Enhancing Night and Day Circadian Contrast through Sleep Education in Prediabetes and Type 2 Diabetes Mellitus: A Randomized Controlled Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. Diabetes Atlas, 10th ed.; 2021; Available online: https://diabetesatlas.org/data/en/ (accessed on 22 February 2022).
- Laxy, M.; Hunger, M.; Thorand, B.; Meisinger, C.; Kirchberger, I.; Holle, R. The Intermediate Burden of Diabetes Mellitus in Patients with Cardiovascular Disease (Cvd): A Quality Adjusted Life Year (Qaly)-Analysis Based on Primary Longitudinal Data. Value Health 2014, 17, A494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed]
- King, P.; Peacock, I.; Donnelly, R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. ClinPharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef]
- Stolar, M.W.; Hoogwerf, B.J.; Gorshow, S.M.; Boyle, P.J.; Wales, D.O. Managing type 2 diabetes: Going beyond glycemic control. J. Manag. Care Pharm. 2008, 14, s2–s19. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Palicka, V. Pathophysiology of Diabetes Mellitus. EJIFCC 2002, 13, 140–144. [Google Scholar] [PubMed]
- Levitt, N.S.; Steyn, K.; Lambert, E.V.; Reagon, G.; Lombard, C.J.; Fourie, J.M.; Rossouw, K.; Hoffman, M. Modifiable risk factors for Type 2 diabetes mellitus in a peri-urban community in South Africa. Diabet. Med. 1999, 16, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, W.Y.; Bergstrom, R.W.; Boyko, E.J.; Kinyoun, J.L.; Leonetti, D.L.; Newell-Morris, L.L.; Robinson, L.R.; Shuman, W.P.; Stolov, W.C.; Tsunehara, C.H.; et al. Diabetes and diabetes risk factors in second and third generation Japanese Americans in Seattle, Washington. Diabetes Res. Clin. Pract. 1994, 24, S43–S52. [Google Scholar] [CrossRef]
- Yadav, D.; Cho, K.H. Total Sleep Duration and Risk of Type 2 Diabetes: Evidence-Based on Clinical and Epidemiological Studies. Curr. Drug Metab. 2018, 19, 979–985. [Google Scholar] [CrossRef]
- Arora, T.; Taheri, S. Sleep Optimization and Diabetes Control: A Review of the Literature. Diabetes Ther. 2015, 6, 425–468. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Knutson, K.; Leproult, R.; Tasali, E.; Van Cauter, E. Sleep loss: A novel risk factor for insulin resistance and Type 2 Diabetes. J. Appl. Physiol. 2005, 99, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of glucocorticoid-induced insulin resistance: Focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.Y.; Tadi, P. Physiology, Obesity Neurohormonal Appetite and Satiety Control; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Cunha, M.C.; Zanetti, M.L.; Hass, V.J. Sleep quality in type 2 diabetics. Rev. Lat. Am. Enfermagem 2008, 16, 850–855. [Google Scholar] [CrossRef]
- Lou, P.; Qin, Y.; Zhang, P.; Chen, P.; Zhang, L.; Chang, G.; Li, T.; Qiao, C.; Zhang, N. Association of sleep quality and quality of life in type 2 diabetes mellitus: A cross-sectional study in China. Diabetes Res. Clin. Pract. 2015, 107, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Araujo, A.B.; McKinlay, J.B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006, 29, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of Sleep Time with Diabetes Mellitus and Impaired Glucose Tolerance. Arch. Intern. Med. 2005, 165, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Després, J.P.; Bouchard, C.; Astrup, A.; Tremblay, A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: Analyses of the Quebec Family Study. Sleep Med. 2009, 10, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Sleep Medicine and Research; Colten, H.R.; Altevogt, B.M. (Eds.) Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications fortype 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.C.; Ordovás, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611, Erratum in Int. J. Obes. 2013, 37, 624. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.M.; Healy, U.; Sreenan, S.; McDermott, J.; Coogan, A.N. An exploratory study of associations between sleep timing variability and cardiometabolic health in middle-aged adults with type 2 diabetes mellitus. Chronobiol. Int. 2022, 39, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Schipper, S.B.J.; Van Veen, M.M.; Elders, P.J.M.; van Straten, A.; Van Der Werf, Y.D.; Knutson, K.L.; Rutters, F. Sleep disorders in people with type 2 diabetes and associated health outcomes: A review of the literature. Diabetologia 2021, 64, 2367–2377. [Google Scholar] [CrossRef]
- Natale, P.; Ruospo, M.; Saglimbene, V.M.; Palmer, S.C.; Strippoli, G.F. Interventions for improving sleep quality in people with chronic kidney disease. Cochrane Database Syst. Rev. 2019, 5, CD012625. [Google Scholar] [CrossRef]
- Pereira, F.H.; Trevisan, D.D.; Lourenço, D.S.; Silva, J.B.; Lima, M.H. Effect of educational strategies on the sleep quality of people with diabetes: Randomized clinical trial. Aquichan 2019, 19, e1932. [Google Scholar] [CrossRef]
- Yang, B.; Xu, J.; Xue, Q.; Wei, T.; Xu, J.; Ye, C.; Mei, C.; Mao, Z. Non-pharmacological interventions for improving sleep quality in patients on dialysis: Systematic review and meta-analysis. Sleep Med. Rev. 2015, 23, 68–82. [Google Scholar] [CrossRef]
- Soleimani, F.; Motaarefi, H.; Hasanpour-Dehkordi, A. Effect of Sleep Hygiene Education on Sleep Quality in Hemodialysis Patients. J. Clin. Diagn. Res. 2016, 10, LC01–LC04. [Google Scholar] [CrossRef]
- García, J. Guía de Diabetes Tipo 2 Para Clínicos; Fundación redGDPS: Sabadell, España, 2018; pp. 16–18. [Google Scholar]
- American Academy of Sleep Medicine; American Alliance for Healthy Sleep. Healthy Sleep Habits. Available online: http://sleepeducation.org/essentials-in-sleep/healthy-sleep-habits (accessed on 10 March 2022).
- National Health Service. How to Get to Sleep. Sleep and Tiredness. Available online: https://www.nhs.uk/live-well/sleep-and-tiredness/self-help-tips-to-fight-fatigue/ (accessed on 2 April 2022).
- Generalitat de Catalunya—Departament de Salut. 10 Consells per Dormir Saludablement. Scientia. Dipòsit d’informació Digital del Departament de Salut. 29 May 2015. Available online: https://scientiasalut.gencat.cat/handle/11351/1514 (accessed on 10 August 2020).
- Larcher, S.; Gauchez, A.S.; Lablanche, S.; Pépin, J.L.; Benhamou, P.Y.; Borel, A.L. Impact of sleep behavior on glicemic control in type 1 diabetes: The role of Social jetlag. Eur. J. Endocrinol. 2016, 175, 411–419. [Google Scholar] [CrossRef]
- Kline, C.E.; Hall, M.H.; Buysse, D.J.; Earnest, C.P.; Church, T.S. Poor Sleep Quality is Associated with Insulin Resistance in Postmenopausal Women with and Without Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 183–189. [Google Scholar] [CrossRef]
- Buysse, D.J.; Germain, A.; Moul, D.E.; Franzen, P.L.; Brar, L.K.; Fletcher, M.E.; Begley, A.; Houck, P.R.; Mazumdar, S.; Reynolds, C.F., 3rd; et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch. Intern. Med. 2011, 171, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping hours: What is the ideal number and how does age impact this? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Chen, P.; Zhang, L.; Zhang, P.; Yu, J.; Zhang, N.; Wu, H.; Zhao, J. Relation of sleep quality and sleep duration to type 2 diabetes: A population-based cross-sectional survey. BMJ Open 2012, 2, e000956. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.D.; Schroder, E.A.; England, J.H.; Senn, N.J.; Westgate, P.M.; Esser, K.A.; Kern, P.A. Temperature as a Circadian Marker in Older Human Subjects: Relationship to Metabolic Syndrome and Diabetes. J. Endocr. Soc. 2017, 1, 843–851. [Google Scholar] [CrossRef]
- Ajala, O.; English, P. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef]
- Ahrén, B.; Johnson, S.L.; Stewart, M.; Cirkel, D.T.; Yang, F.; Perry, C.; Feinglos, M.N.; HARMONY 3 Study Group. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014, 37, 2141–2148. [Google Scholar] [CrossRef]
- Kattah, W.; Coral, P.; Mendez, F. Assessment of a treatment and education program in reducing the glucosilated haemoglobine levels in diabetic patients. Acta Med. Colomb. 2007, 32, 206–211. [Google Scholar]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Brath, H.; Paldánius, P.M.; Bader, G.; Kolaczynski, W.M.; Nilsson, P.M. Differences in glycemic control across world regions: A post-hoc analysis in patients with type 2 diabetes mellitus on dual antidiabetes drug therapy. Nutr. Diabetes 2016, 6, e217. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Gladstone, B.P.; Lübeck, M.; Lindekilde, N.; Upton, D.; Vach, W. Motivational interventions in the management of HbA1c levels: A systematic review and meta-analysis. Prim. Care Diabetes 2014, 8, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Creedy, D.; Lin, H.S.; Wollin, J. Effects of motivational interviewing intervention on self- management, psychological and glycemic outcomes in type 2 diabetes: A randomized controlled trial. Int. J. Nurs. Stud. 2012, 49, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Jemere, T.; Mossie, A.; Berhanu, H.; Yeshaw, Y. Poor sleep quality and its predictors among type 2 diabetes mellitus patients attending Jimma University Medical Center, Jimma, Ethiopia. BMC Res. Notes 2019, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Tan, S.; Gluckman, P.D.; Godfrey, K.M.; Saw, S.M.; Teoh, O.H.; Chong, Y.S.; Meaney, M.J.; Kramer, M.S.; Gooley, J.J.; et al. Sleep Quality and Nocturnal Sleep Duration in Pregnancy and Risk of Gestational Diabetes Mellitus. Sleep 2017, 40, zsw058. [Google Scholar] [CrossRef]
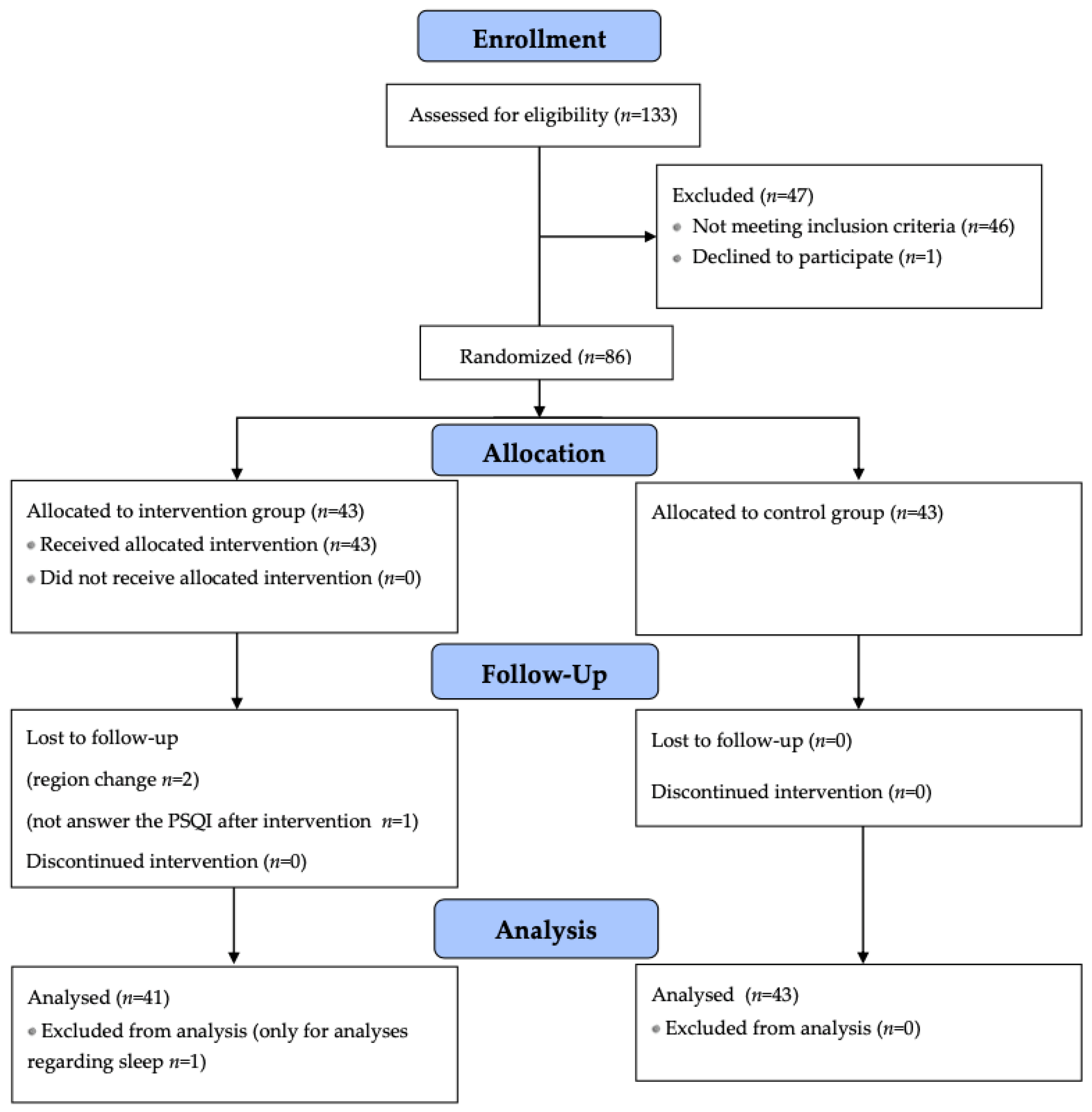

| Variable | Control Group (n = 31) | Intervention Group (n = 38) | Total (n = 69) |
|---|---|---|---|
| Age (years) | 64.5 (12.9) | 66.5 (11.3) | 65.6 (12.0) |
| Body Mass Index (Kg/m2) | 31.0 (5.6) | 29.9 (4.1) | 30.4 (4.8) |
| Time from blood test to intervention (days) | 46.0 [19.5;108] | 101 [15.0;138] | 62.0 [18.0;133] |
| Sex (woman) | 15 (48.4%) | 22 (57.9%) | 37 (53.6%) |
| Diagnosis (T2DM) | 29 (93.5%) | 33 (86.8%) | 62 (89.9%) |
| Antidiabetic pharmacological treatment (yes) | 25 (80.6%) | 31 (81.6%) | 56 (81.2) |
| Benzodiazepine intake (yes) | 5 (16.1%) | 10 (26.3%) | 15 (21.7%) |
| Preintervention sleep time > 6 h (yes) | 9 (29.0%) | 11 (28.9%) | 20 (29.0%) |
| Diet change (yes) | 2 (6.45%) | 4 (10.5%) | 6 (8.70%) |
| Physical activity change (yes) | 3 (9.68%) | 1 (2.63%) | 4 (5.80%) |
| Response Variables | Pre Values | Post Values | Change | |||||
|---|---|---|---|---|---|---|---|---|
| Control Group | Intervention Group | p Value | Control Group | Intervention Group | p Value | Control Group | Intervention Group | |
| Sleep | ||||||||
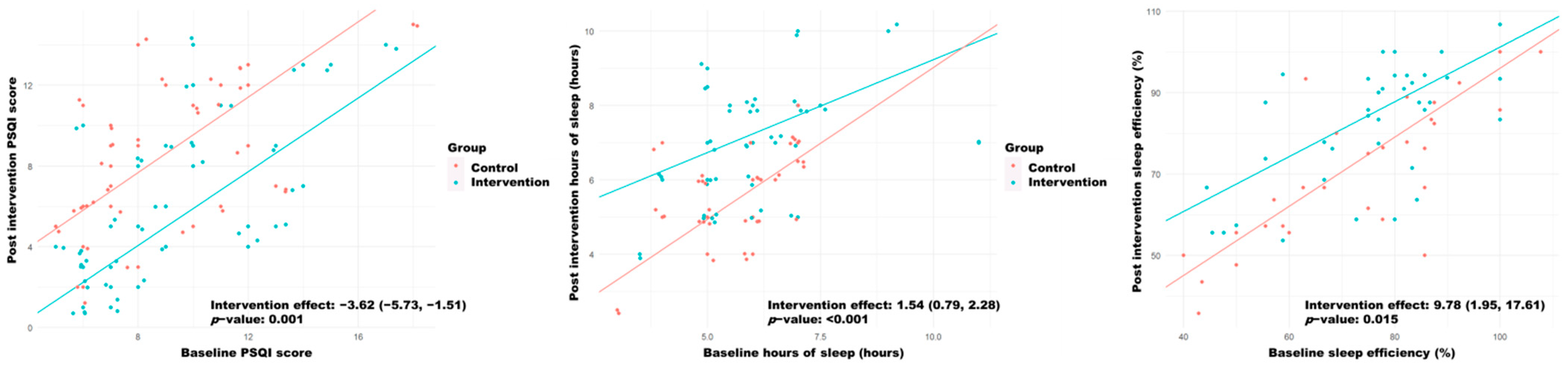
| PSQI | 8.00 [6.00;11.0] | 8.00 [6.00;10.8] | 0.98 | 8.00 [6.00;11.0] | 5.00 [3.00;9.00] | 0.008 | −0.61 (3.11) | −2.97 (2.93) ** |
| Hours of sleep | 6.00 [5.00;6.75] | 6.00 [5.00;6.50] | 0.76 | 6.00 [5.00;6.50] | 7.00 [6.00;8.00] | 0.002 | 0.00 [−0.50;1.00] | 1.00 [0.00;2.00] * |
| Sleep efficiency | 75.0 [58.0;86.3] | 77.3 [66.7;84.0] | 0.809 | 66.7 [57.1;84.5] | 85.7 [71.4;93.3] | 0.007 | −1.65 (12.0) | 6.74 (12.9) ** |
| T2DM management | ||||||||
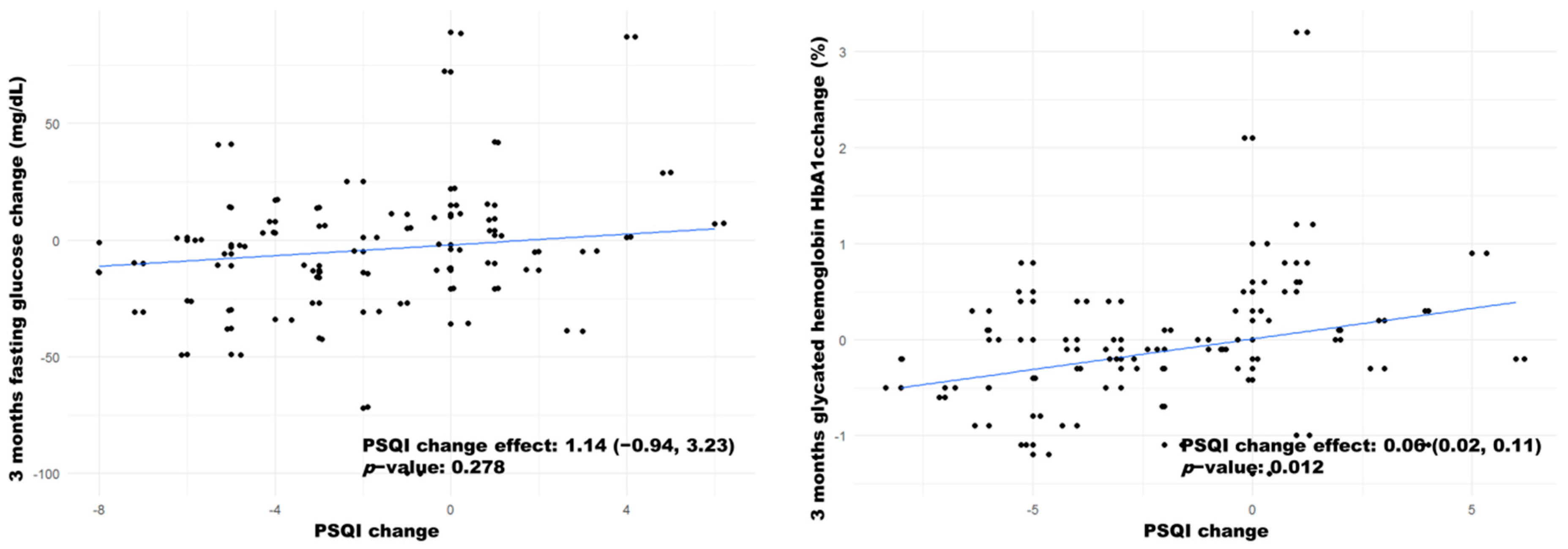
| Fasting glucose (3 months) | 122 [106;134] | 126 [112;154] | 0.249 | 127 [112;138] | 121 [102;137] | 0.473 | 3.00 [−10.50;13.0] | −12.50 [−27.00;1.00] * |
| Fasting glucose (6 months) | 122 [106;134] | 126 [112;154] | 0.249 | 122 [114;140] | 120 [106;151] | 0.766 | 4.43 (22.1) | −4.08 (25.8) |
| HbA1c (3 months) | 6.40 [5.85;7.10] | 6.45 [5.90;7.15] | 0.443 | 6.40 [6.00;7.10] | 6.25 [5.82;7.15] | 0.476 | 0.20 [−0.20;0.55] | −0.20 [−0.50;−0.02] * |
| HbA1c (6 months) | 6.40 [5.85;7.10] | 6.45 [5.90;7.15] | 0.443 | 6.75 [6.00;7.43] | 6.30 [5.95;7.02] | 0.315 | 0.42 (0.58) ** | −0.06 (0.64) |
| Response Variables | Control Group (n = 31) | Intervention Group (n = 38) | Total | p Value |
|---|---|---|---|---|
| Sleep hygiene change (yes) | 5 (16.1%) | 32 (84.2%) | 37 (53.6%) | <0.001 |
| PSQI change ≤ −3 (yes) | 9 (29.0%) | 22 (59.5%) | 31 (45.6%) | 0.024 |
| Post intervention sleep time > 6 h (yes) | 9 (29.0%) | 21 (56.8%) | 30 (44.1%) | 0.041 |
| 3 months HbA1c change ≤ −0.5% (yes) | 4 (12.9%) | 13 (34.2%) | 17 (24.6%) | 0.078 |
| 6 months HbA1c change ≤ −0.5% (yes) | 1 (3.57%) | 10 (29.4%) | 11 (17.7%) | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Serrano, C.; Pujol Salud, J.; Aran-Solé, L.; Sol, J.; Ortiz-Congost, S.; Artigues-Barberà, E.; Ortega-Bravo, M. Enhancing Night and Day Circadian Contrast through Sleep Education in Prediabetes and Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Biology 2022, 11, 893. https://doi.org/10.3390/biology11060893
García-Serrano C, Pujol Salud J, Aran-Solé L, Sol J, Ortiz-Congost S, Artigues-Barberà E, Ortega-Bravo M. Enhancing Night and Day Circadian Contrast through Sleep Education in Prediabetes and Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Biology. 2022; 11(6):893. https://doi.org/10.3390/biology11060893
Chicago/Turabian StyleGarcía-Serrano, Cristina, Jesús Pujol Salud, Lidia Aran-Solé, Joaquim Sol, Sònia Ortiz-Congost, Eva Artigues-Barberà, and Marta Ortega-Bravo. 2022. "Enhancing Night and Day Circadian Contrast through Sleep Education in Prediabetes and Type 2 Diabetes Mellitus: A Randomized Controlled Trial" Biology 11, no. 6: 893. https://doi.org/10.3390/biology11060893
APA StyleGarcía-Serrano, C., Pujol Salud, J., Aran-Solé, L., Sol, J., Ortiz-Congost, S., Artigues-Barberà, E., & Ortega-Bravo, M. (2022). Enhancing Night and Day Circadian Contrast through Sleep Education in Prediabetes and Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Biology, 11(6), 893. https://doi.org/10.3390/biology11060893






