Effects of Cannabis Use on the Protein and Lipid Profile of Olfactory Neuroepithelium Cells from Schizophrenia Patients Studied by Synchrotron-Based FTIR Spectroscopy
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects and Study Design
2.2. Clinical and Functional Assessment
2.3. Nasal Exfoliation and Cell Culture
2.4. Synchrotron-Radiation Fourier Transform Infrared (SR-FTIR) Spectroscopy
2.5. Statistical Analyses
3. Results
3.1. Demographic, Socioeconomic, and Functional Characteristics of the Sample
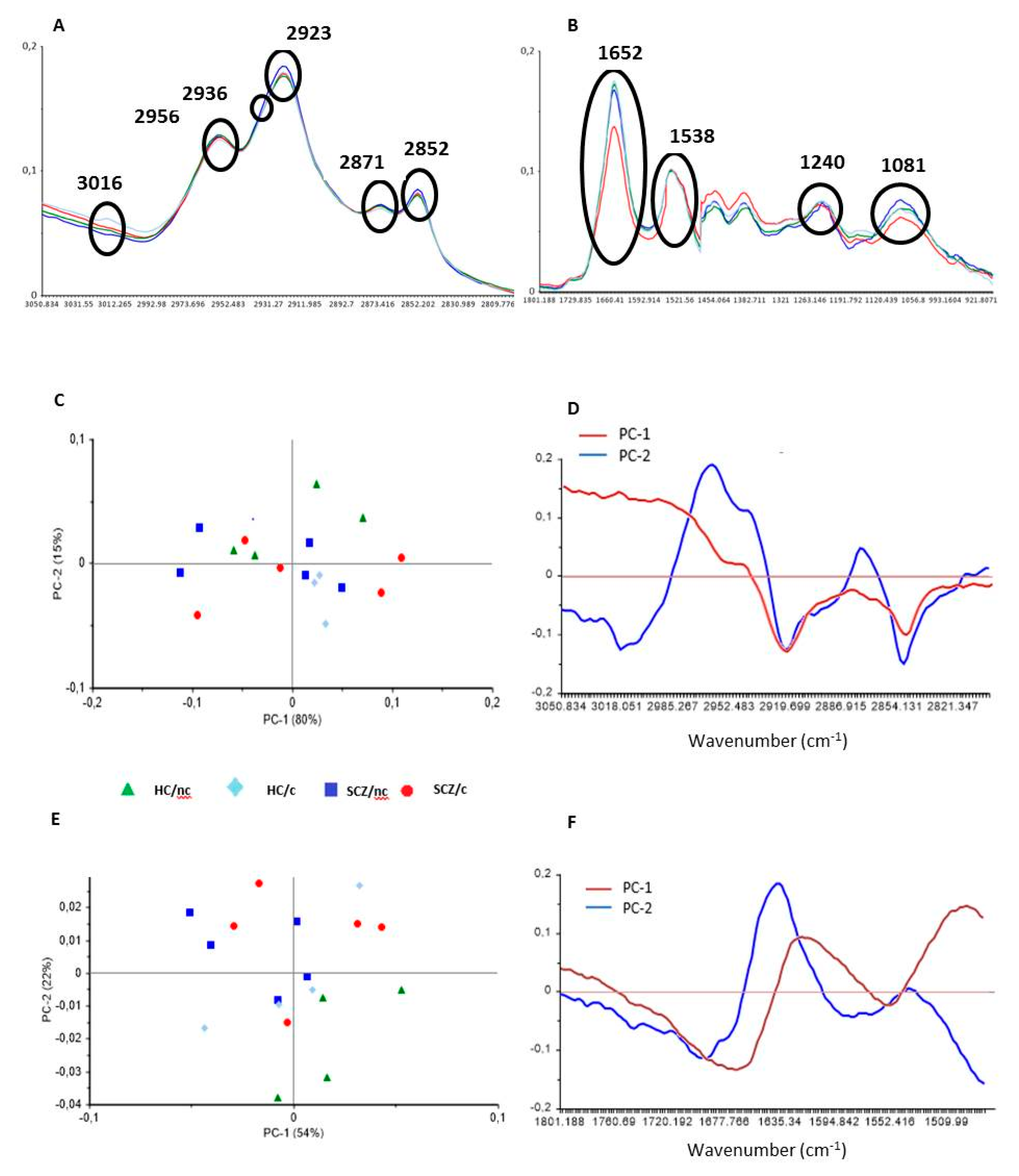
3.2. FTIR Spectrometry Principal Component Analyses
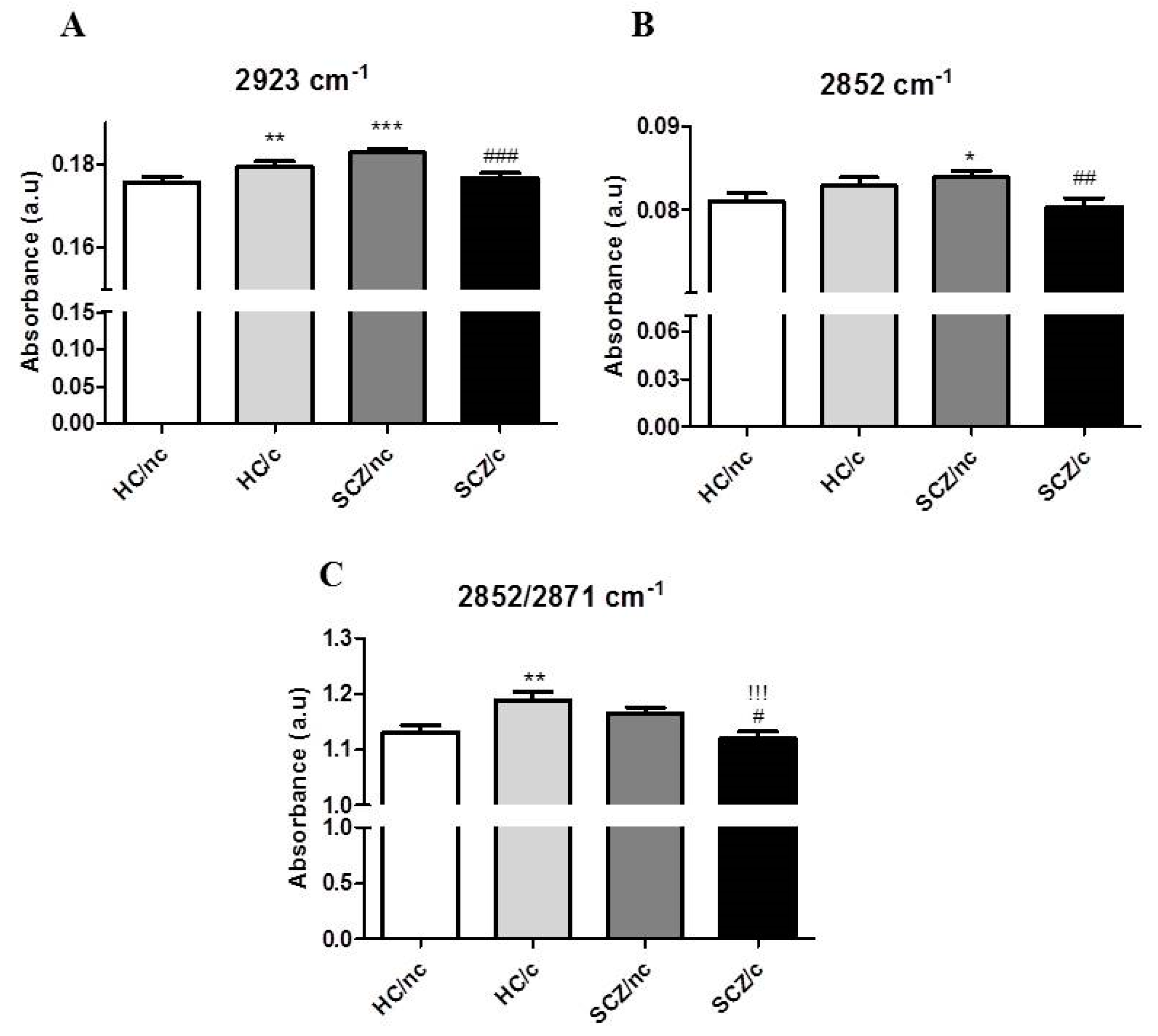
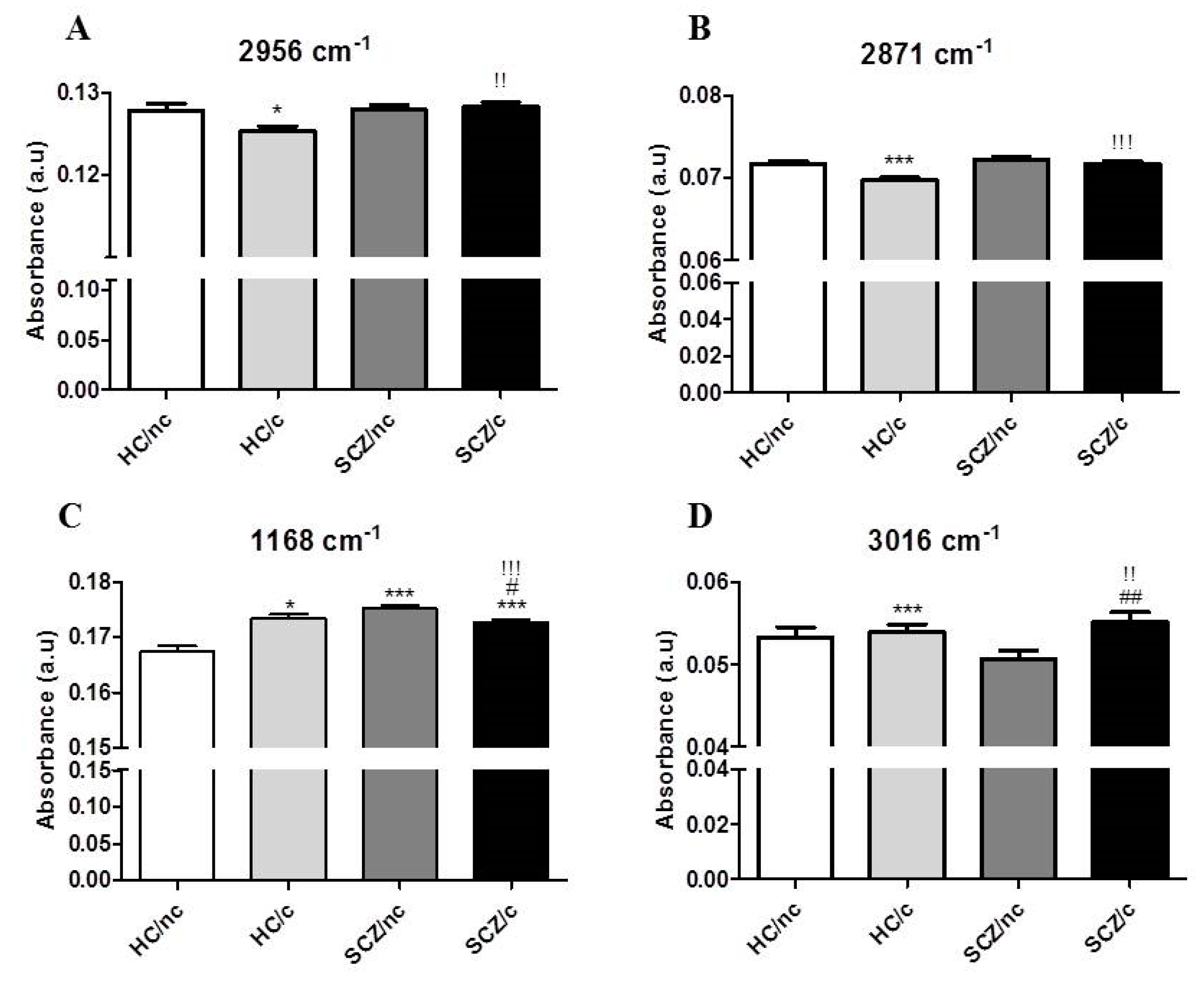
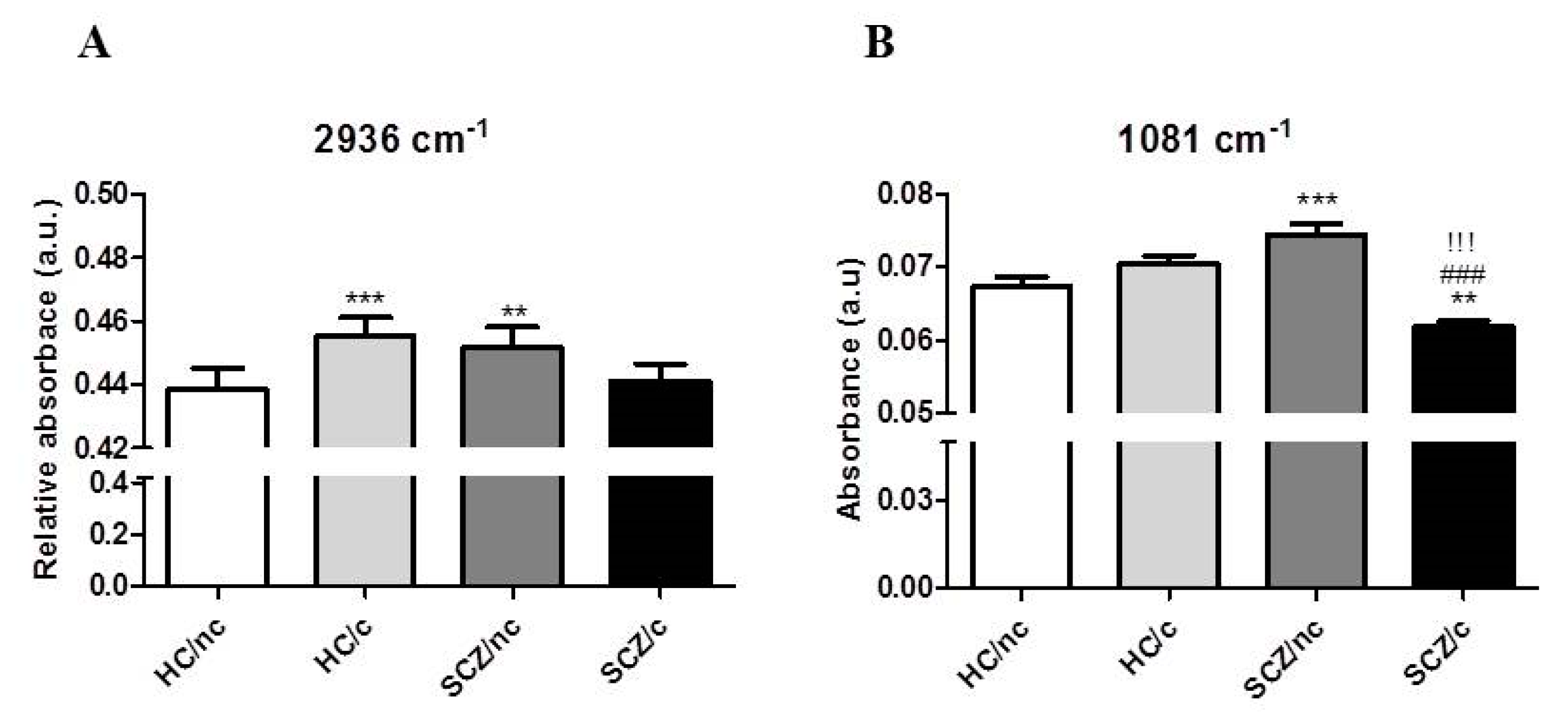
3.3. FTIR Spectrometry Analysis in the Lipid Region
3.4. FTIR Spectrometry Analyses of DNA Methylation
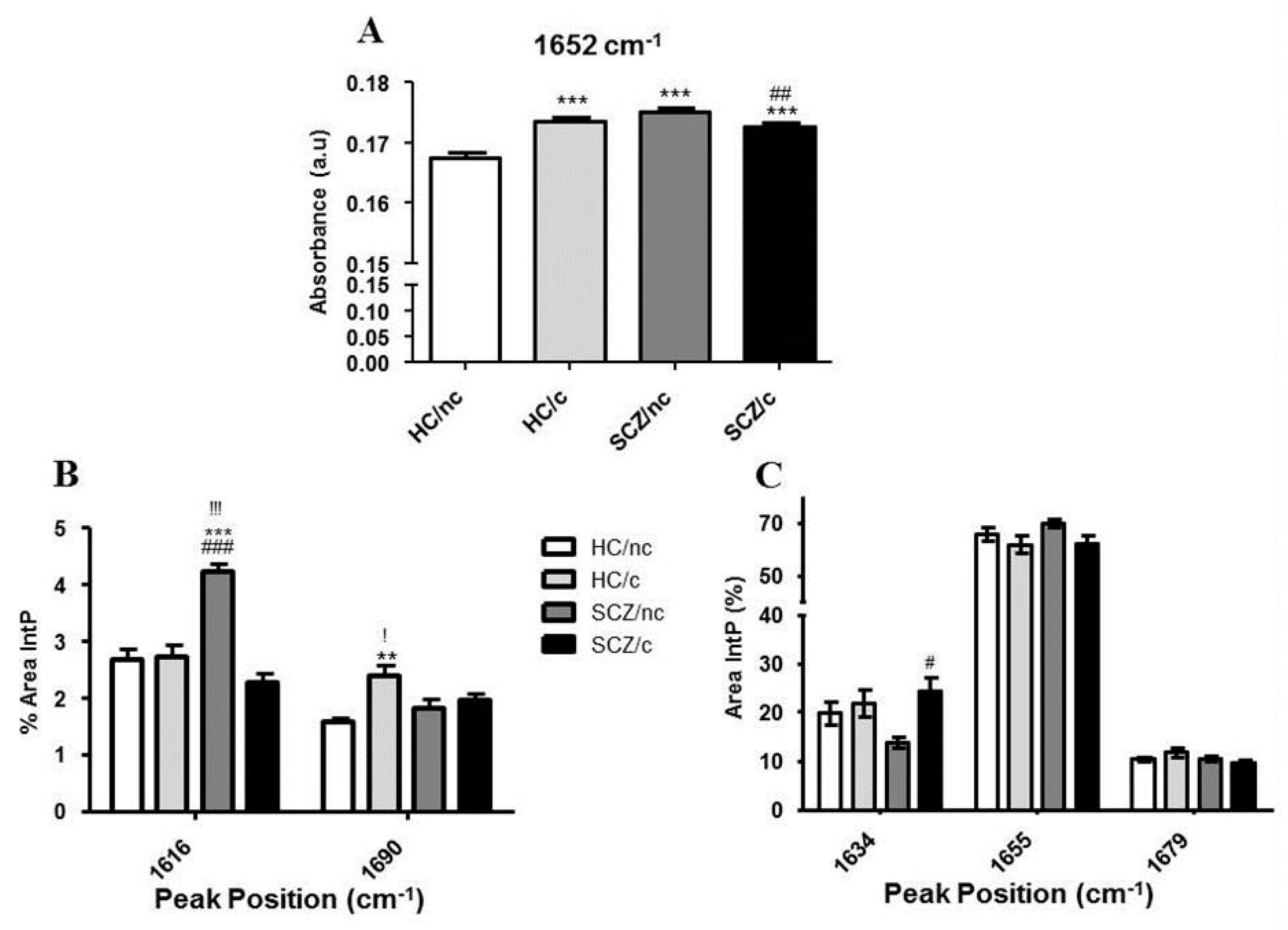
3.5. FTIR Spectrometry Analysis in the Protein Region
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schizophrenia. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia (accessed on 21 January 2020).
- Chong, H.Y.; Teoh, S.L.; Wu, D.B.C.; Kotirum, S.; Chiou, C.F.; Chaiyakunapruk, N. Global Economic Burden of Schizophrenia: A Systematic Review. Neuropsychiatr. Dis. Treat. 2016, 12, 357–373. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.A.; Levitt, P. Schizophr. as a Disorder of Neurodevelopment. Annu. Rev. Neurosci. 2002, 25, 409–432. [Google Scholar] [CrossRef]
- Owen, M.J.; Craddock, N.; O’Donovan, M.C. Schizophrenia: Genes at Last? Trends Genet. 2005, 518–525. [Google Scholar] [CrossRef]
- Owen, M.J.; Williams, N.M.; O’Donovan, M.C. The Molecular Genetics of Schizophrenia: New Findings Promise New Insights. Mol. Psychiatry 2004, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Korth, C. Aggregated Proteins in Schizophrenia and Other Chronic Mental Diseases: DISC1opathies. Prion 2012, 6, 134–141. [Google Scholar] [CrossRef]
- Khan, M.M.; Evans, D.R.; Gunna, V.; Scheffer, R.E.; Parikh, V.V.; Mahadik, S.P. Reduced Erythrocyte Membrane Essential Fatty Acids and Increased Lipid Peroxides in Schizophrenia at the Never-Medicated First-Episode of Psychosis and after Years of Treatment with Antipsychotics. Schizophr. Res. 2002, 58, 1–10. [Google Scholar] [CrossRef]
- Solberg, D.K.; Bentsen, H.; Refsum, H.; Andreassen, O.A. Lipid Profiles in Schizophrenia Associated with Clinical Traits: A Five Year Follow-up Study. BMC Psychiatry 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Dietrich-Muszalska, A.; Kontek, B. Lipid Peroxidation in Patients with Schizophrenia. Psychiatry Clin. Neurosci. 2010, 64, 469–475. [Google Scholar] [CrossRef]
- Davalieva, K.; Kostovska, I.M.; Dwork, A.J. Proteomics Research in Schizophrenia. Front. Cell. Neurosci. 2016, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Maynard, T.M.; Sikich, L.; Lieberman, J.A.; LaMantia, A.S. Neural Development, Cell-Cell Signaling, and the “Two-Hit” Hypothesis of Schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.; Degenhardt, L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry 2008, 7, 68–71. [Google Scholar] [CrossRef]
- Mounteney, J.; Griffiths, P.; Sedefov, R.; Noor, A.; Vicente, J.; Simon, R. The Drug Situation in Europe: An Overview of Data Available on Illicit Drugs and New Psychoactive Substances from European Monitoring in 2015. Addiction 2016, 111, 34–48. [Google Scholar] [CrossRef]
- Ameri, A. The Effects of Cannabinoids on the Brain. Prog. Neurobiol. 1999, 58, 315–348. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Ganesh, S.; Cortes-Briones, J.; Campbell, M.H.; Emmanuel, M.K. Characterizing Psychosis-Relevant Phenomena and Cognitive Function in a Unique Population with Isolated, Chronic and Very Heavy Cannabis Exposure. Psychol. Med. 2019, 1–8. [Google Scholar] [CrossRef]
- Scholes, K.E.; Martin-Iverson, M.T. Cannabis Use and Neuropsychological Performance in Healthy Individuals and Patients with Schizophrenia. Psychol. Med. 2010, 40, 1635–1646. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Cascio, M.G.; Di Marzo, V. The Endocannabinoid System: A General View and Latest Additions. Br. J. Pharmacol. 2004, 141, 765–774. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; Van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1. [Google Scholar] [CrossRef]
- Lavoie, J.; Sawa, A.; Ishizuka, K. Application of Olfactory Tissue and Its Neural Progenitors to Schizophrenia and Psychiatric Research. Curr. Opin. Psychiatry 2017, 176–183. [Google Scholar] [CrossRef]
- Leung, C.T.; Coulombe, P.A.; Reed, R.R. Contribution of Olfactory Neural Stem Cells to Tissue Maintenance and Regeneration. Nat. Neurosci. 2007, 10, 720–726. [Google Scholar] [CrossRef]
- Matigian, N.; Abrahamsen, G.; Sutharsan, R.; Cook, A.L.; Vitale, A.M.; Nouwens, A.; Bellette, B.; An, J.; Anderson, M.; Beckhouse, A.G.; et al. Disease-Specific, Neurosphere-Derived Cells as Models for Brain Disorders. DMM Dis. Model. Mech. 2010, 3, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Galindo, L.; Moreno, E.; Lopez-Armenta, F.; Guinart, D.; Cuenca-Royo, A.; Izquierdo-Serra, M.; Xicota, L.; Fernandez, C.; Menoyo, E.; Fernandez-Fernandez, J.M.; et al. Cannabis Users Show Enhanced Expression of CB1-5HT2A Receptor Heteromers in Olfactory Neuroepithelium Cells. Mol. Neurobiol. 2018, 55, 6347–6361. [Google Scholar] [CrossRef] [Green Version]
- Pijanka, J.; Sockalingum, G.D.; Kohler, A.; Yang, Y.; Draux, F.; Parkes, G.; Lam, K.P.; Collins, D.; Dumas, P.; Sandt, C.; et al. Synchrotron-Based FTIR Spectra of Stained Single Cells. Towards a Clinical Application in Pathology. Lab. Investig. 2010, 90, 797–807. [Google Scholar] [CrossRef] [Green Version]
- Dorling, K.M.; Baker, M.J. Highlighting Attenuated Total Reflection Fourier Transform Infrared Spectroscopy for Rapid Serum Analysis. Trends Biotechnol. 2013, 31, 327–328. [Google Scholar] [CrossRef]
- Sandt, C.; Frederick, J.; Dumas, P. Profiling Pluripotent Stem Cells and Organelles Using Synchrotron Radiation Infrared Microspectroscopy. J. Biophotonics 2013, 6, 60–72. [Google Scholar] [CrossRef]
- First, M.B.; Williams-Janet, B.W.; Spitzer, R.L.; Gibbon, M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT); Biometrics Research, New York State Psychiatric Institute: New York, NY, USA, 2007. [Google Scholar]
- Hollingshead, A.B.; Redlich, F.C. Social Class and Mental Illness: A Community Study. Am. J. Public Health 2007, 97, 1756–1757. [Google Scholar] [CrossRef]
- Torrens, M.; Serrano, D.; Astals, M.; Pérez-Domínguez, G.; Martín-Santos, R. Diagnosing Comorbid Psychiatric Disorders in Substance Abusers: Validity of the Spanish Versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Benitez-King, G.; Valdes-Tovar, M.; Trueta, C.; Galvan-Arrieta, T.; Argueta, J.; Alarcon, S.; Lora-Castellanos, A.; Solis-Chagoyan, H. The Microtubular Cytoskeleton of Olfactory Neurons Derived from Patients with Schizophrenia or with Bipolar Disorder: Implications for Biomarker Characterization, Neuronal Physiology and Pharmacological Screening. Mol. Cell. Neurosci. 2016, 73, 84–95. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [Green Version]
- Ami, D.; Natalello, A.; Mereghetti, P.; Neri, T.; Zanoni, M.; Monti, M.; Doglia, S.M.; Redi, C.A. FT-IR Spectroscopy Supported by PCA-LDA Analysis for the Study of Embryonic Stem Cell Differentiation. Spectroscopy 2010, 24, 89–97. [Google Scholar] [CrossRef]
- Rodríguez-Casado, A.; Alvarez, I.; Toledano, A.; De Miguel, E.; Carmona, P. Amphetamine Effects on Brain Protein Structure and Oxidative Stress as Revealed by FTIR Microspectroscopy. Biopolymers 2007, 86, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Gazi, E.; Dwyer, J.; Lockyer, N.P.; Miyan, J.; Gardner, P.; Hart, C.; Brown, M.; Clarke, N.W. Fixation Protocols for Subcellular Imaging by Synchrotron-Based Fourier Transform Infrared Microspectroscopy. Biopolymers 2005, 77, 18–30. [Google Scholar] [CrossRef]
- Clède, S.; Policar, C.; Sandt, C. Fourier Transform Infrared (FT-IR) Spectromicroscopy to Identify Cell Organelles: Correlation with Fluorescence Staining in MCF-7 Breast Cancer Cells. Appl. Spectrosc. 2014, 68, 113–117. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Vibrational Spectroscopy of Lipids. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- Ushasree, U.V.; Jaleeli, K.A.; Ahmad, A. Study on infrared spectroscopy of human blood. Int. J. Sci. Environ. Technol. 2006, 5, 1189–1192. [Google Scholar]
- Malek, K.; Wood, B.R.; Bambery, K.R. FTIR Imaging of Tissues: Techniques and Methods of Analysis. Chem. Soc. Rev. 2014, 45, 419–473. [Google Scholar] [CrossRef]
- Yoshida, S.; Zhang, Q.Z.; Sakuyama, S.; Matsushima, S. Metabolism of Fatty Acids and Lipid Hydroperoxides in Human Body Monitoring with Fourier Transform Infrared Spectroscopy. Lipids Health Dis. 2009, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Bindukumar, B.; Mahajan, S.D.; Reynolds, J.L.; Hu, Z.; Sykes, D.E.; Aalinkeel, R.; Schwartz, S.A. Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res. 2008, 1191, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wolff, V.; Schlagowski, A.I.; Rouyer, O.; Charles, A.L.; Singh, F.; Auger, C.; Schini-Kerth, V.; Marescaux, C.; Raul, J.S.; Zoll, J.; et al. Tetrahydrocannabinol Induces Brain Mitochondrial Respiratory Chain Dysfunction and Increases Oxidative Stress: A Potential Mechanism Involved in Cannabis-Related Stroke. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Fraile, M.V.; Patrón-Gallardo, B.; López-Rodríguez, G.; Carmona, P. FT-IR Study of Multilamellar Lipid Dispersions Containing Cholesteryl Linoleate and Dipalmitoylphosphatidylcholine. Chem. Phys. Lipids 1999, 97, 119–128. [Google Scholar] [CrossRef]
- Inouye, M.; Mio, T.; Sumino, K. Formation of 9-Hydroxy Linoleic Acid as a Product of Phospholipid Peroxidation in Diabetic Erythrocyte Membranes. Biochim. Biophys. Acta 1999, 1438, 204–212. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar] [CrossRef]
- Dyall, S.C. Interplay Between N-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900. [Google Scholar] [CrossRef]
- Morgan, C.J.A.; Page, E.; Schaefer, C.; Chatten, K.; Manocha, A.; Gulati, S.; Curran, H.V.; Brandner, B.; Leweke, F.M. Cerebrospinal Fluid Anandamide Levels, Cannabis Use and Psychotic-like Symptoms. Br. J. Psychiatry 2013, 202, 381–382. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, A.; Leweke, F.M.; Gerth, C.W.; Schreiber, D.; Koethe, D.; Faulhaber, J.; Klosterkötter, J.; Piomelli, D. Cerebrospinal Anandamide Levels Are Elevated in Acute Schizophrenia and Are Inversely Correlated with Psychotic Symptoms. Neuropsychopharmacology 2004, 29, 2108–2114. [Google Scholar] [CrossRef] [Green Version]
- Grayson, D.R.; Guidotti, A. The Dynamics of DNA Methylation in Schizophrenia and Related Psychiatric Disorders. Neuropsychopharmacology 2013, 38, 138–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C.F. Common Core Structure of Amyloid Fibrils by Synchrotron X-ray Diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR Spectroscopic Imaging of Protein Aggregation in Living Cells. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef] [Green Version]
- Dučić, T.; Stamenković, S.; Lai, B.; Andjus, P.; Lučić, V. Multimodal Synchrotron Radiation Microscopy of Intact Astrocytes from the HSOD1 G93A Rat Model of Amyotrophic Lateral Sclerosis. Anal. Chem. 2019, 91, 1460–1471. [Google Scholar] [CrossRef]
- De Marchi, N.; De Petrocellis, L.; Orlando, P.; Daniele, F.; Fezza, F.; Di Marzo, V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Muguruza, C.; Lehtonen, M.; Aaltonen, N.; Morentin, B.; Meana, J.J.; Callado, L.F. Quantification of endocannabinoids in postmortem brain of schizophrenic subjects. Schizophr. Res. 2013, 148, 145–150. [Google Scholar] [CrossRef]
- Muguruza, C.; Morentin, B.; Meana, J.J.; Alexander, S.P.H.; Callado, L.F. Endocannabinoid system imbalance in the postmortem prefrontal cortex of subjects with schizophrenia. J. Psychopharmacol. 2019, 33, 1132–1140. [Google Scholar] [CrossRef]
- Bioque, M.; Mas, S.; Costanzo, M.C.; Cabrera, B.; Lobo, A.; González-Pinto, A.; Rodriguez-Toscano, E.; Corripio, I.; Vieta, E.; Baeza, I.; et al. Gene-environment interaction between an endocannabinoid system genetic polymorphism and cannabis use in first episode of psychosis. Eur. Neuropsychopharmacol. 2019, 29, 786–794. [Google Scholar] [CrossRef]
- Roth, T.L.; Lubin, F.D.; Sodhi, M.; Kleinman, J.E. Epigenetic mechanisms in schizophrenia. Biochim. Biophys. Acta 2009, 1790, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shorter, K.R.; Miller, B.H. Epigenetic mechanisms in schizophrenia. Prog. Biophys. Mol. Biol. 2015, 118, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wockner, L.F.; Noble, E.P.; Lawford, B.R.; Young, R.M.D.; Morris, C.P.; Whitehall, V.L.J.; Voisey, J. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl. Psychiatry 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Healthy Controls (Non-Cannabis Users) | Healthy Controls (Cannabis Users) | Schizophrenia Patients (Non-Cannabis Users) | Schizophrenia Patients (Cannabis Users) | |
|---|---|---|---|---|
| Age (years) † | 30 (27.5–36.5) | 29 (22.5–41-5) | 39 (26.5–46-5) | 41 (36–47) |
| Gender; male (%) | 2 (40.0) | 4 (100.0) | 2 (40.0) | 5 (100.0) |
| BMI (kg/m2) † | 21.8 (19.9–25.37) | 20.2 (12–25.35) | 30.1 (23.54–41.45) | 25.35 (24.1–29.23) |
| History of nasal trauma/surgery; n (%) | 0 (0) | 1 (25) | 1 (20) | 1 (20) |
| History of rhinitis; n (%) | 1 (20) | 0 (0) | 0 (0) | 0 (0) |
| Tobacco Use | ||||
| Users–n, (%) | 5 (100) | 2 (50) | 3 (60) | 5 (100) |
| Units per week † | 49 (22.5–122-5) | 21 (14–28) | 140 (105–210) | 140 (87.5–210) |
| Length of use (years) † | 12 (8–16.5) | 17 | 9 (4–16) | 23 (15.5–33.5) |
| Cannabis Use | ||||
| Age first use (years) ‡ | n/a | 17 (2.16) | n/a | 17.2 (3.00) |
| Units per week † | n/a | 5.5 (3.25–17.5) | n/a | 21 (6.5–31.5) |
| Length of use (years) † | n/a | 6 (1–13) | n/a | 22 (16–31.5) * |
| Hollingshead-Redlich Score † | 45.5 (38.75–47) | 57 (49–62.5) | 52 (16.5–60,5) | 52 (41–56) |
| Global Assessment of Functioning (GAF) † | 100 (97.5–100) | 100 (92.5–100) | 65 (60–75) * | 65 (60–65) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saladrigas-Manjón, S.; Dučić, T.; Galindo, L.; Fernández-Avilés, C.; Pérez, V.; de la Torre, R.; Robledo, P. Effects of Cannabis Use on the Protein and Lipid Profile of Olfactory Neuroepithelium Cells from Schizophrenia Patients Studied by Synchrotron-Based FTIR Spectroscopy. Biomolecules 2020, 10, 329. https://doi.org/10.3390/biom10020329
Saladrigas-Manjón S, Dučić T, Galindo L, Fernández-Avilés C, Pérez V, de la Torre R, Robledo P. Effects of Cannabis Use on the Protein and Lipid Profile of Olfactory Neuroepithelium Cells from Schizophrenia Patients Studied by Synchrotron-Based FTIR Spectroscopy. Biomolecules. 2020; 10(2):329. https://doi.org/10.3390/biom10020329
Chicago/Turabian StyleSaladrigas-Manjón, Sergi, Tanja Dučić, Liliana Galindo, Cristina Fernández-Avilés, Víctor Pérez, Rafael de la Torre, and Patricia Robledo. 2020. "Effects of Cannabis Use on the Protein and Lipid Profile of Olfactory Neuroepithelium Cells from Schizophrenia Patients Studied by Synchrotron-Based FTIR Spectroscopy" Biomolecules 10, no. 2: 329. https://doi.org/10.3390/biom10020329




