Abstract
The essential oil of Eugenia uniflora has been attributed anti-depressive, antinociceptive, antileishmanial, larvicidal, antioxidant, antibacterial, and antifungal activities. It is known that the cultivation of this plant can be affected by seasonality, promoting alteration in the oil composition and its biological activities. This study aims to perform the annual evaluation of the curzerene-type oil of E. uniflora and determine its antioxidant activity. The oil yield from the dry season (1.4 ± 0.6%) did not differ statistically from that of the rainy season (1.8 ± 0.8%). Curzerene, an oxygenated sesquiterpene, was the principal constituent, and its percentage showed no significant difference between the two periods: dry (42.7% ± 6.1) and rainy (40.8 ± 5.9%). Principal component and hierarchical cluster analyses presented a high level of similarity between the monthly samples of the oils. Also, in the annual study, the yield and composition of the oils did not present a significant correlation with the climatic variables. The antioxidant activity of the oils showed inhibition of DPPH radicals with an average value of 55.0 ± 6.6%. The high curzerene content in the monthly oils of E. uniflora suggests their potential for use as a future phytotherapeutic alternative.
1. Introduction
Eugenia L. is included among the other 130 genera of Myrtaceae, and comprises about 1000 species distributed mainly in Central and South America and the African continent [1,2]. In the phytogeographical domains of Brazil, 260 species of Eugenia are described in the Atlantic Forest, 89 in the Amazon Forest, and 86 in the Brazilian Cerrado (Savanna) area [3], i.e., the country’s three main biomes. Among the taxa of Myrtaceae, Eugenia stands out for the diversity of its biological activities, for example, fungicide [4], bactericide [5], antioxidant, and anti-acetylcholinesterase [6].
Eugenia uniflora L. is a fruit tree which occurs naturally throughout South America, with widespread use of its fruits [7,8]. It is popularly known as “Pitanga”, a denomination originating in the Brazilian indigenous Tupi language, which means “dark red fruit”, and is also called “Cereja Brasileira” or “Ginja” [9]. The plant is used by traditional communities to treat diarrhea, cough, and rheumatism, and has presented other biological properties, such as hypothermic, antinociceptive, and antimicrobial activities [10,11]. Fruits of Pitanga are rich in calcium, magnesium, phosphorus, potassium, and vitamin C [12], as well as anthocyanins, flavonols, and carotenoids [13]. The fruits are consumed in their natural form, or in soft drinks, juices, ice-creams, sweets, liqueurs, and jellies, due to their sweet taste [14]. Traditional communities use an infusion of the leaves and barks as a antihypertensive and antipyretic, as well as against influenza, cough, cardiovascular diseases, asthma, sinusitis, colic, diarrhea, dysentery, fever, and rheumatism [10,11].
Eugenia uniflora is one of the most investigated species of the Eugenia genus, including through botanical, phytochemical, and pharmacological studies, due to its wide geographic distribution [15]. In a previously published paper, we reported that the essential oil of a curzerene-rich E. uniflora chemotype showed a significant antiproliferative and cytotoxic effect against some cancer strains [16]. Curzerene is a sesquiterpene hydrocarbon that was originally isolated from Curcuma longa L., a traditional Chinese herbal medicine, which showed anticancer activity in in vitro and in vivo models [17]. Also, curzerene was reported in the literature as a constituent produced by heating during the essential oil extraction and throughout the gas chromatographic analysis, resulting from the sigmatropic rearrangement of furanodiene, a heat-sensitive sesquiterpene compound which is present in the E. uniflora oil [18,19].
The objective of the present work was to evaluate the annual variability of the oil composition of E. uniflora leaves, the chemotype rich in curzerene thermally produced in situ, as a contribution to its possible use as an anticancer agent. To monitor the seasonal variability of the plant, tests were also carried out on the antioxidant activity of the oils produced, in addition to the previously observed cytotoxic effects.
2. Materials and Methods
2.1. Plant Material and Climatic Data
The leaves of E. uniflora were collected from a specimen existing on the Caratateua Island, Belém city, Pará state, Brazil (coordinates: 01°15′04.1” S/48°27′23.7” W). For the seasonal study, the leaves were sampled on day 5 of each month, at 8 am, from October 2017 to September 2018. Plant identification was performed by comparison with an authentic specimen of Eugenia uniflora, and a plant sample (MG 228493) was incorporated into the Herbarium “João Murça Pires” of Museu Paraense Emílio Goeldi, Belém city, State of Pará, Brazil. At the same time, the climatic parameters (radiation, relative air humidity, and rainfall precipitation) of the mentioned area were obtained each month from the website of the Instituto Nacional de Meteorologia (INMET, http://www.inmet.gov.br/portal/), of the Brazilian Government. Meteorological data were recorded through the automatic station A-201, at the Belém city, Pará state, Brazil, which is equipped with a Vaisala system, model MAWS 301 (Vaisala Corporation, Helsinki, Finland).
2.2. Essential Oils Extraction and Composition
The air-dried leaves were ground and subjected to hydrodistillation using a Clevenger-type apparatus (3 h). The oils were dried over anhydrous sodium sulfate, and the dry plant weights were used to calculate their yields. The moisture content of the plant samples was calculated using an infrared moisture balance for water loss measurement. Analysis of oil yield was done in triplicate.
The essential oil was dissolved with n-hexane at a concentration of 1500 µg/mL (3: 500, v/v), and analyzed simultaneously using gas chromatography–flame ionization detector (GC-FID, Shimadzu Corporation, Tokyo, Japan) and gas chromatography–mass spectrometry (GC/MS, Shimadzu Corporation, Tokyo, Japan) systems. The analysis of the oils was performed on a GCMS-QP2010 Ultra system (Shimadzu Corporation, Tokyo, Japan), equipped with an AOC-20i auto-injector and the GCMS-Solution software containing the NIST and FFNSC 2 libraries [20,21]. A Rxi-5ms (30 m × 0.25 mm; 0.25 μm film thickness) silica capillary column (Restek Corporation, Bellefonte, PA, USA) was used. The conditions of analysis were as follows. Injector temperature: 250 °C; Oven temperature programming: 60–240 °C (3 °C/min); Helium as carrier gas, adjusted to a linear velocity of 36.5 cm/s (1.0 mL/min); split mode injection for 1.0 μL of essential oil solution (6 µg of essential oil injected); split ratio 1:20 (300 ng to column for analysis); ionization by electronic impact at 70 eV; ionization source and transfer line temperatures of 200 and 250 °C, respectively. The mass spectra were obtained by automatic scanning every 0.3 s, with mass fragments in the range of 35–400 m/z. The retention index was calculated for all volatile components using a homologous series of C8-C40 n-alkanes (Sigma-Aldrich, Milwaukee, WI, USA), according to the linear equation of Van Den Dool and Kratz [22]. Individual components were identified by comparing their retention indices and mass spectra (molecular mass and fragmentation pattern) with those existing in the GCMS-Solution system libraries [20,21,23]. The quantitative data regarding the volatile constituents were obtained using a GC 2010 Series, operated under similar conditions to those of the GC-MS system. The relative amounts of individual components were calculated by peak-area normalization using a flame ionization detector (GC-FID) [16]. GC-FID and GC-MS analyses were performed in duplicate.
2.3. Antioxidant Assay
The antioxidant activity of the oils of seasonal samples was evaluated by the DPPH radical-scavenging method [24,25]. DPPH is a stable dark violet free radical with maximum absorption at 517 nm (Shimadzu, UV 1800, Shimadzu Corporation, Tokyo, Japan), which is reduced in the presence of antioxidants. A stock solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH; 0.5 mM) was prepared in ethanol. The solution was diluted to approximately 60 µM, measuring an initial absorbance of 0.62 ± 0.02, at 517 nm and room temperature. The absorbance was measured at the start of the reaction, every 5 min during the first 30 min, and then at 30 min intervals until constant absorbance values were observed (plateau of reaction, 2 h). Each essential oil sample from the seasonal study (5.0 µL, 10 mg/mL) was mixed with Tris-HCl buffer (100 mM, 900 µL, pH 7.4), ethanol (40 µL), and Tween 20 solution (0.5%, 50 µL, w/w), and then added to DPPH (0.5 mM, 1 mL) in ethanol. The standard curves were prepared using Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) (Sigma-Aldrich, St. Louis, MO, USA), at concentrations 30, 60, 150, 200, and 250 µg/mL. The results were expressed as milligrams of Trolox (mg TE/g) equivalents per gram of the sample.
2.4. Statistical Analysis
Statistical significance was assessed by a Tukey test (p < 0.05), and the Pearson correlation coefficients (R) were calculated to determine the relationship among the parameters analyzed (solar radiation, relative air humidity, and rainfall precipitation) in GraphPad Prism, version 5.0. A principal component analysis (PCA) was applied to verify the interrelation in the composition of the oils of the leaves (Minitab free 390 version, Minitab Inc., State College, PA, USA). A hierarchical grouping analysis (HCA), considering the Euclidean distance and complete linkage, was used to verify the similarity of the oil samples, based on the distribution of the constituents selected in the PCA analysis.
3. Results and Discussion
3.1. Essential Oil Yield vs Environmental Conditions
Climatic parameters such as solar radiation, precipitation, and relative humidity were monitored for a period of one year (October 2017 to September 2018) to evaluate the influence of seasonality on yield and composition of E. uniflora essential oil. Mean values of solar radiation ranged from 573.4 KJ/m2 (April) to 1450.7 KJ/m2 (December), relative humidity from 74.1% (November) to 85.4% (February), and the average rainfall from 90.0 mm (July) to 664.4 mm (February). Based on the precipitation data, the rainy season occurred from December (360.8 mm) to April (664.4 mm), and the dry season from June (236.6 mm) to November (90.0 mm). May (476.6 mm) was a period of transition between these two seasons (Figure 1).
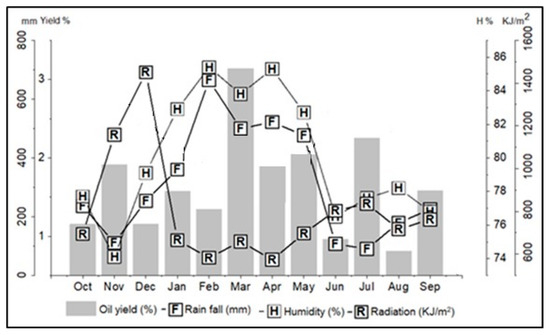
Figure 1.
Relationship between the climatic parameters and the oil yield of Eugenia uniflora during the seasonal study.
The Brazilian Amazonian climate is characterized only by a dry and a rainy season. Due to the permanent humid and warm climate, the Amazon presents spatial and seasonal heterogeneity of rainfall. The city of Belém, the collection site of E. uniflora, located in the Amazon region of northern Brazil, presents a higher rainfall index in December to April (rainy season) and a lower one in June to November (dry period). However, from one year to another, these two seasons may change, depending on the atmospheric phenomena that affect tropical regions [26].
In the annual study, the oil yield of E. uniflora ranged from 0.8% (August) to 3.1% (March), with an average of 1.7 ± 0.6% for the study period (see Table 1). Statistically (Tukey test), there was no significant difference in oil yields in the dry (1.4 ± 0.6%) and rainy (1.8 ± 0.8%) seasons. Additionally, significant correlation (p > 0.05) was not observed between the essential oil content and the climatic parameters, i.e., precipitation (R = 0.31), solar radiation (R = −0.17), and relative humidity (R = 0.32). Therefore, the results indicate that the variation in oil yields of E. uniflora, sampled in Caratateua Island, City of Belém, State of Pará, Brazil, do not correlate with climatic conditions and seasonal period.

Table 1.
Oil-composition of seasonal study of Eugenia uniflora.
3.2. Oil Composition and Environmental Conditions
The constituents of the oils were identified and quantified by GC-MS and GC-FID, respectively. Sixty-one compounds were identified, representing an average of 78.1% of the composition of the total oils (Table 1). Oxygenated sesquiterpenes were predominant (49.6–78.8%), followed by sesquiterpenes hydrocarbons (6.4–27.1%) and monoterpene hydrocarbons (0.5–8.6%).
Curzerene, a germacrane-type oxygenated sesquiterpene, was the principal constituent of this E. uniflora oil during the study period. The percentage of curzerene varied from 34.4% (April) to 53.1% (August). The ion chromatogram of the E. uniflora oil and the curzerene mass spectrum showing the base-peak (108 m/z) fragmentation are depicted in Figure 2 and Figure 3 and the mass spectra and retention index of the main compound are showed in Table S1 (see Supplementary Materials).
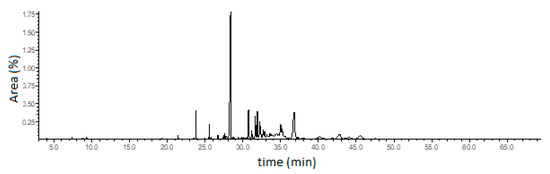
Figure 2.
Ion-chromatogram of the E. uniflora oil.
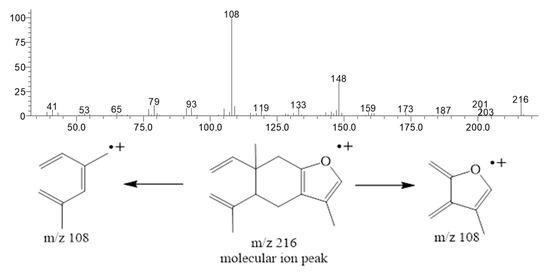
Figure 3.
Curzerene mass spectrum and its base-peak fragmentation (108 m/z).
The average content of curzerene during the dry (42.7% ± 6.1) and rainy (40.8 ± 5.9%) seasons did not present a statistically significant difference (Tukey test, p > 0.05). In addition, the climatic variables relative humidity (R = −0.07), solar radiation (R = −0.19), and precipitation (R = −0.03) showed no significant correlation with curzerene content (p > 0.05).
Other constituents were also identified in significant quantities in the E. uniflora oil (see Figure 4), such as the oxygenated sesquiterpenes germacrone (0.2–10.5%), globulol (1.5–7.4%), spathulenol (0.5–7.0%), and viridiflorol (0.8–6.2%), and the sesquiterpene hydrocarbons germacrene B (0.1–7.5%) and β-elemene (1.8–5.8%).
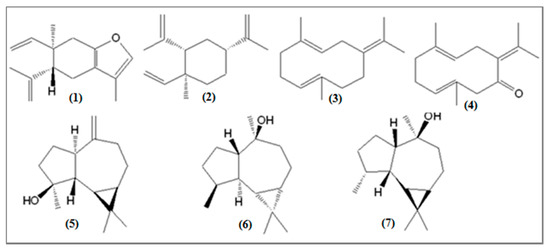
Figure 4.
Other sesquiterpene compounds identified in the oils of Eugenia uniflora: (1) curzerene, (2) β-elemene, (3) germacrene B, (4) germacrone, (5) spathulenol, (6) globulol, (7) viridiflorol.
Based on the Pearson (R) correlation coefficient analysis, there was no statistically significant correlation (p > 0.05) between the main constituents of the oils and the climatic parameters (see Table 2). The results suggest that the variation in the yield and composition of the oils is not related to climatic factors.

Table 2.
Antioxidant capacity of the monthly oils of Eugenia uniflora.
The application of a hierarchical clustering analysis (HCA) provided the dendrogram presented in Figure 5, which shows that the composition of the analyzed oils presented a similarity of 46.58%. HCA and PCA were plotted, with the constituents of oils displaying values above 3%.
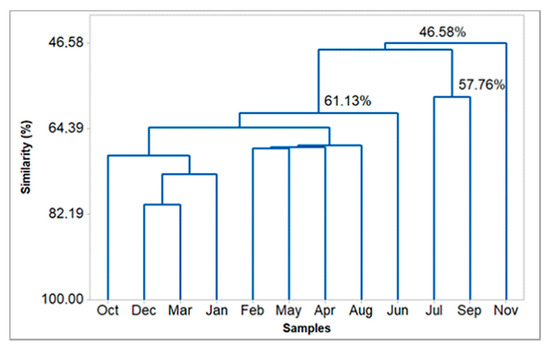
Figure 5.
Hierarchical clustering analysis (HCA) of the Eugenia uniflora oils, based on their main constituents.
In the principal component analysis (PCA), the main components (PC1 and PC2) accounted for 63.4% of the total data variability, of which PC1 explained 39.0% and displayed positive correlations with the variables globulol (R = 0.04), viridiflorol (R = 0.28), β-elemene (R = 0.42), viridiflorene (R = 0.44), γ-elemene (R = 0.41), germacrene B (R = 0.18), (E)-β-ocimene (R = 0.13), and α-cadinol (R = 0.02). The component PC2 explained 24.4% of the variability, and showed a positive correlation with the compounds spathulenol (R = 0.08), curzerene (R = 0.11), globulol (R = 0.57), viridiflorol (R = 0.39), and β-elemene (R = 0.11). The PCA showed that there was no separation between the oil samples from the dry and rainy periods (Figure 6).
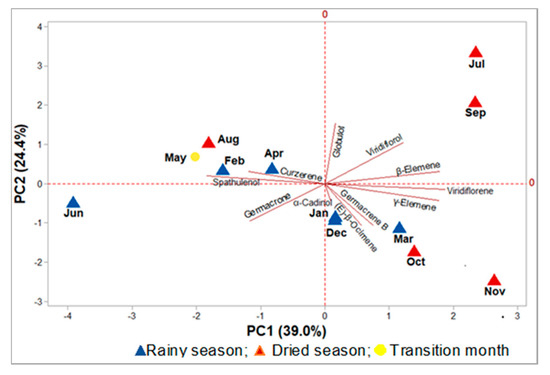
Figure 6.
Principal components analysis (PCA) of the Eugenia uniflora oils, based on their primary constituents.
It is known that in addition to environmental factors, differences in the yield and composition of the oils can be attributed to genetic and geographic factors, invading predators (herbivory and pathogens), as well as soils (edaphic factors) [27]. In the study of specimens of Eugenia lutescens and E. langsdorffii from the Brazilian Midwest, the differences observed in the yield and oil composition were attributed to genetic variation and environmental factors, considered to be responsible for the production and variability of the metabolites identified in these two plants [28]. The leaves of Eugenia neonitida and E. rotundifolia were collected in Rio de Janeiro, Brazil every quarter. The primary compounds of the E. neonitida oil were bicyclogermacrene, germacrene, and β-caryophyllene. In E. rotundifolia oil, the main components were α-pinene, β-pinene, and β-caryophyllene. Seasonality did not significantly interfere in the oils of either species, despite the observed qualitative and quantitative variations [29].
In previous studies, yields of 0.5% and 1.8% for E. uniflora oil were reported in two samples collected in north and south Brazil, respectively [30,31]. A seasonal study of the oil of a specimen of E. uniflora from the Brazilian Midwest indicated that its oil yield in the rainy period (0.3 ± 0.3%) was similar to that of the dry period (0.2 ± 0.2%). On the other hand, it also indicated the existence of two groups of constituents: the group I of samples collected in the dry season, characterized by the presence of spathulenol and caryophyllene oxide; and the group II composed of samples collected in the rainy season, with a predominance of selina-1,3,7(11) trien-8-one epoxide. This work presented different results due to the influence of seasonality in the composition and percentage of the oil constituents [32]. Some Eugenia uniflora oils from the Amazon region showed differences in their composition which were attributed to the environmental factors at the collection sites, leading to the identification of four different chemical types: (1) selin-1,3,7(11)-trien-8-one and selin-1,3,7(11)-trien-8-one epoxide; (2) selin-1,3,7(11)-trien-8-one, selin-1,3,7(11)-trien-8-one epoxide and caryophyllene oxide; (3) curzerene; and (4) germacrene B, curzerene and β-caryophyllene [16].
In the present work with E. uniflora oil, seasonality did not influence curzerene content during the two seasonal periods (dry and rainy) of the Brazilian Amazon, as mentioned (see Figure 5). Curzerene was reported in the literature as a furanediene transformation product, which, as one of the volatile constituents of E. uniflora leaves, undergoes a sigmatropic thermal rearrangement (3,3 Cope rearrangement) upon oil distillation or conventional gas chromatographic analysis [18,19]. In a previous work, furanodiene was isolated by silica column chromatography of young leaf oil from an E. uniflora specimen collected in Rio de Janeiro, Brazil. However, it was not detected after conventional gas chromatography analysis (GC-FID) after heating at the injector port, which means that its conversion to curzerene was complete during the chromatographic run [33]. Also, from the oil of an E. uniflora specimen from Nigeria, Africa, furanodiene and curzerene were identified in almost equal proportions [34]. Furanediene was not identified by 13C-NMR in the oil of the present E. uniflora specimen. On the other hand, using column chromatography, eluted with n-hexane, it was possible to isolate curzerene in high purity, which means that during the oil distillation process, there was a complete transformation of furanediene into curzerene.
3.3. Antioxidant Capacity
Different methods of determining the antioxidant capacity of essential oils and their constituents have been described; each one depends on a specific generator of free radicals and reactions by different mechanisms [35]. For this work, the DPPH radical scavenging method was selected.
The E. uniflora oils, resulting from twelve months of collection of plant samples, showed a DPPH radical scavenging capacity with an average of 55.0 ± 6.6%, as shown in Table 2. The reaction kinetics were considered slow, with an average of 120 min. The highest percentage of inhibition of the DPPH radical was observed for the oils samples resulting from the collection on December (64.0%) and January (64.2%). The total antioxidant capacity was expressed in values equivalent to the standard Trolox. The oil samples from December (435.0 ± 0.6 mg TE/g) and January (436.3 ± 2.3 mg TE/g) showed antioxidant activity only twice as low as Trolox, and therefore, with significant values.
The oils of E. uniflora, which were extracted in June, October, and November, inhibited the DPPH radical in a statistically similar manner (p > 0.05), presenting a similarity level of 46.58% (see Figure 5). Also, the oils obtained in December and January were statistically similar, as justified by the convergent composition observed in the HCA plot, with a similarity of 73.81%. The similarity of the December and January oils was confirmed in the PCA plot, where there is a superposition of both points (see Figure 6). The oils extracted in February and March also showed similar inhibition of the DPPH radical, exhibiting 64.05% similarity in their compositions, as observed in the HCA plot. In April and May, April and July, and April and September, the corresponding oils inhibited the DPPH radical, presenting similarity levels of 68.2%, 48.0%, and 48.0%, respectively. On the other hand, the oil obtained in August did not similarly inhibit the DPPH radical, showing the lowest inhibition (42.6%, 186.9 mg ET/g). In addition, the antioxidant activity showed a moderate and negative correlation with curzerene (R = −0.52) and the monoterpene hydrocarbons (−0.56) contents, as well as a weak or negligible correlation with climatic variables (R < 0.5).
A significant antioxidant activity of curzerene and germacrone against the DPPH radical was previously reported for some Curcuma oils, using the bioautography method [36]. The antioxidant activity of essential oils should not be considered only vis-a-vis its primary constituents. The synergistic action of other minor components contributes to an increase in antioxidant activity [37]. For instance, in two chemotypes of E. uniflora oils, this effect was observed in early reports [16]. One of them showed selin-1,3,7(11)-trien-8-one, selin-1,3,7(11)-trien-8-one epoxide, and caryophyllene oxide as significant constituents of the oil. The other chemotype presented a high curzerene content in the oil. The inhibition rates of the DPPH radical in oils were 45.1% and 42.8% for these two chemotypes, respectively [16]. Another oil of E. uniflora, mainly composed by germacrene B and selin-1,3,7(11)-trien-8-one epoxide, presented a significant DPPH antioxidant activity (IC50 833.3 ± 20.7 μg/mL), based on the DPPH scavenging assay [36]. The antioxidant capacity of the oils of different species of Eugenia has been reported, e.g., E. flavescens oil, with a value of 122.6 ± 6.8 mg TE/mL, E. patrisii oil, with a value of 111.2 ± 12.4 mg TE/mL), and E. egensis oil, with a value of 216.5 ± 11.6 mg TE/mL) value [24].
4. Conclusions
The results showed that climatic variables did not influence the average yield and curzerene content of E. uniflora essential oil. Environmental factors also had little influence on the composition of the oil and its antioxidant potential. The high curzerene content in this E. uniflora sample suggests its potential as a renewable source of this biologically-active compound, in particular as an antioxidant and antitumor agent. The study of this specimen contributes to the knowledge of E. uniflora, which is already widely used for its medicinal potential. Considering that there was a qualitative variation in the composition of the essential oil, with the transformation of furanodiene into curzerene by thermal rearrangement, this situation should be considered in the standardization of the oil, given its use as a possible herbal product.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/10/2/328/s1, Table S1: Mass spectra and retention index of the main compound identified in the essential oils of Eugenia uniflora.
Author Contributions
Formal analysis, J.S.d.C., A.S.B., R.H.V.M.; P.L.B.F., J.K.R.d.S., and J.G.S.M. Writing, proofreading and editing, P.L.B.F. and J.G.S.M. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to CNPq and Capes, institutions for scientific support and scholarship of the Brazilian Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WCSP (2020). World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://wcsp.science.kew.org (accessed on 18 February 2020).
- Van der Merwe, M.M.; Van Wyk, A.E.; Botha, A.M. Molecular phylogenetic analysis of Eugenia L. (Myrtaceae), with emphasis on southern African taxa. Plant. Syst. Evol. 2005, 251, 21–34. [Google Scholar] [CrossRef]
- Eugenia in Flora do Brasil 2020 em Construção. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB10560 (accessed on 18 February 2020).
- Rahimifard, N.; Sabzevari, O.; Shoeibi, S.; Pakzad, S.R.; Ajdary, S.; Hajimehdipoor, H.; Bagheri, F.; Safaee, M. Antifungal activity of the essential oil of Eugenia caryophyllata on Candida albicans, Aspergillus niger and Aspergillus flavus. Biomed. Pharmacol. J. Vol. 2008, 1, 43–46. [Google Scholar]
- Lawal, O.A.; Ogunwande, I.A.; Owolabi, M.S.; Opoku, A.R.; Oyedeji, A.O. Chemical Composition, Antibacterial Activity, and Brine Shrimp Lethality Test of Essential Oil from the Leaves of Eugenia natalitia. Chem. Nat. Compd. 2016, 52, 731–733. [Google Scholar] [CrossRef]
- Feitosa, C.M.; Barbosa, A.D.R.; Melo, C.H.S.D.; Freitas, R.M.; Fontes, J.E.D.N.; Costa, E.V.; Rashed, K.N.Z.; Costa Júnior, J.S.D. Antioxidant and anticholinesterase activities of the essential oil of Eugenia dysenterica DC. Afr. J. Pharm. Pharmacol. 2017, 11, 241–249. [Google Scholar]
- Wilson, P.G. Conspectus of the genus Eugenia (Myrtaceae) in the Philippines. Gard. Bull. Singapore 2009, 60, 399–410. [Google Scholar]
- Costa, A.G.V.; Garcia-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive compounds and health benefits of exotic tropical red-black berries. J. Funct. Foods 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Gallucci, S.; Neto, A.P.; Porto, C.; Barbizan, D.; Costa, I.; Marques, K.; Benevides, P.; Figueiredo, R. Essential Oil of Eugenia uniflora L.: An Industrial Perfumery Approach. J. Essent. Oil Res. 2010, 22, 176–179. [Google Scholar] [CrossRef]
- Vendruscolo, G.S.; Rates, S.M.K.; Mentz, L.A. Dados químicos e farmacológicos sobre as plantas utilizadas como medicinais pela comunidade do bairro Ponta Grossa, Porto Alegre, Rio Grande do Sul. Rev. Bras. Farmacogn. 2005, 15, 361–372. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- Bender, D.A. Benders’ Dictionary of Nutrition and Food Technology, 8th ed.; Woodhead Publishing: Sawston, UK, 2006; Available online: http://www.sciencedirect.com/science/article/pii/B9781845690519500028 (accessed on 14 February 2020).
- Lima, B.G.; Tietbohl, L.A.C.; Fernandes, C.P.; Cruz, R.A.S.; da Botas, G.S.; Santos, M.G.; Silva-Filho, M.V.; Rocha, L. Chemical composition of essential oils and anticholinesterasic activity of Eugenia sulcata spring ex mart. Lat. Am. J. Pharm. 2012, 31, 152–155. [Google Scholar]
- Coradin, L.; Siminski, A.; Reis, A. Espécies Nativas da Flora Brasileira de Valor Econômico Atual ou Potencial: Plantas para o Futuro—Região Sul, 1st ed.; Ministério do Meio Ambiente: Brasília, Brazil, 2011; pp. 170–177. ISBN 9788577381531. [Google Scholar]
- Queiroz, J.M.G.; Suzuki, M.C.M.; Motta, A.P.R.; Nogueira, J.M.R.; Carvalho, E.M.D. Aspectos populares e científicos do uso de espécies de Eugenia como fitoterápico. Rev. Fitos 2015, 9, 87–100. [Google Scholar]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Guo, J.; Wang, Q.; Zhu, S.; Gao, S.; Yang, C.; Wei, M.; Pan, X.; Zhu, W.; et al. Cytotoxic and tumor effects of curzerene from Curcuma longa. Planta. Med. 2017, 83, 23–29. [Google Scholar] [PubMed]
- Baldovini, N.; Tomi, F.; Casanova, J. Identification and quantitative determination of furanodiene, a heat-sensitive compound, in essential oil by 13C-NMR. Phytochem. Anal. 2001, 12, 58–63. [Google Scholar] [CrossRef]
- Melo, R.M.; Corrêa, V.F.S.; Amorim, A.C.L.; Miranda, A.L.P.; Rezende, C.M. Identification of Impact Aroma Compounds in Eugenia uniflora L. (Brazilian Pitanga) Leaf Essential Oil. J. Braz. Chem. Soc. 2007, 18, 179–183. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). Mass Spectral Library (NIST/EPA/NIH, v.2.0d); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: New York, NY, USA, 2011; ISBN 1118145836. [Google Scholar]
- Van Den Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, EUA, 2007. [Google Scholar]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-icrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Andrade, E.H.A.; Barreto, L.; da Silva, N.; Ribeiro, A.; Montenegro, R.; Maia, J. Chemical Composition of Four Essential Oils of Eugenia from the Brazilian Amazon and Their Cytotoxic and Antioxidant Activity. Medicines 2017, 4, 51. [Google Scholar] [CrossRef]
- Marengo, J.A.; Nobre, C.A. Clima da região Amazônica. In Tempo e Clima no Brasil, 1st ed.; Cavalcanti, I.F.A., Ferreira, N.J., Silva, M.G.A.J.D., Dias, M.A.F.D.S., Eds.; Oficina de Textos: São Paulo, Brazil, 2009. [Google Scholar]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Ribeiro, P.H.S.; Dos Santos, M.L.; Da Camara, C.A.G.; Born, F.S.; Fagg, C.W. Seasonal chemical compositions of the essential oils of two Eugenia species and their acaricidal properties. Quim. Nova 2016, 39, 38–43. [Google Scholar]
- Defaveri, A.C.A.; Sato, A.; Borré, L.B.; Aguiar, D.L.M.; San Gil, R.A.S.; Arruda, R.C.O.; Riehl, C.A.S. Eugenia neonitida Sobral and Eugenia rotundifolia Casar. (Cyrtaceae) essential oils: Composition, seasonality influence, antioxidant activity and leaf histochemistry. J. Braz. Chem. Soc. 2011, 22, 1531–1538. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; da Silva, M.H.L.; Zoghbi, M.G.B. A new chemotype of Eugenia uniflora L. from North Brazil. J. Essent. Oil Res. 1999, 11, 727–729. [Google Scholar] [CrossRef]
- Becker, N.A.; Volcão, L.M.; Camargo, T.M.; Freitag, R.A.; Ribeiro, G.A. Biological properties of Eugenia uniflora L. essential oil: Chemical composition and antimicrobial activity against gram negative bacteria. VITTALLE Rev. Ciências da Saúde 2017, 29, 22–30. [Google Scholar] [CrossRef]
- Costa, D.P.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Seasonal variability of essential oils of Eugenia uniflora leaves. J. Braz. Chem. Soc. 2009, 20, 1287–1293. [Google Scholar] [CrossRef]
- Santos, F.R.; Braz-Filho, R.; Castro, R.N. Influência da idade das folhas de Eugenia uniflora L. na composição química do óleo essencial. Quim. Nova 2015, 38, 762–768. [Google Scholar]
- Ogunwande, I.A.; Olawore, N.O.; Ekundayo, O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Victoria, F.N.; Lenardão, E.J.; Savegnago, L.; Perin, G.; Jacob, R.G.; Alves, D.; Silva, W.P.D.; Motta, A.D.S.D.; Nascente, P.D.S. Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).