Living at the Wrong Time: Effects of Unmatching Official Time in Portugal and Western Spain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
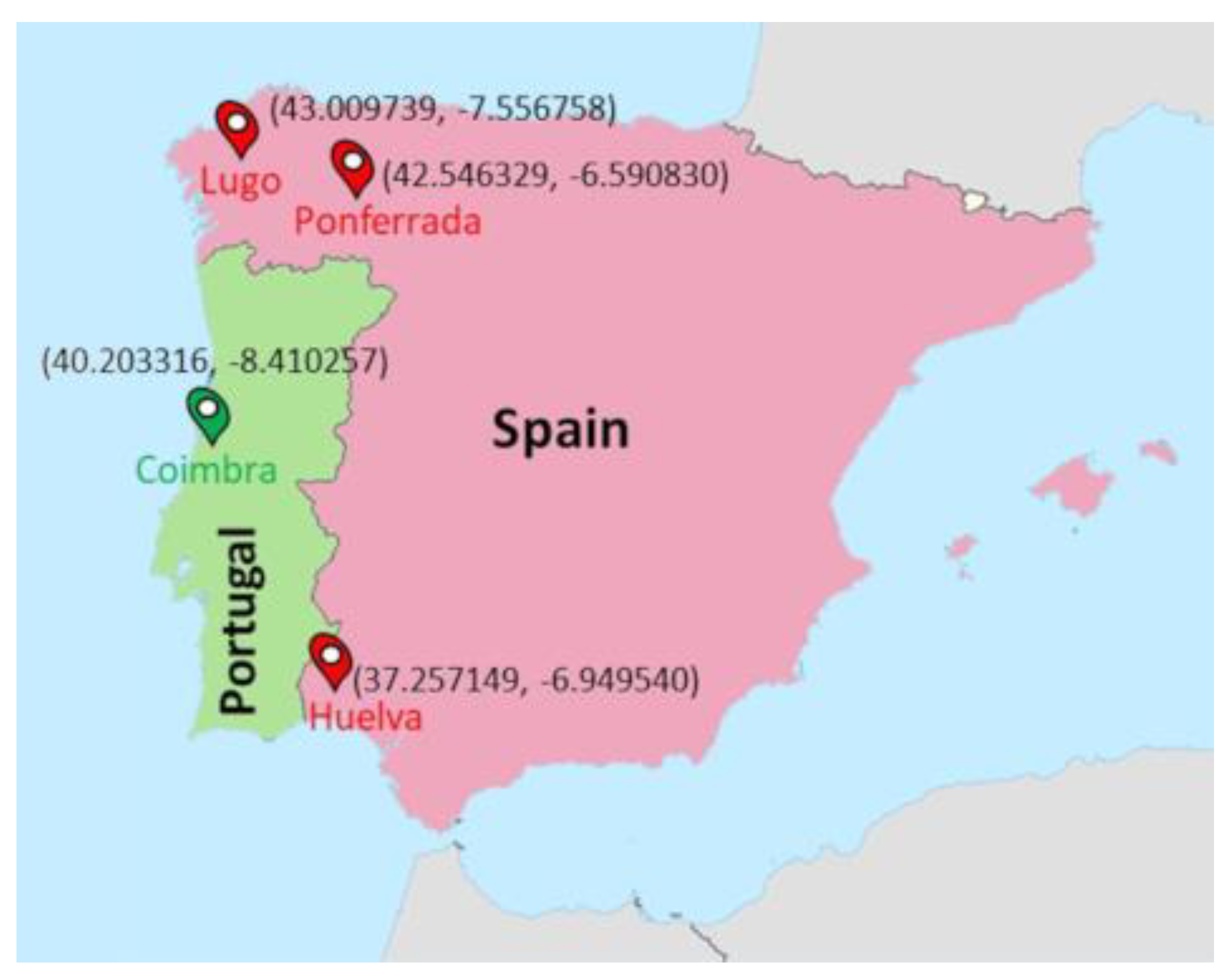
2.2. Locations and Period of Recording
2.3. Ambulatory Circadian Monitoring Device
2.4. Automatic Detection of Sleep and Wake States
2.5. Circadian Parameters
2.6. Internal Desynchronization
2.7. Reported Meal and Sleep Times
2.8. Social Jet Lag Calculation
2.9. Local (Official) Time Correction
2.10. Statistical Analyses
3. Results
3.1. Patterns of Ambulatorily Monitored Circadian Variables
3.2. Circadian Robustness
3.3. Circadian Desynchronization
3.4. Objectively Inferred Sleep Duration
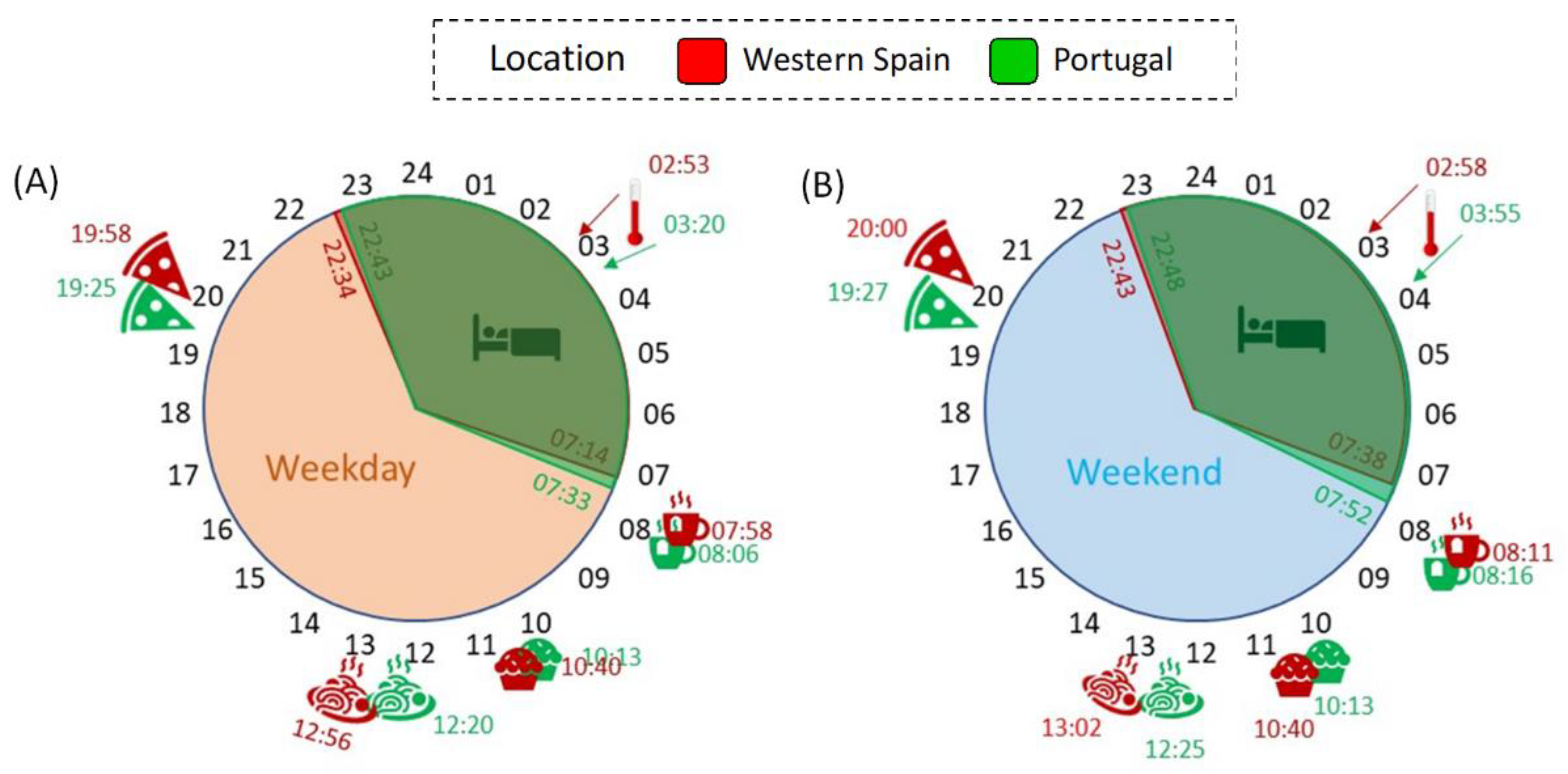
3.5. Daily Schedule: Bed and Meal Times
3.6. Influence of Sleep and Meal Schedules on Circadian Desynchronization
3.7. Social Jet Lag
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roenneberg, T.; Winnebeck, E.C.; Klerman, E.B. Daylight saving time and artificial time zones—A battle between biological and social times. Front. Physiol. 2019, 10, 944. [Google Scholar] [CrossRef] [Green Version]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Madrid, J.A. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv. Drug Deliv. Rev. 2010, 62, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Arguelles-Prieto, R.; Martinez-Madrid, M.J.; Reiter, R.; Hardeland, R.; Rol, M.A.; Madrid, J.A. Protecting the melatonin rhythm through circadian healthy light exposure. Int. J. Mol. Sci. 2014, 15, 23448–23500. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [Green Version]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peirson, S.; Foster, R.G. Melanopsin: Another way of signaling light. Neuron 2006, 49, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Belenky, M.A.; Smeraski, C.A.; Provencio, I.; Sollars, P.J.; Pickard, G.E. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J. Comp. Neurol. 2003, 460, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Mien, I.H.; Hilaire, M.A.S.; Yeo, S.; Chua, E.C.; Van Reen, E.; Hanley, C.J.; Hull, J.T.; Czeisler, C.A.; Lockley, S.W. Melanopsin and rod—Cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J. Neurosci. 2012, 32, 14242–14253. [Google Scholar] [CrossRef] [PubMed]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Arguelles-Prieto, R.; Bonmati-Carrion, M.-A.; Rol, M.A.; Madrid, J.A. Determining light intensity, timing and type of visible and circadian light from an ambulatory circadian monitoring device. Front. Physiol. 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Albares, J.; Segarra, F.; Campos, M.; Estivill, E.; Rol, M.A.; Madrid, J.A. Ambulatory circadian monitoring (ACM) based on thermometry, motor activity and body position (TAP): A comparison with polysomnography. Physiol. Behav. 2014, 126, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Circadian phase assessment by ambulatory monitoring in humans: Correlation with dim light melatonin onset. Chronobiol. Int. 2014, 31, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Nicolas, A.; Ortiz-Tudela, E.; Rol, M.A.; Madrid, J.A. Uncovering different masking factors on wrist skin temperature rhythm in free-living subjects. PLoS ONE 2013, 8, e61142. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Abbott, S.M.; Carroll, J.E.; Davis, C.J.; Dijk, D.-J.; Dinges, D.F.; Gehrman, P.R.; Ginsburg, G.S.; Gozal, D.; Haack, M.; et al. Developing biomarker arrays predicting sleep and circadian-coupled risks to health. Sleep 2016, 39, 727–736. [Google Scholar] [CrossRef]
- Borisenkov, M.F. Latitude of residence and position in time zone are predictors of cancer incidence, cancer mortality, and life expectancy at birth. Chronobiol. Int. 2011, 28, 155–162. [Google Scholar] [CrossRef]
- Gu, F.; Xu, S.; Devesa, S.S.; Zhang, F.; Klerman, E.B.; Graubard, B.I.; Caporaso, N.E. Longitude position in a time zone and cancer risk in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Vopham, T.; Weaver, M.D.; Vetter, C.; Hart, J.E.; Tamimi, R.M.; Laden, F.; Bertrand, K.A. Circadian misalignment and hepatocellular carcinoma incidence in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.G. Circadian disruption and breast cancer: From melatonin to clock genes. Epidemiology 2005, 16, 254–258. [Google Scholar] [CrossRef]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A.J.L. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wefers, J.; Van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; Van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHill, A.; Wright, K. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18 (Suppl. S1), 15–24. [Google Scholar] [CrossRef]
- Maury, E. Off the clock: From circadian disruption to metabolic disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Metabolic Consequences in Humans of Prolonged Sleep Restriction Combined with Circadian Disruption. Sci. Transl. Med. 2012, 4, 129ra43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horne, J.A.; Östberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Haraszti, R.Á.; Ella, K.; Gyöngyösi, N.; Roenneberg, T.; Káldi, K. Social jetlag negatively correlates with academic performance in undergraduates. Chronobiol. Int. 2014, 31, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Giuntella, O.; Mazzonna, F. Sunset time and the economic effects of social jetlag: Evidence from US time zone borders. J. Health Econ. 2019, 65, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Madrid-Navarro, C.J.; Puertas Cuesta, F.J.; Escamilla-Sevilla, F.; Campos, M.; Ruiz Abellán, F.; Rol, M.A.; Madrid, J.A. Validation of a Device for the Ambulatory Monitoring of Sleep Patterns: A Pilot Study on Parkinson’s Disease. Front. Neurol. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.Á.; Madrid, J.A. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, G.A.; Hubbard, E.M.; Mastick, J.; Luxenberg, J.S.; Burr, R.L.; Van Someren, E.J.W. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int. Psychogeriatr. 2005, 17, 221–236. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.L.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Validation of an innovative method, based on tilt sensing, for the assessment of activity and body position. Chronobiol. Int. 2015, 32, 701–710. [Google Scholar] [CrossRef]
- Zornoza-Moreno, M.; Fuentes-Hernández, S.; Sánchez-Solis, M.; Rol, M.Á.; Larqué, E.; Madrid, J.A. Assessment of circadian rhythms of both skin temperature and motor activity in infants during the first 6 months of life. Chronobiol. Int. 2011, 28, 330–337. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.A.; Hild, K.; Isherwood, C.; Sweeney, S.J.; Revell, V.L.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Relationship between human pupillary light reflex and circadian system status. PLoS ONE 2016, 11, e0162476. [Google Scholar] [CrossRef] [Green Version]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and social jetlag: A (self-) critical review. Biology 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Tudela, E.; Innominato, P.F.; Rol, M.A.; Lévi, F.; Madrid, J.A. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, M.; Giarrusso, R. Aging and family life: A decade review. J. Marriage Fam. 2010, 72, 1039–1058. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Colten, H.R.; Altevogt, B.M. Sleep physiology. In Sleep Disorders and Seep Deprivation: An Unmet Public Health Problem; National Academies Press (US): Washington, DC, USA, 2006. [Google Scholar]
- Mota, M.; Silva, C.; Balieiro, L.; Gonçalves, B.; Fahmy, W.; Crispim, C. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS ONE 2019, 14, e0212126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandín, C.; Scheer, F.; Luque, A.; Ávila-Gandía, V.; Zamora, S.; Madrid, J.; Gómez-Abellán, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin. Nutr. 2018, 37, 1133. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Nutrients 2019, 11, 2624–2639. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin effects on glucose metabolism: Time to unlock the controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef] [PubMed]



| a | ||||||||||||
| Wrist Skin Temperature | ||||||||||||
| Whole week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 12:53 ± 0:08 | 14:07 ± 0:12 | 4.57 | <0.0001 | 12:47 ± 0:09 | 13:51 ± 0:12 | 3.77 | 0.000 | 13:22 ± 0:09 | 14:25 ± 0:15 | 3.07 | 0.002 |
| NPM | 2:45 ± 0:10 | 3:25 ± 0:10 | 3.88 | 0.000 | 2:52 ± 0:10 | 3:20 ± 0:11 | 2.58 | 0.010 | 2:58 ± 0:10 | 3:54 ± 0:15 | 3.10 | 0.002 |
| V-day | 31.33 ± 0.04 | 31.18 ± 0.06 | −2.06 | 0.040 | 31.29 ± 0.04 | 31.10 ± 0.06 | −2.40 | 0.017 | 31.35 ± 0.04 | 31.28 ± 0.07 | −1.14 | 0.257 |
| V-night | 33.88 ± 0.04 | 33.99 ± 0.07 | 1.58 | 0.115 | 33.92 ± 0.05 | 34.04 ± 0.07 | 1.68 | 0.094 | 34.01 ± 0.05 | 34.22 ± 0.06 | 2.67 | 0.008 |
| RAN | 0.51 ± 0.01 | 0.56 ± 0.02 | 2.47 | 0.014 | 0.53 ± 0.01 | 0.59 ± 0.02 | 2.87 | 0.004 | 0.53 ± 0.01 | 0.59 ± 0.02 | 2.59 | 0.010 |
| IS | 0.56 ± 0.01 | 0.57 ± 0.02 | 0.69 | 0.494 | 0.61 ± 0.01 | 0.63 ± 0.02 | 1.36 | 0.176 | 0.75 ± 0.01 | 0.75 ± 0.01 | 0.39 | 0.701 |
| CFI | 0.65 ± 0.01 | 0.67 ± 0.01 | 1.52 | 0.129 | 0.67 ± 0.01 | 0.69 ± 0.01 | 2.19 | 0.030 | 0.72 ± 0.00 | 0.73 ± 0.01 | 1.64 | 0.102 |
| CI | 0.59 ± 0.01 | 0.60 ± 0.02 | 0.57 | 0.568 | 0.56 ± 0.01 | 0.57 ± 0.02 | 0.28 | 0.781 | 0.48 ± 0.01 | 0.51 ± 0.02 | 1.34 | 0.182 |
| b | ||||||||||||
| Motor Activity | ||||||||||||
| Whole week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 13:10 ± 0:04 | 13:39 ± 0:06 | 3.30 | 0.001 | 13:02 ± 0:04 | 13:26 ± 0:07 | 2.70 | 0.007 | 13:27 ± 0:04 | 14:07 ± 0:09 | 3.58 | <0.0001 |
| NPM | 3:03 ± 0:04 | 3:12 ± 0:07 | 1.10 | 0.270 | 2:57 ± 0:05 | 3:07 ± 0:07 | 1.31 | 0.190 | 3:18 ± 0:05 | 3:18 ± 0:09 | −0.28 | 0.779 |
| V-day | 19.39 ± 0.36 | 17.27 ± 0.45 | −2.95 | 0.003 | 19.60 ± 0.36 | 17.54 ± 0.46 | −2.94 | 0.004 | 19.43 ± 0.41 | 17.71 ± 0.48 | −1.72 | 0.086 |
| V-night | 2.26 ± 0.04 | 1.98 ± 0.05 | −4.48 | <0.0001 | 2.22 ± 0.04 | 1.93 ± 0.05 | −4.56 | <0.0001 | 2.11 ± 0.04 | 1.84 ± 0.04 | −3.75 | <0.0001 |
| RAN | 0.49 ± 0.01 | 0.44 ± 0.01 | −2.55 | 0.011 | 0.49 ± 0.01 | 0.45 ± 0.01 | −2.45 | 0.015 | 0.49 ± 0.01 | 0.45 ± 0.01 | −1.33 | 0.183 |
| IS | 0.39 ± 0.00 | 0.37 ± 0.01 | −2.64 | 0.009 | 0.45 ± 0.01 | 0.44 ± 0.01 | −1.13 | 0.258 | 0.65 ± 0.01 | 0.64 ± 0.01 | −1.94 | 0.054 |
| CFI | 0.67 ± 0.00 | 0.65 ± 0.01 | −2.59 | 0.010 | 0.69 ± 0.00 | 0.68 ± 0.01 | −1.92 | 0.056 | 0.76 ± 0.00 | 0.75 ± 0.01 | −1.39 | 0.166 |
| CI | 0.59 ± 0.01 | 0.69 ± 0.01 | 6.74 | <0.0001 | 0.56 ± 0.01 | 0.64 ± 0.01 | 5.24 | <0.0001 | 0.49 ± 0.01 | 0.59 ± 0.01 | 6.07 | <0.0001 |
| c | ||||||||||||
| Time in Movement | ||||||||||||
| Whole week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 13:16 ± 0:04 | 13:51 ± 0:07 | 3.91 | <0.0001 | 13:10 ± 0:04 | 13:43 ± 0:07 | 3.50 | <0.0001 | 13:33 ± 0:05 | 14:18 ± 0:09 | 3.82 | <0.0001 |
| NPM | 3:06 ± 0:04 | 3:11 ± 0:07 | 0.91 | 0.365 | 2:54 ± 0:04 | 3:04 ± 0:07 | 0.87 | 0.387 | 3:14 ± 0:05 | 3:18 ± 0:08 | 0.28 | 0.779 |
| V-day | 30.72 ± 0.35 | 30.65 ± 0.58 | 0.23 | 0.820 | 30.95 ± 0.35 | 30.89 ± 0.57 | 0.15 | 0.885 | 31.03 ± 0.39 | 31.54 ± 0.63 | 0.84 | 0.402 |
| V-night | 1.48 ± 0.05 | 1.51 ± 0.09 | −0.46 | 0.647 | 1.43 ± 0.05 | 1.39 ± 0.08 | −0.51 | 0.613 | 1.22 ± 0.04 | 1.22 ± 0.07 | −0.22 | 0.825 |
| RAN | 0.73 ± 0.01 | 0.73 ± 0.01 | 0.15 | 0.881 | 0.74 ± 0.01 | 0.73 ± 0.01 | 0.28 | 0.783 | 0.74 ± 0.01 | 0.75 ± 0.02 | 0.80 | 0.427 |
| IS | 0.50 ± 0.01 | 0.48 ± 0.01 | −1.55 | 0.123 | 0.55 ± 0.01 | 0.55 ± 0.01 | −0.44 | 0.658 | 0.72 ± 0.01 | 0.70 ± 0.01 | −2.13 | 0.034 |
| CFI | 0.76 ± 0.00 | 0.75 ± 0.01 | −1.14 | 0.255 | 0.78 ± 0.00 | 0.78 ± 0.01 | −0.51 | 0.611 | 0.84 ± 0.00 | 0.83 ± 0.00 | −1.59 | 0.113 |
| CI | 0.66 ± 0.01 | 0.75 ± 0.01 | 6.38 | <0.0001 | 0.64 ± 0.01 | 0.71 ± 0.01 | 5.32 | <0.0001 | 0.58 ± 0.01 | 0.66 ± 0.01 | 5.26 | <0.0001 |
| d | ||||||||||||
| Sleep Probability | ||||||||||||
| Whole Week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 14:16 ± 0:06 | 14:25 ± 0:09 | 0.95 | 0.344 | 13:58 ± 0:07 | 14:14 ± 0:09 | 1.45 | 0.147 | 13:52 ± 0:07 | 14:16 ± 0:10 | 2.53 | 0.012 |
| NPM | 3:07 ± 0:04 | 3:18 ± 0:07 | 1.08 | 0.281 | 3:01 ± 0:05 | 3:11 ± 0:07 | 0.79 | 0.428 | 3:14 ± 0:05 | 3:22 ± 0:09 | 0.61 | 0.542 |
| V-day | 0.06 ± 0.00 | 0.04 ± 0.01 | −4.40 | <0.0001 | 0.06 ± 0.00 | 0.04 ± 0.00 | −4.12 | <0.0001 | 0.06 ± 0.00 | 0.05 ± 0.01 | −3.13 | 0.002 |
| V-night | 0.84 ± 0.00 | 0.86 ± 0.01 | 1.69 | 0.091 | 0.85 ± 0.01 | 0.86 ± 0.01 | 2.05 | 0.041 | 0.86 ± 0.00 | 0.87 ± 0.01 | 0.91 | 0.365 |
| RAN | 0.78 ± 0.01 | 0.81 ± 0.01 | 3.41 | 0.001 | 0.79 ± 0.01 | 0.82 ± 0.01 | 4.03 | <0.0001 | 0.80 ± 0.01 | 0.82 ± 0.01 | 2.02 | 0.045 |
| IS | 0.61 ± 0.01 | 0.62 ± 0.01 | 2.02 | 0.044 | 0.65 ± 0.01 | 0.67 ± 0.01 | 2.44 | 0.015 | 0.77 ± 0.01 | 0.77 ± 0.01 | 0.36 | 0.723 |
| CFI | 0.74 ± 0.00 | 0.77 ± 0.01 | 3.88 | 0.000 | 0.76 ± 0.00 | 0.79 ± 0.01 | 4.09 | <.0001 | 0.80 ± 0.00 | 0.82 ± 0.01 | 2.58 | 0.010 |
| CI | 0.70 ± 0.01 | 0.76 ± 0.01 | 5.02 | <0.0001 | 0.68 ± 0.01 | 0.74 ± 0.01 | 5.29 | <0.0001 | 0.60 ± 0.01 | 0.63 ± 0.01 | 1.42 | 0.157 |
| e | ||||||||||||
| Total Light Exposure | ||||||||||||
| Whole Week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 13:14 ± 0:03 | 13:27 ± 0:05 | 2.76 | 0.006 | 13:10 ± 0:04 | 13:24 ± 0:06 | 2.63 | 0.009 | 13:24 ± 0:04 | 13:46 ± 0:06 | 3.25 | 0.001 |
| NPM | 2:42 ± 0:04 | 2:51 ± 0:07 | 0.71 | 0.480 | 2:28 ± 0:04 | 2:37 ± 0:07 | 0.45 | 0.656 | 2:32 ± 0:05 | 2:39 ± 0:08 | 0.60 | 0.548 |
| V-day | 1.41 ± 0.03 | 1.64 ± 0.04 | 4.09 | <0.0001 | 1.42 ± 0.03 | 1.66 ± 0.04 | 4.27 | <0.0001 | 1.42 ± 0.03 | 1.66 ± 0.05 | 3.71 | <0.0001 |
| V-night | 0.01 ± 0.00 | 0.01 ± 0.00 | −0.09 | 0.932 | 0.01 ± 0.00 | 0.01 ± 0.00 | −0.74 | 0.458 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.32 | 0.751 |
| RAN | 0.47 ± 0.01 | 0.54 ± 0.01 | 3.99 | <0.0001 | 0.47 ± 0.01 | 0.55 ± 0.01 | 4.20 | <0.0001 | 0.47 ± 0.01 | 0.55 ± 0.02 | 3.65 | <0.0001 |
| IS | 0.55 ± 0.01 | 0.60 ± 0.01 | 3.17 | 0.002 | 0.60 ± 0.01 | 0.65 ± 0.01 | 3.55 | <0.0001 | 0.75 ± 0.01 | 0.76 ± 0.01 | 1.50 | 0.134 |
| CFI | 0.83 ± 0.00 | 0.84 ± 0.00 | 3.08 | 0.002 | 0.85 ± 0.00 | 0.86 ± 0.00 | 3.62 | <0.0001 | 0.89 ± 0.00 | 0.90 ± 0.00 | 1.82 | 0.070 |
| CI | 0.72 ± 0.01 | 0.79 ± 0.01 | 4.75 | <0.0001 | 0.70 ± 0.01 | 0.76 ± 0.01 | 4.22 | <0.0001 | 0.63 ± 0.01 | 0.71 ± 0.01 | 4.58 | <0.0001 |
| f | ||||||||||||
| Blue Light Exposure | ||||||||||||
| Whole week | Weekdays | Weekend | ||||||||||
| Western spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 13:08 ± 0:03 | 13:21 ± 0:05 | 2.46 | 0.014 | 13:06 ± 0:03 | 13:17 ± 0:05 | 1.90 | 0.058 | 13:17 ± 0:04 | 13:41 ± 0:06 | 3.40 | 0.001 |
| NPM | 2:36 ± 0:04 | 2:46 ± 0:07 | 0.76 | 0.448 | 2:19 ± 0:05 | 2:37 ± 0:08 | 1.36 | 0.176 | 2:27 ± 0:06 | 2:30 ± 0:09 | 0.17 | 0.862 |
| V-day | 1.05 ± 0.03 | 1.30 ± 0.04 | 4.92 | <0.0001 | 1.05 ± 0.03 | 1.31 ± 0.04 | 5.04 | <0.0001 | 1.06 ± 0.03 | 1.32 ± 0.05 | 4.24 | <0.0001 |
| V-night | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.31 | 0.754 | 0.00 ± 0.00 | 0.01 ± 0.00 | 1.02 | 0.307 | 0.00 ± 0.00 | 0.00 ± 0.00 | −0.25 | 0.800 |
| RAN | 0.35 ± 0.01 | 0.43 ± 0.01 | 4.92 | <0.0001 | 0.35 ± 0.01 | 0.44 ± 0.01 | 4.99 | <0.0001 | 0.35 ± 0.01 | 0.44 ± 0.02 | 4.24 | <0.0001 |
| IS | 0.51 ± 0.01 | 0.56 ± 0.01 | 4.25 | <0.0001 | 0.56 ± 0.01 | 0.62 ± 0.01 | 4.40 | <0.0001 | 0.72 ± 0.01 | 0.74 ± 0.01 | 1.99 | 0.047 |
| CFI | 0.81 ± 0.00 | 0.83 ± 0.00 | 4.28 | <0.0001 | 0.83 ± 0.00 | 0.85 ± 0.00 | 4.78 | <0.0001 | 0.88 ± 0.00 | 0.89 ± 0.00 | 2.38 | 0.018 |
| CI | 0.70 ± 0.01 | 0.77 ± 0.01 | 4.97 | <0.0001 | 0.67 ± 0.01 | 0.74 ± 0.01 | 4.41 | <0.0001 | 0.60 ± 0.01 | 0.68 ± 0.01 | 4.86 | <0.0001 |
| g | ||||||||||||
| Infrared Exposure | ||||||||||||
| Whole Week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DPM | 13:03 ± 0:03 | 13:10 ± 0:05 | 1.47 | 0.144 | 13:01 ± 0:03 | 13:09 ± 0:06 | 1.65 | 0.099 | 13:17 ± 0:04 | 13:30 ± 0:04 | 2.71 | 0.007 |
| NPM | 2:42 ± 0:05 | 2:53 ± 0:07 | 0.94 | 0.349 | 2:30 ± 0:05 | 2:43 ± 0:08 | 0.64 | 0.526 | 2:36 ± 0:05 | 2:37 ± 0:09 | −0.13 | 0.899 |
| V-day | 186.00 ± 12.00 | 242.00 ± 20.00 | 3.59 | <0.0001 | 179.00 ± 12.00 | 238.00 ± 21.00 | 3.63 | <0.0001 | 214.00 ± 17.00 | 267.00 ± 26.00 | 3.03 | 0.003 |
| V-night | 0.06 ± 0.01 | 0.05 ± 0.01 | −0.32 | 0.752 | 0.06 ± 0.01 | 0.05 ± 0.01 | −0.51 | 0.608 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.17 | 0.868 |
| RAN | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.67 | 0.502 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.17 | 0.242 | 0.99 ± 0.00 | 1.00 ± 0.00 | 0.91 | 0.360 |
| IS | 0.20 ± 0.00 | 0.21 ± 0.01 | 2.38 | 0.018 | 0.28 ± 0.01 | 0.30 ± 0.01 | 1.77 | 0.077 | 0.54 ± 0.01 | 0.56 ± 0.01 | 0.95 | 0.341 |
| CFI | 0.63 ± 0.00 | 0.64 ± 0.00 | 2.00 | 0.046 | 0.66 ± 0.00 | 0.67 ± 0.00 | 2.35 | 0.019 | 0.74 ± 0.00 | 0.76 ± 0.01 | 1.47 | 0.142 |
| CI | 0.38 ± 0.01 | 0.45 ± 0.01 | 5.12 | <0.0001 | 0.35 ± 0.01 | 0.41 ± 0.01 | 4.20 | <0.0001 | 0.31 ± 0.01 | 0.34 ± 0.01 | 2.88 | 0.004 |
| Internal Desynchronization | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole Week | Weekdays | Weekend | ||||||||||
| Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | Western Spain | Portugal | Z | Pr | |
| DI WT/TM | 0.16 ± 0.01 | 0.10 ± 0.01 | −2.67 | 0.008 | 0.16 ± 0.01 | 0.10 ± 0.01 | −2.21 | 0.028 | 0.18 ± 0.01 | 0.17 ± 0.02 | −0.64 | 0.524 |
| DI WT/TL | 0.16 ± 0.01 | 0.11 ± 0.01 | −1.67 | 0.097 | 0.17 ± 0.01 | 0.11 ± 0.01 | −2.51 | 0.013 | 0.18 ± 0.01 | 0.18 ± 0.02 | −0.31 | 0.757 |
| DI WT/BL | 0.16 ± 0.01 | 0.12 ± 0.01 | −1.29 | 0.198 | 0.18 ± 0.01 | 0.12 ± 0.01 | −2.25 | 0.025 | 0.19 ± 0.01 | 0.18 ± 0.02 | −0.62 | 0.536 |
| DI WT/IL | 0.16 ± 0.01 | 0.10 ± 0.01 | −2.47 | 0.014 | 0.17 ± 0.01 | 0.12 ± 0.01 | −2.31 | 0.022 | 0.18 ± 0.01 | 0.18 ± 0.02 | 0.15 | 0.883 |
| DI TM/TL | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.91 | 0.366 | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.13 | 0.894 | 0.08 ± 0.01 | 0.07 ± 0.01 | −1.61 | 0.109 |
| DI TM/BL | 0.06 ± 0.00 | 0.07 ± 0.01 | 1.50 | 0.133 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.26 | 0.798 | 0.09 ± 0.01 | 0.08 ± 0.01 | −1.24 | 0.215 |
| DI TM/IL | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.43 | 0.664 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.44 | 0.661 | 0.09 ± 0.01 | 0.07 ± 0.01 | −1.64 | 0.102 |
| Mid-in Bed | Mid-Intake | Mid-in Bed—WTiO | Mid-Intake—WTiO | Breakfast Time | Lunch Time | Dinner Time | Breakfast —WTiO | Lunch —WTiO | Dinner —WTiO | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | (GMT0, h) | ||
| DI WT-TM | R | −0.073 | 0.030 | 0.173 | 0.200 | 0.024 | −0.019 | 0.005 | 0.210 | 0.188 | 0.177 |
| p | 0.038 | 0.410 | <0.0001 | <0.0001 | 0.508 | 0.606 | 0.898 | <0.0001 | <0.0001 | <0.0001 | |
| DI WT-TL | R | −0.062 | −0.016 | 0.168 | 0.189 | 0.008 | −0.012 | −0.057 | 0.208 | 0.187 | 0.158 |
| p | 0.080 | 0.668 | <0.0001 | <0.0001 | 0.827 | 0.749 | 0.115 | <0.0001 | <0.0001 | <0.0001 | |
| DI WT-BL | R | −0.056 | 0.008 | 0.173 | 0.204 | 0.044 | 0.002 | −0.045 | 0.230 | 0.203 | 0.168 |
| p | 0.111 | 0.832 | <0.0001 | <0.0001 | 0.221 | 0.964 | 0.211 | <0.0001 | <.0001 | <0.0001 | |
| DI WT-IL | R | −0.053 | −0.017 | 0.145 | 0.159 | 0.016 | −0.018 | −0.060 | 0.177 | 0.158 | 0.134 |
| p | 0.135 | 0.648 | <0.0001 | <0.0001 | 0.662 | 0.632 | 0.104 | <0.0001 | <0.0001 | <0.0001 | |
| DI TM-TL | R | −0.010 | 0.019 | 0.076 | 0.066 | 0.050 | 0.016 | −0.031 | 0.092 | 0.083 | 0.005 |
| p | 0.771 | 0.605 | 0.035 | 0.078 | 0.166 | 0.660 | 0.394 | 0.001 | 0.272 | 0.219 | |
| DI TM-BL | R | −0.029 | 0.039 | 0.056 | 0.067 | 0.060 | 0.012 | 0.003 | 0.088 | 0.078 | 0.044 |
| p | 0.402 | 0.288 | 0.122 | 0.072 | 0.097 | 0.749 | 0.938 | 0.018 | 0.039 | 0.236 | |
| DI TM-IL | R | 0.001 | 0.038 | 0.064 | 0.050 | 0.067 | −0.032 | −0.012 | 0.073 | 0.037 | 0.027 |
| p | 0.967 | 0.303 | 0.077 | 0.185 | 0.066 | 0.385 | 0.738 | 0.049 | 0.332 | 0.460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonmatí-Carrión, M.-Á.; Casado-Ramirez, E.; Moreno-Casbas, M.-T.; Campos, M.; ModulEN Consortium; Madrid, J.A.; Rol, M.-A. Living at the Wrong Time: Effects of Unmatching Official Time in Portugal and Western Spain. Biology 2022, 11, 1130. https://doi.org/10.3390/biology11081130
Bonmatí-Carrión M-Á, Casado-Ramirez E, Moreno-Casbas M-T, Campos M, ModulEN Consortium, Madrid JA, Rol M-A. Living at the Wrong Time: Effects of Unmatching Official Time in Portugal and Western Spain. Biology. 2022; 11(8):1130. https://doi.org/10.3390/biology11081130
Chicago/Turabian StyleBonmatí-Carrión, María-Ángeles, Elvira Casado-Ramirez, María-Teresa Moreno-Casbas, Manuel Campos, ModulEN Consortium, Juan Antonio Madrid, and Maria-Angeles Rol. 2022. "Living at the Wrong Time: Effects of Unmatching Official Time in Portugal and Western Spain" Biology 11, no. 8: 1130. https://doi.org/10.3390/biology11081130
APA StyleBonmatí-Carrión, M.-Á., Casado-Ramirez, E., Moreno-Casbas, M.-T., Campos, M., ModulEN Consortium, Madrid, J. A., & Rol, M.-A. (2022). Living at the Wrong Time: Effects of Unmatching Official Time in Portugal and Western Spain. Biology, 11(8), 1130. https://doi.org/10.3390/biology11081130







