The Birth of the Mammalian Sleep
Abstract
Simple Summary
Abstract
1. Introduction
2. Sleep: Behavioral and Electrophysiological
3. Sleep: Monophyletic or Polyphyletic?
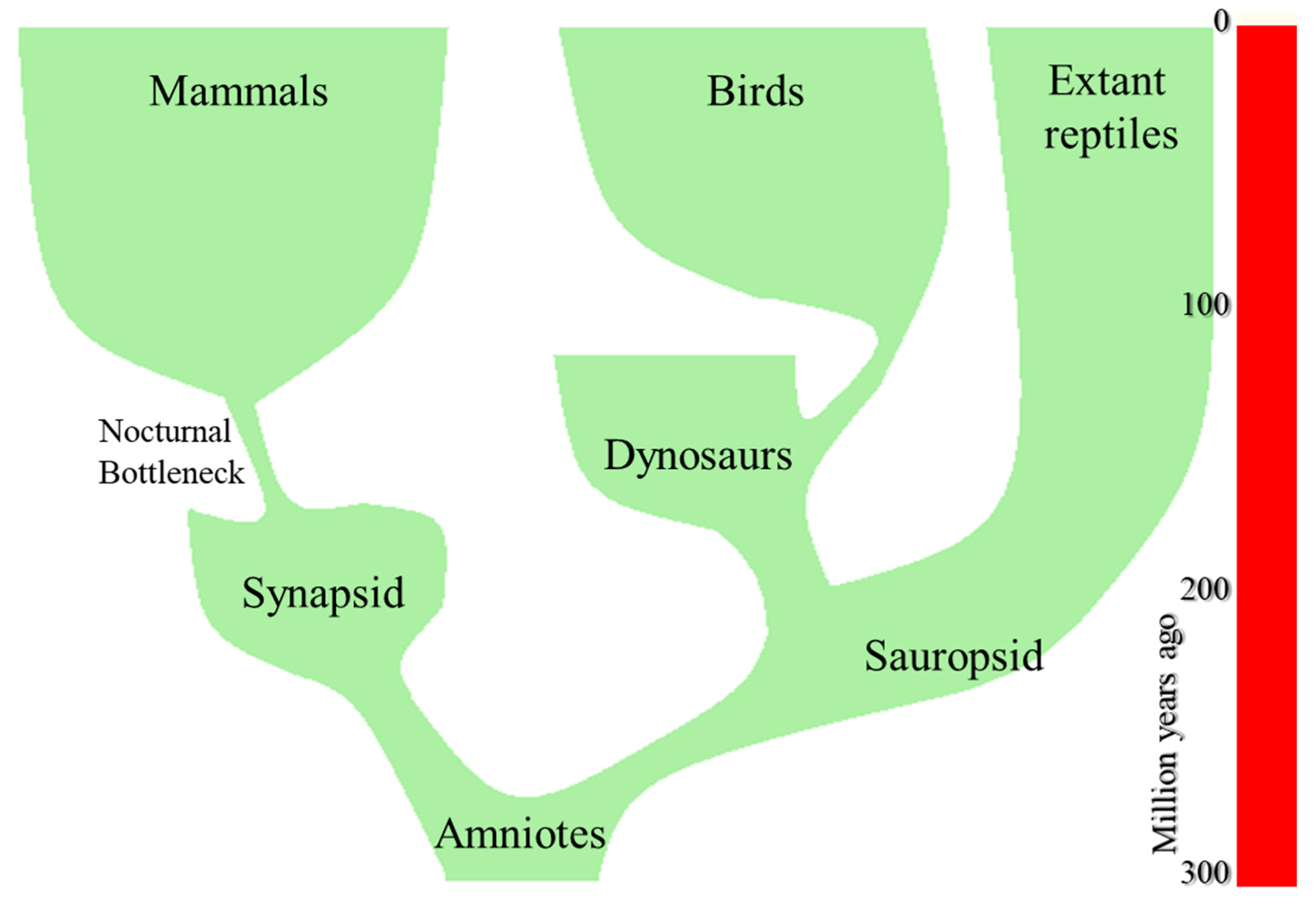
4. The Origin of Mammals
5. How Early Mammals Reached Endothermy
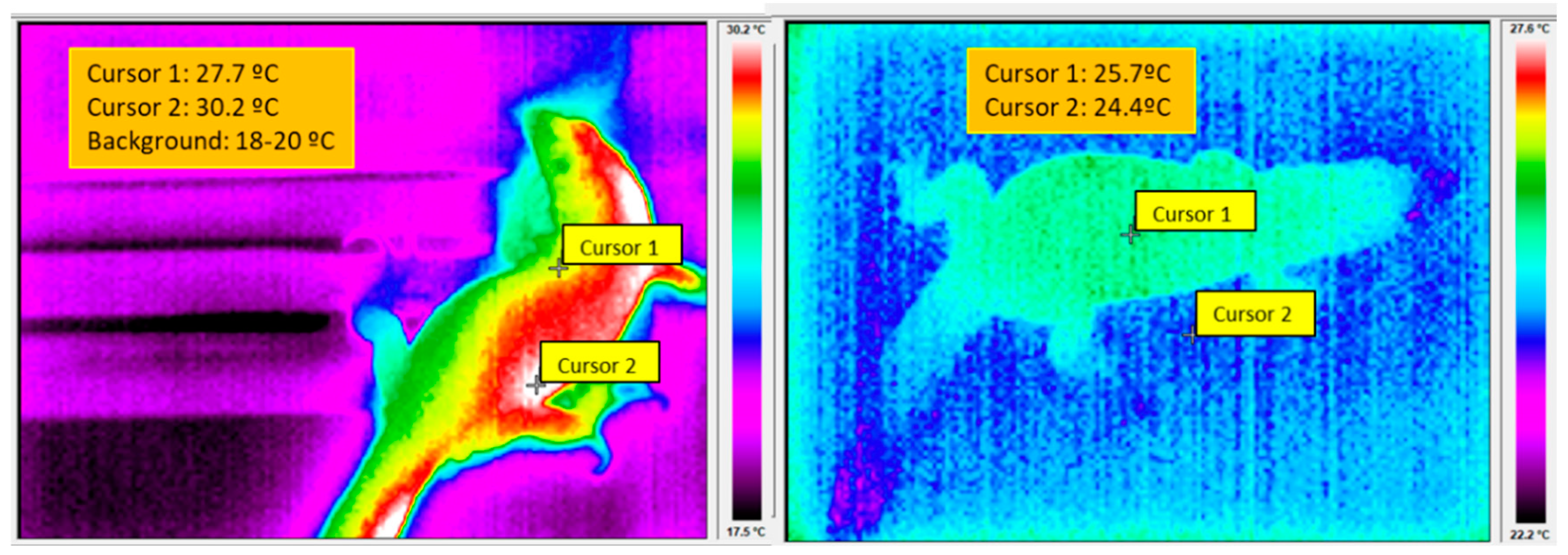
5.1. The Control of BT in Reptiles and Mammals
5.2. Do Reptiles Sleep?
6. The Nocturnal Bottleneck
6.1. Adapting the Reptilian Vision to Nighttime
6.2. Bottleneck: Nocturnal or Crepuscular?
6.3. Adapting Non-Visual Sensory Systems to Nighttime
7. The Birth of Mammalian Sleep
The Origin of the Sleep Regulation
8. The Variability of Sleep and the Evolutionary Pressure
9. Comparing Behavioral Sleep and Rest
9.1. Wakeful Rest and Laziness
9.2. The Principle of Stringency
9.3. The Idling Wakeful Rest
9.4. Pleasure, WI, Sleep, and Homeostatic Regulation
“Is sleep rebound a way to make up for a loss of an otherwise impaired biological process, or is it instead merely a “punishment” phenomenon, evolved to guarantee that a constant, largely species-specific amount of sleep is met?”.[218]
10. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyazaki, S.; Liu, C.Y.; Hayashi, Y. Sleep in vertebrate and invertebrate animals, and insights into the function and evolution of sleep. Neurosci. Res. 2017, 118, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, M.S.; Rattenborg, N.C. Decomposing the evolution of sleep: Comparative and developmental approaches. In Evolution of Nervous Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 523–545. [Google Scholar]
- Hayashi, Y.; Liu, C.Y. The Evolution and Function of Sleep. In Brain Evolution by Design; Springer: Tokyo, Japan, 2017; pp. 343–366. [Google Scholar]
- Keene, A.C.; Duboue, E.R. The origins and evolution of sleep. J. Exp. Biol. 2018, 221, jeb159533. [Google Scholar] [CrossRef] [PubMed]
- Field, J.M.; Bonsall, M.B. The evolution of sleep is inevitable in a periodic world. PLoS ONE 2018, 13, e0201615. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.S.; Varela, S.A.; Gardner, A. The social evolution of sleep: Sex differences, intragenomic conflicts and clinical pathologies. Proc. R. Soc. B 2019, 286, 20182188. [Google Scholar] [CrossRef] [PubMed]
- Lesku, J.A.; Aulsebrook, A.E.; Kelly, M.L.; Tisdale, R.K. Evolution of sleep and adaptive sleeplessness. Handb. Behav. Neurosci. 2019, 30, 299–316. [Google Scholar]
- Ungurean, G.; van der Meij, J.; Rattenborg, N.C.; Lesku, J.A. Evolution and plasticity of sleep. Curr. Opin. Physiol. 2020, 15, 111–119. [Google Scholar] [CrossRef]
- Anafi, R.C.; Kayser, M.S.; Raizen, D.M. Exploring phylogeny to find the function of sleep. Nat. Rev. Neuro-Sci. 2019, 20, 109–116. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Hayashi, Y. The existence of two states of sleep as a common trait in various animals and its molecu-lar and neuronal mechanisms. Curr. Opin. Physiol. 2020, 15, 197–202. [Google Scholar] [CrossRef]
- Jha, V.M.; Jha, S.K. Sleep: Evolution and Functions; Springer: Singapore, 2020; pp. 61–78. [Google Scholar]
- Piéron, H. Le Problem Physiologique du Sommeil; Masson et Cie: Paris, France, 1913. [Google Scholar]
- Flanigan, W.F., Jr. Sleep and Wakefulness in Iguanid Lizards, Ctenosaura pectinata and Iguana iguana. Brain Behav. Evol. 1973, 8, 417–436. [Google Scholar] [CrossRef]
- Durie, D.J.B. Sleep in animals. In Psychopharmacology of Sleep; Wheatley, D., Ed.; Raven Press: New York, NY, USA, 1981; pp. 1–18. [Google Scholar]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- Rial, R.; Canellas, F.; Gamundí, A.; Akaârir, M.; Nicolau, M. Pleasure: The missing link in the regulation of sleep. Neurosci. Biobehav. Rev. 2018, 88, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.A.; Bedford, J.D.; Newcombe, C.P. The role of thermoregulation in lizard biology: Predatory efficiency in a temperate diurnal basker. Behav. Ecol. Sociobiol. 1982, 11, 261–267. [Google Scholar] [CrossRef]
- Ezcurra, M.D.; Butler, R.J. The rise of the ruling reptiles and ecosystem recovery from the Permo-Triassic mass extinction. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180361. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T. At the roots of the mammalian family tree. Nature 1999, 398, 283–284. [Google Scholar] [CrossRef]
- Kielan-Jaworowska, Z.; Cifelli, R.L.; Luo, Z.X. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure; Columbia University Press: New York, NY, USA, 2005. [Google Scholar]
- Bakker, R.T. Dinosaur Physiology and the Origin of Mammals. Evolution 1971, 25, 636. [Google Scholar] [CrossRef]
- Kemp, T.S. The Origin and Evolution of Mammals; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Tattersall, G.J. Reptile thermogenesis and the origins of endothermy. Zoology 2016, 119, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Pörtner, H.-O. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 2010, 85, 703–727. [Google Scholar] [CrossRef]
- Greer, A.E.; Lazell, J.D.; Wright, R.M. Anatomical Evidence for a Counter-current Heat Exchanger in the Leatherback Turtle (Dermochelys coriacea). Nature 1973, 244, 181. [Google Scholar] [CrossRef]
- Frair, W.; Ackman, R.G.; Mrosovsky, N. Body Temperature of Dermochelys coriacea: Warm Turtle from Cold Water. Science 1972, 177, 791–793. [Google Scholar] [CrossRef]
- Davenport, J.; Holland, D.L.; East, J. Thermal and biochemical characteristics of the lipids of the leatherback turtleDermochelys coriacea: Evidence of endothermy. J. Mar. Biol. Assoc. United Kingd. 1990, 70, 33–41. [Google Scholar] [CrossRef]
- Martin, T.; Marugán-Lobón, J.; Vullo, R.; Martín-Abad, H.; Luo, Z.X.; Buscalioni, A.D. A Cretaceous eutriconodont and integument evolution in early mammals. Nature 2015, 526, 380. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.M.; Sharp, J.A.; Nicholas, K.R. Evolution of Lactation: Ancient Origin and Extreme Adaptations of the Lactation System. Annu. Rev. Genom. Hum. Genet. 2010, 11, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Grigg, G.C.; Beard, L.A.; Augee, M.L. The Evolution of Endothermy and Its Diversity in Mammals and Birds. Physiol. Biochem. Zool. 2004, 77, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Garrick, D. Body surface temperature and length in relation to the thermal biology of lizards. Biosci. Horizons 2008, 1, 136–142. [Google Scholar] [CrossRef]
- Kluger, M.J.; Tarr, R.S.; Heath, J.E. Posterior Hypothalamic Lesions and Disturbances in Behavioral Thermoregulation in the Lizard Dipsosaurus dorsalis. Physiol. Zool. 1973, 46, 79–84. [Google Scholar] [CrossRef]
- Berk, M.L.; Heath, J.E. Effects of preoptic, hypothalamic, and telencephalic lesions on thermoregulation in the lizard, Dipsosaurus dorsalis. J. Therm. Biol. 1976, 1, 65–78. [Google Scholar] [CrossRef]
- Bicego, K.; Branco, L.G.S. Discrete electrolytic lesion of the preoptic area prevents LPS-induced behavioral fever in toads. J. Exp. Biol. 2002, 205, 3513–3518. [Google Scholar] [CrossRef]
- Bicego, K.C.; Barros, R.C.; Branco, L. Physiology of temperature regulation: Comparative aspects. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 147, 616–639. [Google Scholar] [CrossRef]
- Liu, C.; Li, R.; Liu, Z.; Yin, S.; Wang, Z. The role of prostaglandins and the hypothalamus in thermoregulation in the lizard, Phrynocephalus przewalskii (Agamidae). J. Comp. Physiol. B 2005, 176, 321–328. [Google Scholar] [CrossRef]
- Piercy, J.; Rogers, K.H.; Reichert, M.; Andrade, D.V.; Abe, A.S.; Tattersall, G.; Milsom, W.K. The relationship between body temperature, heart rate, breathing rate, and rate of oxygen consumption, in the tegu lizard (Tupinambis merianae) at various levels of activity. J. Comp. Physiol. B 2015, 185, 891–903. [Google Scholar] [CrossRef]
- Rose, B. Factors Affecting Activity in Sceloporus Virgatus. Ecology 1981, 62, 706–716. [Google Scholar] [CrossRef]
- Merker, G.P.; Nagy, K.A. Energy Utilization by Free-Ranging Sceloporus Virgatus Lizards. Ecology 1984, 65, 575–581. [Google Scholar] [CrossRef]
- Lister, B.C.; Aguayo, A.G. Seasonality, Predation, and the Behaviour of a Tropical Mainland Anole. J. Anim. Ecol. 1992, 61, 717. [Google Scholar] [CrossRef]
- Clark, T.D.; Butler, P.J.; Frappell, P.B. Factors influencing the prediction of metabolic rate in a reptile. Funct. Ecol. 2006, 20, 105–113. [Google Scholar] [CrossRef]
- Alam, M.N.; McGinty, D.; Szymusiak, R. Preoptic/anterior hypothalamic neurons: Thermosensitivity in rapid eye movement sleep. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R1250–R1257. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, R.; Gvilia, I.; McGinty, D. Hypothalamic control of sleep. Sleep Med. 2007, 8, 291–301. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Shen, W.L. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef]
- Chen, K.-S.; Xu, M.; Zhang, Z.; Chang, W.-C.; Gaj, T.; Schaffer, D.V.; Dan, Y. A Hypothalamic Switch for REM and Non-REM Sleep. Neuron 2018, 97, 1168–1176.e4. [Google Scholar] [CrossRef]
- Egan, G.F.; Johnson, J.; Farrell, M.; McAllen, R.; Zamarripa, F.; McKinley, M.J.; Lancaster, J.; Denton, D.; Fox, P.T. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: A positron-emission tomography study. Proc. Natl. Acad. Sci. USA 2005, 102, 5262–5267. [Google Scholar] [CrossRef]
- Milsom, W.K.; Andrade, D.V.; Brito, S.P.; Toledo, L.F.; Wang, T.; Abe, A.S. Seasonal Changes in Daily Metabolic Patterns of Tegu Lizards (Tupinambis merianae) Placed in the Cold (17°C) and Dark. Physiol. Biochem. Zool. 2008, 81, 165–175. [Google Scholar] [CrossRef]
- Cadena, V.; Tattersall, G.J. The Effect of Thermal Quality on the Thermoregulatory Behavior of the Bearded Dragon Pogona vitticeps: Influences of Methodological Assessment. Physiol. Biochem. Zool. 2009, 82, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Rusch, T.W. Methods and pitfalls of measuring thermal preference and tolerance in lizards. J. Therm. Biol. 2017, 68, 63–72. [Google Scholar] [CrossRef]
- Regal, P.J. Voluntary Hypothermia in Reptiles. Science 1967, 155, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Rismiller, P.D.; Heldmaier, G. The effect of photoperiod on temperature selection in the European green lizard, Lacerta viridis. Oecologia 1982, 53, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Engbretson, G.A.; Hutchison, V.H. Parietalectomy and thermal selection in the lizardSceloporus magister. J. Exp. Zool. 1976, 198, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.; Frye, F.L.; Murphy, J.B. Introduction: Health and welfare of captive reptiles. In Health and Welfare of Captive Reptiles; Springer: Dordrecht, The Netherland, 1995; pp. 1–4. [Google Scholar]
- Tracy, C.R.; Flack, K.M.; Zimmerman, L.C.; Espinoza, R.E.; Tracy, C.R. Herbivory imposes constraints on voluntary hypothermia in lizards. Copeia 2005, 2005, 12–19. [Google Scholar] [CrossRef]
- Saber, S.A. Preferred body temperature of free-ranging Starred Agama Laudakia stellio (Linnaeus, 1758) (Agamidae) from Egypt. Russ. J. Herpetol. 2012, 19, 171–176. [Google Scholar]
- Licht, P. Effects of Temperature on Heart Rates of Lizards during Rest and Activity. Physiol. Zool. 1965, 38, 129–137. [Google Scholar] [CrossRef]
- Pough, F.H. Recommendations for the Care of Amphibians and Reptiles in Academic Institutions. ILAR J. 1991, 33, S1–S21. [Google Scholar] [CrossRef]
- Herbert, J.; Coulson, T.; Coulson, R. Growth rates of Chinese and American alligators. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 909–916. [Google Scholar] [CrossRef]
- Brien, M.L.; Webb, G.J.; Gienger, C.M.; Lang, J.W.; Christian, K.A. Thermal preferences of hatchling saltwater crocodiles (Crocodylus porosus) in response to time of day, social aggregation and feeding. J. Therm. Biol. 2012, 37, 625–630. [Google Scholar] [CrossRef]
- Wilhoft, D.C. The Effect of Temperature on Thyroid Histology and Survival in the Lizard, Sceloporus occidentalis. Copeia 1958, 1958, 265. [Google Scholar] [CrossRef]
- Beuchat, C.A. Temperature effects during gestation in a viviparous lizard. J. Therm. Biol. 1988, 13, 135–142. [Google Scholar] [CrossRef]
- Hutchison, V.H.; Maness, J.D. The Role of Behavior in Temperature Acclimation and Tolerance in Ectotherms. Am. Zool. 1979, 19, 367–384. [Google Scholar] [CrossRef]
- Shein-Idelson, M.; Ondracek, J.M.; Liaw, H.-P.; Reiter, S.; Laurent, G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 2016, 352, 590–595. [Google Scholar] [CrossRef]
- Libourel, P.-A.; Barrillot, B.; Arthaud, S.; Massot, B.; Morel, A.-L.; Beuf, O.; Herrel, A.; Luppi, P.-H. Partial homologies between sleep states in lizards, mammals, and birds suggest a complex evolution of sleep states in amniotes. PLoS Biol. 2018, 16, e2005982. [Google Scholar] [CrossRef]
- Libourel, P.-A.; Barrillot, B. Is there REM sleep in reptiles? A key question, but still unanswered. Curr. Opin. Physiol. 2020, 15, 134–142. [Google Scholar] [CrossRef]
- Andry, M.L.; Luttges, M.W.; Gamow, R.I. Temperature effects on spontaneous and evoked neural activity in the garter snake. Exp. Neurol. 1971, 311, 32–44. [Google Scholar] [CrossRef]
- Parsons, L.C.; Huggins, S.E. Effects of Temperature on Electroencephalogram of the Caiman. Proc. Soc. Exp. Biol. Med. 1965, 1202, 422–426. [Google Scholar] [CrossRef]
- De Vera, L.; González, J.; Rial, R.V. Reptilian waking EEG: Slow waves, spindles and evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 298–303. [Google Scholar] [CrossRef]
- Sanders, C.E.; Tattersall, G.J.; Reichert, M.; Andrade, D.V.; Abe, A.S.; Milsom, W.K. Daily and annual cycles in thermoregulatory behaviour and cardio-respiratory physiology of black and white tegu lizards. J. Comp. Physiol. B 2015, 185, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.E.; Olszowka, J.S.; Subramanian, S. Electroencephalographic and neurological correlates of deep hypo-thermia and circulatory arrest in infants. Ann. Thorac. Surg. 1977, 23, 238–244. [Google Scholar] [CrossRef]
- CDCP (Centers for Disease Control and Prevention). Hypothermia-related deaths—United States, 2003–2004. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 173–175. [Google Scholar]
- Fischbeck, K.H.; Simon, R.P. Neurological manifestations of accidental hypothermia. Ann. Neurol. 1981, 10, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Jolly, B.T.; Ghezzi, K.T. Accidental hypothermia. Emerg. Med. Clin. North Am. 1992, 10, 311–327. [Google Scholar] [CrossRef]
- Hobson, J.A. Sleep; Scientific American Library, W.H. Freeman: New York, NY, USA, 1989. [Google Scholar]
- Pelayo, R.; Dement, W.C. History of Sleep Physiology and Medicine. In Principles and Practice of Sleep Medicine, 6th ed.; Kryger, M.H., Roth, T., Dement, E.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Walls, G.L. The vertebrate eye and its adaptive radiation. In Cranbrook Institute of Science; Cranbrook Press Bulletin: Bloom Hills, MI, USA, 1942; p. 19. [Google Scholar]
- Chakraborty, R.; Nei, M. Bottleneck effects on average heterozygosity and genetic distance with the stepwise mutation model. Evolution 1977, 31, 347–356. [Google Scholar] [CrossRef]
- Menaker, M.; Moreira, L.; Tosini, G. Evolution of circadian organization in vertebrates. Braz. J. Med Biol. Res. 1997, 30, 305–313. [Google Scholar] [CrossRef]
- Heesy, C.P. Ecomorphology of Orbit Orientation and the Adaptive Significance of Binocular Vision in Primates and Other Mammals. Brain Behav. Evol. 2007, 71, 54–67. [Google Scholar] [CrossRef]
- Heesy, C.P.; Hall, M.I. The Nocturnal Bottleneck and the Evolution of Mammalian Vision. Brain Behav. Evol. 2010, 75, 195–203. [Google Scholar] [CrossRef]
- Hall, M.I.; Kamilar, J.; Kirk, C. Eye shape and the nocturnal bottleneck of mammals. Proc. R. Soc. B Boil. Sci. 2012, 279, 4962–4968. [Google Scholar] [CrossRef]
- Gerkema, M.P.; Davies, W.; Foster, R.G.; Menaker, M.; Hut, R.A. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc. R. Soc. B Boil. Sci. 2013, 280, 20130508. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Yang, H.-J.; Oel, A.P.; Brooks, M.J.; Jia, L.; Plachetzki, D.C.; Li, W.; Allison, W.T.; Swaroop, A. Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals. Dev. Cell 2016, 37, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Maor, R.; Dayan, T.; Ferguson-Gow, H.; Jones, K.E. Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat. Ecol. Evol. 2017, 1, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Hadly, E.A. Invasion of Ancestral Mammals into Dim-light Environments Inferred from Adaptive Evolution of the Phototransduction Genes. Sci. Rep. 2017, 7, srep46542. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Johnson, W.E.; O’Brien, S.J.; Gomes, C.; Heesy, C.P.; Antunes, A. Adaptive genomic evolution of opsins reveals that early mammals flourished in nocturnal environments. BMC Genom. 2018, 19, 121. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of colour vision in vertebrates. Eye 1998, 12, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, J.K. Evolution of vertebrate visual pigments. Vis. Res. 2008, 48, 2022–2041. [Google Scholar] [CrossRef]
- Collin, S.P.; Davies, W.; Hart, N.S.; Hunt, D.M. The evolution of early vertebrate photoreceptors. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2925–2940. [Google Scholar] [CrossRef]
- Hunt, D.M.; Carvalho, L.S.; Cowing, J.A.; Davies, W.L. Evolution and spectral tuning of visual pigments in birds and mammals. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2941–2955. [Google Scholar] [CrossRef]
- Sui, G.-Y.; Liu, G.-C.; Gao, Y.-Y.; Deng, Y.; Wang, W.-Y.; Tong, S.-H.; Wang, L. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br. J. Ophthalmol. 2012, 97, 389–394. [Google Scholar] [CrossRef]
- Jacobs, G.H. Losses of functional opsin genes, short-wavelength cone photopigments, and color vision—A significant trend in the evolution of mammalian vision. Vis. Neurosci. 2013, 30, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Kelber, A.; Jacobs, G.H. Evolution of color vision. In Human Color Vision; Springer: Cham, Germany, 2016; pp. 317–354. [Google Scholar]
- Peichl, L. Diversity of mammalian photoreceptor properties: Adaptations to habitat and lifestyle? Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. Off. Publ. Am. Assoc. Anat. 2005, 287, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.H. Evolution of color vision and its reflections in contemporary mammals. In Handbook of Color Psychology; Elliott, A.J., Fairchild, M.D., Franklin, A., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 110–130. [Google Scholar]
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104. [Google Scholar] [CrossRef]
- Ringvold, A.; Anderssen, E.; Kjønniksen, I. Ascorbate in the corneal epithelium of diurnal and nocturnal species. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2774–2777. [Google Scholar]
- De Vera Mudry, M.C.; Kronenberg, S.; Komatsu, S.I.; Aguirre, G.D. Blinded by the light: Retinal phototoxicity in the context of safety studies. Toxicol. Pathol. 2013, 41, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.V.; Coyle, F.P.; O’Stben, W. Retinal degeneration produced by low-intensity colored light. Exp. Neurol. 1972, 35, 233–238. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Darrow, R.M.; I Jiang, Y.; E Marak, G.; Blanks, J.C. Protection by dimethylthiourea against retinal light damage in rats. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1599–1609. [Google Scholar]
- Wasowicz, M.; Morice, C.; Ferrari, P.; Callebert, J.; Versaux-Botteri, C. Long-term effects of light damage on the retina of albino and pigmented rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 813–820. [Google Scholar]
- Marco-Gomariz, M.A.; Hurtado-Montalbán, N.; Vidal-Sanz, M.; Lund, R.D.; Villegas-Pérez, M.P. Phototoxic-induced photo-receptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J. Comp. Neurol. 2006, 498, 163–179. [Google Scholar] [CrossRef]
- Taylor, H.R. Ultraviolet radiation and the eye: An epidemiologic study. Trans. Am. Ophthalmol. Soc. 1989, 87, 802–853. [Google Scholar]
- Cruickshanks, K.J.; Klein, R.; Klein, B.E. Sunlight and age-related macular degeneration: The Beaver Dam Eye Study. Arch. Ophthalmol. 1993, 111, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yoder, A.D.; Yamashita, N.; Li, W.-H. Evidence from opsin genes rejects nocturnality in ancestral primates. Proc. Natl. Acad. Sci. USA 2005, 102, 14712–14716. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.I.L.; Collin, S.P.; Hunt, D.M. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 2012, 21, 3121–3158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chi, H.; Li, L.; Rossiter, S.J.; Zhang, S. Molecular Data Support an Early Shift to an Intermediate-Light Niche in the Evolution of Mammals. Mol. Biol. Evol. 2018, 35, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Y.; Chi, H.; Xia, Y.; Liu, H.; Rossiter, S.J.; Zhang, S. Scotopic rod vision in tetrapods arose from multiple early adaptive shifts in the rate of retinal release. Proc. Natl. Acad. Sci. USA 2019, 116, 12627–12628. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.H.; Martin, R.D.; Verrelli, B.C. Signatures of Functional Constraint at Aye-aye Opsin Genes: The Potential of Adaptive Color Vision in a Nocturnal Primate. Mol. Biol. Evol. 2007, 24, 1963–1970. [Google Scholar] [CrossRef]
- Emerling, C.A.; Springer, M.S. Genomic evidence for rod monochromacy in sloths and armadillos suggests early subterranean history for Xenarthra. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142192. [Google Scholar] [CrossRef]
- Henriksson, J.T.; Bergmanson, J.P.; Walsh, J.E. Ultraviolet radiation transmittance of the mouse eye and its individual media components. Exp. Eye Res. 2010, 90, 382–387. [Google Scholar] [CrossRef]
- Cooper, G.F.; Robson, J.G. The yellow colour of the lens of man and other primates. J. Physiol. 1969, 203, 411–417. [Google Scholar] [CrossRef]
- Hut, R.A.; Scheper, A.; Daan, S. Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light? J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2000, 186, 707–715. [Google Scholar] [CrossRef]
- Glösmann, M.; Steiner, M.; Peichl, L.; Ahnelt, P.K. Cone photoreceptors and potential UV vision in a subterranean insectivore, the European mole. J. Vis. 2008, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Allin, E.F. Evolution of the mammalian middle ear. J. Morphol. 1975, 147, 403–437. [Google Scholar] [CrossRef] [PubMed]
- Crompton, A.W.; Parker, P. Evolution of the mammalian masticatory apparatus: The fossil record shows how mam-mals evolved both complex chewing mechanisms and an effective middle ear, two structures that distinguish them from reptiles. Am. Sci. 1978, 66, 192–201. [Google Scholar]
- Rowe, T.B. The emergence of mammals. In Evolutionary Neuroscience; Academic Press: Austin, TX, USA, 2020; pp. 263–319. [Google Scholar]
- Coleman, M.N.; Boyer, D.M. Inner Ear Evolution in Primates Through the Cenozoic: Implications for the Evolution of Hearing. Anat. Rec. 2012, 295, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Grothe, B.; Pecka, M. The natural history of sound localization in mammals–a story of neuronal inhibition. Front. Neural Circuits 2014, 8, 116. [Google Scholar] [CrossRef]
- Muchlinski, M.N.; Durham, E.L.; Smith, T.D.; Burrows, A.M. Comparative histomorphology of intrinsic vibrissa musculature among primates: Implications for the evolution of sensory ecology and “face touch”. Am. J. Phys. Anthr. 2012, 150, 301–312. [Google Scholar] [CrossRef]
- Zelenitsky, D.K.; Therrien, F.; Ridgely, R.C.; McGee, A.R.; Witmer, L.M. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B Boil. Sci. 2011, 278, 3625–3634. [Google Scholar] [CrossRef]
- Northcutt, R.G. Evolving Large and Complex Brains. Science 2011, 332, 926–927. [Google Scholar] [CrossRef]
- Rowe, T.B.; Shepherd, G.M. Role of ortho-retronasal olfaction in mammalian cortical evolution. J. Comp. Neurol. 2015, 524, 471–495. [Google Scholar] [CrossRef]
- Allman, J. The origin of the neocortex. In Seminars in the Neuroscienccs. Neurosciences 1990, 2, 257–262. [Google Scholar]
- Aboitiz, F.; Montiel, J.F. Olfaction, navigation, and the origin of isocortex. Front. Neurosci. 2015, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Menaker, M. Circadian Rhythm of Body Temperature in an Ectotherm (Iguana iguana. J. Biol. Rhythm. 1995, 10, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.J.; Firth, B.T.; Belan, I. Thermocyclic and photocyclic entrainment of circadian locomotor activity rhythms in sleepy lizards, Tiliqua rugosa. Chronobiol. Int. 2009, 26, 1369–1388. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J. Comparative Physiology: Diurnal Rhythms. Annu. Rev. Physiol. 1963, 25, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C.S.; Minis, D.H. The Entrainment of Circadian Oscillations by Light and Their Role as Photoperiodic Clocks. Am. Nat. 1964, 98, 261–294. [Google Scholar] [CrossRef]
- A Czeisler, C.; Richardson, G.S.; Zimmerman, J.C.; Moore-Ede, M.C.; Weitzman, E.D. Entrainment of human circadian rhythms by light-dark cycles: A reassessment. Photochem. Photobiol. 1981, 34, 239–247. [Google Scholar] [CrossRef]
- Arendt, J.; Broadway, J. Light and Melatonin as Zeitgebers in Man. Chronobiol. Int. 1987, 4, 273–282. [Google Scholar] [CrossRef]
- Van Essen, D.C. Visual Areas of the Mammalian Cerebral Cortex. Annu. Rev. Neurosci. 1979, 2, 227–261. [Google Scholar] [CrossRef]
- Sereno, M.I.; Allman, J.M. Cortical visual areas in mammals. Neural Basis Vis. Funct. 1991, 4, 160–172. [Google Scholar]
- Saper, C.B.; Chou, T.C.; E Scammell, T. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001, 24, 726–731. [Google Scholar] [CrossRef]
- Bastuji, H.; García-Larrea, L. Human auditory information processing during sleep assessed with evoked potentials. In The Physiologic Nature of Sleep; Imperial Colllege Press: London, UK, 2005; pp. 509–534. [Google Scholar]
- Velluti, R.A.; Pedemonte, M. Auditory neuronal networks in sleep and wakefulness. Int. J. Bifurc. Chaos 2010, 20, 403–407. [Google Scholar] [CrossRef]
- Tavakoli, P.; Dale, A.; Boafo, A.; Campbell, K. Evidence of P3a During Sleep, a Process Associated with Intrusions Into Consciousness in the Waking State. Front. Neurosci. 2019, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Parker, P.H.; Sibley, C.G.; Kumar, S. Continental breakup and the ordinal diversification of birds and mammals. Nature 1996, 381, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, E.; Murphy, W.J.; O’Brien, S.J. Molecular dating and biogeography of the early placental mammal radiation. J. Hered. 2001, 92, 212–219. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, J.; Wang, Y.; Li, C. Large Mesozoic mammals fed on young dinosaurs. Nature 2005, 433, 149–152. [Google Scholar] [CrossRef]
- Augustin, F.J.; Matzke, A.T.; Maisch, M.W.; Hinz, J.K.; Pfretzschner, H.U. The smallest eating the largest: The oldest mammalian feeding traces on dinosaur bone from the Late Jurassic of the Junggar Basin (northwestern China). Sci. Nat. 2020, 107, 32. [Google Scholar] [CrossRef]
- Longrich, N.R.; Ryan, M.J. Mammalian tooth marks on the bones of dinosaurs and other Late Cretaceous vertebrates. Palaeontology 2010, 53, 703–709. [Google Scholar] [CrossRef]
- De Valais, S.; Apesteguía, S.; Garrido, A.C. Cretaceous Small Scavengers: Feeding Traces in Tetrapod Bones from Patagonia, Argentina. PLoS ONE 2012, 7, e29841. [Google Scholar] [CrossRef][Green Version]
- Slater, G.J. Phylogenetic evidence for a shift in the mode of mammalian body size evolution at the Cretaceous-Palaeogene boundary. Methods Ecol. Evol. 2013, 4, 734–744. [Google Scholar] [CrossRef]
- Luo, Z.-X. Transformation and diversification in early mammal evolution. Nature 2007, 450, 1011–1019. [Google Scholar] [CrossRef]
- Surridge, A.K.; Osorio, D.; Mundy, N.I. Evolution and selection of trichromatic vision in primates. Trends Ecol. Evol. 2003, 18, 198–205. [Google Scholar] [CrossRef]
- Elewa, A.M. K-Pg mass extinction. In Mass Extinction; Springer: Berlin/Heidelberg, Germany, 2008; pp. 129–131. [Google Scholar]
- Meredith, R.W.; Janečka, J.E.; Gatesy, J.; Ryder, O.A.; Fisher, C.A.; Teeling, E.C.; Goodbla, A.; Eizirik, E.; Simão, T.L.L.; Stadler, T.; et al. Impacts of the Cretaceous Terrestrial Revolution and KPg Extinction on Mammal Diversification. Science 2011, 334, 521–524. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Bloch, J.I.; Flynn, J.J.; Gaudin, T.J.; Giallombardo, A.; Giannini, N.P.; Goldberg, S.L.; Kraatz, B.P.; Luo, Z.-X.; Meng, J.; et al. The Placental Mammal Ancestor and the Post–K-Pg Radiation of Placentals. Science 2013, 339, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Brusatte, S.L.; Butler, R.; Barrett, P.M.; Carrano, M.T.; Evans, D.C.; Lloyd, G.T.; Mannion, P.D.; Norell, M.A.; Peppe, D.; Upchurch, P.; et al. The extinction of the dinosaurs. Biol. Rev. 2014, 90, 628–642. [Google Scholar] [CrossRef]
- Witts, J.D.; Whittle, R.J.; Wignall, P.B.; Crame, J.A.; Francis, J.E.; Newton, R.; Bowman, V.C. Macrofossil evidence for a rapid and severe Cretaceous–Paleogene mass extinction in Antarctica. Nat. Commun. 2016, 7, 11738. [Google Scholar] [CrossRef]
- Canudo, J.I.; Oms, O.; Vila, B.; Galobart, À.; Fondevilla, V.; Pascual, E.P.; Sellés, A.G.; Cruzado-Caballero, P.; Dinarès-Turell, J.; Vicens, E.; et al. The upper Maastrichtian dinosaur fossil record from the southern Pyrenees and its contribution to the topic of the Cretaceous–Palaeogene mass extinction event. Cretac. Res. 2016, 57, 540–551. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Rheindt, F.E.; Lei, F.; Qu, Y.; Wang, Y.; Zhang, Y.; Sullivan, C.; Nie, W.; Wang, J.; et al. Genomic evidence reveals a radiation of placental mammals uninterrupted by the KPg boundary. Proc. Natl. Acad. Sci. USA 2017, 114, E7282–E7290. [Google Scholar] [CrossRef]
- Elgar, M.A.; Pagel, M.D.; Harvey, P.H. Sleep in mammals. Anim. Behav. 1988, 36, 1407–1419. [Google Scholar] [CrossRef]
- Elgar, M.A.; Pagel, M.D.; Harvey, P.H. Sources of variation in mammalian sleep. Anim. Behav. 1990, 40, 991–994. [Google Scholar] [CrossRef]
- Madsen, O.D.; Scally, M.J.; Douady, C.; Kao, D.J.; DeBry, R.W.; Adkins, R.M.; Amrine, H.M.; Stanhope, M.J.; De Jong, W.W.; Springer, M.S. Parallel adaptive radiations in two major clades of placental mammals. Nature 2001, 409, 610–614. [Google Scholar] [CrossRef]
- Smith, F.A.; Boyer, A.G.; Brown, J.H.; Costa, D.P.; Dayan, T.; Ernest, S.K.M.; Evans, A.R.; Fortelius, M.; Gittleman, J.L.; Hamilton, M.J.; et al. The Evolution of Maximum Body Size of Terrestrial Mammals. Science 2010, 330, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J.J.; Boyer, A.G.; Brown, J.H.; Costa, D.; Ernest, M.; Evans, A.; Fortelius, M.; Gittleman, J.L.; Hamilton, M.J.; Harding, L.E.; et al. Patterns of maximum body size evolution in Cenozoic land mammals: Eco-evolutionary processes and abiotic forcing. Proc. R. Soc. B Boil. Sci. 2014, 281, 20132049. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, M.G. The Effect of Selection on Genetic Variability. Am. Nat. 1971, 105, 201–211. [Google Scholar] [CrossRef]
- Herron, J.C.; Freeman, S. Evolutionary Analysis; Pearson: New York, NY, USA, 2014. [Google Scholar]
- Busch, N.A.; VanRullen, R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc. Natl. Acad. Sci. USA 2010, 107, 16048–16053. [Google Scholar] [CrossRef] [PubMed]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Neural Circuits That Mediate Selective Attention: A Comparative Perspective. Trends Neurosci. 2018, 41, 789–805. [Google Scholar] [CrossRef]
- Ng, B.S.W.; Schroeder, T.; Kayser, C. A Precluding But Not Ensuring Role of Entrained Low-Frequency Oscillations for Auditory Perception. J. Neurosci. 2012, 32, 12268–12276. [Google Scholar] [CrossRef]
- Keller, A.S.; Payne, L.; Sekuler, R. Characterizing the roles of alpha and theta oscillations in multisensory attention. Neuropsychologia 2017, 99, 48–63. [Google Scholar] [CrossRef]
- Karapanagiotidis, T.; Vidaurre, D.; Quinn, A.J.; Vatansever, D.; Poerio, G.L.; Turnbull, A.; Ho, N.S.P.; Leech, R.; Bernhardt, B.C.; Jefferies, E.; et al. The psychological correlates of distinct neural states occurring during wakeful rest. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Herbers, J.M. Time resources and laziness in animals. Oecologia 1981, 49, 252–262. [Google Scholar] [CrossRef]
- Wilson, E.O. Sociobiology: The New Synthesis, 25th ed.; Harvard University Press: Cambridge, UK, 2000; pp. 142–143. [Google Scholar]
- Ettinger, A.O.; King, J.R. Time and energy budgets of the Willow Flycatcher (Empidonax traillii) during the breed-ing season. Auk 1980, 97, 533–546. [Google Scholar]
- Wolf, L.L.; Hainsworth, F.R.; Gill, F.B. Foraging efficiencies and time budgets in nectar-feeding birds. Ecology 1975, 56, 117–128. [Google Scholar] [CrossRef]
- Odden, M.; Wegge, P. Kill rates and food consumption of leopards in Bardia National Park, Nepal. Mammal Res. 2009, 54, 23–30. [Google Scholar] [CrossRef]
- Lamp, A.; Cook, M.; Soriano Smith, R.N.; Belenky, G. Exercise, nutrition, sleep, and waking rest? Sleep 2019, 42, 1–2. [Google Scholar] [CrossRef]
- Tofts, C.; Franks, N.R. Doing the right thing: Ants, honeybees and naked mole-rats. Trends Ecol. Evol. 1992, 7, 346–349. [Google Scholar] [CrossRef]
- Breed, M. Why are workers lazy? Insectes Sociaux 2015, 62, 7–8. [Google Scholar] [CrossRef]
- Hasegawa, E.; Ishii, Y.; Tada, K.; Kobayashi, K.; Yoshimura, J. Lazy workers are necessary for long-term sustainability in insect societies. Sci. Rep. 2016, 6, 20846. [Google Scholar] [CrossRef]
- Charbonneau, D.; Sasaki, T.; Dornhaus, A. Who needs ‘lazy’ workers? Inactive workers act as a ‘reserve’ labor force replacing active workers, but inactive workers are not replaced when they are removed. PLoS ONE 2017, 12, e0184074. [Google Scholar] [CrossRef]
- Lieberman, D.E. Is exercise really medicine? An evolutionary perspective. Curr. Sports Med. Rep. 2015, 14, 313–319. [Google Scholar] [CrossRef]
- Lee, M.G.; Hassani, O.K.; Jones, B.E. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. J. Neurosci. 2005, 25, 6716–6720. [Google Scholar] [CrossRef]
- Cheval, B.; Sarrazin, P.; Isoard-Gautheur, S.; Radel, R.; Friese, M. Reflective and impulsive processes explain (in)effectiveness of messages promoting physical activity: A randomized controlled trial. Heal. Psychol. 2015, 34, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cheval, B.; Tipura, E.; Burra, N.; Frossard, J.; Chanal, J.; Orsholits, D.; Radel, R.; Boisgontier, M.P. Avoiding sedentary behaviors requires more cortical resources than avoiding physical activity: An EEG study. Neuropsychologia 2018, 119, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C. Waking rest: A game changer or a name changer? Sleep 2019, 42, zsz172. [Google Scholar] [CrossRef] [PubMed]
- Cabanac, M. Pleasure: The common currency. J. Theor. Biol. 1992, 155, 173–200. [Google Scholar] [CrossRef]
- Cabanac, M. Sensory pleasure optimizes muscular work. Clin. Investig. Med. 2006, 29, 110–116. [Google Scholar]
- Cabanac, M. Pleasure and joy, and their role in human life. In Creating the Productive Workplace; Routledge: London, UK, 2017; pp. 73–82. [Google Scholar]
- Roth, D.A.-E.; Kishon-Rabin, L.; Hildesheimer, M.; Karni, A. A latent consolidation phase in auditory identification learning: Time in the awake state is sufficient. Learn. Mem. 2005, 12, 159–164. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Dewar, M.; Alber, J.; Butler, C.; Cowan, N.; Della Sala, S. Brief Wakeful Resting Boosts New Memories Over the Long Term. Psychol. Sci. 2012, 23, 955–960. [Google Scholar] [CrossRef]
- Brokaw, K.; Tishler, W.; Manceor, S.; Hamilton, K.; Gaulden, A.; Parr, E.; Wamsley, E.J. Resting state EEG correlates of memory consolidation. Neurobiol. Learn. Mem. 2016, 130, 17–25. [Google Scholar] [CrossRef]
- Humiston, G.B.; Tucker, M.A.; Summer, T.; Wamsley, E.J. Resting States and Memory Consolidation: A Preregistered Replication and Meta-Analysis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Humiston, G.; Wamsley, E.J. A brief period of eyes-closed rest enhances motor skill consolidation. Neurobiol. Learn. Mem. 2018, 155, 1–6. [Google Scholar] [CrossRef]
- Wamsley, E.J. Memory Consolidation during Waking Rest. Trends Cogn. Sci. 2019, 23, 171–173. [Google Scholar] [CrossRef] [PubMed]
- A Tucker, M.; Humiston, G.B.; Summer, T.; Wamsley, E. Comparing the Effects of Sleep and Rest on Memory Consolidation. Nat. Sci. Sleep 2020, ume 12, 79–91. [Google Scholar] [CrossRef]
- Klinzing, J.G.; Herbrik, L.; Nienborg, H.; Rauss, K. Binocular disparity-based learning is retinotopically specific and independent of sleep. Philos. Trans. R. Soc. B 2020, 375, 20190463. [Google Scholar] [CrossRef] [PubMed]
- Klinzing, J.G.; Nienborg, H.; Rauss, K. Sleep does not aid the generalisation of binocular disparity-based learning to the other visual hemifield. J. Sleep Res. 2021, 30, e13335. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. Memory Consolidation Is Similar in Waking and Sleep. Curr. Sleep Med. Rep. 2021, 7, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Cordi, M.J.; Rasch, B. How robust are sleep-mediated memory benefits? Curr. Opin. Neurobiol. 2021, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Lemmens, G.; Parkes, D. Pre-sleep behaviour in normal subjects. J. Sleep Res. 1995, 4, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Eban-Rothschild, A.; Rothschild, G.; Giardino, W.; Jones, J.R.; De Lecea, L. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat. Neurosci. 2016, 19, 1356–1366. [Google Scholar] [CrossRef]
- Webb, W.B. Sleep as an Adaptive Response. Percept. Mot. Ski. 1974, 38, 1023–1027. [Google Scholar] [CrossRef]
- Meddis, R. The Sleep Instinct; Routledge: Oxford, UK, 1975. [Google Scholar]
- Rial, R.V.; Nicolau, M.; A Lopez-Garcia, J.; Almirall, H. On the evolution of waking and sleeping. Comp. Biochem. Physiol. Part A Physiol. 1993, 104, 189–193. [Google Scholar] [CrossRef]
- Rial, M.C.; Nicolau, A.; Gamundí, M.; Akaârir, S.; Aparicio, C.; Garau, S.; Tejada, C.; Roca, L.; Gené, D.; Moranta, S.; et al. The trivial function of sleep. Sleep Med. Rev. 2007, 11, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Rial, M.; Akaârir, A.; Gamundí, C.; Nicolau, C.; Garau, S.; Aparicio, S.; Tejada, L.; Gené, J.; González, L.M.; De Vera, A.M. Coenen. Evolution of wakefulness, sleep and hibernation: From reptiles to mammals. Neurosci. Biobehav. Rev. 2010, 34, 1144–1160. [Google Scholar] [CrossRef]
- Siegel, J.M. Sleep viewed as a state of adaptive inactivity. Nat. Rev. Neurosci. 2009, 10, 747–753. [Google Scholar] [CrossRef]
- Siegel, J.M. Sleep in Animals: A State of Adaptive Inactivity. Princ. Pract. 2011, 5, 126–138. [Google Scholar]
- Frank, M.G. Challenging sleep homeostasis. Neurobiol. Sleep Circadian Rhythm. 2021, 10, 100060. [Google Scholar] [CrossRef]
- Benington, J.H. Sleep Homeostasis and the Function of Sleep. Sleep 2000, 23, 1–8. [Google Scholar] [CrossRef]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; DeBoer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Frank, M.G. The mystery of sleep function: Current perspectives and future directions. Rev. Neurosci. 2006, 17, 375–392. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Bergmann, B.M.; Gilliland, M.A.; Bauer, K. Effects of Method, Duration, and Sleep Stage on Rebounds from Sleep Deprivation in the Rat. Sleep 1999, 22, 11–31. [Google Scholar] [CrossRef]
- Gulevich, G.; Dement, W.; Johnson, L. Psychiatric and EEG observations on a case of prolonged (264 hours) wakeful-ness. Arch. Gen. Psychiatry 1966, 15, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kavanau, J. Vertebrates That Never Sleep: Implications For Sleep’s Basic Function. Brain Res. Bull. 1998, 46, 269–279. [Google Scholar] [CrossRef]
- Siegel, J.M. Do all animals sleep? Trends Neurosci. 2008, 31, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M.E. Learned helplessness. Annu. Rev. Med. 1972, 23, 407–412. [Google Scholar] [CrossRef]
- Lyamin, O.; Pryaslova, J.; Kosenko, P.; Siegel, J. Behavioral aspects of sleep in bottlenose dolphin mothers and their calves. Physiol. Behav. 2007, 92, 725–733. [Google Scholar] [CrossRef]
- Pilleri, G. Observation on the behavior of Platanista gangetica in the Indus and Brahmaputra rivers. Investig. Cetacea 1970, 2, 27–60. [Google Scholar]
- Gravett, N.; Bhagwandin, A.; Sutcliffe, R.; Landen, K.; Chase, M.J.; Lyamin, O.; Siegel, J.M.; Manger, P.R. Inactivity/sleep in two wild free-roaming African elephant matriarchs—Does large body size make elephants the shortest mammalian sleepers? PLoS ONE 2017, 12, e0171903. [Google Scholar] [CrossRef]
- Geissmann, Q.; Beckwith, E.J.; Gilestro, G.F. Most sleep does not serve a vital function: Evidence from Drosophila melanogaster. bioRxiv 2018, 361667. [Google Scholar] [CrossRef]
- Geissmann, Q.; Beckwith, E.J.; Gilestro, G.F. Most sleep does not serve a vital function: Evidence from Drosophila melanogaster. Sci. Adv. 2019, 5, eaau9253. [Google Scholar] [CrossRef]
- Olds, J.; Milner, P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954, 47, 419–427. [Google Scholar] [CrossRef]
- Nicholson, A.; Pascoe, P.A. Dopaminergic transmission and the sleep-wakefulness continuum in man. Neuropharmacology 1990, 29, 411–417. [Google Scholar] [CrossRef]
- Boutrel, B.; Koob, G.F. What Keeps Us Awake: The Neuropharmacology of Stimulants and Wakefulness Promoting Medications. Sleep 2004, 27, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Lazarus, M. The control of sleep and wakefulness by mesolimbic dopamine systems. Neurosci. Res. 2017, 118, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wisor, J.P. Dopamine and Wakefulness: Pharmacology, Genetics, and Circuitry. In Sleep-Wake Neurobiology and Pharmacology; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2018; Volume 253, pp. 321–335. [Google Scholar] [CrossRef]
- Berridge, K. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E.; Aldridge, J.W. Dissecting components of reward:‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Overskeid, G. The Role of Emotions in Reinforcement: Response Selection in Humans. Psychol. Rec. 2012, 62, 125–131. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M. Pleasure Systems in the Brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Fadda, P.; Martellotta, M.; DE Montis, M.G.; Gessa, G.; Fratta, W. Dopamine D1 and opioid receptor binding changes in the limbic system of sleep deprived rats. Neurochem. Int. 1992, 20, 153–156. [Google Scholar] [CrossRef]
- Zant, J.C.; Leenaars CH, C.; Kostin, A.; Van Someren EJ, W.; Porkka-Heiskanen, T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011, 1399, 40–48. [Google Scholar] [CrossRef]
- Satterfield, B.C.; Wisor, J.P.; A Schmidt, M.; A Van Dongen, H.P. Time-on-Task Effect During Sleep Deprivation in Healthy Young Adults Is Modulated by Dopamine Transporter Genotype. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Wisor, J.P.; Nishino, S.; Sora, I.; Uhl, G.H.; Mignot, E.; Edgar, D.M. Dopaminergic Role in Stimulant-Induced Wakefulness. J. Neurosci. 2001, 21, 1787–1794. [Google Scholar] [CrossRef]
- Nakajima, T.; Tobe, Y. Self-awakening Technique and Dopamine Agonistic Medication Against the Difficulty of Morning Awakening. J. Int. Soc. Life Inf. Sci. 2015, 33, 80. [Google Scholar]
- Lal, S.; Thavundayil, J.; Nair NP, V.; Etienne, P.; Rastogi, R.; Schwartz, G.; Guyda, H. Effect of sleep deprivation on dopamine receptor function in normal subjects. J. Neural Transm. 1981, 50, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Tomasi, D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Logan, J.; Ferré, S. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J. Neurosci. 2012, 32, 6711–6717. [Google Scholar] [CrossRef] [PubMed]
- Klumpers, U.M.; Veltman, D.J.; van Tol, M.J.; Kloet, R.W.; Boellaard, R.; Lammertsma, A.A.; Hoogendijk, W.J. Neurophysiological effects of sleep deprivation in healthy adults, a pilot study. PLoS ONE 2015, 10, e0116906. [Google Scholar] [CrossRef]
- Emerson, M.J.; Schram, F.R. Theories, patterns, and reality: Game plan for arthropod phylogeny. In Arthropod Relationships; Springer: Dordrecht, The Netherlands, 1998; pp. 67–86. [Google Scholar]
- Edgecombe, G.D. Arthropod phylogeny: An overview from the perspectives of morphology, molecular data and the fossil record. Arthropod Struct. Dev. 2010, 39, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Giribet, G.; Edgecombe, G.D. The phylogeny and evolutionary history of arthropods. Curr. Biol. 2019, 29, R592–R602. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial, R.V.; Canellas, F.; Akaârir, M.; Rubiño, J.A.; Barceló, P.; Martín, A.; Gamundí, A.; Nicolau, M.C. The Birth of the Mammalian Sleep. Biology 2022, 11, 734. https://doi.org/10.3390/biology11050734
Rial RV, Canellas F, Akaârir M, Rubiño JA, Barceló P, Martín A, Gamundí A, Nicolau MC. The Birth of the Mammalian Sleep. Biology. 2022; 11(5):734. https://doi.org/10.3390/biology11050734
Chicago/Turabian StyleRial, Rubén V., Francesca Canellas, Mourad Akaârir, José A. Rubiño, Pere Barceló, Aida Martín, Antoni Gamundí, and M. Cristina Nicolau. 2022. "The Birth of the Mammalian Sleep" Biology 11, no. 5: 734. https://doi.org/10.3390/biology11050734
APA StyleRial, R. V., Canellas, F., Akaârir, M., Rubiño, J. A., Barceló, P., Martín, A., Gamundí, A., & Nicolau, M. C. (2022). The Birth of the Mammalian Sleep. Biology, 11(5), 734. https://doi.org/10.3390/biology11050734







