Pharmacokinetics of Sativex® in Dogs: Towards a Potential Cannabinoid-Based Therapy for Canine Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Analysis of CBD, Δ9-THC and 11-Hydroxy-ΔΔ9-THC
2.3. Recording of the Neurological Status, Body Temperature, Respiratory Rate and Hemodynamic Parameters
2.4. Statistics
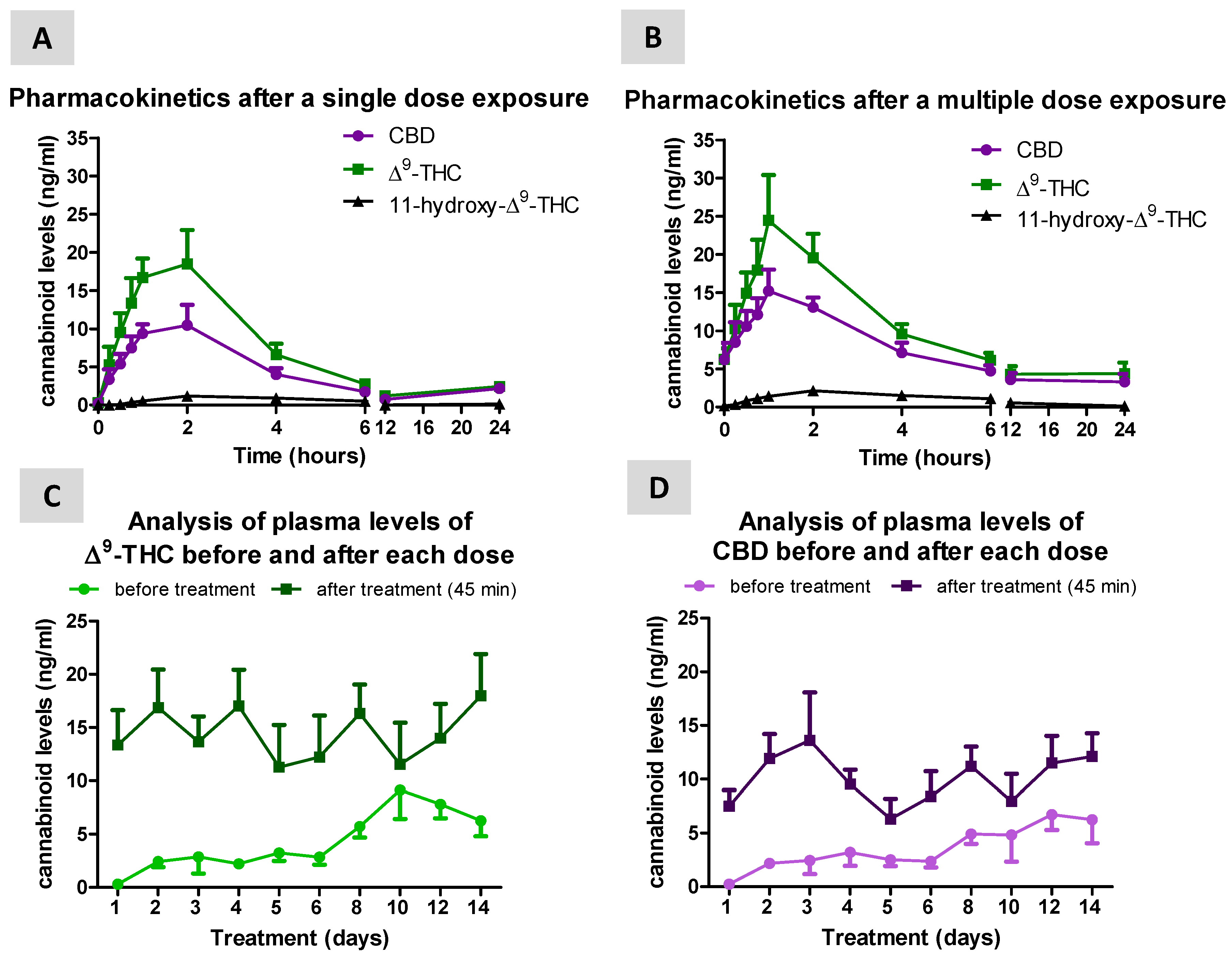
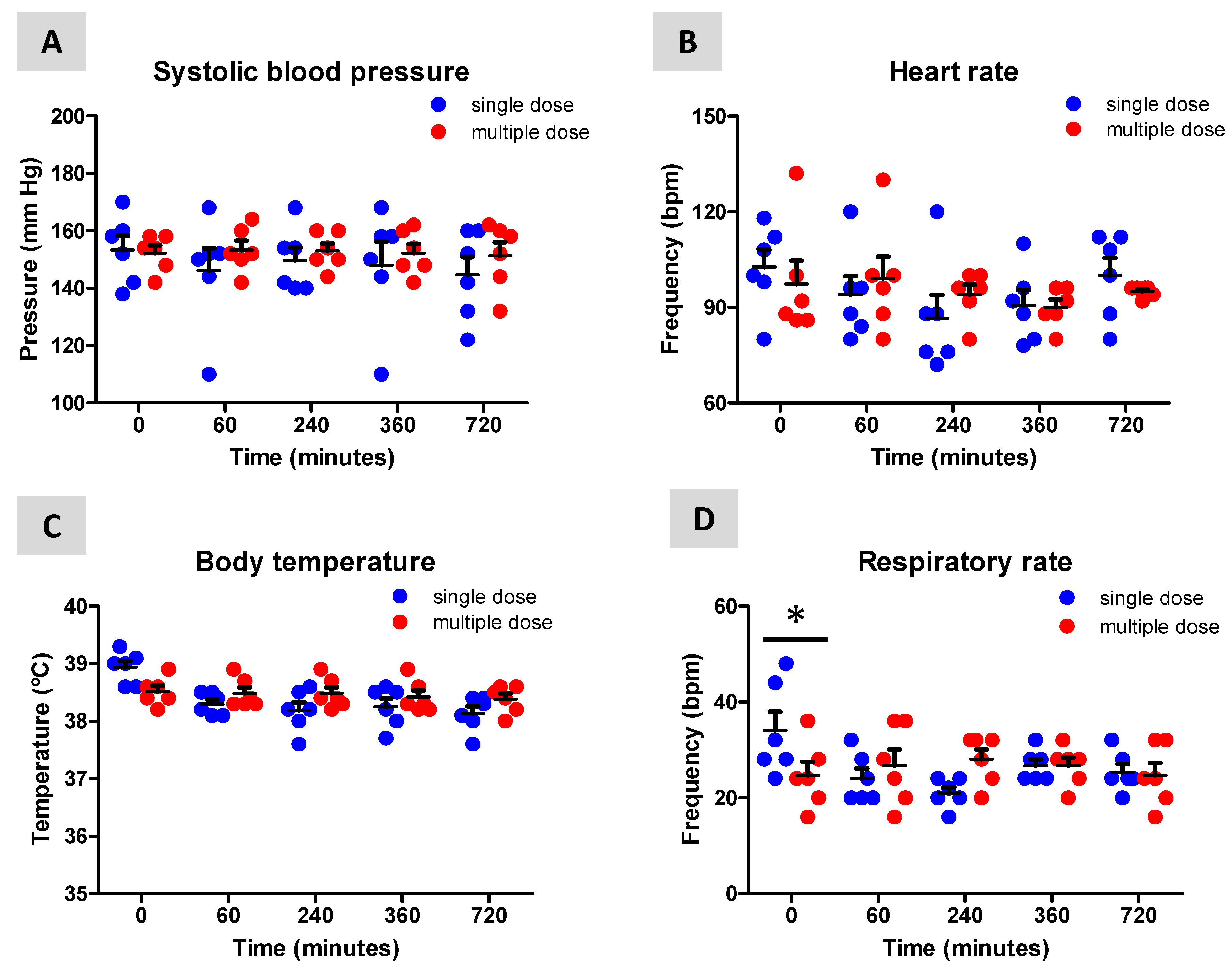
3. Results
3.1. Levels of Cannabinoids after Sativex Treatment
3.2. Neurological, Temperature, Respiratory and Hemodynamic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid. Res. 2016, 62, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sánchez, C.; Guzmán, M. Endocannabinoids and Cancer. Handb. Exp. Pharmacol. 2015, 231, 449–472. [Google Scholar] [PubMed]
- Howlett, A.C.; Johnson, M.R.; Melvin, L.S. Classical and nonclassical cannabinoids: Mechanism of action--brain binding. NIDA Res. Monogr 1990, 96, 100–111. [Google Scholar]
- Howlett, A.C.; Bidaut-Russell, M.; Devane, W.A.; Melvin, L.S.; Johnson, M.R.; Herkenham, M. The cannabinoid receptor: Biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990, 13, 420–423. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef]
- Martin, B.R.; Mechoulam, R.; Razdan, R.K. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999, 65, 573–595. [Google Scholar] [CrossRef]
- Pertwee, R.G. Ligands that target cannabinoid receptors in the brain: From THC to anandamide and beyond. Addict. Biol. 2008, 13, 147–159. [Google Scholar] [CrossRef]
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3353–3363. [Google Scholar] [CrossRef]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Δ9-Tetrahydrocannabinol/Cannabidiol oromucosal spray (Sativex®): A review in multiple sclerosis-related spasticity. Drugs 2017, 77, 563–574. [Google Scholar] [CrossRef]
- Giacoppo, S.; Bramanti, P.; Mazzon, E. Sativex in the management of multiple sclerosis-related spasticity: An overview of the last decade of clinical evaluation. Mult. Scler. Relat. Disord. 2017, 17, 22–31. [Google Scholar] [CrossRef]
- Otero-Romero, S.; Sastre-Garriga, J.; Comi, G.; Hartung, H.P.; Soelberg Sørensen, P.; Thompson, A.J.; Vermersch, P.; Gold, R.; Montalban, X. Pharmacological management of spasticity in multiple sclerosis: Systematic review and consensus paper. Mult. Scler. 2016, 22, 1386–1396. [Google Scholar] [CrossRef]
- Maccarrone, M.; Maldonado, R.; Casas, M.; Henze, T.; Centonze, D. Cannabinoids therapeutic use: What is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert Rev. Clin. Pharmacol. 2017, 10, 443–455. [Google Scholar] [CrossRef]
- Sagredo, O.; Pazos, M.R.; Valdeolivas, S.; Fernandez-Ruiz, J. Cannabinoids: Novel medicines for the treatment of Huntington’s disease. Recent Pat. CNS Drug Discov. 2012, 7, 41–48. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2019, in press. [Google Scholar] [CrossRef]
- Perez, J.; Ribera, M.V. Managing neuropathic pain with Sativex: A review of its pros and cons. Expert Opin. Pharmacother. 2008, 9, 1189–1195. [Google Scholar] [CrossRef]
- Valastro, C.; Campanile, D.; Marinaro, M.; Franchini, D.; Piscitelli, F.; Verde, R.; Di Marzo, V.; Di Bello, A. Characterization of endocannabinoids and related acylethanolamides in the synovial fluid of dogs with osteoarthritis: A pilot study. BMC Vet. Res. 2017, 13, 309. [Google Scholar] [CrossRef]
- Abramo, F.; Campora, L.; Albanese, F.; della Valle, M.F.; Cristino, L.; Petrosino, S.; Di Marzo, V.; Miragliotta, V. Increased levels of palmitoylethanolamide and other bioactive lipid mediators and enhanced local mast cell proliferation in canine atopic dermatitis. BMC Vet. Res. 2014, 10, 21. [Google Scholar] [CrossRef]
- Gesell, F.K.; Zoerner, A.A.; Brauer, C.; Engeli, S.; Tsikas, D.; Tipold, A. Alterations of endocannabinoids in cerebrospinal fluid of dogs with epileptic seizure disorder. BMC Vet. Res. 2013, 9, 262. [Google Scholar] [CrossRef]
- Fernández-Trapero, M.; Espejo-Porras, F.; Rodríguez-Cueto, C.; Coates, J.R.; Pérez-Díaz, C.; de Lago, E.; Fernández-Ruiz, J. Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Dis Model. Mech. 2017, 10, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Freundt-Revilla, J.; Heinrich, F.; Zoerner, A.; Gesell, F.; Beyerbach, M.; Shamir, M.; Oevermann, A.; Baumgärtner, W.; Tipold, A. The endocannabinoid system in canine Steroid-Responsive Meningitis-Arteritis and Intraspinal Spirocercosis. PLoS ONE 2018, 13, e0187197. [Google Scholar] [CrossRef] [PubMed]
- Galiazzo, G.; Giancola, F.; Stanzani, A.; Fracassi, F.; Bernardini, C.; Forni, M.; Pietra, M.; Chiocchetti, R. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem Cell Biol. 2018, 150, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Averill, D.R., Jr. Degenerative myelopathy in the aging German Shepherd dog: Clinical and pathologic findings. J. Am. Vet. Med. Assoc. 1973, 162, 1045–1051. [Google Scholar]
- Coates, J.R.; Wininger, F.A. Canine degenerative myelopathy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 929–950. [Google Scholar] [CrossRef] [PubMed]
- Awano, T.; Johnson, G.S.; Wade, C.M.; Katz, M.L.; Johnson, G.C.; Taylor, J.F.; Perloski, M.; Biagi, T.; Baranowska, I.; Long, S.; et al. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc. Natl. Acad Sci. USA 2009, 106, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Freundt-Revilla, J.; Kegler, K.; Baumgärtner, W.; Tipold, A. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS ONE 2017, 12, e0181064. [Google Scholar] [CrossRef]
- Pirone, A.; Cantile, C.; Miragliotta, V.; Lenzi, C.; Giannessi, E.; Cozzi, B. Immunohistochemical distribution of the cannabinoid receptor 1 and fatty acid amide hydrolase in the dog claustrum. J. Chem. Neuroanat. 2016, 74, 21–27. [Google Scholar] [CrossRef]
- Chiocchetti, R.; Galiazzo, G.; Tagliavia, C.; Stanzani, A.; Giancola, F.; Menchetti, M.; Militerno, G.; Bernardini, C.; Forni, M.; Mandrioli, L. Cellular distribution of canonical and putative cannabinoid receptors in canine cervical dorsal root ganglia. Front. Vet. Sci. 2019, 6, 313. [Google Scholar] [CrossRef]
- Whalley, B.J.; Lin, H.; Bell, L.; Hill, T.; Patel, A.; Gray, R.A.; Elizabeth Roberts, C.; Devinsky, O.; Bazelot, M.; Williams, C.M.; et al. Species-specific susceptibility to cannabis-induced convulsions. Br. J. Pharmacol 2019, 176, 1506–1523. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.lgcgroup.com/pharma (accessed on 10 February 2020).
- Ash, K.; Hayes, G.M.; Goggs, R.; Sumner, J.P. Performance evaluation and validation of the animal trauma triage score and modified Glasgow Coma Scale with suggested category adjustment in dogs: A VetCOT registry study. J. Vet. Emerg. Crit. Care 2018, 28, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Karschner, E.L.; Darwin, W.D.; Goodwin, R.S.; Wright, S.; Huestis, M.A. Plasma cannabinoid pharmacokinetics following controlled oral Δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin. Chem. 2011, 57, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Stott, C.G.; White, L.; Wright, S.; Wilbraham, D.; Guy, G.W. A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur. J. Clin. Pharmacol 2013, 69, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Norén, K.; Sjövall, J.; Halldin, M.M. Determination of Δ1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed. Chromatogr. 1989, 3, 35–38. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Trapero, M.; Pérez-Díaz, C.; Espejo-Porras, F.; de Lago, E.; Fernández-Ruiz, J. Pharmacokinetics of Sativex® in Dogs: Towards a Potential Cannabinoid-Based Therapy for Canine Disorders. Biomolecules 2020, 10, 279. https://doi.org/10.3390/biom10020279
Fernández-Trapero M, Pérez-Díaz C, Espejo-Porras F, de Lago E, Fernández-Ruiz J. Pharmacokinetics of Sativex® in Dogs: Towards a Potential Cannabinoid-Based Therapy for Canine Disorders. Biomolecules. 2020; 10(2):279. https://doi.org/10.3390/biom10020279
Chicago/Turabian StyleFernández-Trapero, María, Carmen Pérez-Díaz, Francisco Espejo-Porras, Eva de Lago, and Javier Fernández-Ruiz. 2020. "Pharmacokinetics of Sativex® in Dogs: Towards a Potential Cannabinoid-Based Therapy for Canine Disorders" Biomolecules 10, no. 2: 279. https://doi.org/10.3390/biom10020279
APA StyleFernández-Trapero, M., Pérez-Díaz, C., Espejo-Porras, F., de Lago, E., & Fernández-Ruiz, J. (2020). Pharmacokinetics of Sativex® in Dogs: Towards a Potential Cannabinoid-Based Therapy for Canine Disorders. Biomolecules, 10(2), 279. https://doi.org/10.3390/biom10020279




