Novel Metabolites as Potential Indicators of Recovery After Large Vessel Occlusion Stroke: A Pilot Study
Abstract
:1. Introduction
2. Methods
2.1. Study Goal and Design
2.2. Metabolome Analysis
2.3. Statistical Analysis
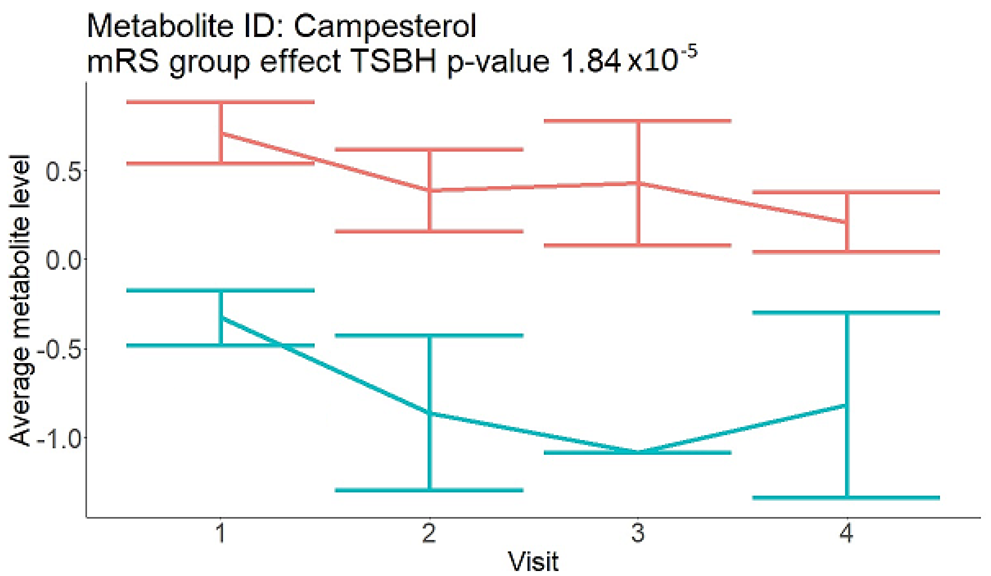
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Sue Nelson, L.W. Projections of Cardiovascular Disease Prevalence and Costs: 2015–2035; RTI International: Research Triangle Park, NC, USA, 2016. [Google Scholar]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, E.; Sanghera, D.K.; Vanamala, J.K.P. Biomarker for ischemic stroke using metabolome: A clinician perspective. J. Stroke 2019, 21, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Baranovicova, E.; Kalenska, D.; Kaplan, P.; Kovalska, M.; Tatarkova, Z.; Lehotsky, J. Blood and brain metabolites after cerebral ischemia. Int. J. Mol. Sci. 2023, 24, 17302. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gui, X.; Wu, L.; Tian, S.; Wang, H.; Xie, L.; Wu, W. Amino acid metabolism, lipid metabolism, and oxidative stress are associated with post-stroke depression: A metabonomics study. BMC Neurol. 2020, 20, 250. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, K.; Li, H.; Dong, X.; Tan, G.; Chai, Y.; Wang, W.; Bi, X. Potential of serum metabolites for diagnosing post-stroke cognitive impairment. Mol. Biosyst. 2015, 11, 3287–3296. [Google Scholar] [CrossRef]
- Chi, N.F.; Chang, T.H.; Lee, C.Y.; Wu, Y.W.; Shen, T.A.; Chan, L.; Chen, Y.R.; Chiou, H.Y.; Hsu, C.Y.; Hu, C.J. Untargeted metabolomics predicts the functional outcome of ischemic stroke. J. Formos. Med. Assoc. 2021, 120, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Harshfield, E.L.; Sands, C.J.; Tuladhar, A.M.; de Leeuw, F.E.; Lewis, M.R.; Markus, H.S. Metabolomic profiling in small vessel disease identifies multiple associations with disease severity. Brain 2022, 145, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, E.V.; Rout, M.; Xu, C.; Jordan, L.; Fields, E.; Apple, B.; Smith, K.; Gordon, D.; Chainakul, J.; Sanghera, D.K. Difference in acute and chronic stage ischemic stroke metabolic markers with controls. J. Stroke Cerebrovasc. Dis. 2023, 32, 107211. [Google Scholar] [CrossRef]
- Sidorov, E.V.; Bejar, C.; Xu, C.; Ray, B.; Gordon, D.; Chainakul, J.; Sanghera, D.K. Novel metabolites as potential indicators of ischemic infarction volume: A pilot study. Transl. Stroke Res. 2020, 12, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Santucci, J.A.; Ross, S.R.; Greenert, J.C.; Aghaei, F.; Ford, L.; Hollabaugh, K.M.; Cornwell, B.O.; Wu, D.H.; Zheng, B.; Bohnstedt, B.N.; et al. Radiological estimation of intracranial blood volume and occurrence of hydrocephalus determines stress-induced hyperglycemia after aneurysmal subarachnoid hemorrhage. Transl. Stroke Res. 2018, 10, 327–337. [Google Scholar] [CrossRef] [PubMed]
- von Kummer, R.; Broderick, J.P.; Campbell, B.C.; Demchuk, A.; Goyal, M.; Hill, M.D.; Treurniet, K.M.; Majoie, C.B.; Marquering, H.A.; Mazya, M.V.; et al. The heidelberg bleeding classification: Classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015, 46, 2981–2986. [Google Scholar] [CrossRef]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef]
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.L.; Mägi, R.; et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Med. 2014, 11, e1001606. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Wurtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Tukiainen, T.; Tynkkynen, T.; Laatikainen, R.; Järvelin, M.R.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; et al. High-throughput serum nmr metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009, 134, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Dehaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of gc/ms and lc/ms metabolomics data into chemical libraries. J. Cheminform. 2010, 2, 9. [Google Scholar] [CrossRef]
- Jung, J.; Park, M.; Park, H.J.; Shim, S.B.; Cho, Y.H.; Kim, J.; Lee, H.S.; Choi, D.; Hwang, G.S. 1h nmr-based metabolic profiling of naproxen-induced toxicity in rats. Toxicol. Lett. 2011, 200, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, L.; Pu, H.; Mao, L.; Hu, X.; Jiang, X.; Xu, N.; Stetler, R.A.; Zhang, F.; Liu, X.; et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef] [PubMed]
- Rout, M.; Vaughan, A.; Sidorov, E.V.; Sanghera, D.K. Improving stroke outcome prediction using molecular and machine learning approaches in large vessel occlusion. J. Clin. Med. 2024, 13, 5917. [Google Scholar] [CrossRef] [PubMed]
- Rout, M.; Vaughan, A.; Blair, A.; Stavrakis, S.; Sidorov, E.V.; Sanghera, D.K. Discovery and validation of circulating stroke metabolites by nmr-based analyses using patients from the miss and uk biobank. Neurochem. Int. 2023, 169, 105588. [Google Scholar] [CrossRef]
- Anroedh, S.; Hilvo, M.; Akkerhuis, K.M.; Kauhanen, D.; Koistinen, K.; Oemrawsingh, R.; Serruys, P.; van Geuns, R.J.; Boersma, E.; Laaksonen, R.; et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 2018, 59, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.; Koster, G.; Douet, L.J.; Scigelova, M.; Woffendin, G.; Ward, J.M.; Smith, A.; Humphries, J.; Burnand, K.G.; Macphee, C.H.; et al. Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase a2 in oxidized human low density lipoprotein. J. Biol. Chem. 2008, 283, 6428–6437. [Google Scholar] [CrossRef]
- Jung, J.I.; Price, A.R.; Ladd, T.B.; Ran, Y.; Park, H.J.; Ceballos-Diaz, C.; Smithson, L.A.; Hochhaus, G.; Tang, Y.; Akula, R.; et al. Cholestenoic acid, an endogenous cholesterol metabolite, is a potent gamma-secretase modulator. Mol. Neurodegener. 2015, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Dammann, C.; Stapelfeld, C.; Maser, E. Expression and activity of the cortisol-activating enzyme 11beta-hydroxysteroid dehydrogenase type 1 is tissue and species-specific. Chem. Biol. Interact. 2019, 303, 57–61. [Google Scholar] [CrossRef]
- Tamura, G.; Ihara, S.; Morota, N. Reversible diffusion weighted imaging hyperintensities during the acute phase of ischemic stroke in pediatric moyamoya disease: A case report. Childs Nerv. Syst. 2016, 32, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Dongmei, W.; Zhenzhou, L.; Yongming, W.U.; Zhong, J.I. Diffusion-weighted imaging hyperintensity is reversible in large middle cerebral artery infarction following thrombectomy: A case report. J. South. Med. Univ. 2020, 40, 459–462. [Google Scholar]
- Li, Y.; Schappell, L.E.; Polizu, C.; DiPersio, J.; Tsirka, S.E.; Halterman, M.W.; Nadkarni, N.A. Evolving clinical-translational investigations of cerebroprotection in ischemic stroke. J. Clin. Med. 2023, 12, 6715. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Cross-Sectional Part; n = 48 | Longitudinal Part; n = 15 1 |
|---|---|---|
| Age | 62.5 (15.5) | 64.73 (14.18) |
| (Sex) Female | 23 (48%) | 7 (46.7%) |
| (Race) White | 34 (71%) | 10 (66.7%) |
| Black | 11 (23%) | 3 (20.0%) |
| Other | 3 (6%) | 2 (13.4%) |
| NIHSS at presentation | 8.85 (7.0) | 14.27 (6.71) |
| Pre-adm mRS | 0.81 (1.16) | 0.33 (0.82) |
| mRS 3 months | 2.81 (1.65) | 2.60 (2.41) |
| Infarction volume | 24.82 (6.77) | 55.63 (57.88) |
| Hx of HTN | 34 (71%) | 10 (66.7%) |
| Hx of HLD | 22 (45.8) | 7 (46.7%) |
| Hx of CAD | 10 (21%) | 3 (20.0%) |
| Cross-Sectional Part Metabolite (NMR) Changes 48–72 h | Corresponding Longitudinal Part Metabolite Derivative Trend (LC-MS) | Trends for Patients with Good (mRS of 0–3) and Poor (mRS of 4–6) Outcomes |
|---|---|---|
| Sphingomyelin (small infarction volume) Decreased in patients with good compared to poor outcomes (R = −17.59 ± 7.28; p = 0.03) | 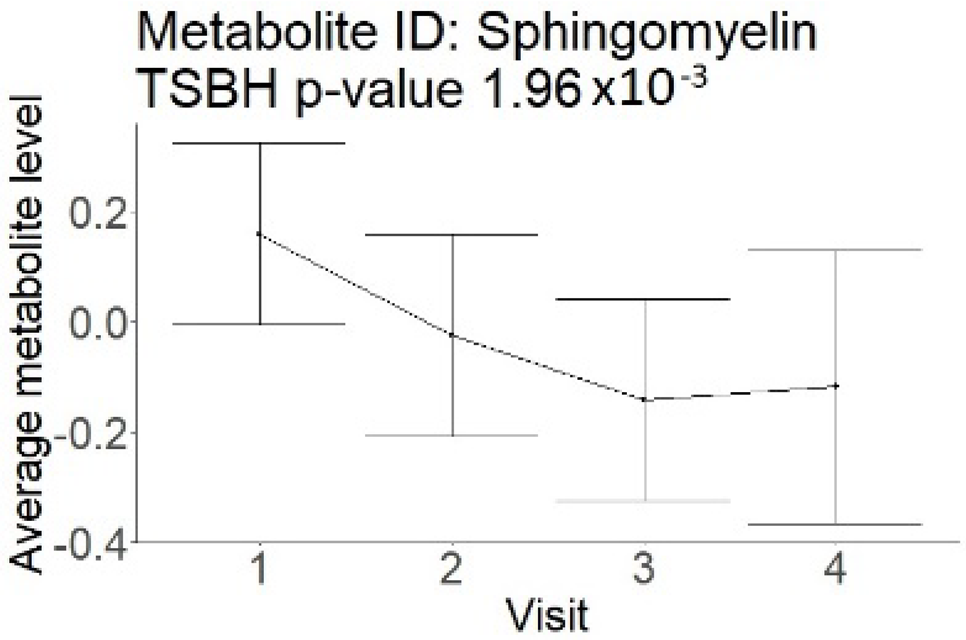 |  |
| Ceramides (not a part of NMR panel) | 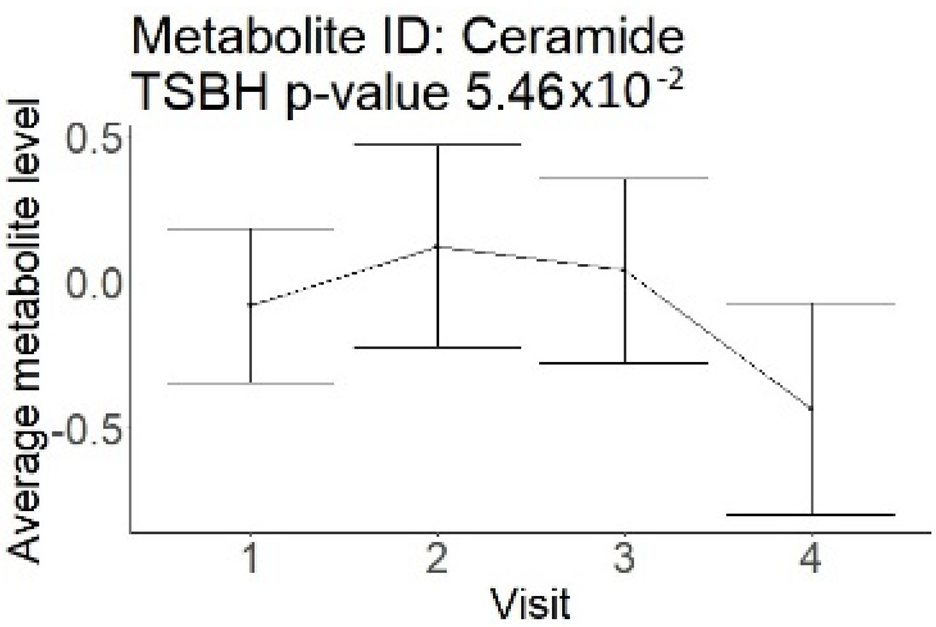 | 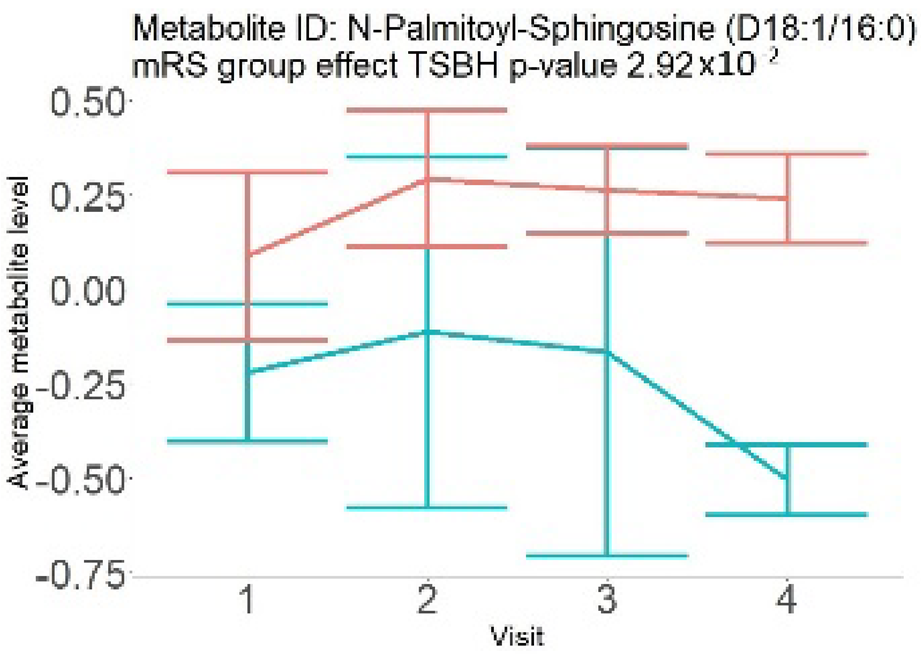 |
| Phosphatidylethanolamine (large infarction volume) Decreased in patients with good compared to poor outcomes (R = − 4.30 ± 1.70; p = 0.02) Phosphoglycerides (large infarction volume) Decreased in patients with good compared to poor outcomes (R = − 4.13 ± 1.61; p = 0.02) | 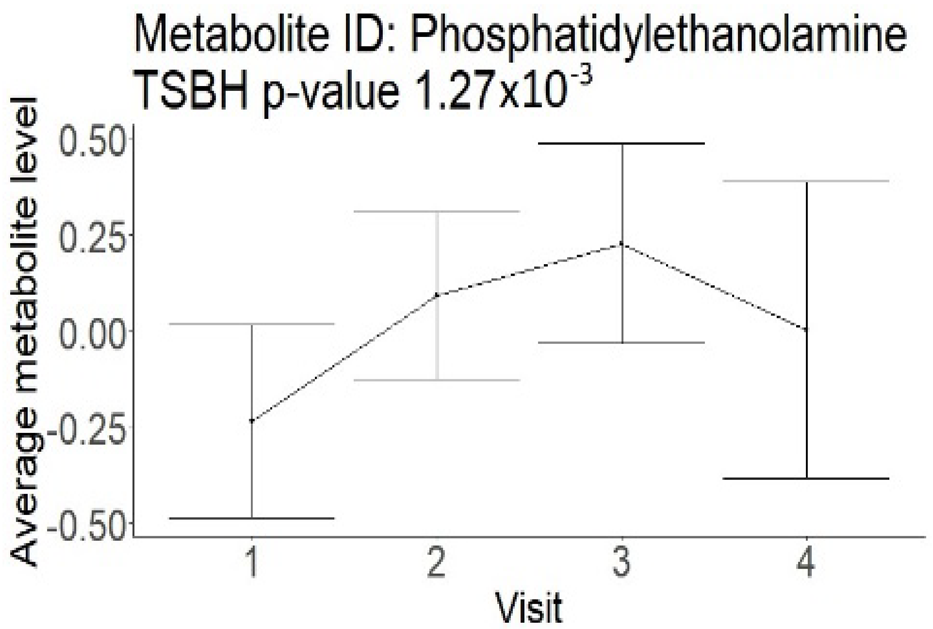 | 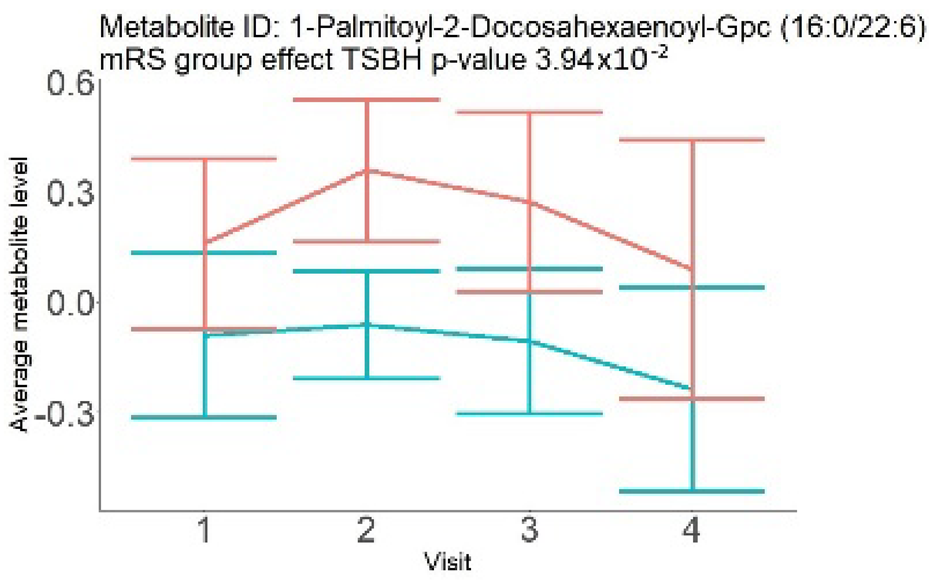 |
 | ||
| Glycerol (large infarction volume) Insignificantly decreased in patients with good compared to poor outcomes (R = − 6.72 ± 4.20; p = 0.12) | 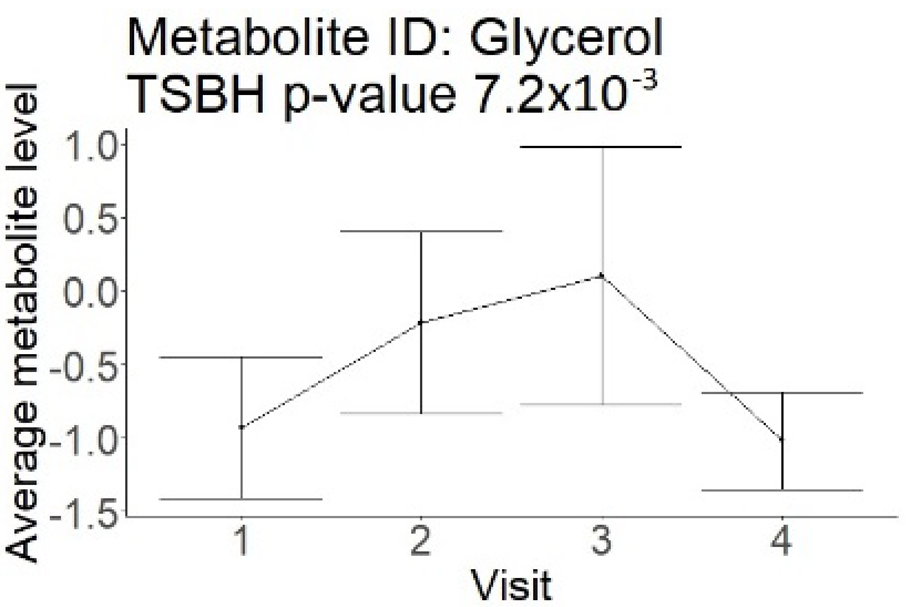 | 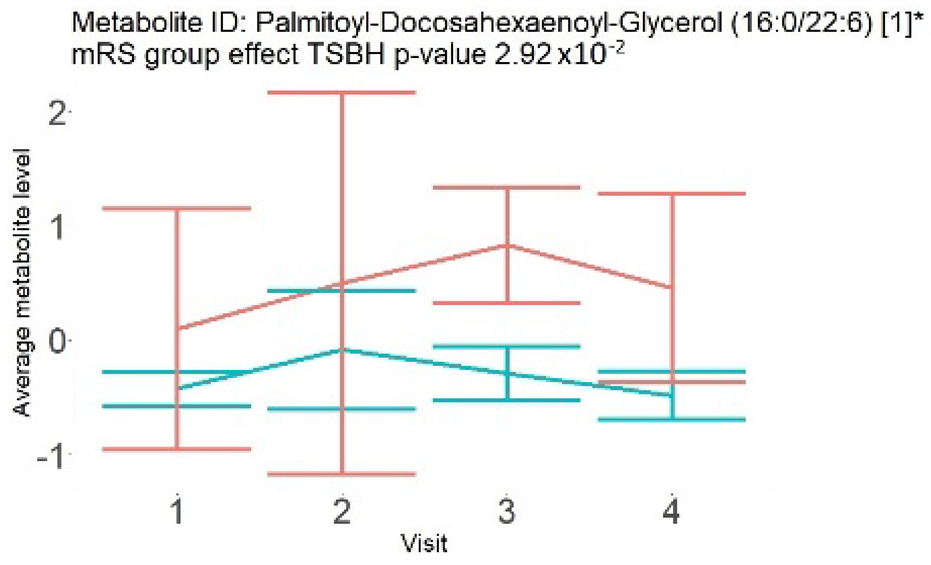 |
| Steroids (not a part of NMR panel) | 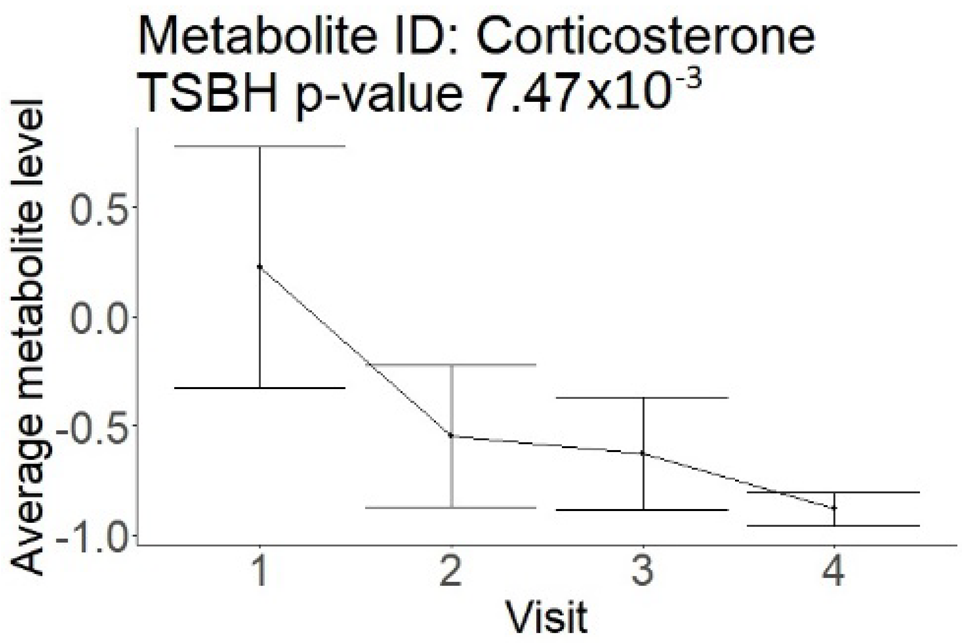 |  |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorov, E.V.; Smith, K.; Xu, C.; Sanghera, D.K. Novel Metabolites as Potential Indicators of Recovery After Large Vessel Occlusion Stroke: A Pilot Study. Neurol. Int. 2025, 17, 30. https://doi.org/10.3390/neurolint17020030
Sidorov EV, Smith K, Xu C, Sanghera DK. Novel Metabolites as Potential Indicators of Recovery After Large Vessel Occlusion Stroke: A Pilot Study. Neurology International. 2025; 17(2):30. https://doi.org/10.3390/neurolint17020030
Chicago/Turabian StyleSidorov, Evgeny V., Kyle Smith, Chao Xu, and Dharambir K. Sanghera. 2025. "Novel Metabolites as Potential Indicators of Recovery After Large Vessel Occlusion Stroke: A Pilot Study" Neurology International 17, no. 2: 30. https://doi.org/10.3390/neurolint17020030
APA StyleSidorov, E. V., Smith, K., Xu, C., & Sanghera, D. K. (2025). Novel Metabolites as Potential Indicators of Recovery After Large Vessel Occlusion Stroke: A Pilot Study. Neurology International, 17(2), 30. https://doi.org/10.3390/neurolint17020030






