Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis
Abstract
1. Introduction
2. Materials and Methods
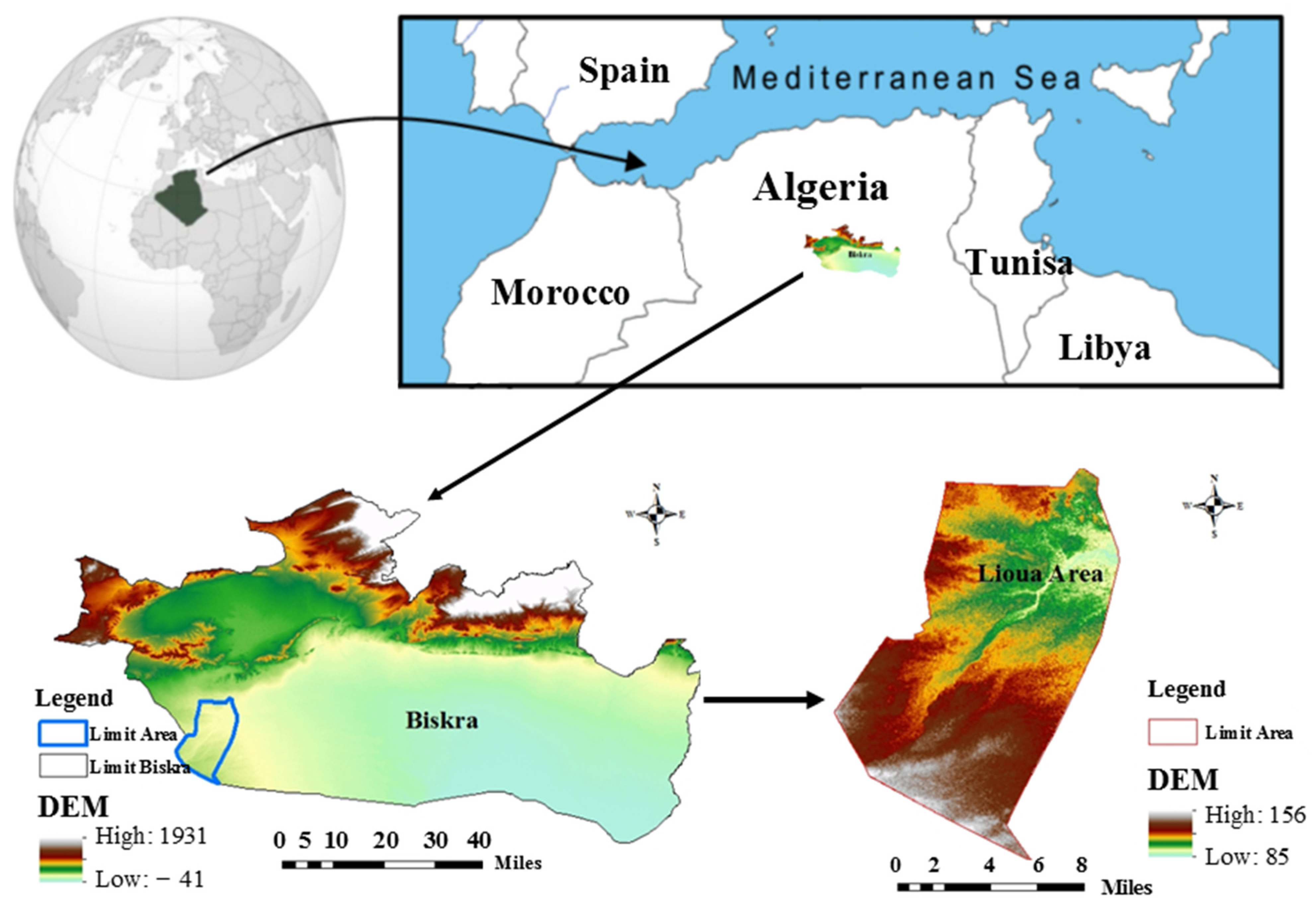
2.1. Study Area
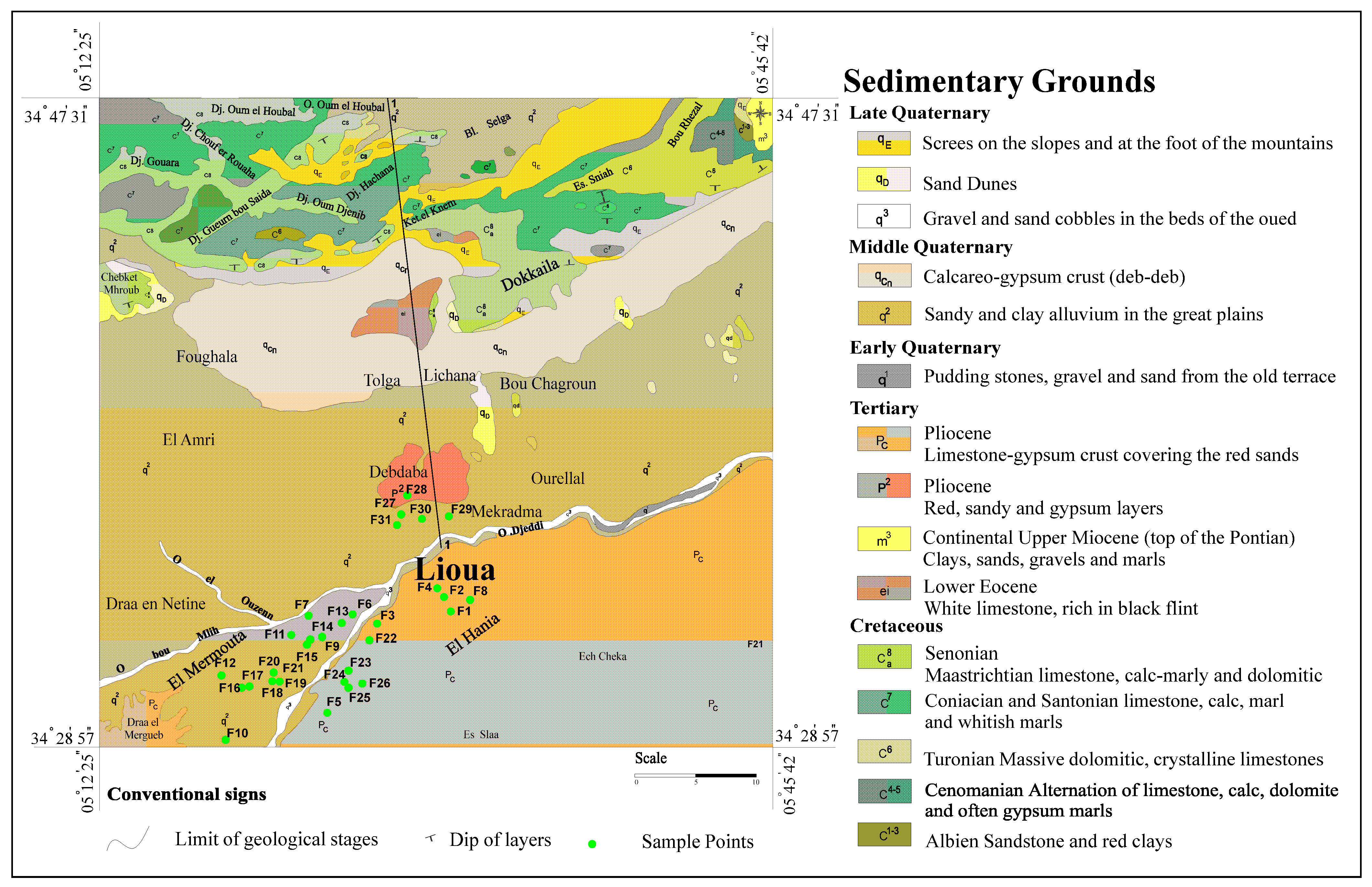
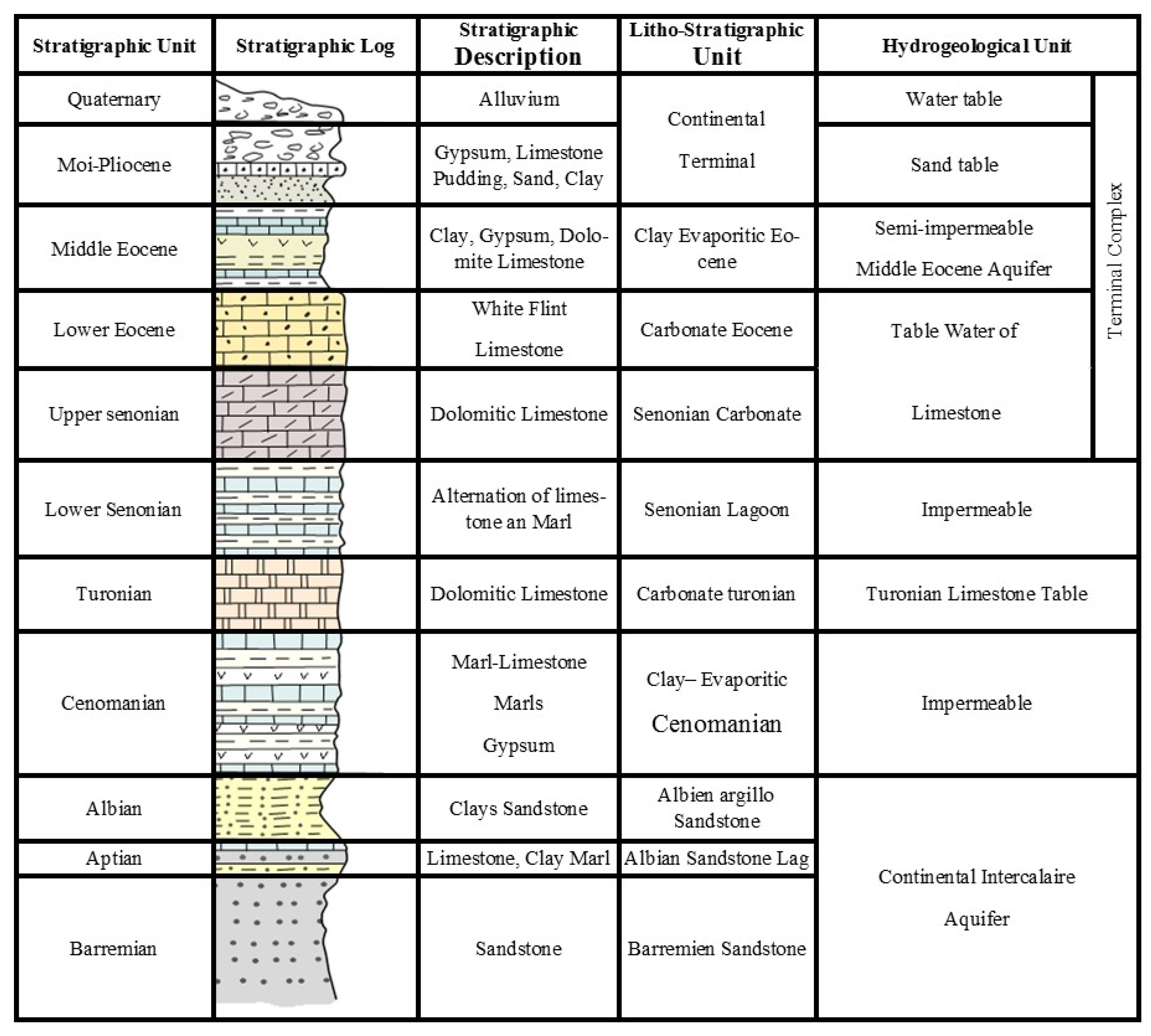
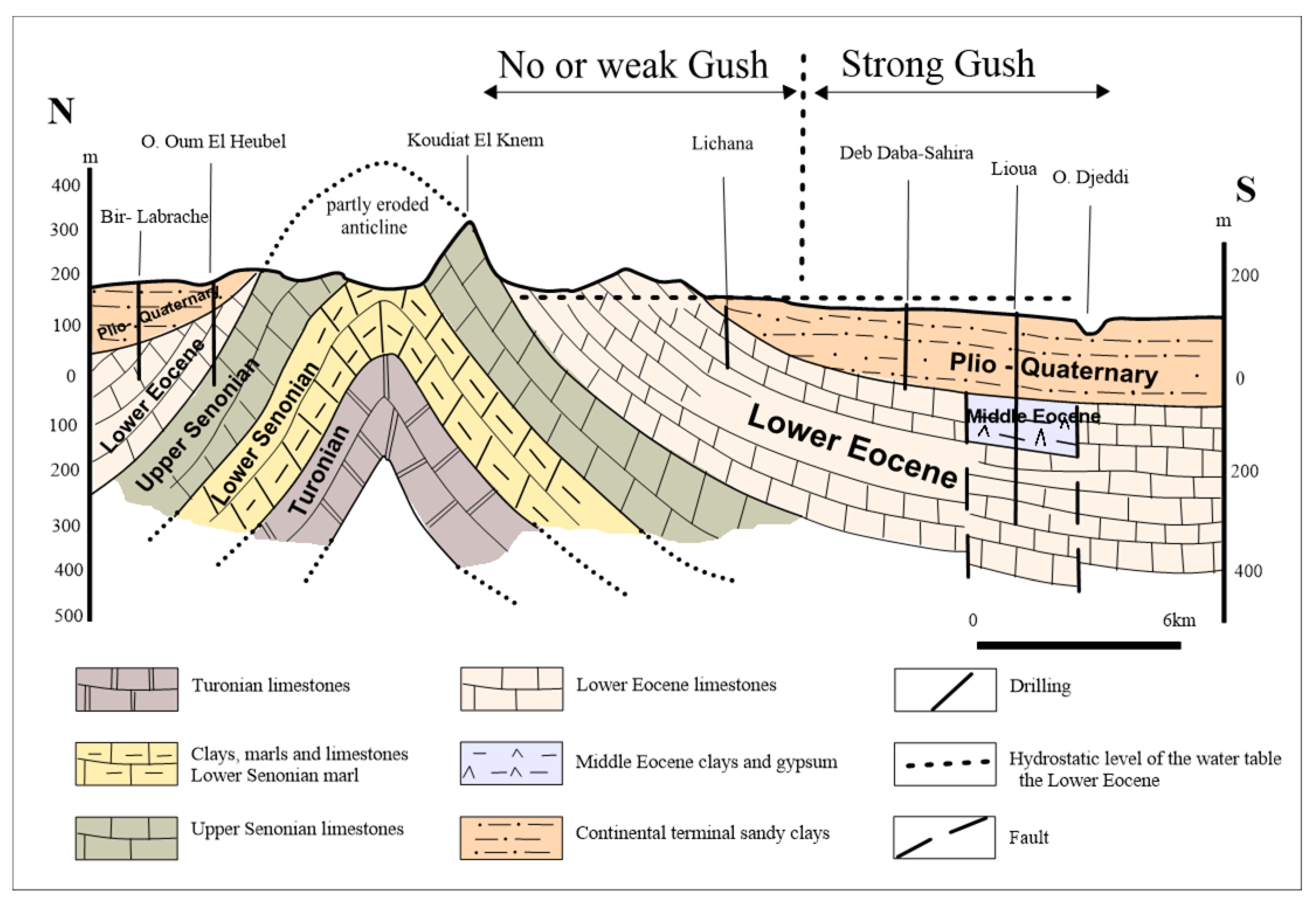
2.2. Geological and Hydrogeological Setting
2.3. Groundwater Sampling and Analysis
2.4. Methodology
2.4.1. Data Treatment and Multivariate Statistical Analysis
2.4.2. Time Series Analysis
3. Results
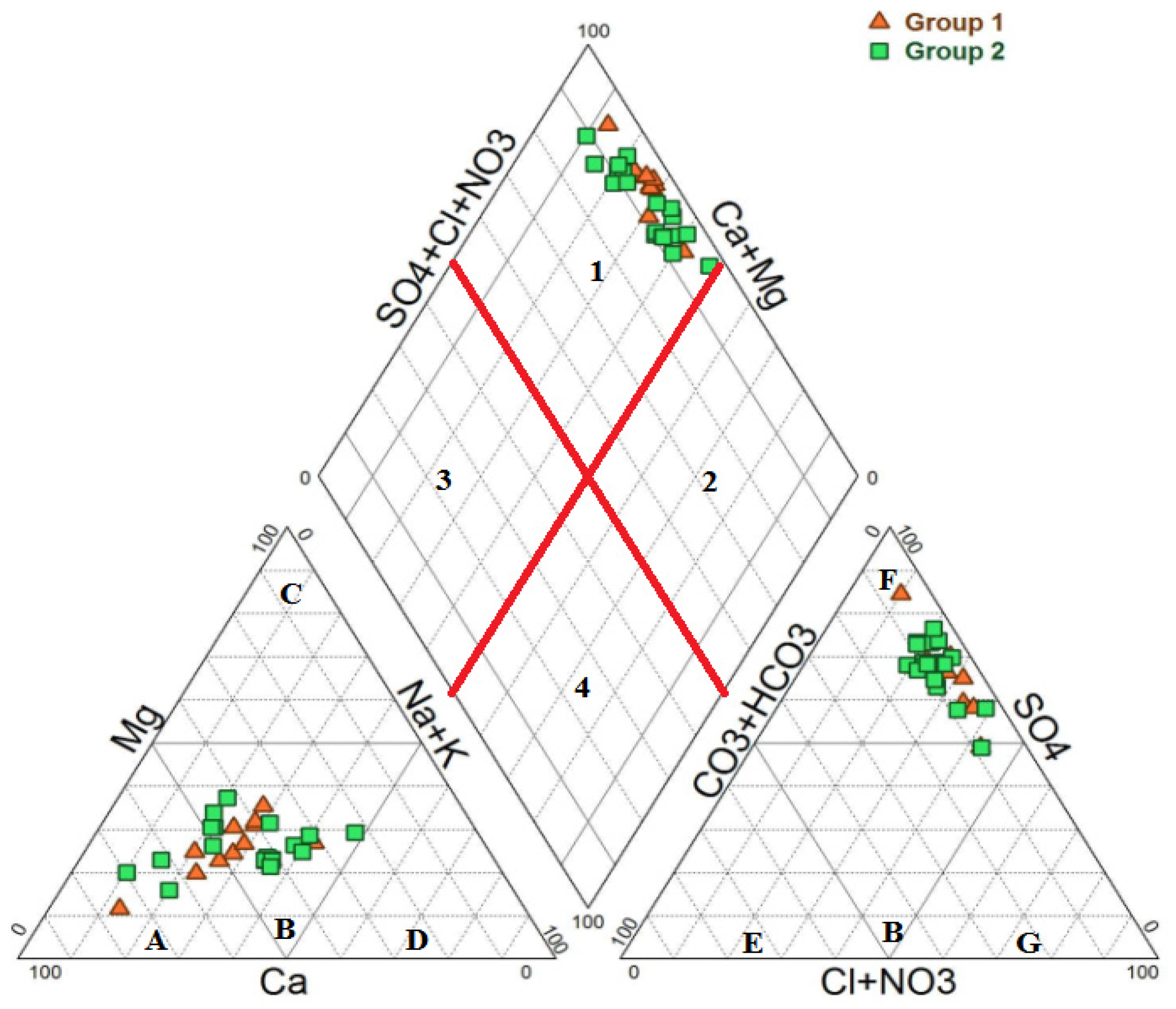
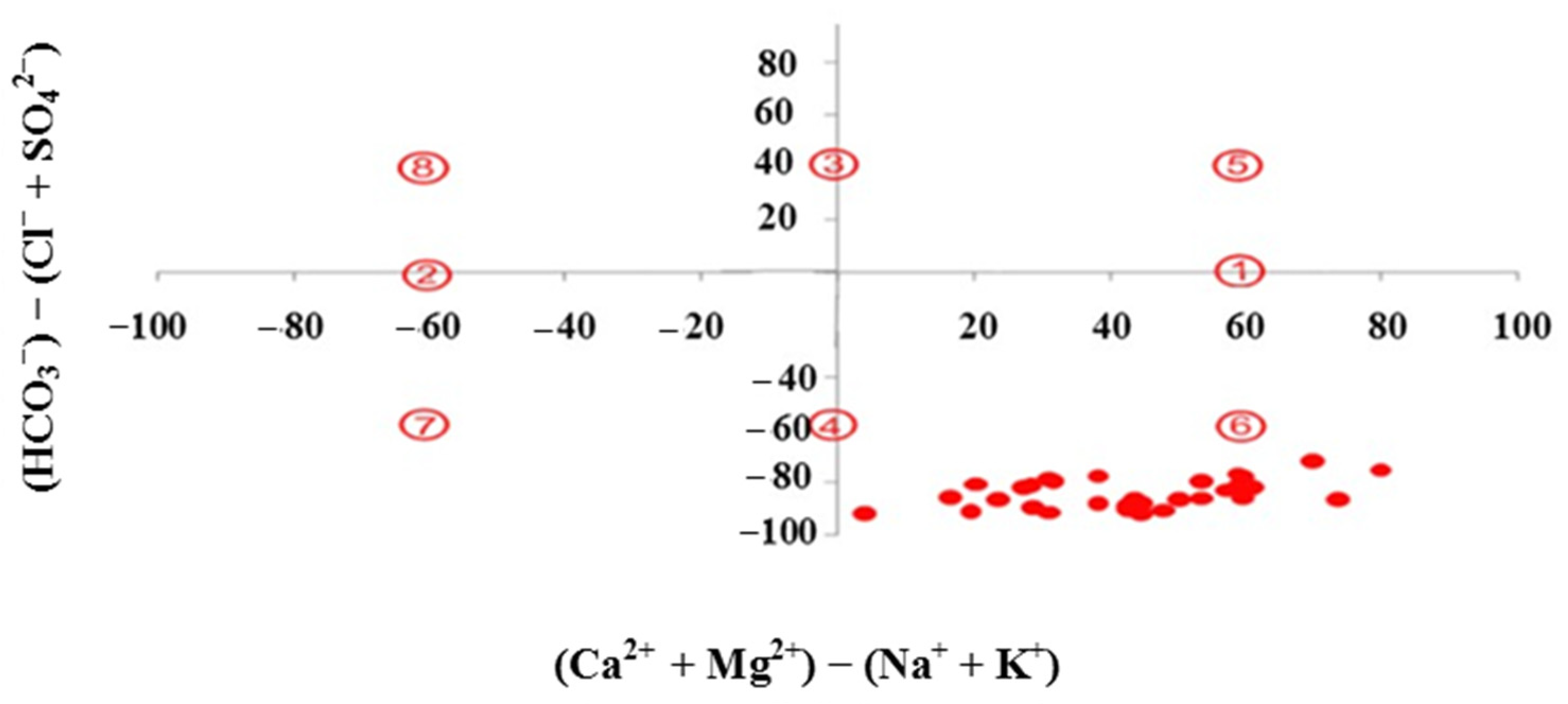
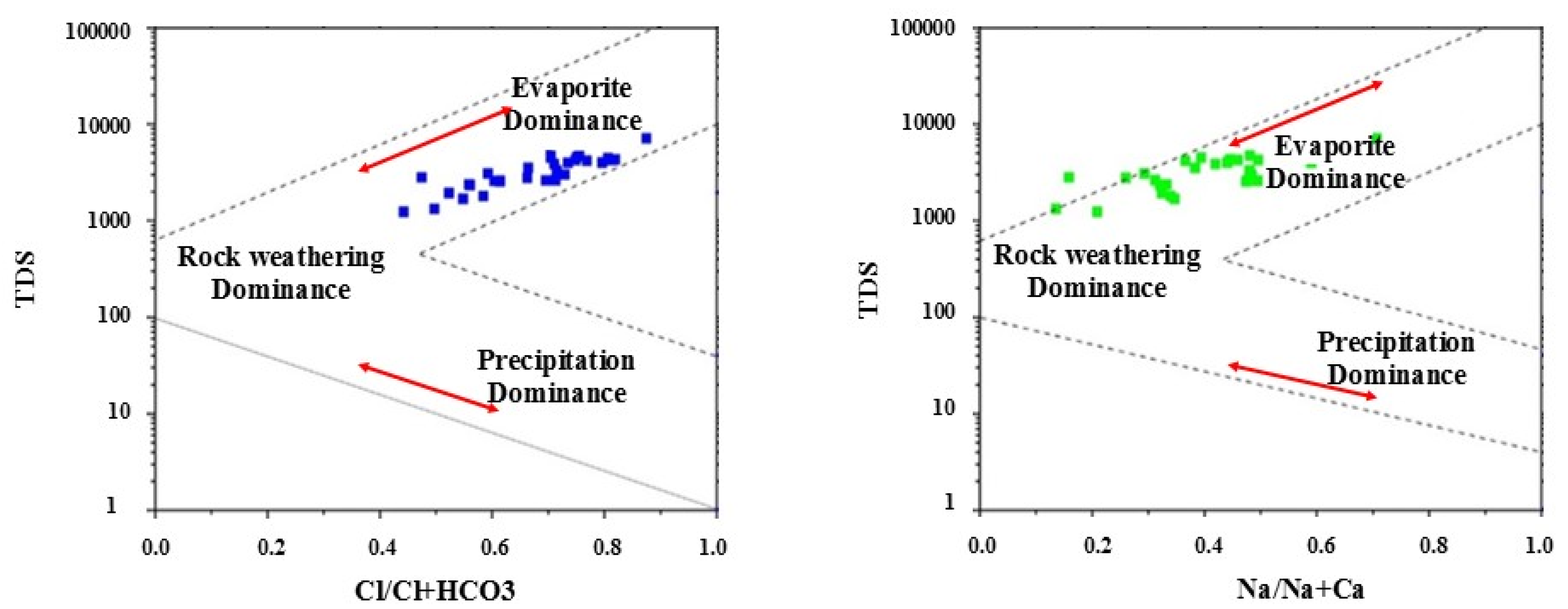
3.1. Hydrochemical Water Types and Groundwater Natural Evolution Mechanisms
3.2. Statistical Analysis
3.2.1. Correlation Analysis and Elementary Statistics
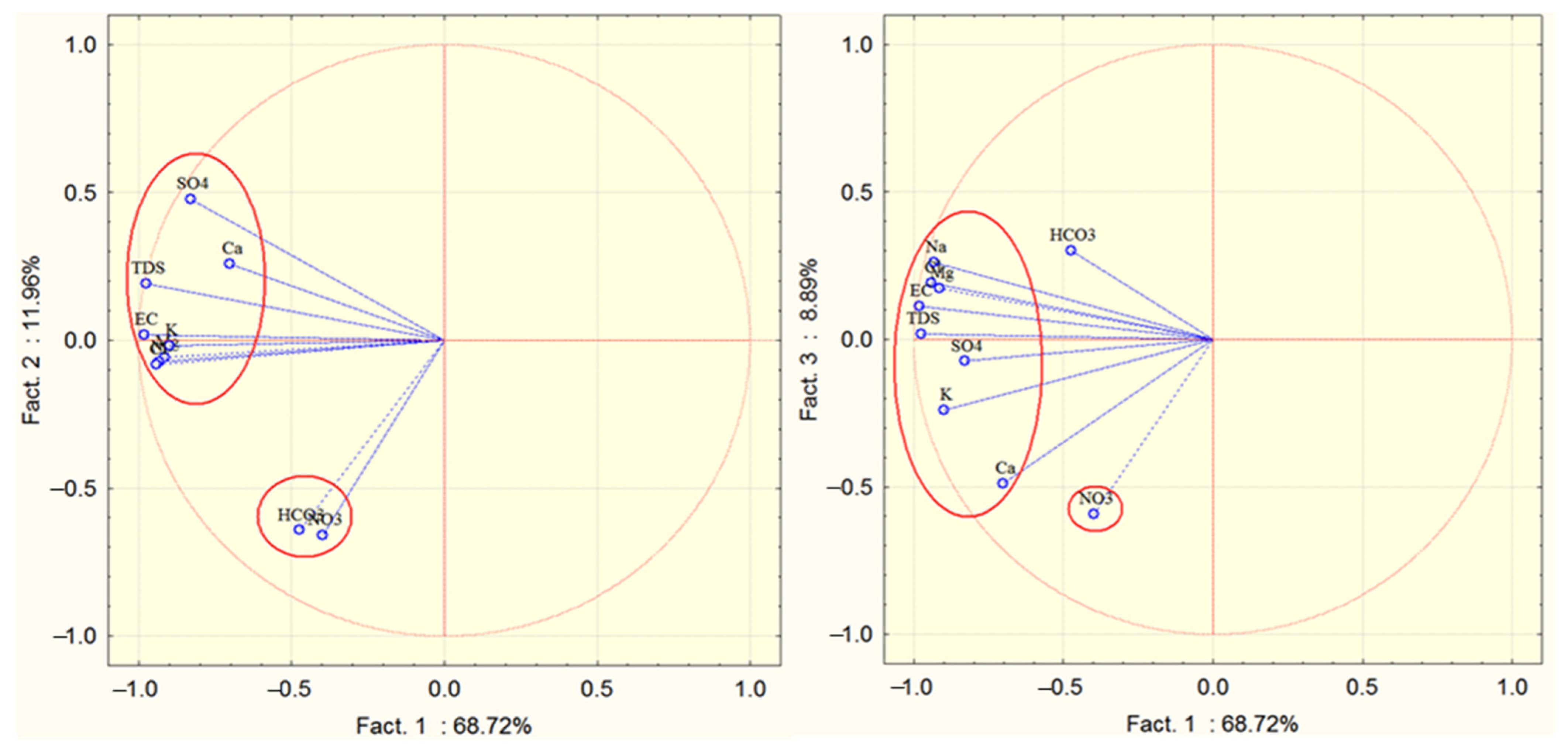
3.2.2. Principal Component Analysis (PCA)
3.2.3. Cluster Analysis (CA)
3.2.4. Time Series Analysis
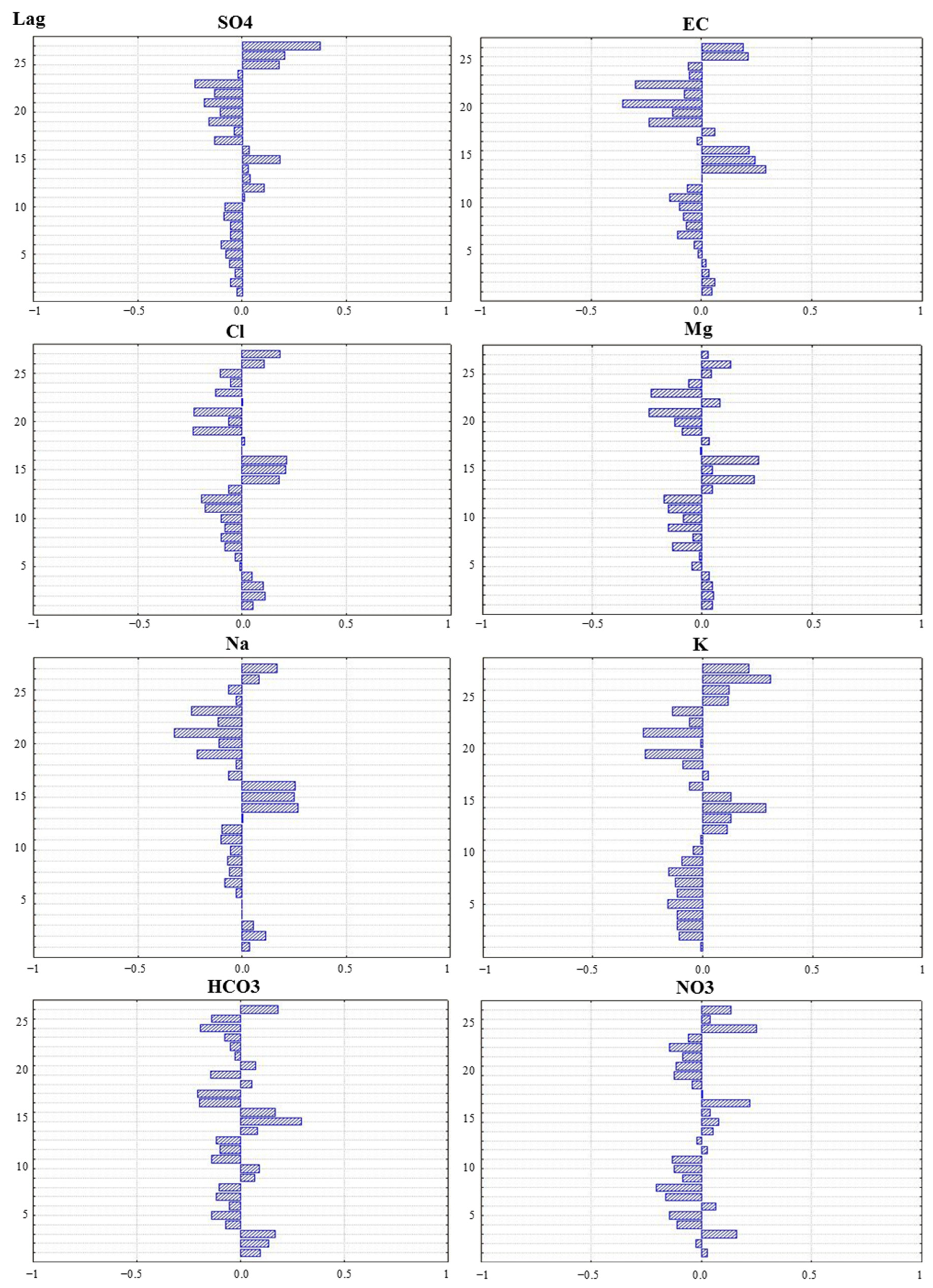
Autocorrelation
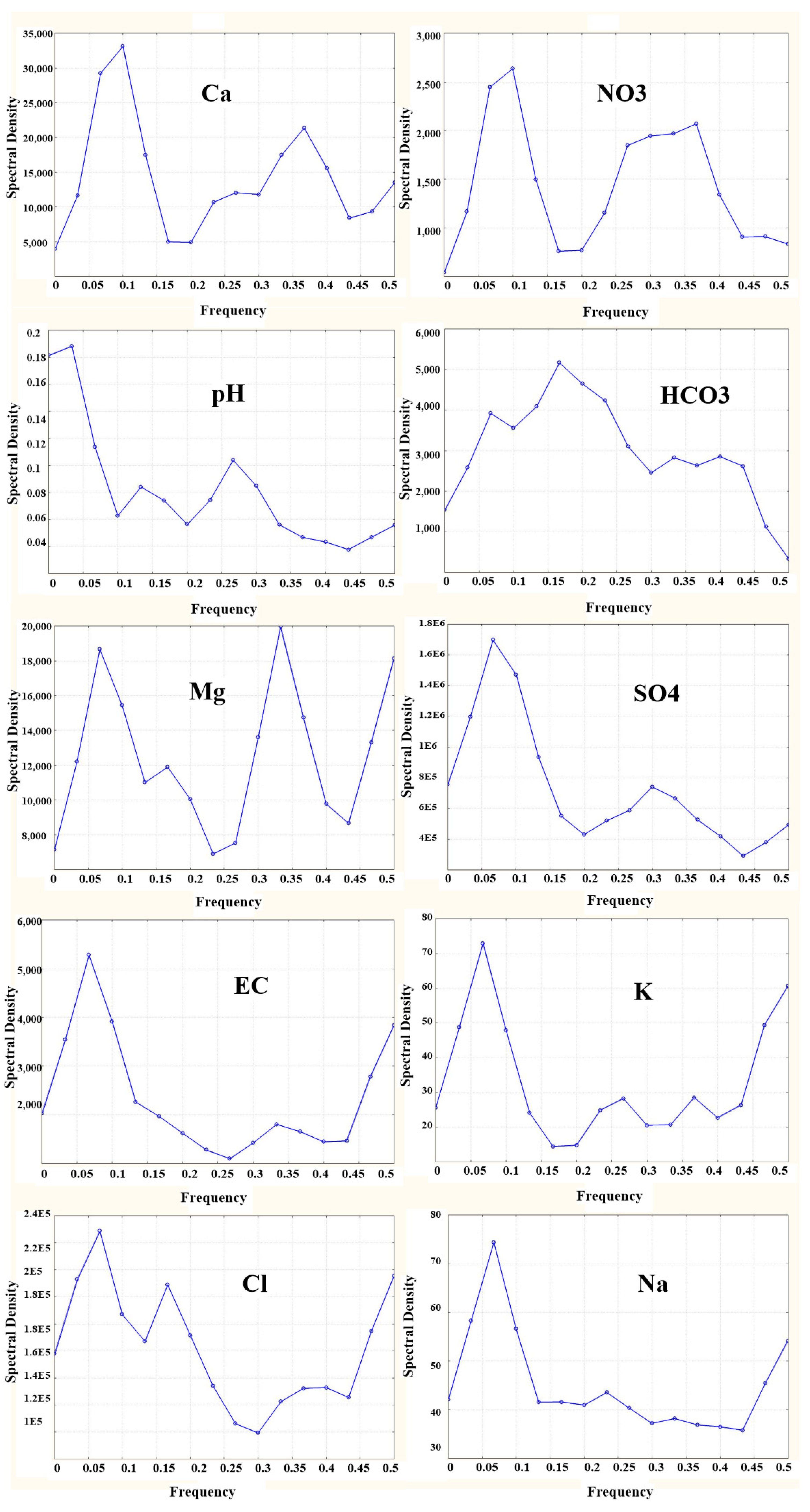
Spectral Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burlakovs, J.; Lacis, D. The Developmental Trends of Groundwater Horizon Surface Depression and Sea Water Intrusion Impact in Liepaja City. In Proceedings of the SGEM2012 Conference Proceedings, Albena, Bulgaria, 12–13 June 2012. [Google Scholar]
- Gul, S.; Gul, H.; Gul, M.; Khattak, R.; Rukh, G.; Khan, M.S.; Aouissi, H.A. Enhanced Adsorption of Rhodamine B on Biomass of Cypress/False Cypress (Chamaecyparis Lawsoniana) Fruit: Optimization and Kinetic Study. Water 2022, 14, 2987. [Google Scholar] [CrossRef]
- Travi, Y. Hydrogéologie et hydrochimie des aquifères du Sénégal. Hydrogéochimie du fluor dans les eaux souterraines. Sci. Géol. Bull. Mém. 1993, 1, 95. [Google Scholar]
- Xu, P.; Zhang, Q.; Qian, H.; Zheng, L. Spatial Distribution Characteristics of Irrigation Water Quality Assessment in the Central-Western Guanzhong Basin, China. IOP Conf. Ser. Earth Environ. Sci. 2021, 647, 012143. [Google Scholar] [CrossRef]
- Athamena, A.; Menani, M.R. Nitrogen Flux and Hydrochemical Characteristics of the Calcareous Aquifer of the Zana Plain, North East of Algeria. Arab. J. Geosci. 2018, 11, 356. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Sachpazidou, V.; Bisters, V.; Klavins, M.; Anne, O.; Grinfelde, I.; Hanc, E.; Hogland, W.; Ibrahim, M.A.; Jani, Y.; et al. Applying Macroalgal Biomass as an Energy Source: Utility of the Baltic Sea Beach Wrack for Thermochemical Conversion. Sustainability 2022, 14, 13712. [Google Scholar] [CrossRef]
- Boudoukha, A.; Athamena, M. Caractérisation des eaux thermales de l’ensemble Sud sétifien. Est. Algérien. Rseau 2012, 25, 103–118. [Google Scholar] [CrossRef][Green Version]
- Belalite, H.; Menani, M.R.; Athamena, A. Calculation of Water Needs of the Main Crops and Water Resources Available in a Semi-Arid Climate, Case of Zana-Gadaïne Plain, Northeastern Algeria. Alger. J. Environ. Sci. Technol. 2022, 8, 2477–2488. [Google Scholar]
- Gaagai, A.; Aouissi, H.A.; Krauklis, A.E.; Burlakovs, J.; Athamena, A.; Zekker, I.; Boudoukha, A.; Benaabidate, L.; Chenchouni, H. Modeling and Risk Analysis of Dam-Break Flooding in a Semi-Arid Montane Watershed: A Case Study of the Yabous Dam, Northeastern Algeria. Water 2022, 14, 767. [Google Scholar] [CrossRef]
- Al-Barakah, F.N.; Aly, A.A.; Abaakhel, E.H.S.; Al-Rizkid, A.M.; Alghamdi, A.G.; Al-Sewailem, M.S. Comparison and Hydrochemical Characterization of Groundwater Resources in the Arabian Peninsula: A Case Study of Al-Baha and Al-Qassim in Saudi Arabia. Water Resour. 2020, 47, 877–891. [Google Scholar] [CrossRef]
- Mfonka, Z.; Kpoumié, A.; Ngouh, A.N.; Mouncherou, O.F.; Nsangou, D.; Rakotondrabe, F.; Takounjou, A.F.; Zammouri, M.; Ngoupayou, J.R.N.; Ndjigui, P.-D. Water Quality Assessment in the Bamoun Plateau, Western-Cameroon: Hydrogeochemical Modelling and Multivariate Statistical Analysis Approach. J. Water Resour. Prot. 2021, 13, 112–138. [Google Scholar] [CrossRef]
- Awasthi, A.; Rishi, M.S.; Panjgotra, S. Groundwater Quality Assessment for Drinking and Industrial Purposes in Transboundary Aquifers of Gurdaspur District, Punjab, India. Int. J. Environ. Anal. Chem. 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Khelifi, W.; Bencedira, S.; Azab, M.; Riaz, M.S.; Abdallah, M.; Abdel Baki, Z.; Krauklis, A.E.; Aouissi, H.A. Conservation Environments’ Effect on the Compressive Strength Behaviour of Wood–Concrete Composites. Materials 2022, 15, 3572. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rishi, M.S.; Kaur, L. Multi-Parametric Analysis of Groundwater Quality to Assess Human Health Risk and Hydrogeochemical Processes in an Agriculturally Intensive Alluvial Aquifer of Northwest India. Int. J. Environ. Anal. Chem. 2022, 1–19. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Kreicbergs, I.; Dreyer, I. Modified Ginstling-Brounshtein Model for Wet Precipitation Synthesis of Hydroxyapatite: Analytical and Experimental Study. Acta Bioeng. Biomech. 2018, 20, 47–57. [Google Scholar]
- Belhadj, M.Z.; Boudoukha, A.; Amroune, A.; Gaagai, A.; Ziani, D. Statistical characterization of groundwater quality of the northern area of the basin of hodna, m’sila. southeastern algeria. Larhyss J. 2017, 31, 177–194. [Google Scholar]
- Herojeet, R.; Rishi, M.S.; Lata, R.; Dolma, K. Quality Characterization and Pollution Source Identification of Surface Water Using Multivariate Statistical Techniques, Nalagarh Valley, Himachal Pradesh, India. Appl. Water Sci. 2017, 7, 2137–2156. [Google Scholar] [CrossRef]
- Rajkumar, H.; Naik, P.K.; Rishi, M.S. Evaluation of Heavy Metal Contamination in Soil Using Geochemical Indexing Approaches and Chemometric Techniques. Int. J. Environ. Sci. Technol. 2019, 16, 7467–7486. [Google Scholar] [CrossRef]
- Aouissi, H.A.; Petrişor, A.-I.; Ababsa, M.; Boştenaru-Dan, M.; Tourki, M.; Bouslama, Z. Influence of Land Use on Avian Diversity in North African Urban Environments. Land 2021, 10, 434. [Google Scholar] [CrossRef]
- Belkhiri, L.; Narany, T.S. Using Multivariate Statistical Analysis, Geostatistical Techniques and Structural Equation Modeling to Identify Spatial Variability of Groundwater Quality. Water Resour. Manag. 2015, 29, 2073–2089. [Google Scholar] [CrossRef]
- Gaagai, A.; Boudoukha, A.; Boumezbeur, A.; Benaabidate, L. Hydrochemical Characterization of Surface Water in the Babar Watershed (Algeria) Using Environmetric Techniques and Time Series Analysis. Int. J. River Basin Manag. 2017, 15, 361–372. [Google Scholar] [CrossRef]
- Meng, S.X.; Maynard, J.B. Use of Statistical Analysis to Formulate Conceptual Models of Geochemical Behavior: Water Chemical Data from the Botucatu Aquifer Is Sao Paulo State, Brazil. J. Hydrol. 2001, 250, 78–97. [Google Scholar] [CrossRef]
- Farnham, I.M.; Stetzenbach, K.J.; Singh, A.K.; Johannesson, K.H. Deciphering Groundwater Flow Systems in Oasis Valley, Nevada, Using Trace Element Chemistry, Multivariate Statistics, and Geographical Information System. Math. Geol. 2000, 32, 943–968. [Google Scholar] [CrossRef]
- Roubil, A.; El Ouali, A.; Bülbül, A.; Lahrach, A.; Mudry, J.; Mamouch, Y.; Essahlaoui, A.; El Hmaidi, A.; El Ouali, A. Groundwater Hydrochemical and Isotopic Evolution from High Atlas Jurassic Limestones to Errachidia Cretaceous Basin (Southeastern Morocco). Water 2022, 14, 1747. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, C.; Chen, J.; Chen, J. Groundwater Recharge and Hydrogeochemical Evolution in Leizhou Peninsula, China. J. Chem. 2015, 2015, e427579. [Google Scholar] [CrossRef]
- Shen, F.; Yang, L.; He, X.; Zhou, C.; Adams, J.M. Understanding the Spatial–Temporal Variation of Human Footprint in Jiangsu Province, China, Its Anthropogenic and Natural Drivers and Potential Implications. Sci. Rep. 2020, 10, 13316. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.C. Time Series Trend Analysis and Prediction of Water Quality in a Managed Canal System, Florida (USA). IOP Conf. Ser. Earth Environ. Sci. 2018, 191, 012013. [Google Scholar] [CrossRef]
- Loucks, D.P.; van Beek, E. Water resources planning and management: An overview. In Water Resource Systems Planning and Management: An Introduction to Methods, Models, and Applications; Loucks, D.P., van Beek, E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–49. ISBN 978-3-319-44234-1. [Google Scholar]
- Aouissi, H.A.; Ababsa, M.; Gaagai, A.; Bouslama, Z.; Farhi, Y.; Chenchouni, H. Does Melanin-Based Plumage Coloration Reflect Health Status of Free-Living Birds in Urban Environments? Avian Res. 2021, 12, 45. [Google Scholar] [CrossRef]
- Koull, N.; Helimi, S.; Mihoub, A.; Mokhtari, S.; Kherraze, M.E.; Aouissi, H.A.; Koull, N.; Helimi, S.; Mihoub, A.; Mokhtari, S.; et al. Integración de SIG y Análisis Jerárquico Multi-Criterio Para Analizar La Idoneidad de La Tierra Para Los Cereales En La Zona Árida de Argelia. Int. J. Agric. Nat. Resour. 2022, 49, 36–50. [Google Scholar] [CrossRef]
- Teegavarapu, R.S.V. Chapter 1—Methods for analysis of trends and changes in hydroclimatological time-series. In Trends and Changes in Hydroclimatic Variables; Teegavarapu, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–89. ISBN 978-0-12-810985-4. [Google Scholar]
- Reghunath, R.; Murthy, T.R.S.; Raghavan, B.R. The Utility of Multivariate Statistical Techniques in Hydrogeochemical Studies: An Example from Karnataka, India. Water Res. 2002, 36, 2437–2442. [Google Scholar] [CrossRef]
- Chorfi, A.; Hafid, H.; Baaloudj, A.; Rizi, H.; Aouissi, H.A.; Chaib, S.; Ababsa, M.; Allaoua, N.; Houhamdi, M. Characterization and Diversity of Macroin-Vertebrates in Groundwater in the Region of Souk-Ahras (North-East of Algeria). Ekológia 2022, 41, 219–227. [Google Scholar] [CrossRef]
- Ahcène, S.; Bachir, H.; Bourafai, S. Hydrochemical Characteristics of Aquifers and Their Predicted Impact on Soil Properties in Biskra Region, Algeria. Egypt. J. Agric. Res. 2021, 99, 205–220. [Google Scholar] [CrossRef]
- Guettaia, S.; Hacini, M.; Boudjema, A.; Zahrouna, A. Vulnerability Assessment of an Aquifer in an Arid Environment and Comparison of the Applied Methods: Case of the Mio-Plio-Quaternary Aquifer. Energy Procedia 2017, 119, 482–489. [Google Scholar] [CrossRef]
- Obeidat, M.; Awawdeh, M. Assessment of Groundwater Quality in the Area Surrounding Al- Zaatari Camp, Jordan, Using Cluster Analysis and Water Quality Index (WQI). JJEES 2021, 12, 187. [Google Scholar]
- Amer Public Health Assn. Standard Methods for the Examination of Water & Wastewater, Centennial Edition, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Franson, M.A.H., Eds.; Amer Public Health Assn: Washington, DC, USA, 2005; ISBN 978-0-87553-047-5. [Google Scholar]
- Singh, G.; Rishi, M.S.; Herojeet, R.; Kaur, L.; Sharma, K. Evaluation of Groundwater Quality and Human Health Risks from Fluoride and Nitrate in Semi-Arid Region of Northern India. Environ. Geochem Health 2020, 42, 1833–1862. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Rishi, M.S.; Herojeet, R.; Kaur, L.; Priyanka; Sharma, K. Multivariate Analysis and Geochemical Signatures of Groundwater in the Agricultural Dominated Taluks of Jalandhar District, Punjab, India. J. Geochem. Explor. 2020, 208, 106395. [Google Scholar] [CrossRef]
- Simeonov, V.; Stratis, J.A.; Samara, C.; Zachariadis, G.; Voutsa, D.; Anthemidis, A.; Sofoniou, M.; Kouimtzis, T. Assessment of the Surface Water Quality in Northern Greece. Water Res. 2003, 37, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Malik, A.; Sinha, S. Water Quality Assessment and Apportionment of Pollution Sources of Gomti River (India) Using Multivariate Statistical Techniques—A Case Study. Anal. Chim. Acta 2005, 538, 355–374. [Google Scholar] [CrossRef]
- Vieira, J.S.; Pires, J.C.M.; Martins, F.G.; Vilar, V.J.P.; Boaventura, R.A.R.; Botelho, C.M.S. Surface Water Quality Assessment of Lis River Using Multivariate Statistical Methods. Water Air Soil Pollut. 2012, 223, 5549–5561. [Google Scholar] [CrossRef]
- Simeonov, V.; Simeonova, P.; Tzimou-Tsitouridou, R. Chemometric Quelity Assessment of Surface Waters: Two Case Studies. Chem. Inżynieria Ekol. 2004, 11, 449–469. [Google Scholar]
- Yang, K.; Xu, C.; Chi, M.; Wang, P. Analytical Analysis of the Groundwater Drawdown Difference Induced by Foundation Pit Dewatering with a Suspended Waterproof Curtain. Appl. Sci. 2022, 12, 10301. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Devkota, J.U. Multivariate Analysis of COVID-19 for Countries with Limited and Scarce Data: Examples from Nepal. J. Environ. Public Health 2021, 2021, 8813505. [Google Scholar] [CrossRef] [PubMed]
- Wai, W.W.; AlKarkhi, A.F.M.; Easa, A.M. Comparing Biosorbent Ability of Modified Citrus and Durian Rind Pectin. Carbohydr. Polym. 2010, 79, 584–589. [Google Scholar] [CrossRef]
- Swanson, S.K.; Bahr, J.M.; Schwar, M.T.; Potter, K.W. Two-Way Cluster Analysis of Geochemical Data to Constrain Spring Source Waters. Chem. Geol. 2001, 179, 73–91. [Google Scholar] [CrossRef]
- Danielsson, Å.; Cato, I.; Carman, R.; Rahm, L. Spatial Clustering of Metals in the Sediments of the Skagerrak/Kattegat. Appl. Geochem. 1999, 14, 689–706. [Google Scholar] [CrossRef]
- Fovell, R.G.; Fovell, M.-Y.C. Climate Zones of the Conterminous United States Defined Using Cluster Analysis. J. Clim. 1993, 6, 2103–2135. [Google Scholar] [CrossRef]
- Taheri Tizro, A.; Ghashghaie, M.; Georgiou, P.; Voudouris, K. Time Series Analysis of Water Quality Parameters. J. Appl. Res. Water Wastewater 2014, 1, 40–50. [Google Scholar]
- Mangin, A. The use of autocorrelation and spectral analyses to obtain a better understanding of hydrological systems. J. Hydrol. 1984, 67, 25–43. [Google Scholar] [CrossRef]
- Padilla, A.; Pulido-Bosch, A.; Mangin, A. Relative Importance of Baseflow and Quickflow from Hydrographs of Karst Spring. Groundwater 1994, 32, 267–277. [Google Scholar] [CrossRef]
- Chung, S.Y.; Venkatramanan, S.; Park, N.; Rajesh, R.; Ramkumar, T.; Kim, B.W. An Assessment of Selected Hydrochemical Parameter Trend of the Nakdong River Water in South Korea, Using Time Series Analyses and PCA. Environ. Monit. Assess. 2015, 187, 4192. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, K.-K. Use of Hydrologic Time Series Data for Identification of Recharge Mechanism in a Fractured Bedrock Aquifer System. J. Hydrol. 2000, 229, 190–201. [Google Scholar] [CrossRef]
- Box, G.E.P. Time Series Analysis: Forecasting and Control, 3rd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1994; ISBN 978-0-13-060774-4. [Google Scholar]
- WHO. WHO Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2017; Volume 1, ISBN 978-92-4-154995-0.
- Jain, C.K.; Sharma, S.K.; Singh, S. Physico-Chemical Characteristics and Hydrogeological Mechanisms in Groundwater with Special Reference to Arsenic Contamination in Barpeta District, Assam (India). Environ. Monit. Assess. 2018, 190, 417. [Google Scholar] [CrossRef] [PubMed]
- A Graphic Procedure in the Geochemical Interpretation of Water-Analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [CrossRef]
- Thilagavathi, R.; Chidambaram, S.; Prasanna, M.V.; Thivya, C.; Singaraja, C. A Study on Groundwater Geochemistry and Water Quality in Layered Aquifers System of Pondicherry Region, Southeast India. Appl. Water Sci. 2012, 2, 253–269. [Google Scholar] [CrossRef]
- Chadha, D.K. A Proposed New Diagram for Geochemical Classification of Natural Waters and Interpretation of Chemical Data. Hydrogeol. J. 1999, 7, 431–439. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Fisher, R.S.; William, F.; Mullican, I.I.I. Hydrochemical Evolution of Sodium-Sulfate and Sodium-Chloride Groundwater Beneath the Northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol. J. 1997, 2, 4–16. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the Hydrogeochemical Processes and Assessment of Groundwater Quality Using Classic Integrated Geochemical Methods in the Southeastern Part of Ordos Basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef]
- Dalton, M.G.; Upchurch, S.B. Interpretation of Hydrochemical Facies by Factor Analysis. Groundwater 1978, 16, 228–233. [Google Scholar] [CrossRef]
- Güler, C.; Thyne, G.D.; McCray, J.E.; Turner, K.A. Evaluation of Graphical and Multivariate Statistical Methods for Classification of Water Chemistry Data. Hydrogeol. J. 2002, 10, 455–474. [Google Scholar] [CrossRef]
- Amin, M.; Yousuf, M.; Ahmad, N.; Attaullah, M.; Ahmad, S.; Zekker, I.; Latif, M.; Buneri, I.D.; Khan, A.A.; Ali, H.; et al. Application of Alkaline Phosphatase to Assess the Health of Oreochromis Niloticus Exposed to Organophosphates and Synthetic Pyrethroid Pesticides In Vivo. J. Hazard. Toxic Radioact. Waste 2022, 26, 04022029. [Google Scholar] [CrossRef]
- Grochowska, J. Assessment of Water Buffer Capacity of Two Morphometrically Different, Degraded, Urban Lakes. Water 2020, 12, 1512. [Google Scholar] [CrossRef]
- Cocomazzi, G.; Grieco, G.; Sinojmeri, A.; Cavallo, A.; Bussolesi, M.; Ferrari, E.S.; Destefanis, E. Buffering Copper Tailings Acid Mine Drainage: Modeling and Testing at Fushë Arrëz Flotation Plant, Albania. Water 2022, 14, 2398. [Google Scholar] [CrossRef]
- Ghodbane, M.; Benaabidate, L.; Boudoukha, A.; Gaagai, A.; Adjissi, O.; Chaib, W.; Aouissi, H.A. Analysis of groundwater quality in the lower Soummam Valley, North-East of Algeria. J. Water Land Dev. 2022, 54, 1–12. [Google Scholar] [CrossRef]
- Davis, S.N.; De Weist, R.J.M. Hydrogeology; John Wiley and Sons: Hoboken, NJ, USA, 1966; 463p. [Google Scholar]
- Tang, H.; Zhong, H.; Pan, Y.; Zhou, Q.; Huo, Z.; Chu, W.; Xu, B. A New Group of Heterocyclic Nitrogenous Disinfection Byproducts (DBPs) in Drinking Water: Role of Extraction PH in Unknown DBP Exploration. Environ. Sci. Technol. 2021, 55, 6764–6772. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Aouissi, H.A.; Bencedira, S.; Burlakovs, J.; Zekker, I.; Bute, I.; Klavins, M. Influence of Environmental Parameters and Fiber Orientation on Dissolution Kinetics of Glass Fibers in Polymer Composites. J. Compos. Sci. 2022, 6, 210. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G.; Paktunc, D.; Gould, W.D.; Johnson, D.B. 11.5—The geochemistry of acid mine drainage. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 131–190. ISBN 978-0-08-098300-4. [Google Scholar]
- Sujatha, D.; Reddy, B.R. Quality Characterization of Groundwater in the South-Eastern Part of the Ranga Reddy District, Andhra Pradesh, India. Environ. Geol. 2003, 44, 579–586. [Google Scholar] [CrossRef]
- Selvam, S.; Venkatramanan, S.; Chung, S.Y.; Singaraja, C. Identification of Groundwater Contamination Sources in Dindugal District of Tamil Nadu, India Using GIS and Multivariate Statistical Analyses. Arab. J. Geosci. 2016, 5, 407. [Google Scholar] [CrossRef]
- Mokadem, N.; Dennis, R.; Dennis, I. Hydrochemical and Stable Isotope Data of Water in Karst Aquifers during Normal Flow in South Africa. Environ. Earth Sci 2021, 80, 519. [Google Scholar] [CrossRef]
- Rishi, M.S.; Kaur, L.; Sharma, S. Groundwater Quality Appraisal for Non-Carcinogenic Human Health Risks and Irrigation Purposes in a Part of Yamuna Sub-Basin, India. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 2716–2736. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Knibbe, W.-J.; Feng, C.; Liu, W.; Medema, G.; van der Meer, W. Potential Impacts of Changing Supply-Water Quality on Drinking Water Distribution: A Review. Water Res. 2017, 116, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Svensson, T.; Kylin, H.; Montelius, M.; Sandén, P.; Bastviken, D. Chlorine Cycling and the Fate of Cl in Terrestrial Environments. Environ. Sci. Pollut. Res. 2021, 28, 7691–7709. [Google Scholar] [CrossRef] [PubMed]
- Mokadem, N.; Demdoum, A.; Hamed, Y.; Bouri, S.; Hadji, R.; Boyce, A.; Laouar, R.; Sâad, A. Hydrogeochemical and Stable Isotope Data of Groundwater of a Multi-Aquifer System: Northern Gafsa Basin—Central Tunisia. J. Afr. Earth Sci. 2016, 114, 174–191. [Google Scholar] [CrossRef]
- Guerzou, M.; Aouissi, H.A.; Guerzou, A.; Burlakovs, J.; Doumandji, S.; Krauklis, A.E. From the Beehives: Identification and Comparison of Physicochemical Properties of Algerian Honey. Resources 2021, 10, 94. [Google Scholar] [CrossRef]
- Das, N.; Sarma, K.P.; Patel, A.K.; Deka, J.P.; Das, A.; Kumar, A.; Shea, P.J.; Kumar, M. Seasonal Disparity in the Co-Occurrence of Arsenic and Fluoride in the Aquifers of the Brahmaputra Flood Plains, Northeast India. Environ. Earth Sci. 2017, 76, 183. [Google Scholar] [CrossRef]
- Pant, D.; Keesari, T.; Rishi, M.S.; Sharma, D.A.; Jaryal, A.; Kamble, S.N.; Sinha, U.K. Hydrochemical Evolution of Groundwater in the Waterlogged Area of Southwest Punjab. Arab. J. Geosci. 2020, 13, 773. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: Hoboken, NJ, USA, 1996; ISBN 978-0-471-51184-7. [Google Scholar]
- Li, P.; Wu, J.; Qian, H. Preliminary Assessment of Hydraulic Connectivity between River Water and Shallow Groundwater and Estimation of Their Transfer Rate during Dry Season in the Shidi River, China. Environ. Earth Sci. 2016, 75, 99. [Google Scholar] [CrossRef]
- Chebotarev, I.I. Metamorphism of Natural Waters in the Crust of Weathering—1. Geochim. Cosmochim. Acta 1955, 8, 22–48. [Google Scholar] [CrossRef]
- Appelo, C.A.J. Geochemistry, Groundwater and Pollution, 2nd ed.; Appelo, C.A.J., Appelo, C.A.J., Postma, D., Postma, D., Eds.; CRC Press: London, UK, 2005; ISBN 978-0-429-15232-0. [Google Scholar]
- Kumari, R.; Datta, P.S.; Rao, M.S.; Mukherjee, S.; Azad, C. Anthropogenic Perturbations Induced Groundwater Vulnerability to Pollution in the Industrial Faridabad District, Haryana, India. Environ. Earth Sci. 2018, 77, 187. [Google Scholar] [CrossRef]
- Hardie, L.W.A.; Eugster, H.P. The evolution of closed-basin brines. Min. Soc. Spec. Pap. 1970, 3, 273–290. [Google Scholar]
- Xiao, J.; Jin, Z.D.; Wang, J.; Zhang, F. Hydrochemical Characteristics, Controlling Factors and Solute Sources of Groundwater within the Tarim River Basin in the Extreme Arid Region, NW Tibetan Plateau. Quat. Int. 2015, 380–381, 237–246. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, X.; Rioual, P.; Qin, X.; Liu, Z.; Xiong, H.; Yu, J. Hydrogeochemistry of Three Watersheds (the Erlqis, Zhungarer and Yili) in Northern Xinjiang, NW China. Appl. Geochem. 2011, 26, 1535–1548. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global Silicate Weathering and CO2 Consumption Rates Deduced from the Chemistry of Large Rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Aouissi, H.A.; Hamimes, A.; Ababsa, M.; Bianco, L.; Napoli, C.; Kebaili, F.K.; Krauklis, A.E.; Bouzekri, H.; Dhama, K. Bayesian Modeling of COVID-19 to Classify the Infection and Death Rates in a Specific Duration: The Case of Algerian Provinces. Int. J. Environ. Res. Public Health 2022, 19, 9586. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Ramanathan, A.L. Geochemical Assessment of Groundwater Quality in Vicinity of Bhalswa Landfill, Delhi, India, Using Graphical and Multivariate Statistical Methods. Environ. Geol. 2008, 53, 1509–1528. [Google Scholar] [CrossRef]
- Kraiem, Z.; Zouari, K.; Chkir, N.; Agoune, A. Geochemical Characteristics of Arid Shallow Aquifers in Chott Djerid, South-Western Tunisia. J. Hydro-Environ. Res. 2014, 8, 460–473. [Google Scholar] [CrossRef]
- Byrne, M.P.; Tobin, J.T.; Forrestal, P.J.; Danaher, M.; Nkwonta, C.G.; Richards, K.; Cummins, E.; Hogan, S.A.; O’Callaghan, T.F. Urease and Nitrification Inhibitors—As Mitigation Tools for Greenhouse Gas Emissions in Sustainable Dairy Systems: A Review. Sustainability 2020, 12, 6018. [Google Scholar] [CrossRef]
- Halecki, W.; Stachura, T.; Fudała, W.; Rusnak, M. Evaluating the Applicability of MESS (Matrix Exponential Spatial Specification) Model to Assess Water Quality Using GIS Technique in Agricultural Mountain Catchment (Western Carpathian). Environ. Monit. Assess. 2018, 191, 26. [Google Scholar] [CrossRef]
- Aflatooni, M. Time Series Analysis of Ground Water Table Fluctuations Due to Temperature and Rainfall Change in Shiraz Plain. Int. J. Water Resour. Environ. Eng. 2011, 3, 176–188. [Google Scholar]
- Alam, S.; Khan, M.S.; Bibi, W.; Zekker, I.; Burlakovs, J.; Ghangrekar, M.M.; Bhowmick, G.D.; Kallistova, A.; Pimenov, N.; Zahoor, M. Preparation of Activated Carbon from the Wood of Paulownia Tomentosa as an Efficient Adsorbent for the Removal of Acid Red 4 and Methylene Blue Present in Wastewater. Water 2021, 13, 1453. [Google Scholar] [CrossRef]
- Alam, S.; Ullah, B.; Khan, M.S.; Rahman, N.U.; Khan, L.; Shah, L.A.; Zekker, I.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; et al. Adsorption Kinetics and Isotherm Study of Basic Red 5 on Synthesized Silica Monolith Particles. Water 2021, 13, 2803. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Taussi, M.; Gozzi, C.; Vaselli, O.; Cabassi, J.; Menichini, M.; Doveri, M.; Romei, M.; Ferretti, A.; Gambioli, A.; Nisi, B. Contamination Assessment and Temporal Evolution of Nitrates in the Shallow Aquifer of the Metauro River Plain (Adriatic Sea, Italy) after Remediation Actions. Int. J. Environ. Res. Public Health 2022, 19, 12231. [Google Scholar] [CrossRef] [PubMed]
- Gaagai, A. Etude de L’évolution de la Qualité des Eaux du Barrage de Babar (Sud-Est Algérien) et L’impact de la Rupture de la Digue sur L’environnement. Ph.D. Thesis, University of Batna 2, Batna, Algeria, 2017. [Google Scholar] [CrossRef]

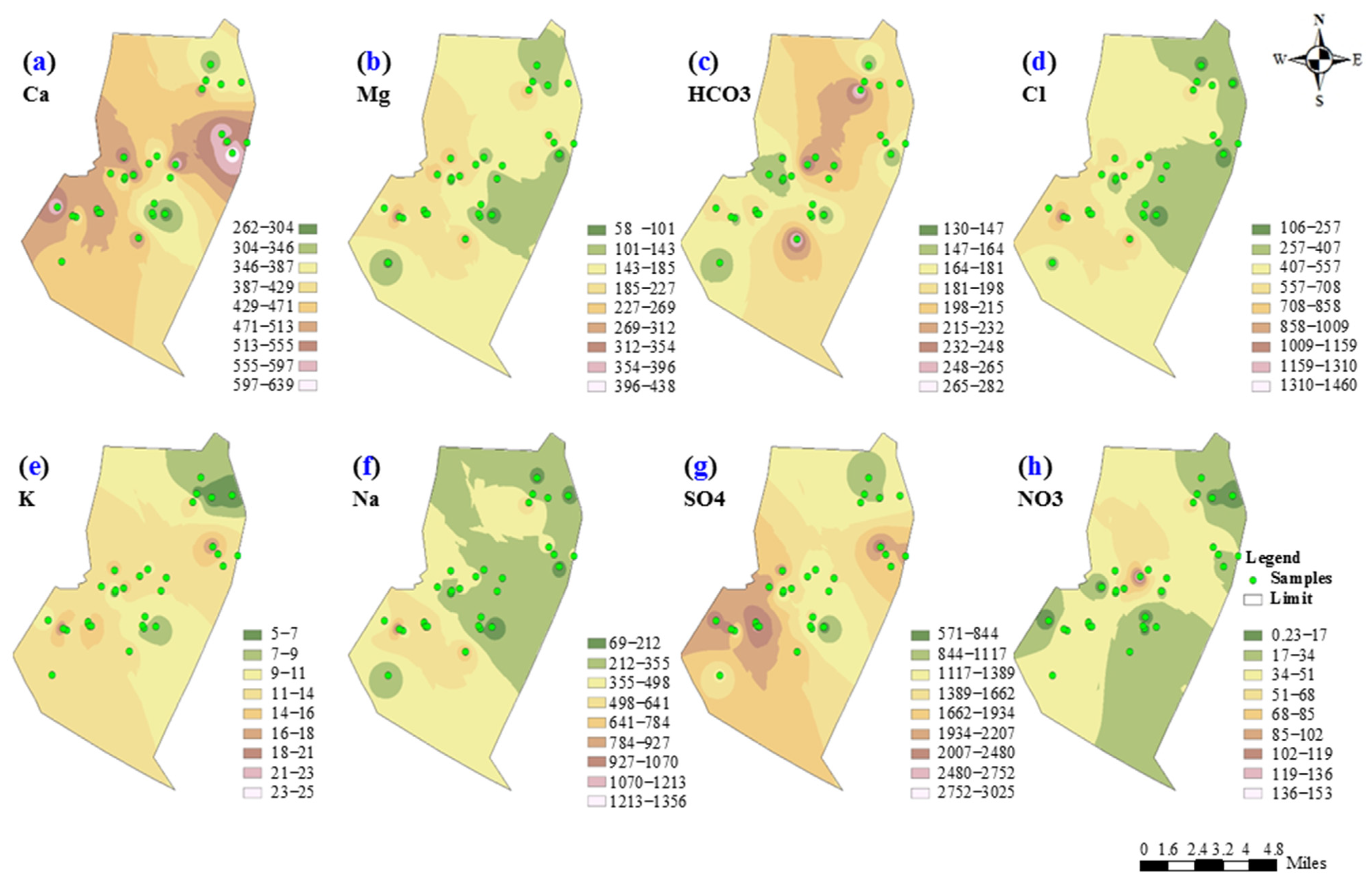


| Parameters | Min | Mean | Max | St Dev | Coef. of Variation (CV, %) | WHO (2017) Second Addendum 2021 |
|---|---|---|---|---|---|---|
| Ca2+ | 252 | 452.71 | 640 | 117.89 | 0.26 | 150 |
| Mg2+ | 52.8 | 172.61 | 439.2 | 84.66 | 0.49 | 70 |
| Na+ | 47.73 | 373.16 | 1358.54 | 260.41 | 0.7 | 200 |
| K+ | 5.01 | 12.02 | 25.63 | 4.66 | 0.39 | 12 |
| HCO3− | 129.32 | 187.6 | 283.04 | 43.32 | 0.23 | 500 |
| SO42− | 571.43 | 1636.22 | 3028.57 | 647.01 | 0.4 | 250 |
| Cl− | 106.5 | 462.07 | 1462.6 | 277.65 | 0.6 | 250 |
| NO3− | 0.2 | 37.13 | 153.44 | 30.31 | 0.82 | 50 |
| pH | 7.03 | 7.64 | 8.16 | 0.3 | 0.04 | 6.5–8.5 |
| EC | 2000 | 4370.97 | 10000 | 1617.88 | 0.37 | 1500 |
| TDS | 1232.00 | 3333.45 | 7145.00 | 1269.12 | 0.38 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athamena, A.; Gaagai, A.; Aouissi, H.A.; Burlakovs, J.; Bencedira, S.; Zekker, I.; Krauklis, A.E. Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis. Sustainability 2023, 15, 20. https://doi.org/10.3390/su15010020
Athamena A, Gaagai A, Aouissi HA, Burlakovs J, Bencedira S, Zekker I, Krauklis AE. Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis. Sustainability. 2023; 15(1):20. https://doi.org/10.3390/su15010020
Chicago/Turabian StyleAthamena, Ali, Aissam Gaagai, Hani Amir Aouissi, Juris Burlakovs, Selma Bencedira, Ivar Zekker, and Andrey E. Krauklis. 2023. "Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis" Sustainability 15, no. 1: 20. https://doi.org/10.3390/su15010020
APA StyleAthamena, A., Gaagai, A., Aouissi, H. A., Burlakovs, J., Bencedira, S., Zekker, I., & Krauklis, A. E. (2023). Chemometrics of the Environment: Hydrochemical Characterization of Groundwater in Lioua Plain (North Africa) Using Time Series and Multivariate Statistical Analysis. Sustainability, 15(1), 20. https://doi.org/10.3390/su15010020











