol. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef]
- Kunikata, H.; Ida, T.; Sato, K.; Aizawa, N.; Sawa, T.; Tawarayama, H.; Murayama, N.; Fujii, S.; Akaike, T.; Nakazawa, T. Metabolomic profiling of reactive persulfides and polysulfides in the aqueous and vitreous humors. Sci. Rep. 2017, 7, 41984. [Google Scholar] [CrossRef]
- Numakura, T.; Sugiura, H.; Akaike, T.; Ida, T.; Fujii, S.; Koarai, A.; Yamada, M.; Onodera, K.; Hashimoto, Y.; Tanaka, R.; et al. Production of reactive persulfide species in chronic obstructive pulmonary disease. Thorax 2017, 72, 1074–1083. [Google Scholar] [CrossRef]
- Ikeda, M.; Ishima, Y.; Shibata, A.; Chuang, V.T.G.; Sawa, T.; Ihara, H.; Watanabe, H.; Xian, M.; Ouchi, Y.; Shimizu, T.; et al. Quantitative determination of polysulfide in albumins, plasma proteins and biological fluid samples using a novel combined assays approach. Anal. Chim. Acta 2017, 969, 18–25. [Google Scholar] [CrossRef]
- Peng, H.; Shen, J.; Edmonds, K.A.; Luebke, J.L.; Hickey, A.K.; Palmer, L.D.; Chang, F.J.; Bruce, K.A.; Kehl-Fie, T.E.; Skaar, E.P.; et al. Sulfide homeostasis and nitroxyl intersect via formation of reactive sulfur species in Staphylococcus aureus. mSphere 2017, 2, e00082–e17. [Google Scholar] [CrossRef]
- Khan, S.; Fujii, S.; Matsunaga, T.; Nishimura, A.; Ono, K.; Ida, T.; Ahmed, K.A.; Okamoto, T.; Tsutsuki, H.; Sawa, T.; et al. Reactive persulfides from Salmonella typhimurium downregulate autophagy-mediated innate immunity in macrophages by inhibiting electrophilic signaling. Cell Chem. Biol. 2018, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Walsh, B.J.C.; Flores-Mireles, A.L.; Peng, H.; Zhang, Y.; Zhang, Y.; Trinidad, J.C.; Hultgren, S.J.; Giedroc, D.P. Hydrogen sulfide sensing through reactive sulfur species (RSS) and nitroxyl (HNO) in Enterococcus faecalis. ACS Chem. Biol. 2018, 13, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ono, K.; Tsutsuki, H.; Ihara, H.; Islam, W.; Akaike, T.; Sawa, T. Enhanced cellular polysulfides negatively regulate TLR4 signaling and mitigate lethal endotoxin shock. Cell Chem. Biol. 2019, 26, 686–698. [Google Scholar] [CrossRef]
- Hidese, R.; Mihara, H.; Esaki, N. Bacterial cysteine desulfurases: Versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl. Microbiol. Biotechnol. 2011, 91, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Crack, J.C.; Subramanian, S.; Green, J.; Thomson, A.J.; Le Brun, N.E.; Johnson, M.K. Reversible cycling between cysteine persulfide-ligated [2Fe-2S] and cysteine-ligated [4Fe-4S] clusters in the FNR regulatory protein. Proc. Natl. Acad. Sci. USA 2012, 109, 15734–15739. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Wei, F.Y.; Watanabe, S.; Hirayama, M.; Ohuchi, Y.; Fujimura, A.; Kaitsuka, T.; Ishii, I.; Sawa, T.; Nakayama, H.; et al. Reactive sulfur species regulate tRNA methylthiolation and contribute to insulin secretion. Nucleic Acids Res. 2017, 45, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Tsukuda, A.; Tsuchiya, Y.; Akaike, T.; Watanabe, Y. The active-site cysteine residue of Ca2+/calmodulin-dependent protein kinase I is protected from irreversible modification via generation of polysulfidation. Nitric Oxide 2019, 86, 68–75. [Google Scholar] [CrossRef]
- Takata, T.; Araki, S.; Tsuchiya, Y.; Watanabe, Y. Persulfide signaling in stress-initiated CaM kinase response. Antioxid. Redox Signal. 2020, in press. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small molecule signaling agents: The integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012, 25, 769–793. [Google Scholar] [CrossRef]
- Rudyk, O.; Rowan, A.; Prysyazhna, O.; Krasemann, S.; Hartmann, K.; Zhang, M.; Shah, A.M.; Ruppert, C.; Weiss, A.; Schermuly, R.T.; et al. Oxidation of PKGIα mediates an endogenous adaptation to pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2019, 116, 13016–13025. [Google Scholar] [CrossRef]
- Francoleon, N.E.; Carrington, S.J.; Fukuto, J.M. The reaction of H2S with oxidized thiols: Generation of persulfides and implications to H2S biology. Arch. Biochem. Biophys. 2011, 516, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.A.; Tanaka, A.; Ida, T.; Nishimura, A.; Matsunaga, T.; Fujii, S.; Morita, M.; Sawa, T.; Fukuto, J.M.; Nagy, P.; et al. Polysulfide stabilization by tyrosine and hydroxyphenyl-containing derivatives that is important for a reactive sulfur metabolomics analysis. Redox Biol. 2019, 21, 101096. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Akaike, T.; Sawa, T.; Kumagai, Y.; Wink, D.A.; Tantillo, D.J.; Hobbs, A.J.; Nagy, P.; Xian, M.; Lin, J.; et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014, 77, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat. Rev. Drug Discov. 2013, 12, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Kyogoku, Y.; Sugiura, H.; Ichikawa, T.; Numakura, T.; Koarai, A.; Yamada, M.; Fujino, N.; Tojo, Y.; Onodera, K.; Tanaka, R.; et al. Nitrosative stress in patients with asthma-chronic obstructive pulmonary disease overlap. J. Allergy Clin. Immunol. 2019, 144, 972–983 e14. [Google Scholar] [CrossRef]
- Postma, D.S.; Rabe, K.F. The Asthma-COPD Overlap Syndrome. N. Engl. J. Med. 2015, 373, 1241–1249. [Google Scholar] [CrossRef]
- Conrady, D.G.; Brescia, C.C.; Horii, K.; Weiss, A.A.; Hassett, D.J.; Herr, A.B. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. USA 2008, 105, 19456–19461. [Google Scholar] [CrossRef]
- Takagi, H.; Ohtsu, I. L-Cysteine Metabolism and Fermentation in Microorganisms. Adv. Biochem. Eng. Biotechnol. 2017, 159, 129–151. [Google Scholar]
- Miles, E.W.; Kraus, J.P. Cystathionine β-synthase: Structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 2004, 279, 29871–29874. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem. 1993, 62, 715–748. [Google Scholar] [CrossRef]
- Woese, C.R.; Olsen, G.J.; Ibba, M.; Soll, D. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol. Mol. Biol. Rev. 2000, 64, 202–236. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yang, X.L.; Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 2010, 11, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Christian, T.; Newberry, K.J.; Perona, J.J.; Hou, Y.M. Zinc-mediated amino acid discrimination in cysteinyl-tRNA synthetase. J. Mol. Biol. 2003, 327, 911–917. [Google Scholar] [CrossRef]
- Hallmann, K.; Zsurka, G.; Moskau-Hartmann, S.; Kirschner, J.; Korinthenberg, R.; Ruppert, A.K.; Ozdemir, O.; Weber, Y.; Becker, F.; Lerche, H.; et al. A homozygous splice-site mutation in CARS2 is associated with progressive myoclonic epilepsy. Neurology 2014, 83, 2183–2187. [Google Scholar] [CrossRef]
- Coughlin, C.R., 2nd; Scharer, G.H.; Friederich, M.W.; Yu, H.C.; Geiger, E.A.; Creadon-Swindell, G.; Collins, A.E.; Vanlander, A.V.; Coster, R.V.; Powell, C.A.; et al. Mutations in the mitochondrial cysteinyl-tRNA synthase gene, CARS2, lead to a severe epileptic encephalopathy and complex movement disorder. J. Med. Genet. 2015, 52, 532–540. [Google Scholar] [CrossRef]
- Doka, E.; Pader, I.; Biro, A.; Johansson, K.; Cheng, Q.; Ballago, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef]
- Jung, M.; Kasamatsu, S.; Matsunaga, T.; Akashi, S.; Ono, K.; Nishimura, A.; Morita, M.; Abdul Hamid, H.; Fujii, S.; Kitamura, H.; et al. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016, 480, 180–186. [Google Scholar] [CrossRef]
- Zivanovic, J.; Kouroussis, E.; Kohl, J.B.; Adhikari, B.; Bursac, B.; Schott-Roux, S.; Petrovic, D.; Miljkovic, J.L.; Thomas-Lopez, D.; Jung, Y.; et al. Selective persulfide detection reveals evolutionarily conserved antiaging effects of S-sulfhydration. Cell Metab. 2019, 30, 1152–1170. [Google Scholar] [CrossRef]
- Heppner, D.E.; Hristova, M.; Ida, T.; Mijuskovic, A.; Dustin, C.M.; Bogdandi, V.; Fukuto, J.M.; Dick, T.P.; Nagy, P.; Li, J.; et al. Cysteine perthiosulfenic acid (Cys-SSOH): A novel intermediate in thiol-based redox signaling? Redox Biol. 2018, 14, 379–385. [Google Scholar] [CrossRef]
- Nishimura, A.; Shimoda, K.; Tanaka, T.; Toyama, T.; Nishiyama, K.; Shinkai, Y.; Numaga-Tomita, T.; Yamazaki, D.; Kanda, Y.; Akaike, T.; et al. Depolysulfidation of Drp1 induced by low-dose methylmercury exposure increases cardiac vulnerability to hemodynamic overload. Sci. Signal. 2019, 12, eaaw1920. [Google Scholar] [CrossRef] [PubMed]
- Doka, E.; Ida, T.; Dagnell, M.; Abiko, Y.; Luong, N.C.; Balog, N.; Takata, T.; Espinosa, B.; Nishimura, A.; Cheng, Q.; et al. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020, 6, eaax8358. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-induced sulfhydration: Biological function and detection methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhao, S.; Xie, L.; Han, Y.; Ji, Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018, 175, 1146–1156. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Chen, Z.; Zhao, R.; Wang, Q.; Ran, M.; Xia, Y.; Hu, X.; Liu, J.; Xian, M.; et al. Using resonance synchronous spectroscopy to characterize the reactivity and electrophilicity of biologically relevant sulfane sulfur. Redox Biol. 2019, 24, 101179. [Google Scholar] [CrossRef]
- Everett, S.A.; Wardman, P. Perthiols as antioxidants: Radical-scavenging and prooxidative mechanisms. Methods Enzymol. 1995, 251, 55–69. [Google Scholar]
- Ihara, H.; Kasamatsu, S.; Kitamura, A.; Nishimura, A.; Tsutsuki, H.; Ida, T.; Ishizaki, K.; Toyama, T.; Yoshida, E.; Abdul Hamid, H.; et al. Exposure to electrophiles impairs reactive persulfide-dependent redox signaling in neuronal cells. Chem. Res. Toxicol. 2017, 30, 1673–1684. [Google Scholar] [CrossRef]
- Sawa, T.; Zaki, M.H.; Okamoto, T.; Akuta, T.; Tokutomi, Y.; Kim-Mitsuyama, S.; Ihara, H.; Kobayashi, A.; Yamamoto, M.; Fujii, S.; et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 2007, 3, 727–735. [Google Scholar] [CrossRef]
- Nishida, M.; Sawa, T.; Kitajima, N.; Ono, K.; Inoue, H.; Ihara, H.; Motohashi, H.; Yamamoto, M.; Suematsu, M.; Kurose, H.; et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Wang, Y.; Carrazzone, R.J.; Matson, J.B. A persulfide donor responsive to reactive oxygen species: Insights into reactivity and therapeutic potential. Angew. Chem. Int. Ed. 2018, 57, 6324–6328. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, B.; Li, Z.; Yuan, Z.; Organ, C.L.; Trivedi, R.K.; Wang, S.; Lefer, D.J.; Wang, B. An esterase-sensitive prodrug approach for controllable delivery of persulfide species. Angew. Chem. Int. Ed. Engl. 2017, 56, 11749–11753. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xu, S.; Radford, M.N.; Zhang, W.; Kelly, S.S.; Day, J.J.; Xian, M. O→S relay deprotection: A general approach to controllable donors of reactive sulfur species. Angew. Chem. Int. Ed. 2018, 57, 5893–5897. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Venkatesh, Y.; Das, J.; Gangopadhyay, M.; Maiti, T.K.; Singh, N.D.P. One- and two-photon-activated cysteine persulfide donors for biological targeting. J. Org. Chem. 2019, 84, 11441–11449. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Anuar, F.B.; Murch, O.; Bhatia, M.; Moore, P.K.; Thiemermann, C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005, 146, 498–505. [Google Scholar] [CrossRef]
- Wang, X.L.; Pan, L.L.; Long, F.; Wu, W.J.; Yan, D.; Xu, P.; Liu, S.Y.; Qin, M.; Jia, W.W.; Liu, X.H.; et al. Endogenous hydrogen sulfide ameliorates NOX4 induced oxidative stress in LPS-stimulated macrophages and mice. Cell Physiol. Biochem. 2018, 47, 458–474. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Young, S.H.; Ye, J.; Frazer, D.G.; Shi, X.; Castranova, V. Molecular mechanism of tumor necrosis factor-α production in 1→3-β-glucan (zymosan)-activated macrophages. J. Biol. Chem. 2001, 276, 20781–20787. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, R.; Wei, H.; Tian, Z. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc. Natl. Acad. Sci. USA 2005, 102, 17077–17082. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to nano therapeutics. Front. Physiol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.U.; Batool, M.; Choi, S. TLR4-targeting therapeutics: Structural basis and computer-aided drug discovery approaches. Molecules 2020, 25, 627. [Google Scholar] [CrossRef]
- Bachtell, R.; Hutchinson, M.R.; Wang, X.; Rice, K.C.; Maier, S.F.; Watkins, L.R. Targeting the toll of drug abuse: The translational potential of toll-like receptor 4. CNS Neurol. Disord. Drug Targets 2015, 14, 692–699. [Google Scholar] [CrossRef]
- Liu, J.; Buisman-Pijlman, F.; Hutchinson, M.R. Toll-like receptor 4: Innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front. Neurosci. 2014, 8, 309. [Google Scholar] [CrossRef]
- De Paola, M.; Sestito, S.E.; Mariani, A.; Memo, C.; Fanelli, R.; Freschi, M.; Bendotti, C.; Calabrese, V.; Peri, F. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol. Res. 2016, 103, 180–187. [Google Scholar] [CrossRef]
- Thakur, K.K.; Saini, J.; Mahajan, K.; Singh, D.; Jayswal, D.P.; Mishra, S.; Bishayee, A.; Sethi, G.; Kunnumakkara, A.B. Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol. Res. 2017, 115, 224–232. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawa, T.; Motohashi, H.; Ihara, H.; Akaike, T. Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides. Biomolecules 2020, 10, 1245. https://doi.org/10.3390/biom10091245
Sawa T, Motohashi H, Ihara H, Akaike T. Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides. Biomolecules. 2020; 10(9):1245. https://doi.org/10.3390/biom10091245
Chicago/Turabian StyleSawa, Tomohiro, Hozumi Motohashi, Hideshi Ihara, and Takaaki Akaike. 2020. "Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides" Biomolecules 10, no. 9: 1245. https://doi.org/10.3390/biom10091245
APA StyleSawa, T., Motohashi, H., Ihara, H., & Akaike, T. (2020). Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides. Biomolecules, 10(9), 1245. https://doi.org/10.3390/biom10091245





