Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of B5 Culture Medium
2.3. Establishment and Culture of Taxus cuspidata Callus
2.3.1. Collection and Pretreatment of Explants
2.3.2. Disinfection of Explants
2.3.3. Inoculation of Explants
2.3.4. Subculture of Callus
2.4. Cell Suspension Culture and Subculture of Taxus cuspidata
2.5. Ultrasonic Extraction Experiment
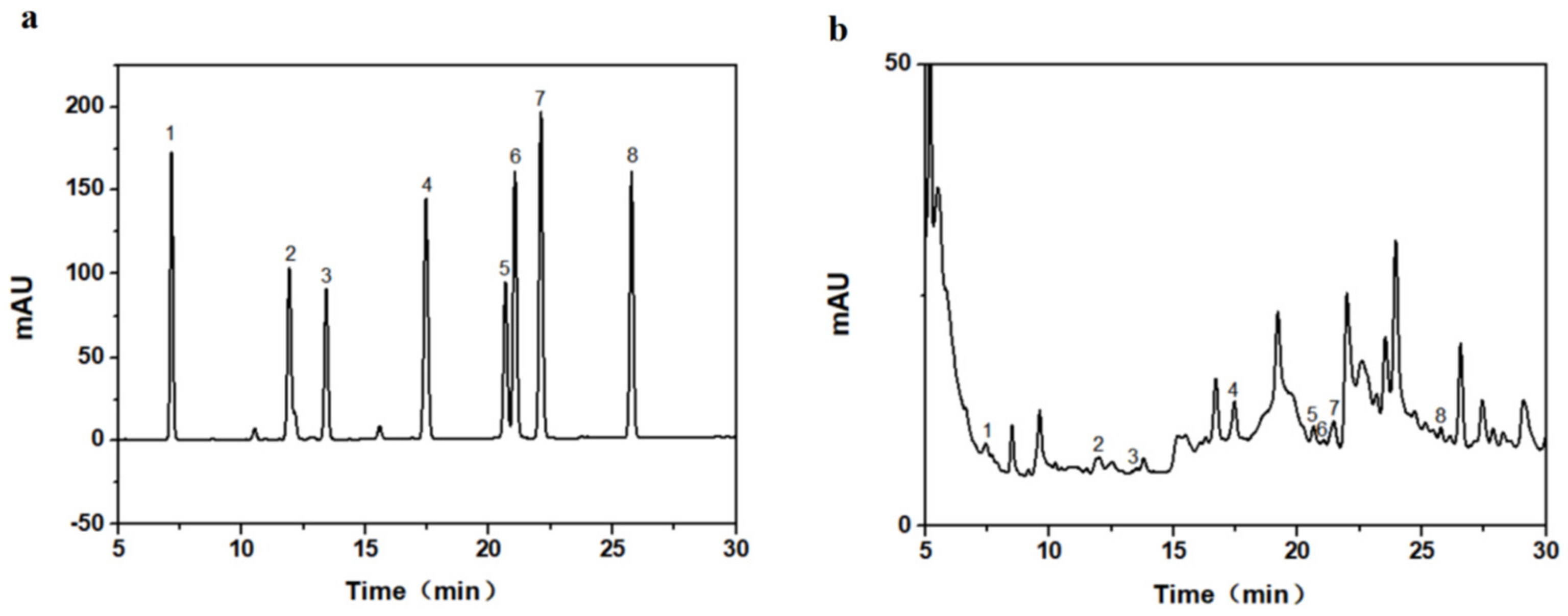
2.6. HPLC Detection of Taxane Yields
2.7. Single-Factor Experiment
2.8. RSM Experiment
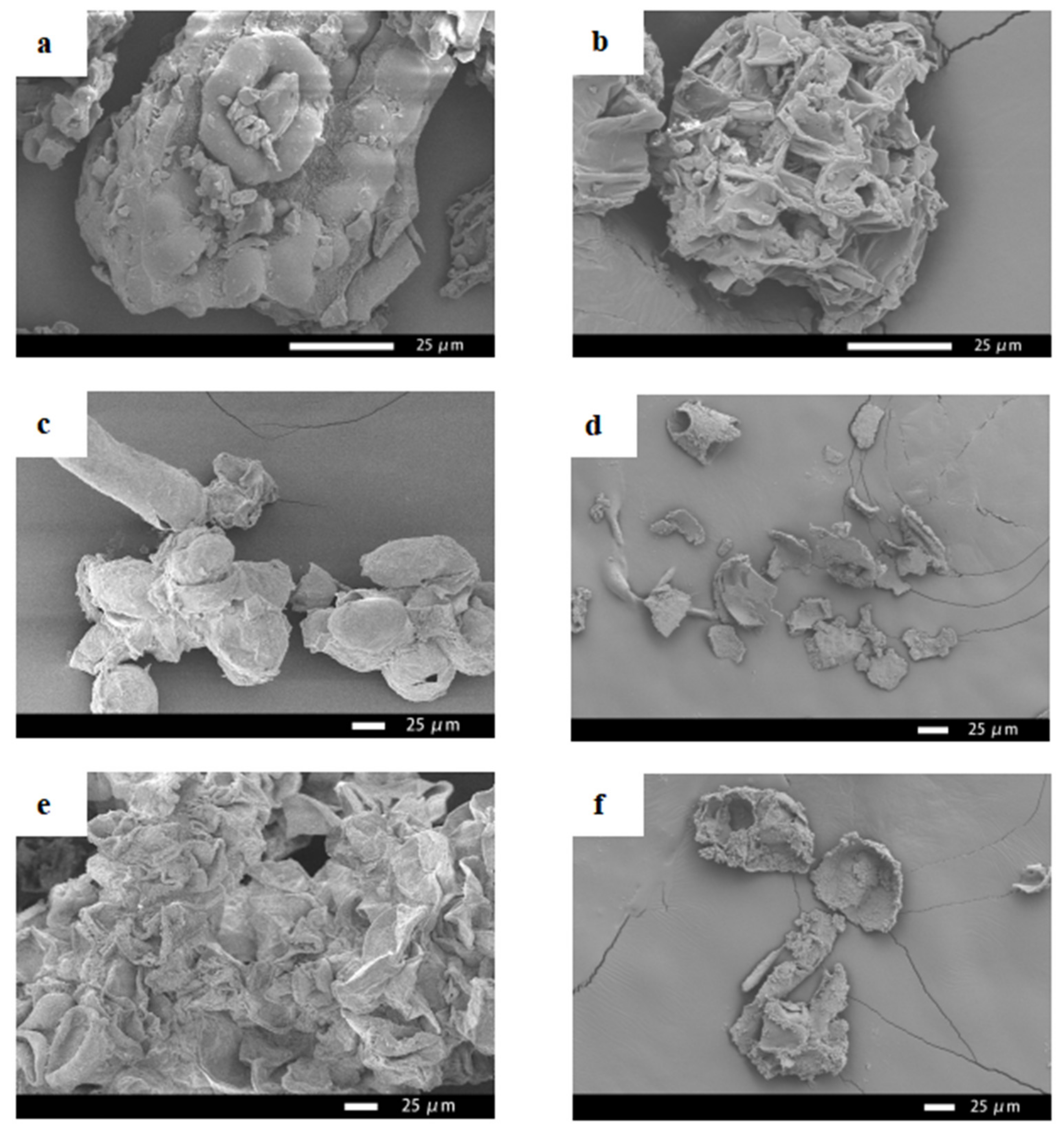
2.9. SEM
3. Results
3.1. Drawing of Standard Curve
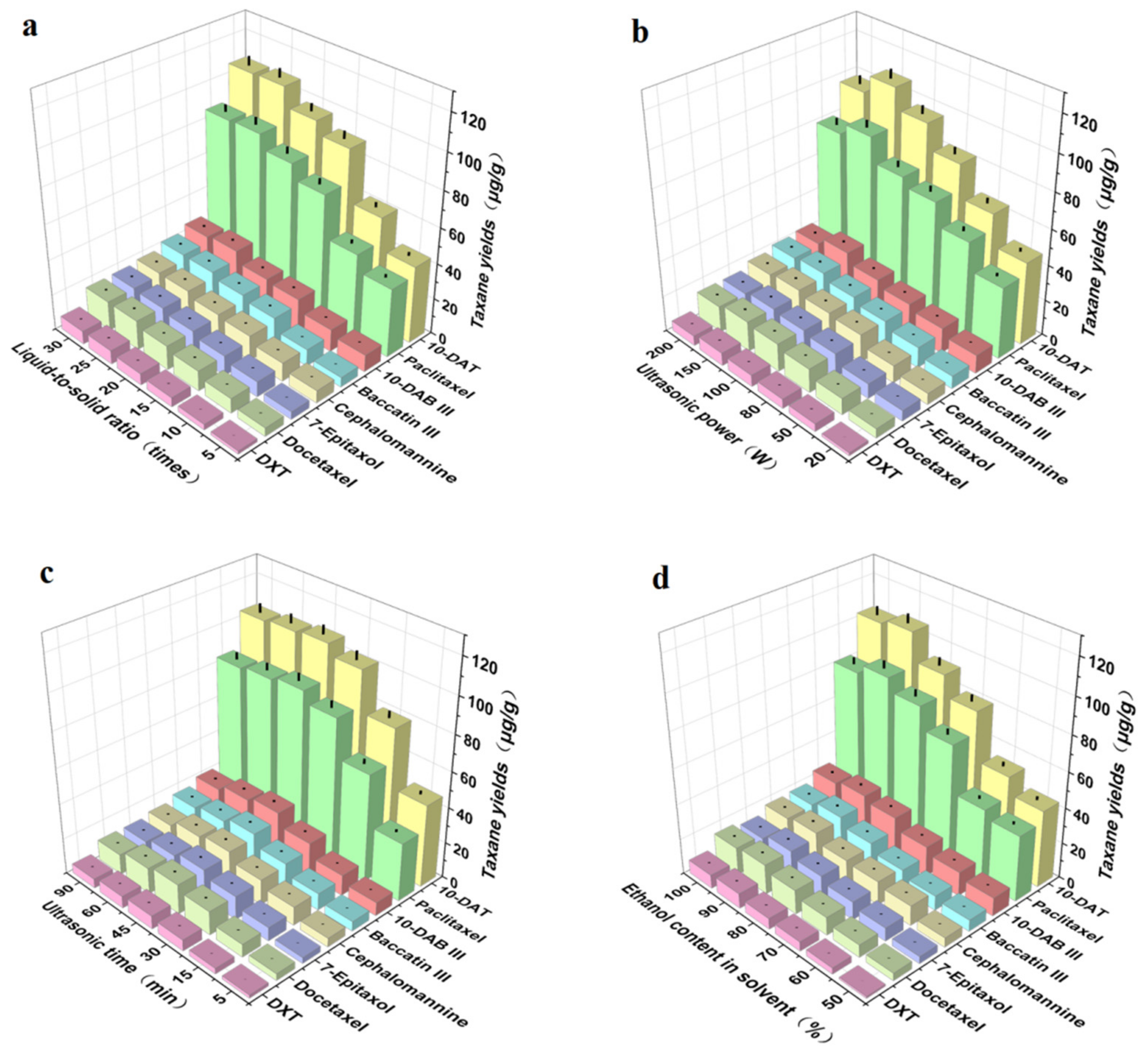
3.2. Single-Factor Experiments
3.2.1. Liquid-to-Solid Ratio
3.2.2. Ultrasonic Power
3.2.3. Ultrasonic Time
3.2.4. Ethanol Content in Solvent
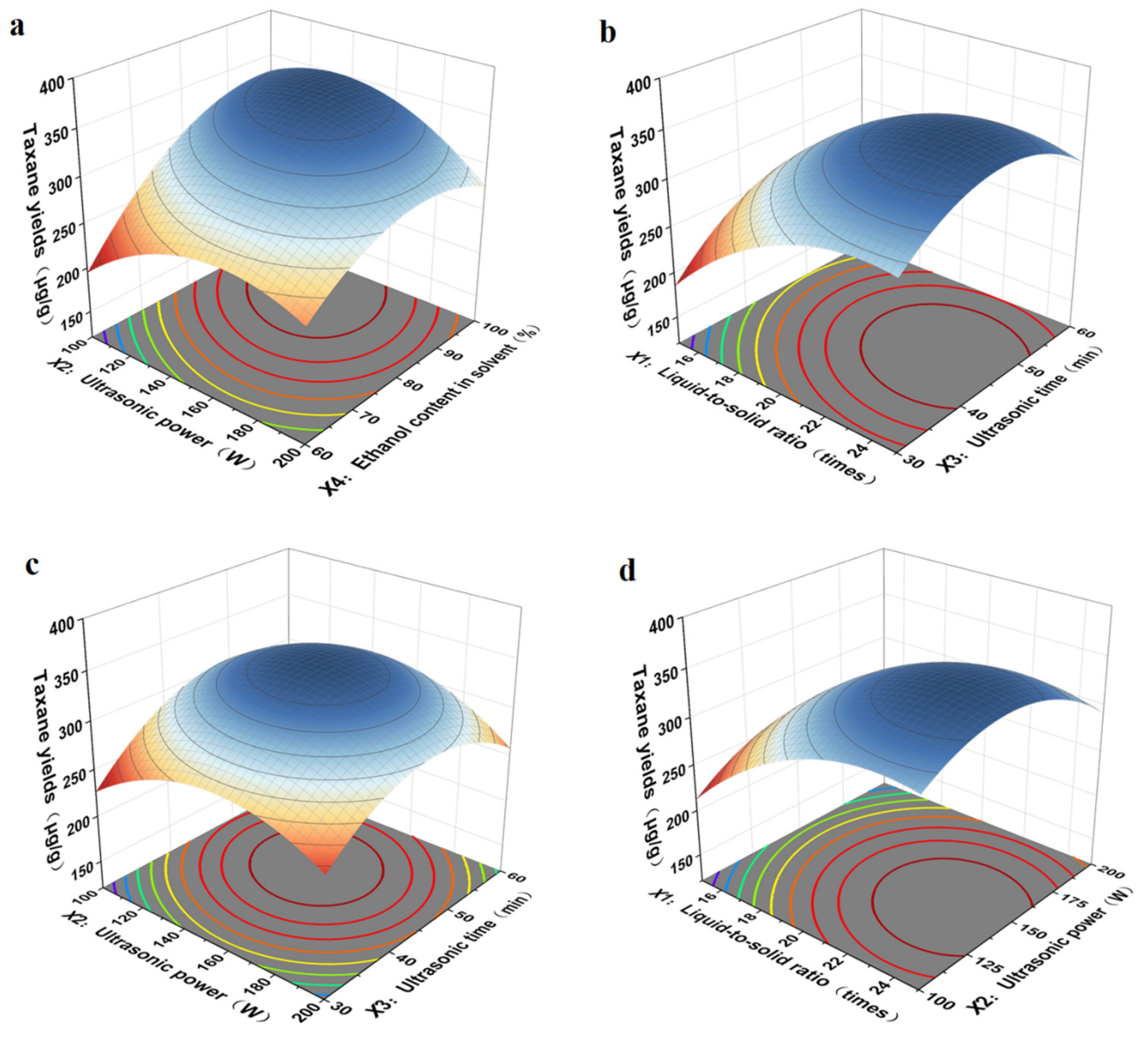
3.3. Construction of RSM Model and Conditions Optimization
− 11.46X2X3 − 26.03X2X4 − 3.18X3X4 − 37.68X12 − 41.94X22 − 45.55X32 − 37.61X42
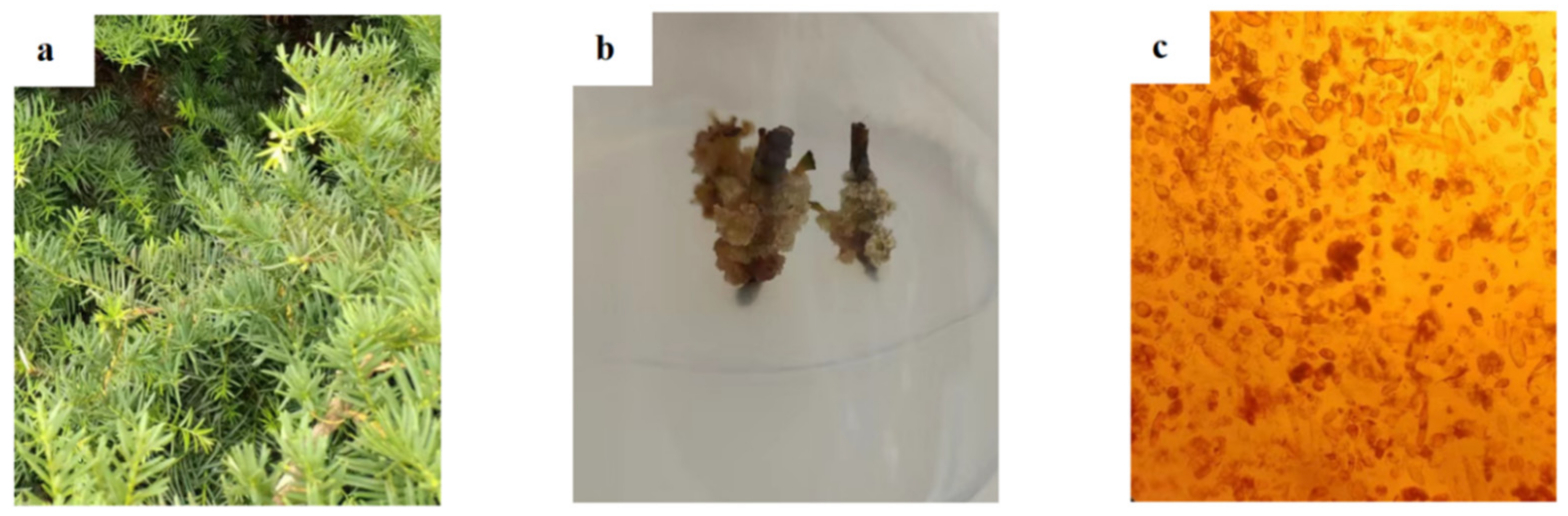
3.4. SEM Analysis of Needles, Callus and Suspension Cells of Taxus cuspidata
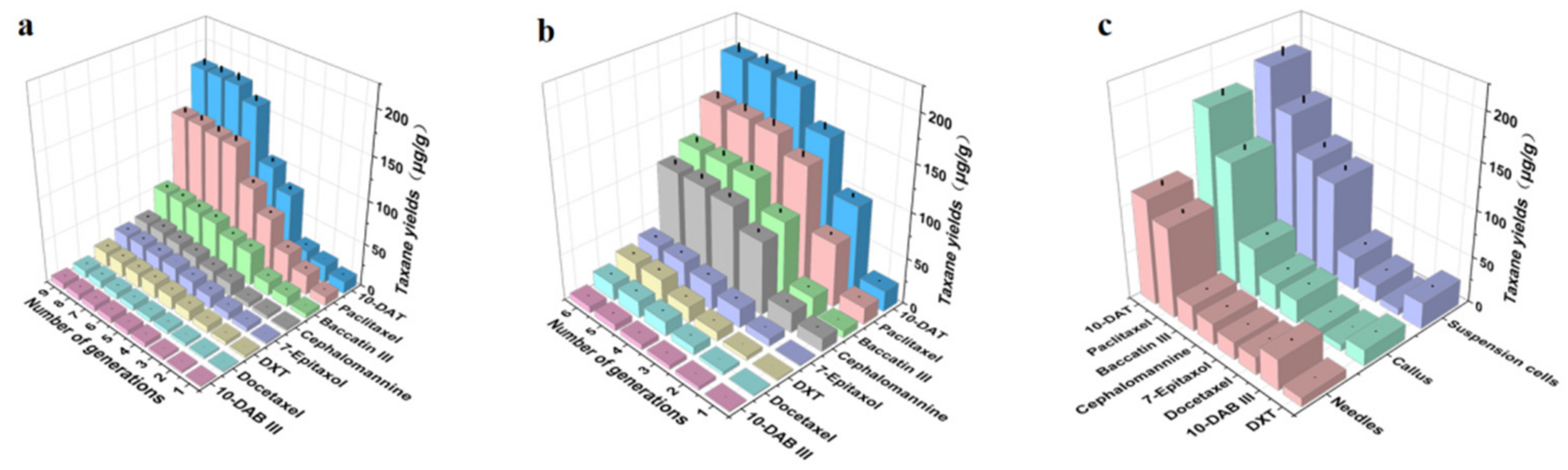
3.5. Determination of Taxane Yields in Needles, Callus and Suspension Cells of Taxus cuspidata
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, T.; Wu, X.; Wang, Y.; Chen, J.; Zang, R. The population status and threats of Taxus cuspidata, a plant species with extremely small populations in China. Glob. Ecol. Conserv. 2021, 26, e01495. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Globa, E.B.; Demidova, E.V.; Gaisinsky, V.V.; Galishev, B.A.; Kolotyrkina, N.G. Occurrence of 14-hydroxylated taxoids in the plant in vitro cell cultures of different yew species (Taxus spp.). Dokl. Biochem. Biophys. 2017, 476, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Li, G.; Zheng, Z.; Su, W. Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. Fems. Immunol. Med. Microbiol. 2001, 31, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Yu, X.M.; Guo, W.L.; Li, Y.D.; Liu, X.D.; Wang, N.N.; Liu, B. Genomic diversity within Taxus cuspidata var. nana revealed by random amplified polymorphic DNA markers. Russ. J. Plant Physiol. 2006, 53, 684–688. [Google Scholar] [CrossRef]
- Lenka, S.K.; Nims, N.E.; Vongpaseuth, K.; Boshar, R.A.; Roberts, S.C.; Walker, E.L. Jasmonate-responsive expression of paclitaxel biosynthesis genes in Taxus cuspidata cultured cells is negatively regulated by the bHLH transcription factors TcJAMYC1, TcJAMYC2, and TcJAMYC4. Front. Plant Sci. 2015, 6, 115–128. [Google Scholar] [CrossRef]
- Kishiko, O.; Sanro, T.; Masaya, S. Electron Microscopic Observation of Plastid Containing Taxol-like Substances in Callus Cells of Taxus cuspidata var. Nana. Pak. J. Biol. Sci. 2004, 7, 1028–1034. [Google Scholar] [CrossRef][Green Version]
- Tong, L.; Zhi, Q.Z. Characteristics of natural Japanese yew population in Muling Nature Reserve of Heilongjiang Province, China. J. For. Res. 2006, 17, 132–134. [Google Scholar] [CrossRef]
- Shi, Q.W.; Oritani, T.; Sugiyama, T. Three novel bicyclic taxane diterpenoids with verticillene skeleton from the needles of Chinese yew, Taxus chinensis var. mairei. Planta Med. 1999, 62, 1114–1118. [Google Scholar] [CrossRef]
- Yao, X.; Da, B.U.; Chen, J.W.; Xiang, L.I.; Tang, B. Tissue culture and analysis on taxane diterpenoids in callus of Taxus chinensis var. mairei. Chin. Tradit. Herb. Drugs 2014, 45, 2696–2702. [Google Scholar] [CrossRef]
- Fan, X.H.; Wang, L.T.; Chang, Y.H.; An, J.Y.; Fu, Y.J. Application of green and recyclable menthol-based hydrophobic deep eutectic solvents aqueous for the extraction of main taxanes from Taxus chinensis needles. J. Mol. Liq. 2020, 326, 114–122. [Google Scholar] [CrossRef]
- Fang, W.; Wu, Y.; Zhou, J.; Chen, W.; Fang, Q. Qualitative and quantitative determination of taxol and related compounds in Taxus cuspidata Sieb et Zucc. Phytochem. Anal. 2010, 4, 115–119. [Google Scholar] [CrossRef]
- Muth, M.; Ojara, F.W.; Kloft, C.; Joerger, M. Role of TDM-based dose adjustments for taxane anticancer drugs. Br. J. Clin. Pharmacol. 2021, 87, 306–316. [Google Scholar] [CrossRef]
- Falah, M.; Rayan, M.; Rayan, A. A Novel Paclitaxel Conjugate with Higher Efficiency and Lower Toxicity: A New Drug Candidate for Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 4965. [Google Scholar] [CrossRef]
- Szenajch, J.; Szabelska, B.A.; Wiercz, A.; Zyprych, W.J.; Handschuh, L. Transcriptome Remodeling in Gradual Development of Inverse Resistance between Paclitaxel and Cisplatin in Ovarian Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9218. [Google Scholar] [CrossRef]
- Yang, X.Y.; Li, Y.X.; Li, M.; Zhang, L.; Feng, L.X.; Zhang, N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef]
- Levit, S.L.; Gade, N.R.; Roper, T.D.; Yang, H.; Tang, C. Self-Assembly of pH-Labile Polymer Nanoparticles for Paclitaxel Prodrug Delivery: Formulation, Characterization, and Evaluation. Int. J. Mol. Sci. 2020, 21, 9292. [Google Scholar] [CrossRef]
- Thalkari, A.B.; Karwa, P.N.; Zambare, K.K.; Tour, N.S.; Chopane, P.S. Paclitaxel Against Cancer: A new trademarked drug. Res. J. Pharmacogn. Phytochem. 2019, 11, 123. [Google Scholar] [CrossRef]
- Azerbaijan, M.H.; Bahmani, E.; Jouybari, M.H.; Hassaniazardaryani, A.; Irani, M. Electrospun gold nanorods/graphene oxide loaded-core-shell nanofibers for local delivery of paclitaxel against lung cancer during photo-chemotherapy method. Eur. J. Pharm. Sci. 2021, 164, 105914. [Google Scholar] [CrossRef]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Quan, V.V. Isolation and Maximisation of Extraction of Mangiferin from the Root of Salacia chinensis L. Separations 2019, 6, 44. [Google Scholar] [CrossRef]
- Ali, S.; Albekairi, N.; Wang, X.M.; Patrikeeva, S.; Nanovskaya, T.N.; Ahmed, M.S.; Rytting, E. Determination of the Transplacental Transfer of Paclitaxel and Antipyrine by High Performance Liquid Chromatography Coupled with Photodiode Array Detector. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 232–238. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Ming, Z.; Jiang, L.; Zhou, Y. Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography–Mass Spectrometry. Separations. 2021, 9, 2297–2310. [Google Scholar] [CrossRef]
- Zheng, N.; Lian, B.; Du, W.; Xu, G.; Ji, J. Extraction protocol and liquid chromatography/tandem mass spectrometry method for determining micelle-entrapped paclitaxel at the cellular and subcellular levels: Application to a cellular uptake and distribution study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 10, 347–354. [Google Scholar] [CrossRef]
- Tong, X.; Zhou, J.; Tan, Y. Determination of Paclitaxel in Rat Plasma by LC--MS--MS. J. Chromatogr. Sci. 2006, 44, 266–271. [Google Scholar] [CrossRef][Green Version]
- Wang, C.C.; Sheu, S.R.; Yau, H.T.; Jang, M.J. Effect of coffee reduction on constituent concentration in an energy-efficient process of ultrasonic extraction. Therm. Sci. 2015, 19, 1373–1377. [Google Scholar] [CrossRef]
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Hydroglycerolic Solvent and Ultrasonication Pretreatment: A Green Blend for High-Efficiency Extraction of Salvia fruticosa Polyphenols. Sustainability 2020, 12, 4048. [Google Scholar] [CrossRef]
- Zhang, R.; Grimi, N.; Marchal, L.; Lebovka, N.; Vorobiev, E. Effect of ultrasonication, high pressure homogenization and their combination on efficiency of extraction of bio-molecules from microalgae Parachlorella kessleri. Algal Res. 2019, 40, 101–114. [Google Scholar] [CrossRef]
- Xiao, H.F.; Chen, D.; Xu, J.; Guo, S.F. Defects identification using the improved ultrasonic measurement model and support vector machines. NDT. E Int. 2020, 111, 102223. [Google Scholar] [CrossRef]
- Feliu, J.X.; Cubarsi, R.; Villaverde, A. Optimized release of recombinant proteins by ultrasonication of E. coli cells. Biotechnol. Bioeng. 1998, 58, 536–540. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Shi, J.T. Preparation of Sub-Micron Bi Alloy Powers with the Ultrasonic Mixed Crushing. Mater. Sci. Forum. 2021, 10, 217–226. [Google Scholar] [CrossRef]
- Boni, M.R.; Amato, E.D.; Polettini, A.; Pomi, R.; Rossi, A. Effect of ultrasonication on anaerobic degradability of solid waste digestate. Waste Manag. 2016, 48, 209–217. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, W. The Establishment of Extraction Method of Taxol from Taxus brevifolia. Lett. Biotechnol. 2007, 53, 482–483. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Bae, H. Use of Ultrasonication Technology for the Increased Production of Plant Secondary Metabolites. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Escrich, A.; Almagro, L.; Moyano, E.; Cusido, R.M.; Palazon, J. Improved biotechnological production of paclitaxel in Taxus media cell cultures by the combined action of coronatine and calix [8]arenes. Plant Physiol. Biochem. 2021, 163, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, D.; Yi, R.; Patel, B.; Zhang, J.; Kong, N. A sensitive HPLC-MS/MS method for the detection, resolution and quantitation of cathinone enantiomers in horse blood plasma and urine. Anal. Bioanal. Chem. 2021, 413, 1618–1632. [Google Scholar] [CrossRef]
- Sun, Q.; Du, B.; Wang, C.; Xu, W.; Zhang, H. Ultrasound-Assisted Ionic Liquid Solid–Liquid Extraction Coupled with Aqueous Two-Phase Extraction of Naphthoquinone Pigments in Arnebia euchroma (Royle) Johnst. Chromatographia 2019, 82, 1777–1789. [Google Scholar] [CrossRef]
- Cai, Z.; Han, M.; Zhang, X.; Gao, X.; Wang, F.; Pang, M. Extraction of Anthraquinone Compounds from Chinese Chestnut by Using Ultrasonic-assisted Technology. Sci. Publ. Group 2019, 7, 43–47. [Google Scholar] [CrossRef]
- Xi, H.; Liu, Y.; Guo, L.; Hu, R. Effect of ultrasonic power on drying process and quality properties of far-infrared radiation drying on potato slices. Food Sci. Biotechnol. 2019, 29, 93–101. [Google Scholar] [CrossRef]
- Usui, H.; Ishibashi, T.; Matsuo, H.; Watanabe, K.; Ando, K. Visualization of Acoustic Waves and Cavitation in Ultrasonic Water Flow. Solid State Phenom. 2021, 314, 186–191. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of Ultrasonic-Assisted Extraction of Flavonoid Compounds and Antioxidants from Alfalfa Using Response Surface Method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Tong, L.; Li, C.; Zhong, X. The selection and stability analysis of stable and high Taxol-producing cell lines from Taxus cuspidata. J. For. Res. 2018, 29, 65–71. [Google Scholar] [CrossRef]
- Cheng, J.S.; Lei, C.; Wu, J.C.; Yuan, Y.J. Expression of arabinogalactan proteins involved in Taxol production by immobilized Taxus cuspidata cells. J. Biotechnol. 2008, 133, 96–102. [Google Scholar] [CrossRef]
| Medium Composition | Dosage (mg/L) | Medium Composition | Dosage (mg/L) |
|---|---|---|---|
| Vitamin B1 | 20 | NaH2PO4·H2O | 150 |
| Vitamin B6 | 3 | (NH4)2SO4 | 134 |
| Inositol | 200 | MgSO4·7H2O | 500 |
| Niacin | 2 | ZnSO4·7H2O | 2 |
| Glycine | 2 | MnSO4·H2O | 10 |
| Hydrolyzed milk protein | 500 | FeSO4·7H2O | 27.8 |
| Agar | 7000 | Na2EDTA | 37.3 |
| Sucrose | 20,000 | H3PO3 | 3 |
| α-Naphthalene Acetic Acid | 2 | KI | 0.75 |
| KNO3 | 3000 | CuSO4·5H2O | 0.025 |
| CaCl2·2H2O | 150 | CoCl2·6H2O | 0.025 |
| Na2MoO4·2H2O | 0.25 |
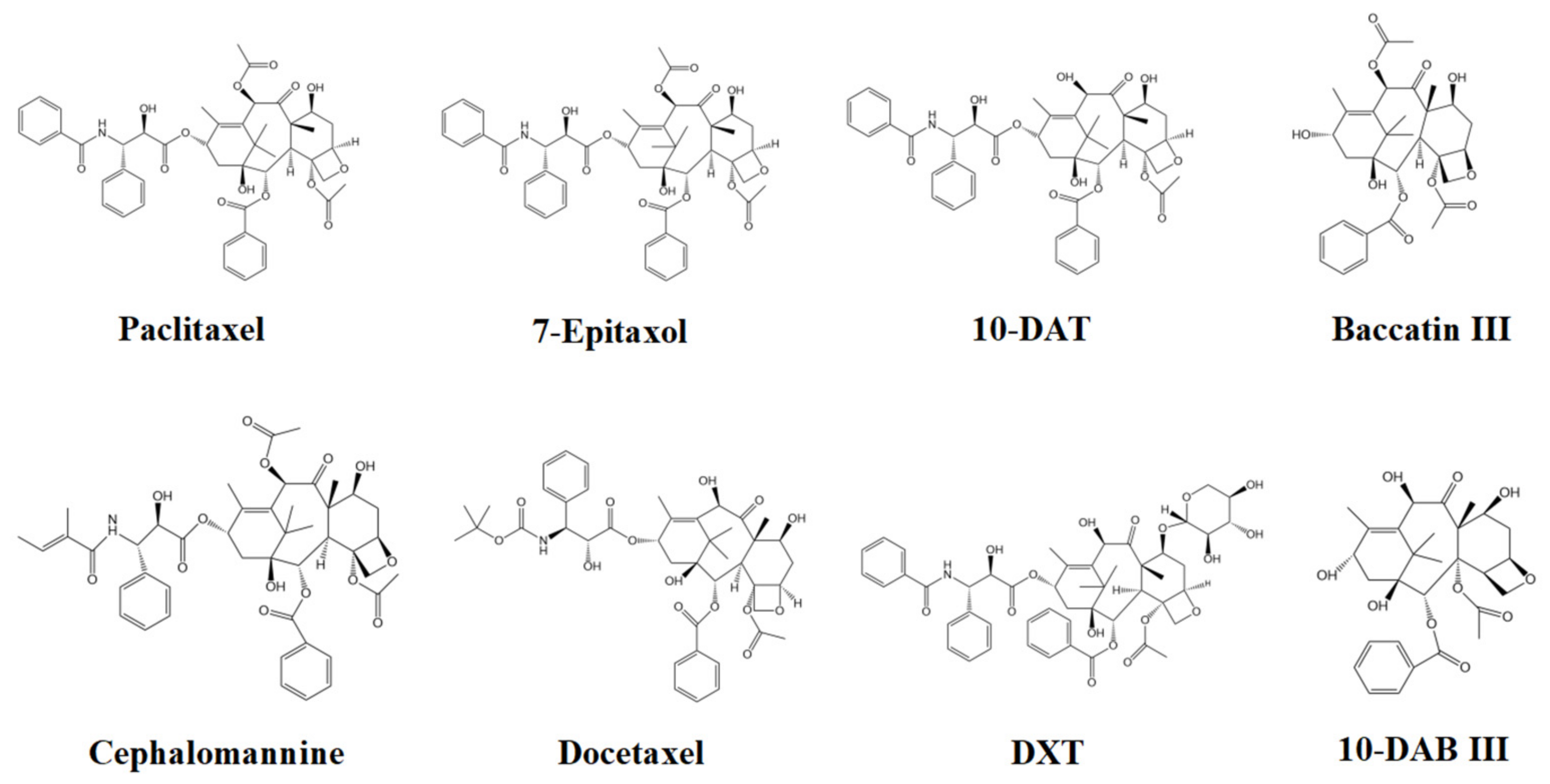
| Order | Compounds | Regression Equation | R2 | Linear Range (μg/mL) | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|---|
| 1 | 10-DAB III | Y = 0.1805X − 0.1085 | 0.9999 | 1.25–125 | C29H36O10 | 544.590 |
| 2 | Baccatin III | Y = 0.1607X − 0.0991 | 0.9998 | 1.25–125 | C31H38O11 | 586.627 |
| 3 | DXT | Y = 0.1218X − 0.0793 | 0.9999 | 1.25–125 | C50H57NO17 | 943.984 |
| 4 | 10-DAT | Y = 0.2195X − 0.1441 | 0.9999 | 1.25–125 | C45H49NO13 | 811.870 |
| 5 | Docetaxel | Y = 0.124X + 0.0486 | 0.9999 | 1.25–125 | C43H53NO14 | 807.879 |
| 6 | Cephalomannine | Y = 0.2259X − 0.1266 | 0.9999 | 1.25–125 | C45H53NO14 | 831.901 |
| 7 | Paclitaxel | Y = 0.27X − 0.1652 | 0.9999 | 1.25–125 | C47H51NO14 | 853.906 |
| 8 | 7-Epitaxol | Y = 0.2141X − 0.1053 | 0.9999 | 1.25–125 | C47H51NO14 | 853.906 |
| Run | X1 | X2 | X3 | X4 | Taxane Yields (μg/g) |
|---|---|---|---|---|---|
| 1 | 20.00 | 150.00 | 45.00 | 80.00 | 343.537 |
| 2 | 20.00 | 100.00 | 45.00 | 60.00 | 193.494 |
| 3 | 20.00 | 200.00 | 60.00 | 80.00 | 249.139 |
| 4 | 25.00 | 150.00 | 45.00 | 100.00 | 349.785 |
| 5 | 20.00 | 150.00 | 30.00 | 100.00 | 284.831 |
| 6 | 20.00 | 200.00 | 45.00 | 100.00 | 269.831 |
| 7 | 25.00 | 150.00 | 60.00 | 80.00 | 303.128 |
| 8 | 20.00 | 150.00 | 60.00 | 60.00 | 239.811 |
| 9 | 15.00 | 150.00 | 45.00 | 60.00 | 178.533 |
| 10 | 20.00 | 150.00 | 45.00 | 80.00 | 339.437 |
| 11 | 20.00 | 150.00 | 60.00 | 100.00 | 314.728 |
| 12 | 20.00 | 150.00 | 45.00 | 80.00 | 346.372 |
| 13 | 15.00 | 200.00 | 45.00 | 80.00 | 235.761 |
| 14 | 20.00 | 100.00 | 45.00 | 100.00 | 327.823 |
| 15 | 20.00 | 200.00 | 30.00 | 80.00 | 241.681 |
| 16 | 15.00 | 150.00 | 45.00 | 100.00 | 273.363 |
| 17 | 20.00 | 100.00 | 60.00 | 80.00 | 289.482 |
| 18 | 15.00 | 150.00 | 30.00 | 80.00 | 178.617 |
| 19 | 20.00 | 150.00 | 45.00 | 80.00 | 340.933 |
| 20 | 20.00 | 150.00 | 30.00 | 60.00 | 197.179 |
| 21 | 25.00 | 200.00 | 45.00 | 80.00 | 299.256 |
| 22 | 20.00 | 150.00 | 45.00 | 80.00 | 331.621 |
| 23 | 15.00 | 100.00 | 45.00 | 80.00 | 207.189 |
| 24 | 15.00 | 150.00 | 60.00 | 80.00 | 244.472 |
| 25 | 20.00 | 200.00 | 45.00 | 60.00 | 239.605 |
| 26 | 25.00 | 100.00 | 45.00 | 80.00 | 308.517 |
| 27 | 25.00 | 150.00 | 30.00 | 80.00 | 289.781 |
| 28 | 20.00 | 100.00 | 30.00 | 80.00 | 236.182 |
| 29 | 25.00 | 150.00 | 45.00 | 60.00 | 263.575 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 77,956.23 | 14 | 5568.30 | 108.61 | <0.0001 | *** |
| X1 | 20,510.18 | 1 | 20,510.18 | 400.06 | <0.0001 | *** |
| X2 | 62.63 | 1 | 62.63 | 1.22 | 0.2877 | |
| X3 | 3762.63 | 1 | 3762.63 | 73.39 | <0.0001 | *** |
| X4 | 21,519.22 | 1 | 21,519.22 | 419.74 | <0.0001 | *** |
| X1X2 | 357.83 | 1 | 357.83 | 6.98 | 0.0193 | * |
| X1X3 | 689.27 | 1 | 689.27 | 13.44 | 0.0025 | ** |
| X1X4 | 18.58 | 1 | 18.58 | 0.36 | 0.5568 | |
| X2X3 | 525.37 | 1 | 525.37 | 10.25 | 0.0064 | ** |
| X2X4 | 2709.36 | 1 | 2709.36 | 52.85 | <0.0001 | *** |
| X3X4 | 40.55 | 1 | 40.55 | 0.79 | 0.3889 | |
| X12 | 9210.87 | 1 | 9210.87 | 179.66 | <0.0001 | *** |
| X22 | 11,406.84 | 1 | 11,406.84 | 222.49 | <0.0001 | *** |
| X32 | 13,458.92 | 1 | 13,458.92 | 262.52 | <0.0001 | *** |
| X42 | 9175.40 | 1 | 9175.40 | 178.97 | <0.0001 | *** |
| Residual | 717.75 | 14 | 51.27 | |||
| Lack of fit | 593.97 | 10 | 59.40 | 1.92 | 0.2772 | |
| Pure error | 123.79 | 4 | 30.95 | |||
| Total | 78,673.98 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, Z.; Meng, H.; Li, W.; Wang, S. Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology. Separations 2022, 9, 193. https://doi.org/10.3390/separations9080193
Zhang Y, Zhao Z, Meng H, Li W, Wang S. Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology. Separations. 2022; 9(8):193. https://doi.org/10.3390/separations9080193
Chicago/Turabian StyleZhang, Yajing, Zirui Zhao, Huiwen Meng, Wenlong Li, and Shujie Wang. 2022. "Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology" Separations 9, no. 8: 193. https://doi.org/10.3390/separations9080193
APA StyleZhang, Y., Zhao, Z., Meng, H., Li, W., & Wang, S. (2022). Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology. Separations, 9(8), 193. https://doi.org/10.3390/separations9080193





