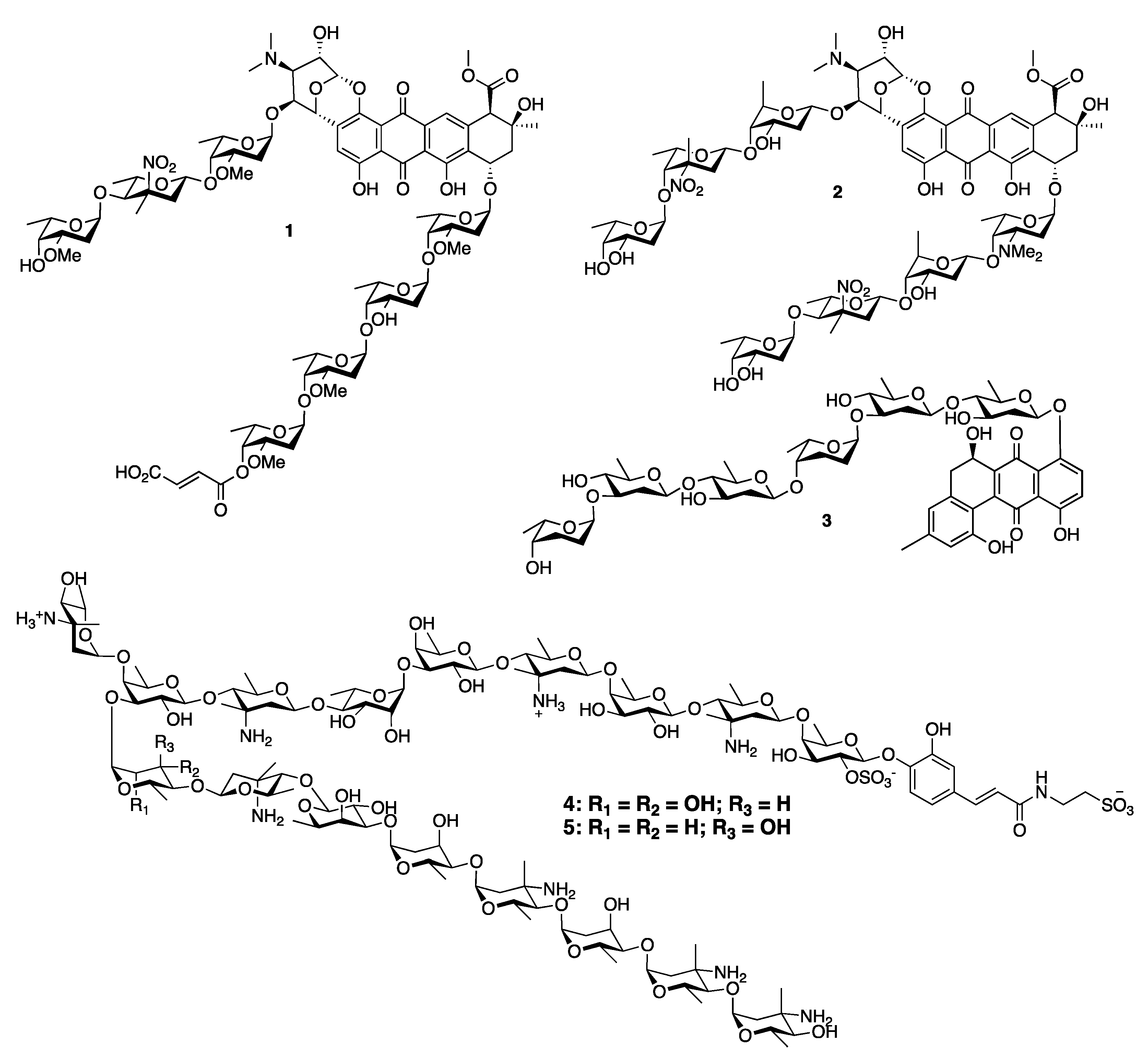
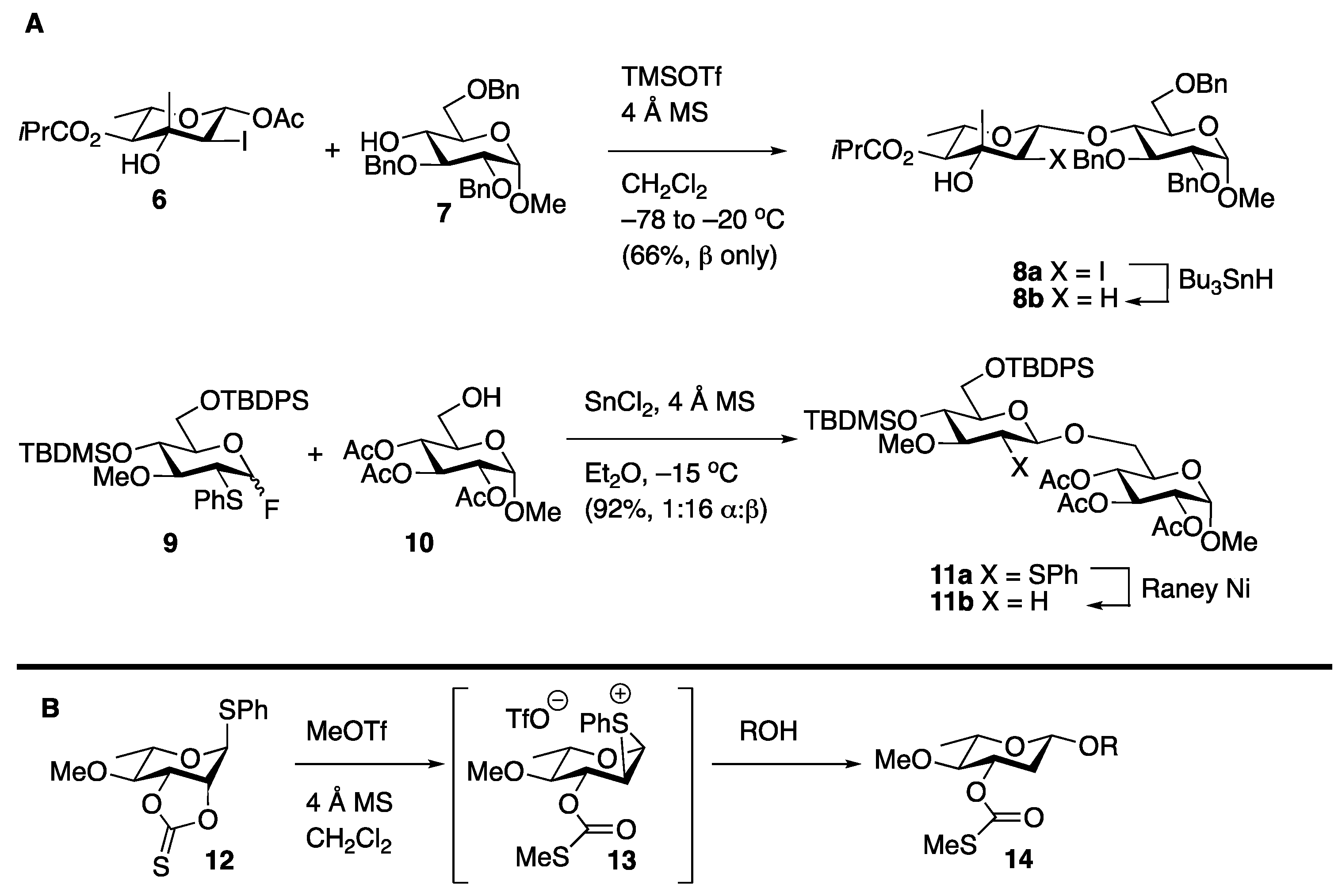
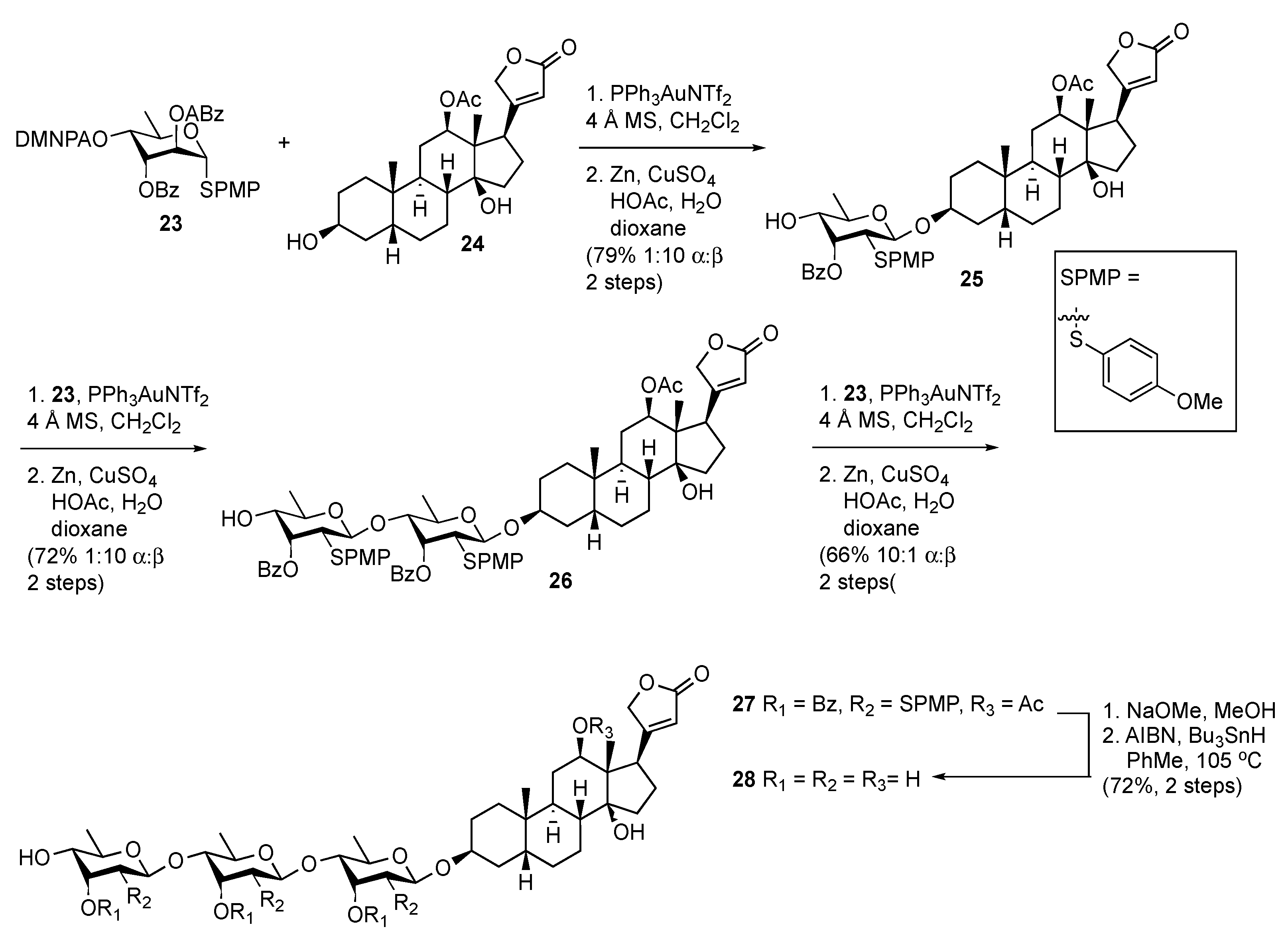
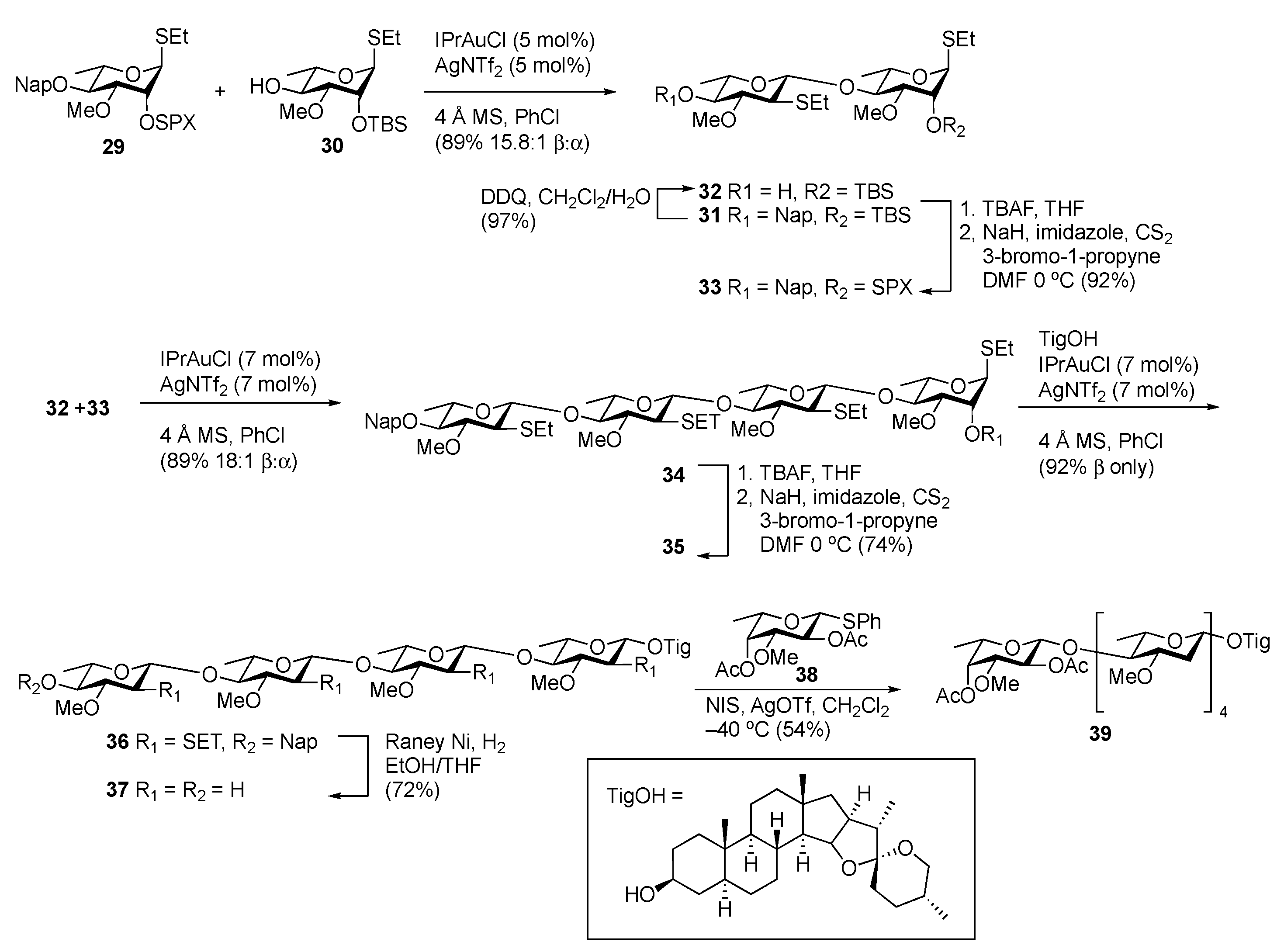
Stereoselective Approaches to β-Linked 2-Deoxy Sugars
Abstract
:1. Introduction
2. Classical Approaches to Control Stereochemistry
3. Directing Group-Based Approaches
4. Catalytic Approaches
5. Reagent-Controlled Approaches
6. Anomeric O-Alkylation and Umpolung
7. De Novo Approaches
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elshahawi, S.I.; Shaaban, K.A.; Kharel, M.K.; Thorson, J.S. A Comprehensive Review of Glycosylated Bacterial Natural Products. Chem. Soc. Rev. 2015, 44, 7591–7697. [Google Scholar] [PubMed]
- Shimosaka, A.; Kawai, H.; Hayakawa, H.; Komeshima, M.; Nakagawa, M.; Seto, H.; Otake, N. Arugomycin, a New Anthracycline Antibiotic. III. Biological Activities of Arugomycin and its Analogues Obtained by Chemical Degradation and Modification. J. Antibiot. 1987, 40, 1283–1291. [Google Scholar] [CrossRef]
- Adnani, N.; Chevrette, M.G.; Adibhatla, S.N.; Zhang, F.; Yu, Q.; Braun, D.R.; Nelson, J.; Simpkins, S.W.; McDonald, B.R.; Myers, C.L.; et al. Coculture of Marine Invertebrate-Associated Bacteria and Interdisciplinary Technologies Enable Biosynthesis and Discovery of a New Antibiotic, Keyicin. ACS Chem. Biol. 2017, 12, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Langenhan, J.M.; Griffith, B.R.; Thorson, J.S. Neoglycorandomization and Chemoenzymatic Glycorandomization: Two Complementary Tools for Natural Product Diversification. J. Nat. Prod. 2005, 68, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Langenhan, J.M.; Peters, N.R.; Guzei, I.A.; Hoffman, F.M.; Thorson, J.S. Enhancing the Anticancer Properties of Cardiac Glycosides by Neoglycorandomization. Proc. Natl. Acad. Sci. USA 2005, 32, 12305–12310. [Google Scholar] [CrossRef]
- Iyer, A.K.V.; Zhou, M.; Azad, N.; Elbaz, H.; Wang, L.; Rogalsky, D.K.; Rojanasakul, Y.; O’Doherty, G.A.; Langenhan, J.M. A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides. ACS Med. Chem. Lett. 2010, 1, 326–330. [Google Scholar] [CrossRef]
- Marzabadi, C.H.; Franck, R.W. The Synthesis of 2-Deoxyglycosides: 1998–1999. Tetrahedron 2000, 56, 8385–8417. [Google Scholar] [CrossRef]
- Hou, D.; Lowary, T.L. Recent Advances in the Synthesis of 2-Deoxy-Glycosides. Carbohydr. Res. 2009, 344, 1911–1940. [Google Scholar] [CrossRef]
- Bennett, C.S.; Galan, M.C. Methods for 2-Deoxyglycoside Synthesis. Chem. Rev. 2018, 118, 7931–7985. [Google Scholar] [CrossRef]
- Ling, J.; Bennett, C.S. Recent Developments in Stereoselective Chemical Glycosylations. Asian J. Org. Chem. 2019, 8, 802–813. [Google Scholar] [CrossRef]
- Yadav, Y.; Sagar, R. Glycals as Chiral Synthons in Organic Synthesis of Privileged Molecular Scaffolds. Chem. Asian J. 2025, e202401773. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.T.; Woerpel, K.A. The Effect of Electrostatic Interactions on Conformational Equilibria of Multiply Substituted Tetrahydropyran Oxocarbenium Cations. J. Org. Chem. 2009, 74, 545–553. [Google Scholar]
- Krumper, J.R.; Salamant, W.A.; Woerpel, K.A. Continuum of Mechanisms for Nucleophilic Substitutions of Cyclic Acetals. Org. Lett. 2008, 10, 4907–4910. [Google Scholar]
- McDonald, F.E.; Reddy, K.S. Convergent Synthesis of Digitoxin: Stereoselective Synthesis and Glycosylation of the Digoxin Trisaccharide Glycal. Angew. Chem. Int. Ed. 2001, 40, 3653–3655. [Google Scholar]
- Chun, Y.; Remmerswaal, W.A.; Codée, J.D.C.; Woerpel, K.A. Neighboring-Group Participation by C-2 Acyloxy Groups: Influence of the Nucleophile and Acyl Group on the Stereochemical Outcome of Acetal Substitution Reactions. Chem. Eur. J. 2023, 29, e202301894. [Google Scholar]
- Basu, P.; Crich, D. The Stereoselectivity of Neighboring Group-Directed Glycosylation is Concentration Dependent. J. Am. Chem. Soc. 2025, 147, 5808–5818. [Google Scholar] [PubMed]
- Gervay, J.; Danishefsky, S. A Stereospecific Route to 2-Deoxy-β-glycosides. J. Org. Chem. 1991, 58, 5448–5451. [Google Scholar] [CrossRef]
- Bock, K.; Lundt, I.; Pedersen, C. 2-Bromo-2-Deoxy Sugars as Starting Materials for the Synthesis of α- or β-Glycosides of 2-Deoxy Sugars. Carbohydr. Res. 1984, 130, 125–134. [Google Scholar]
- Roush, W.R.; Gung, B.W.; Bennet, C.E. 2-Deoxy-2-iodo- and 2-Deoxy-2-bromo-α-glucopyranosyl Trichloroacetimidates: Highly Reactive and Stereoselective Donors for the Synthesis of 2-Deoxy-β-glycosides. Org. Lett. 1999, 1, 891–893. [Google Scholar]
- Nicolaou, K.C.; Ladduwahetty, T.; Randall, J.L.; Chucholowski, A. Stereospecific 1,2-Migrations in Carbohydrates. Stereocontrolled Synthesis of α- and β-2-Deoxyglycosides. J. Am. Chem. Soc. 1986, 108, 2466–2467. [Google Scholar]
- Roush, W.R.; Bennet, C.E. A Highly Stereoselective Synthesis of the Landomycin A Hexasaccharide Unit. J. Am. Chem. Soc. 2000, 122, 6124–6125. [Google Scholar] [CrossRef]
- Yang, X.; Fu, B.; Yu, B. Total Synthesis of Landomycin A, a Potent Antitumor Angucycline Antibiotic. J. Am. Chem. Soc. 2011, 133, 12433–12435. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, Z. Stereoselective Synthesis of 2-S-Phenyl-2-deoxy-β-glycosides Using Phenyl-2,3-O-Thiocarbonyl-1-thioglycoside Donors via 1,2-Migration and Concurrent Glycosidation. Org. Lett. 2001, 2, 377–379. [Google Scholar] [CrossRef]
- Yu, B.; Wang, P. Efficient Synthesis of the Hexasaccharide Fragment of Landomycin A: Using Phenyl 2,3-O-Thionocarbonyl-1-thioglycosides as 2-Deoxy-β-glycoside Precursors. Org. Lett. 2002, 4, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.-i.; Sano, A.; Sakamoto, H.; Nakajima, M.; Yanagiya, Y.; Ikegami, S. An Attempt at the Direct Construction of 2-Deoxy-β-glycosidic Linkages Capitalizing on 2-Deoxyglycopyranosyl Diethyl Phosphates as Glycosyl Donors. Synett 1995, 1271–1273. [Google Scholar]
- Guo, Y.; Sulikowski, G.A. Synthesis of the Hexasaccharide Fragment of Landomycin A: Application of Glycosyl Tetrazoles and Phosphites in the Synthesis of a Deoxyoligosaccharide. J. Am. Chem. Soc. 1998, 120, 1392–1397. [Google Scholar] [CrossRef]
- Pongdee, R.; Wu, B.; Sulikowski, G.A. One-Pot Synthesis of 2-Deoxy-β-oligosaccharides. Org. Lett. 2001, 3, 3523–3525. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshizawa, A.; Takahashi, T. Direct and Stereoselective Synthesis of β-Linked 2,6-Deoxyoligosaccharides. Angew. Chem. Int. Ed. 2007, 46, 2505–2507. [Google Scholar]
- Tanaka, H.; Yamaguchi, S.; Yoshizawa, A.; Takagi, M.; Shin-ya, K.; Takahashi, T. Combinatorial Synthesis of Deoxyhexasaccharides Related to the Landomycin A Sugar Moiety, Based on an Orthogonal Deprotection Strategy. Chem. Asian J. 2010, 5, 1407–1424. [Google Scholar] [CrossRef]
- Crich, D.; Vinogradova, O. On the Influence of the C2-O2 and C3-O3 Bonds in the 4,6-O-Benzylidene-Directed-β-Mannopyranosylation and α-Glucopyranosylation. J. Org. Chem. 2006, 71, 8473–8480. [Google Scholar] [CrossRef]
- Liao, J.-X.; Li, Z.-Q.; Qiu, Y.; Gao, X.-Y.; Lv, X.; Tu, Y.-H.; Sun, J.-S. Stereoselective Synthesis of 2-Deoxy Glycosides via a Novel O-Resided (o-Alkynyl)benzoate-Initiated 1,2 Sulfur Migration. Glycosylation and Desulfurization Protocol as well as Mechanism Elucidation. Chin. J. Chem. 2025, 43, 783–790. [Google Scholar]
- Liu, H.; Zhou, S.-Y.; Wen, G.-E.; Liu, X.-X.; Liu, D.-Y.; Zhang, Q.-J.; Schmidt, R.R.; Sun, J.-S. The 2,2-dimethyl-2-(ortho-nitrophenyl)ace-tyl (DMNPA) group: A novel protecting group in carbohydrate chemistry. Org. Lett. 2019, 21, 8049–8052. [Google Scholar]
- Codee, J.D.C.; Schmidt, R.R.; Sun, J.-S. Dual-participation protecting group solves the anomeric stereocontrol problems in glycosylation reactions. Org. Lett. 2019, 21, 8713–8717. [Google Scholar]
- Liu, H.; Zhou, S.-Y.; Liao, J.-X.; Tu, Y.-H.; Sun, J.-S. Highly Efficient Synthesis of Digoxin. Synlett 2021, 32, 810–813. [Google Scholar]
- Wang, X.; Ding, H.; Guo, A.; Song, X.; Wang, P.; Song, N.; Yu, B.; Xu, P.; Liu, X.-W.; Li, M. Gold(I)-Catalyzed 2-Deoxy-β-glycosylation via 1,2-Alkyl/Aryllthio Migration: Synthesis of Velutinoside A Pentasaccharide. J. Am. Chem. Soc. 2025, 147, 4469–4481. [Google Scholar] [PubMed]
- Hou, D.; Taha, H.A.; Lowary, T.L. 2,3-Anhydrosugars in Glycoside Bond Synthesis: Mechanism of 2-Deoxy-2-thioaryl Glycoside Formation. J. Am. Chem. Soc. 2009, 131, 12937–12948. [Google Scholar]
- Crich, D. En Route to Transformation of Glycoscience: A Chemist’s Perspective on Internal and External Crossroads in Glycochemistry. J. Am. Chem. Soc. 2021, 143, 17–34. [Google Scholar]
- Ruei, J.-H.; Venukumar, P.; Ingle, A.B.; Mong, K.-K.T. C6 Picoloyl Protection: A Remote Stereodirecting Group for 2-Deoxy-β-Glycoside Formation. Chem. Commun. 2015, 51, 5394–5397. [Google Scholar]
- Yasomanee, J.P.; Demchanko, A.V. Effect of Remote Picolinyl and Picoloyl Substituents on the Stereoselectivity of Chemical Glycosylation. J. Am. Chem. Soc. 2012, 134, 20097–20102. [Google Scholar]
- Barton, D.H.R.; McCombie, S.W. A New Methods for the Deoxygenation of Secondary Alcohols. J. Chem. Soc. Perkin Trans. 1 1975, 16, 1574–1585. [Google Scholar]
- Liu, X.; Lin, Y.; Liu, A.; Sun, Q.; Sun, H.; Xu, P.; Li, G.; Song, Y.; Kie, W.; Sun, H.; et al. 2-Diphenylphosphinoyl-acetal as a Remote Directing Group for the Highly Stereoselective Synthesis of β-Glycosides. Chin. J. Chem. 2022, 40, 443–452. [Google Scholar]
- Liu, X.; Lin, Y.; Peng, W.; Zhang, Z.; Gao, L.; Zhou, Y.; Song, Z.; Wang, Y.; Xu, P.; Yu, B.; et al. Direct Synthesis of 2,6-Dideoxy-β-glycosides and β-Rhamnosides with a Stereodirecting 2-(Diphenylphosphinoyl)acetyl Group. Angew. Chem. Int. Ed. 2022, 61, e202206128. [Google Scholar]
- Yu, B.; Tao, H. Glycosyl Trifluoroacetimidates. Part 1: Preparation and Application as New glycosyl Donors. Tetrahedron Lett. 2001, 42, 2405–2407. [Google Scholar]
- Li, Y.; Yang, Y.; Yu, B. An Efficient Glycosylation Protocol with Glycosyl ortho-Alkynylbenzoates as Donors Under the Catalysis of Ph3PAuOTf. Tetrahedron Lett. 2008, 49, 3604–3608. [Google Scholar]
- Zhang, Z.; Wu, R.; Cao, S.; Li, J.; Huang, G.; Wang, H.; Yang, T.; Tang, W.; Xu, P.; Yu, B. Merging Total Synthesis and NMR Technology for Deciphering the Realistic Structure of Natural 2,6-Dideoxyglycosides. Sci. Adv. 2024, 10, eand1305. [Google Scholar]
- Grundler, G.; Schmidt, R.R. Glycosylimidate, 13. Anwendung des Trichloracetimidat-Verhahrens auf 2-Azidoglucose- und 2-Azidogalactose-Derivate. Liebigs Ann. Chem. 1984, 1826–1847. [Google Scholar]
- Beale, T.M.; Moon, P.J.; Taylor, M.S. Organoboron-Catalyzed Regio- and Stereoselective Formation of β-2-Deoxyglycosidic Linkages. Org. Lett. 2014, 16, 3604–3607. [Google Scholar]
- Gouliaras, C.; Lee, D.; Chen, L.; Taylor, M.S. Regioselective Activation of Glycosyl Acceptors by a Diarylborinic Acid-Derived Catalyst. J. Am. Chem. Soc. 2011, 133, 13926–13929. [Google Scholar]
- Niggemann, J.; Lindhorst, T.K.; Walfort, M.; Laupichler, L.; Sajus, H.; Thiem, J. Synthetic Approaches to 2-Deoxygycosyl Phosphates. Carbohydr. Res. 1993, 246, 173–183. [Google Scholar]
- D’Angelo, K.A.; Taylor, M.S. Borinic Acid Catalyzed Stereo- and Regioselective Couplings of Glycosyl Mesylates. J. Am. Chem. Soc. 2016, 138, 11058–11066. [Google Scholar]
- Park, Y.; Harper, K.C.; Kuhl, N.; Kwan, E.E.; Liu, R.Y.; Jacobsen, E.N. Macrocyclic Bis-Thioureas Catalyze Stereospecific Glycosylation Reactions. Science 2017, 355, 162–166. [Google Scholar] [PubMed]
- Kwan, E.E.; Park, Y.; Besser, H.A.; Anderson, T.L.; Jacobsen, E.N. Sensitive and Accurate 13C Kinetic Isotope Effect Measurements Enabled by Polarization Transfer. J. Am. Chem. Soc. 2017, 139, 43–46. [Google Scholar] [PubMed]
- Beyer, P.D.; Nielsen, M.M.; Picazo, E.; Jacobsen, E.N. β-Selective 2-Deoxy and 2,6-Dideoxyglucosylations Catalyzed by Bis-Thioureas. J. Am. Chem. Soc. 2024, 146, 27318–27323. [Google Scholar] [CrossRef]
- Deng, L.-F.; Wang, Y.; Xu, S.; Zhu, H.; Zhang, S.; Zhang, X.; Niu, D. Palladium Catalysis Enables Cross-Coupling-Like SN2-Glycosylation of Phenols. Science 2023, 382, 928–935. [Google Scholar]
- Ma, X.; Zhang, Y.; Wei, Y.; Zhang, L. Directed SN2 Glycosylation Employing and Amide-Functionalized 1-Naphthoate Platform Featuring a Selectivity-Safeguarding Mechanism. J. Am. Chem. Soc. 2023, 145, 11921–11926. [Google Scholar] [PubMed]
- Cheng, C.-W.; Zhou, Y.; Pan, W.-H.; Dey, S.; Wu, C.-Y.; Hsu, W.-L.; Wong, C.-H. Hierarchical and Programmable One-Pot Synthesis of Oligosaccharides. Nat. Commun. 2018, 9, 5202. [Google Scholar]
- Chang, C.-W.; Wu, C.-H.; Lin, M.-H.; Liao, P.-H.; Chang, C.-C.; Chuang, H.-H.; Lin, S.-C.; Lam, S.; Verma, V.P.; Hsu, C.-P.; et al. Establishment of Guidelines for the Control of Glycosylation Reactions and Intermediates by Quantitative Assessment of Reactivity. Angew. Chem. Int. Ed. 2019, 58, 16775–16779. [Google Scholar]
- Lu, S.-R.; Lai, Y.-H.; Chen, J.-H.; Liu, C.-Y.; Mong, K.-K.T. Dimethylformamide: An Unusual Glycosylation Modulator. Angew. Chem. Int. Ed. 2011, 50, 7315–7320. [Google Scholar]
- Liu, K.-M.; Wang, P.-Y.; Guo, Z.-Y.; Xiong, D.-C.; Qin, X.-J.; Liu, M.; Liu, M.; Xue, W.-Y.; Ye, X.-S. Iterative Synthesis of 2-Deoxyoligosaccharides Enabled by Stereoselective Visible-Light-Promoted Glycosylation. Angew. Chem. Int. Ed. 2022, 20, e20214626. [Google Scholar]
- Koto, S.; Morishima, N.; Owa, M.; Zen, S. A Stereoselective α-Glucosylation by Use of a Mixture of 4-Nitrobenzenesulfonyl Chloride, Silver Trifluoromethanesulfonate, N,N-Dimethyacetamide, and Triethylamine. Carbohydr. Res. 1984, 130, 73–83. [Google Scholar]
- Kaneko, M.; Herzon, S.B. Scope and Limitations of 2-Deoxy- and 2,6-Dideoxyglycosyl Bromides as Donors for the Synthesis of β-2-Deoxy- and β-26-Dideoxyglycosides. Org. Lett. 2014, 16, 2776–2779. [Google Scholar] [PubMed]
- Xu, Z.; DiBello, M.; Wang, Z.; Rose, J.A.; Chen, L.; Li, X.; Herzon, S.B. Stereocontrolled Synthesis of the Fully Glycosylated Monomeric Unit of Lomaiviticin A. J. Am. Chem. Soc. 2022, 144, 16199–16205. [Google Scholar]
- Kaneko, M.; Li, Z.; Burk, M.; Colis, L.; Herzon, S.B. Synthesis and Biological Evaluation of (2S,2′S)-Lomaiviticin. J. Am. Chem. Soc. 2021, 143, 1126–1132. [Google Scholar]
- Issa, J.P.; Lloyd, D.; Steliotes, E.; Bennett, C.S. Reagent Controlled β-Specific Dehydrative Glycosylation Reactions with 2-Deoxy-Sugars. Org. Lett. 2013, 15, 4170–4173. [Google Scholar] [PubMed]
- Issa, J.P.; Bennett, C.S. A Reagent Controlled SN2-Glycosylation for the Direct Synthesis of β-Linked 2-Deoxy-Sugars. J. Am. Chem. Soc. 2014, 136, 5740–5744. [Google Scholar] [PubMed]
- Lloyd, D.; Bennett, C. An Improved Approach to the Direct Construction of 2-Deoxy-β-Linked Sugars: Applications to Oligosaccharide Synthesis. Chem. Eur. J. 2018, 24, 7610–7614. [Google Scholar]
- Xie, T.; Zheng, C.; Chen, K.; He, H.; Gao, S. Asymmetric Total Synthesis of the Complex Polycyclic Xanthone FD-594. Angew. Chem. Int. Ed. 2020, 59, 4360–4364. [Google Scholar]
- Mizia, J.C.; Bennett, C.S. Reagent Controlled Direct Dehydrative Glycosylation with 2-Deoxy Sugars: Construction of the Saquayamycin Z Pentasaccharide. Org. Lett. 2019, 21, 5922–5927. [Google Scholar]
- Yalamanchili, S.; Lloyd, D.; Bennett, C.S. Synthesis of the Hexasaccharide Fragment of Landomycin A Using a Mild, Reagent Controlled Approach. Org. Lett. 2019, 21, 3674–3677. [Google Scholar]
- Lloyd, D.; Bylsma, M.; Bright, D.K.; Chen, X.; Bennett, C.S. Mild Method for 2-Naphthylmethyl Ether Protecting Group Removal Using a Combination of 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone and β-Pinene. J. Org. Chem. 2017, 82, 3926–3934. [Google Scholar]
- Cattaneo, V.; Oldrini, D.; Corrado, A.; Berti, F.; Adamo, R. Orthogonal Cleavage of the 2-Naphthylmethyl Group in the Presence of the p-Methoxy Phenyl-Protected Anomeric Position and its use in Carbohydrate Synthesis. Org. Chem. Front. 2016, 3, 753–758. [Google Scholar]
- Mizia, J.C.; Seyed, U.M.; Bennett, C.S. Synthesis of the α-Linked Digitoxose Trisaccharide Fragment of Kijanimicin: An Unexpected Application of Glycosyl Sulfonates. Org. Lett. 2022, 24, 731–735. [Google Scholar] [PubMed]
- Bylsma, M.; Bennett, C.S. Stereospecific Synthesis of the Saccharosamine-Rhamnose-Fucose Fragment Present in Saccharomicin B. Org. Lett. 2018, 20, 4695–4698. [Google Scholar]
- Garreffi, B.P.; Kowk, R.W.; Marianski, M.; Bennett, C.S. Origins of Selectivity in Glycosylation Reactions with Saccharosamine Donors. Org. Lett. 2023, 25, 8856–8860. [Google Scholar] [CrossRef]
- Zhou, M.; Wilbur, D.J.; Kwan, E.E.; Bennett, C.S. Matching Glycosyl Donor Reactivity to its Leaving Group Ability Permits SN2 Glycosylations. J. Am. Chem. Soc. 2019, 141, 16743–16754. [Google Scholar]
- Zeng, J.; Sun, G.; Yao, W.; Zhu, R.; Cai, L.; Lie, K.; Zhang, Q.; Liu, X.-W.; Wan, Q. 3-Aminodeoxypyranoses in Glycosylation: Diversity-Oriented Synthesis and Assembly in Oligosaccharides. Angew. Chem. Int. Ed. 2017, 19, 5227–5231. [Google Scholar]
- Zeng, J.; Wang, R.; Zhang, S.; Fang, J.; Liu, S.; Sun, G.; Xu, B.; Xiao, Y.; Fu, D.; Zhang, W.; et al. Hydrogen-Bonding-Assisted Exogenous Nucleophilic Reagent Effect for β-Selective Glycosylation of Rare 3-Amino Sugars. J. Am. Chem. Soc. 2019, 141, 8509–8515. [Google Scholar]
- Schmidt, R.R.; Michel, J. Direct O-Glycosyl Trichloroacetimidate Formation. Nucleophilicity of the Oxygen Atom. Tetrahedron Lett. 1984, 25, 821–824. [Google Scholar]
- Morris, W.J.; Shair, M.D. Setereoselective Synthesis of 2-Deoxy-β-glycosides Using Anomeric O-Alkylation/Arylation. Org. Lett. 2009, 11, 9–12. [Google Scholar]
- Zhu, D.; Baryal, K.N.; Adhikari, S.; Zhu, J. Direct Synthesis of 2-Deoxy-β-Glycosides via Anomeric O-Alkylation with Secondary Electrophiles. J. Am. Chem. Soc. 2014, 136, 3172–3175. [Google Scholar] [CrossRef]
- Li, X.; Woodward, J.; Hourani, A.; Zhu, D.; Ayoub, S.; Zhu, J. Synthesis of the 2-Deoxy Trisaccharide Glycal of the Antitumor Antibiotics Landomycins A and E. Carbohydr. Res. 2016, 430, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Adhikari, S.; Baryal, K.N.; Abdullah, B.N.; Zhu, J. Stereoselective Synthesis of α-Digitoxosides and α-Boivinosides via Chelation-Controlled Anomeric O-Alkylation. J. Carbohydr. Chem. 2014, 33, 438–451. [Google Scholar] [CrossRef]
- Baryal, K.N.; Zhu, D.; Li, X.; Zhu, J. Umpolung Reactivity in the Stereoselective Synthesis of S-Linked 2-Deoxyglycosides. Angew. Chem. Int. Ed. 2013, 52, 8012–8016. [Google Scholar] [CrossRef] [PubMed]
- Baryal, K.N.; Zhu, J. Stereoselective Synthesis of S-Linked Hexasaccharide of Landomycin A via Umpolung S-Glycosylation. Org. Lett. 2015, 17, 4530–4533. [Google Scholar] [CrossRef]
- Hoang, K.M.; Lees, N.R.; Herzon, S.B. Programmable Synthesis of 2-Deoxyglycosides. J. Am. Chem. Soc. 2019, 141, 8098–8103. [Google Scholar] [CrossRef]
- Hoang, K.M.; Lees, N.R.; Herzon, S.B. General Method for the Synthesis of α- or β-Deoxyaminoglycosides Bearing Basic Nitrogen. J. Am. Chem. Soc. 2021, 143, 2777–2783. [Google Scholar] [CrossRef]
- Hoang, K.M.; Zheng, X.; Herzon, S.B. Synthesis of 2-Deoxyglycosides bearing Free Hydroxyl Substituents on the Glycosyl Donor. J. Org. Chem. 2022, 87, 10768–10790. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.A.; O’Doherty, G.A. A Palladium-Catalyzed Glycosylation Reaction: The de Novo Synthesis of Natural and Unnatural Glycosides. J. Am. Chem. Soc. 2003, 125, 12406–12407. [Google Scholar] [CrossRef]
- Zhou, M.; O’Doherty, G.A. A Stereoselective Synthesis of Digitoxin and Digitoxigen Mono- and Bisdigitoxoside from Digitoxigenin via a Palladium-Catalyzed Glycosylation. Org. Lett. 2006, 8, 4339–4342. [Google Scholar] [CrossRef]
- Zhou, M.; O’Doherty, G.A. De Novo Synthesis of the Trisaccharide Subunit of Landomycins A and E. Org. Lett. 2008, 10, 2283–2286. [Google Scholar] [CrossRef]
- Luche, J.L. Lanthanides in Organic Chemistry. 1. Selective 1m2 Reductions of Conjugated Ketones. J. Am. Chem. Soc. 1978, 100, 2226–2227. [Google Scholar]
- Myers, A.G.; Zheng, B. New and Stereospecific Synthesis of Allenes in a Single Step from Propargylic Alcohols. J. Am. Chem. Soc. 1996, 118, 4492–4493. [Google Scholar]
- Lim, W.; Kim, J.; Rhee, Y.H. Pd-Catalyzed Asymmetric Intermolecular Hydroalkoxylation of Allene: An Entry to Cyclic Acetals with Activating Group-Free and Flexible Anomeric Control. J. Am. Chem. Soc. 2014, 136, 13618–13621. [Google Scholar] [PubMed]
- Lee, J.; Kang, S.; Kim, J.; Moon, D.; Rhee, Y.H. A Convergent Synthetic Strategy towards Oligosaccharides Containing 2,3,6-Trideoxypyranosides. Angew. Chem. Int. Ed. 2019, 58, 628–631. [Google Scholar]
- Seo, K.; Rhee, Y.H. Ruthernium-Catalyzed Regioselective Olefin Migration of Diydropyran Acetals: A De Novo Strategy toward β-2,6-Dideoxyhexopyranosidss. Org. Lett. 2020, 22, 2178–2181. [Google Scholar]
- Barpuzary, B.; Kim, M.; Rheem, Y.H. Synthetic Study toward Saccharomicin Based upon Asymmetric Metal Catalysis. Org. Lett. 2021, 15, 5969–5972. [Google Scholar]
- Lee, J.; Kang, J.; Lee, S.; Rhee, Y.H. Flexible Total Synthesis of 11-Deoxylandomycins and Their Non-Natural Analogues by Way of Asymmetric Metal Catalysis. Angew. Chem. Int. Ed. 2020, 59, 2349–2353. [Google Scholar]
- Kim, H.; Men, H.; Lee, C. Stereoselective Palladium-Catalyzed O-Glycosylation Using Glycals. J. Am. Chem. Soc. 2004, 126, 1336–1337. [Google Scholar] [PubMed]


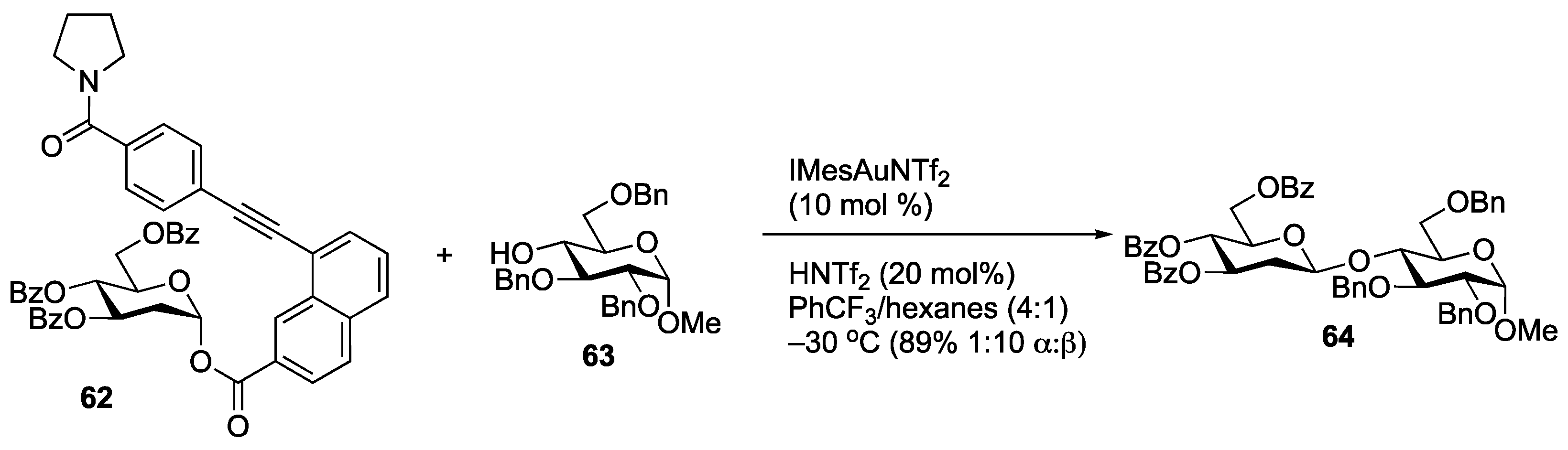
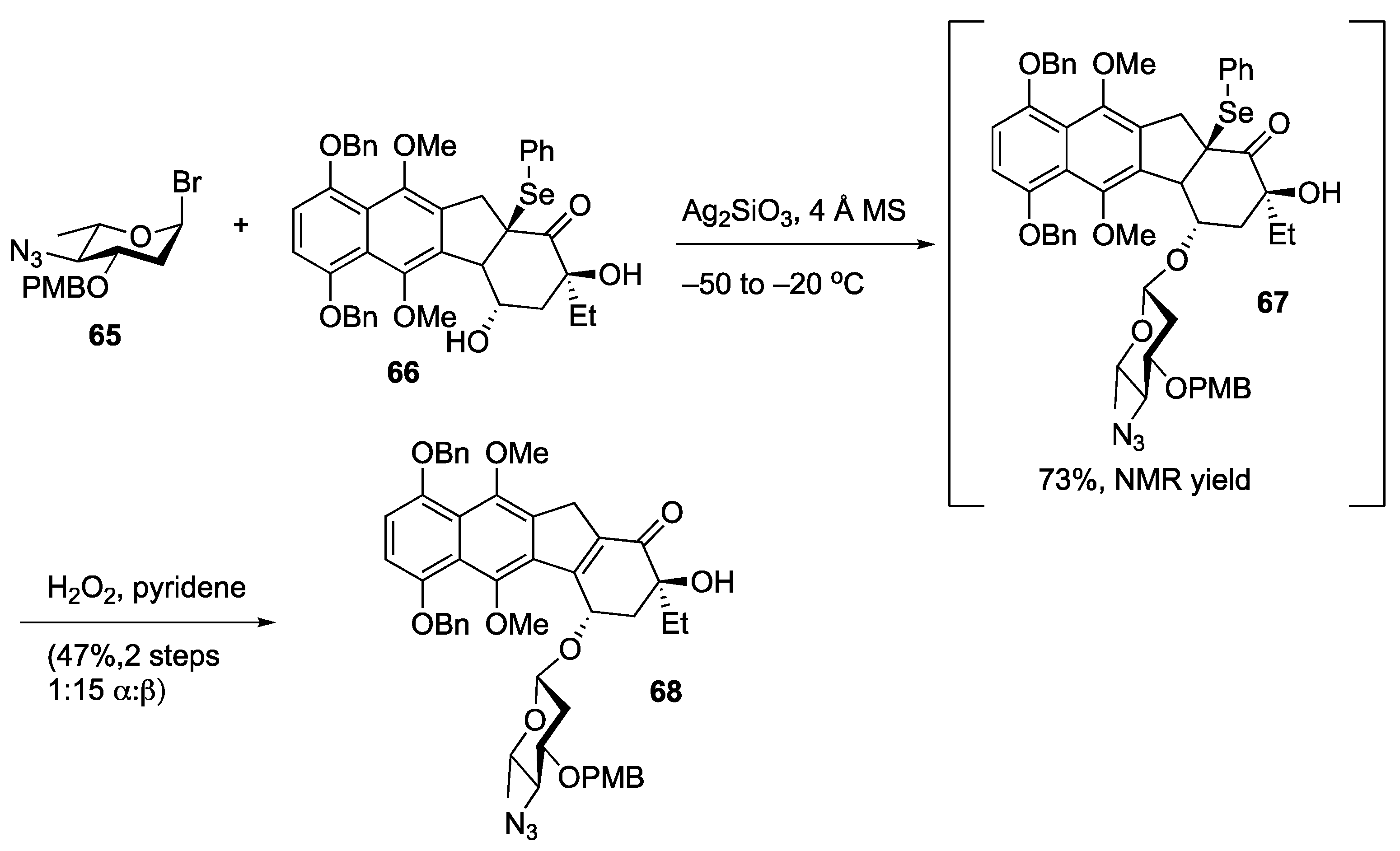
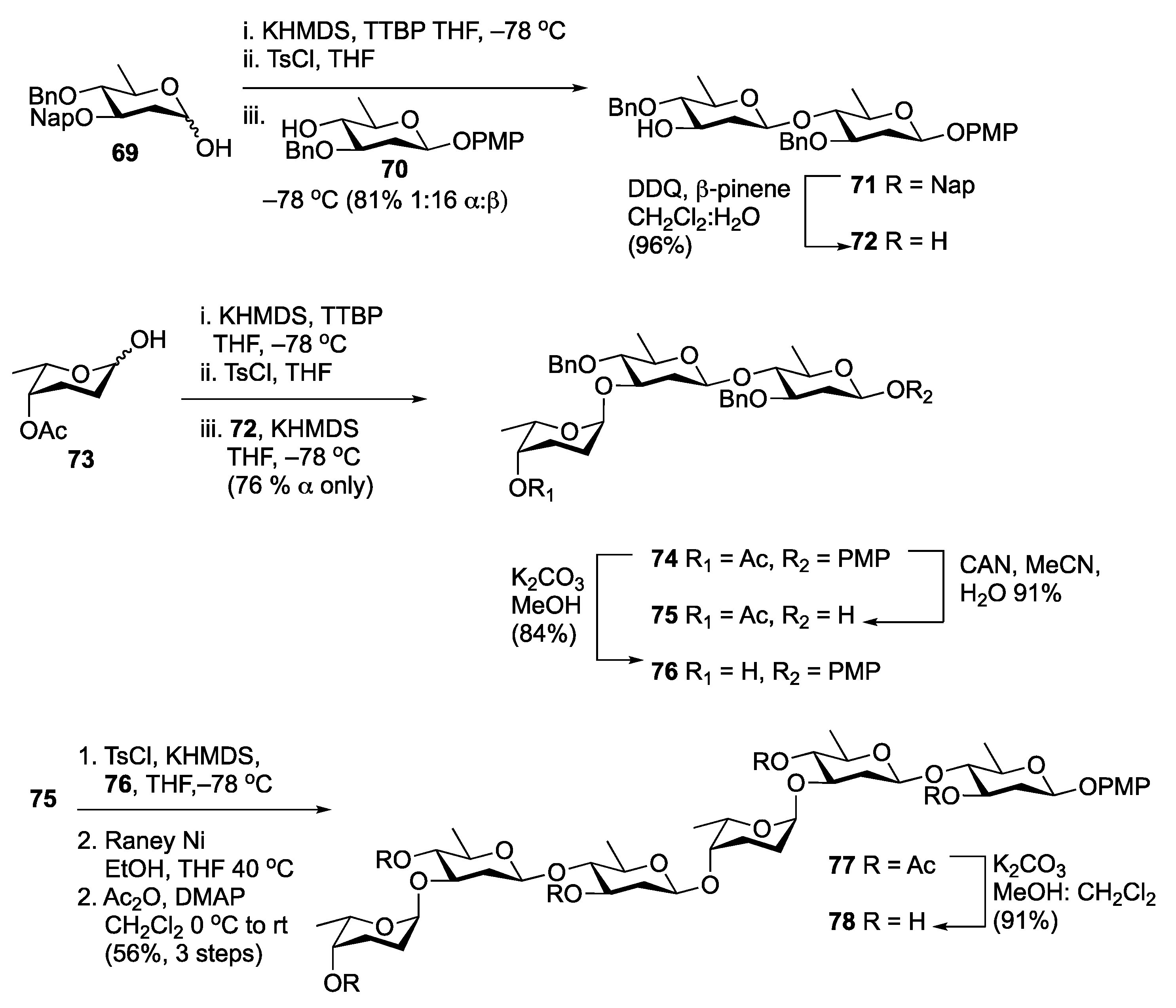
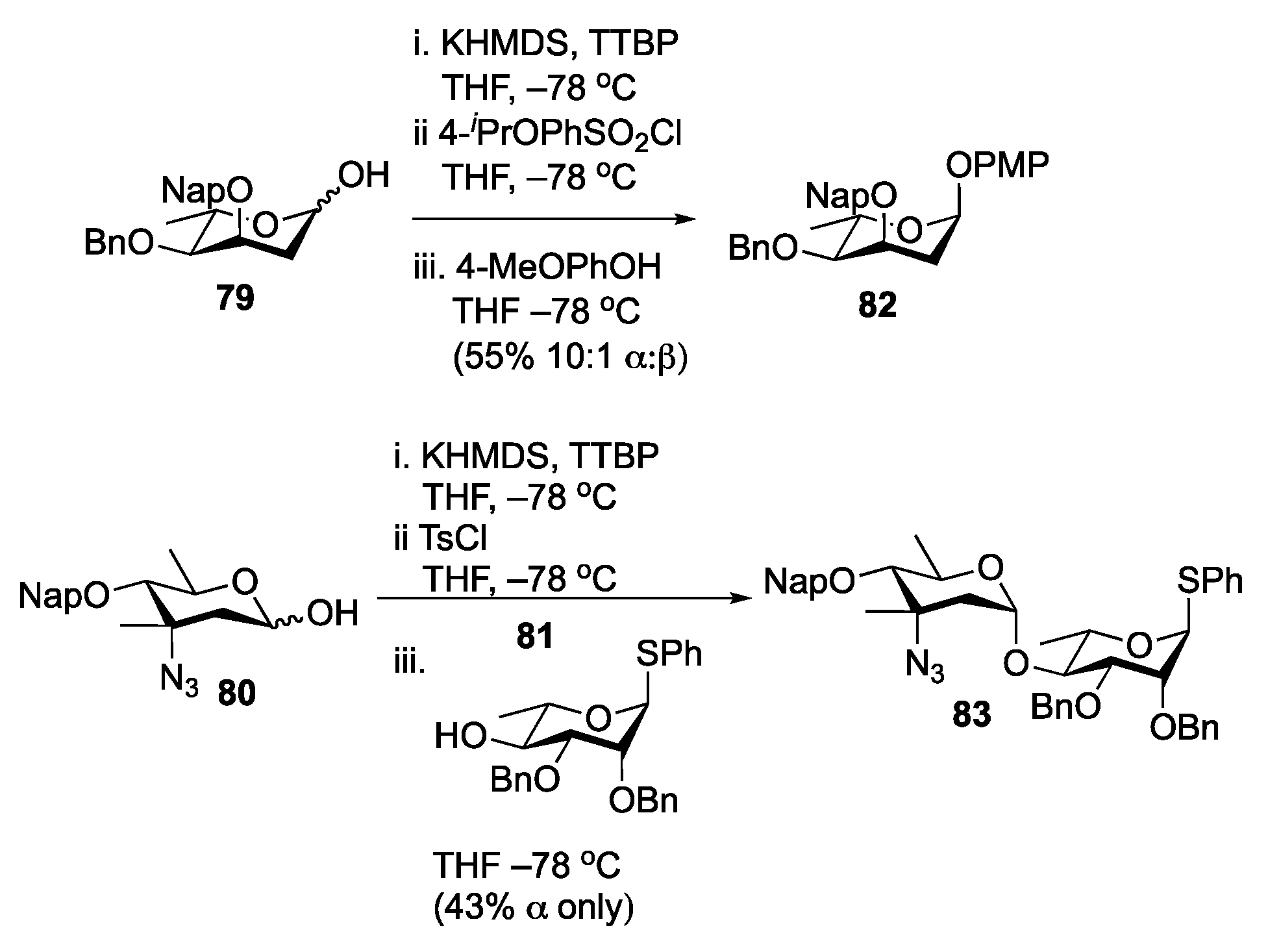


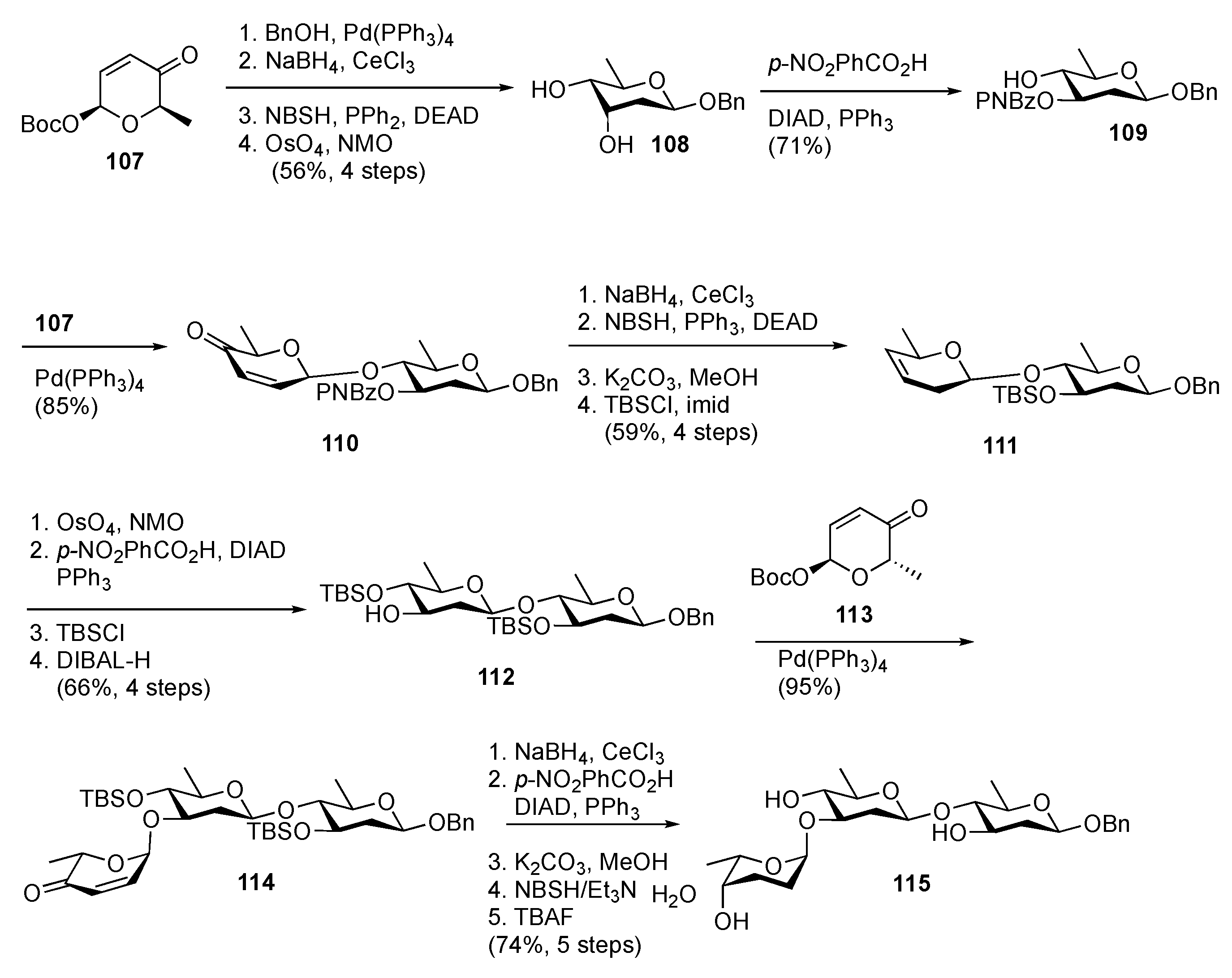
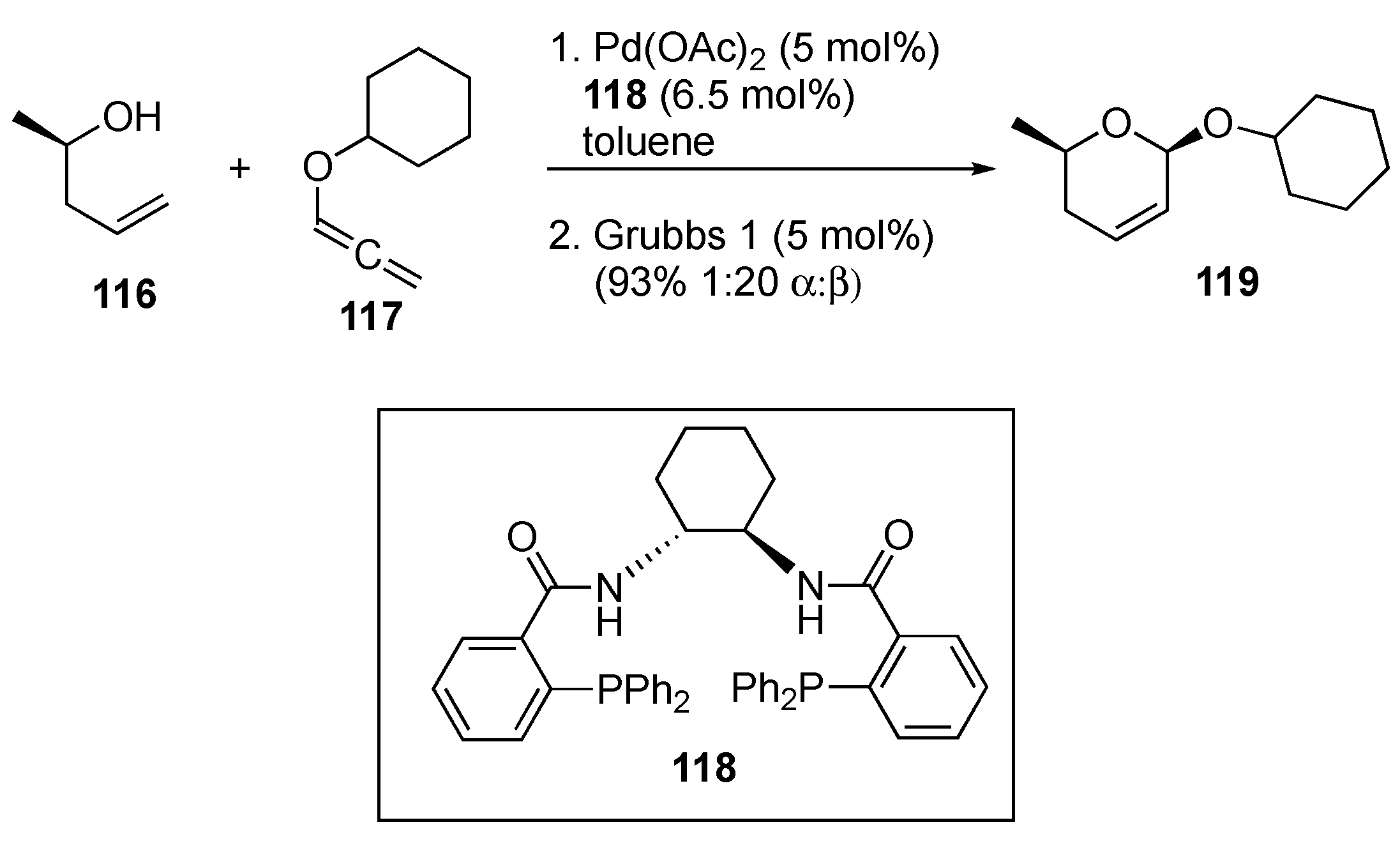
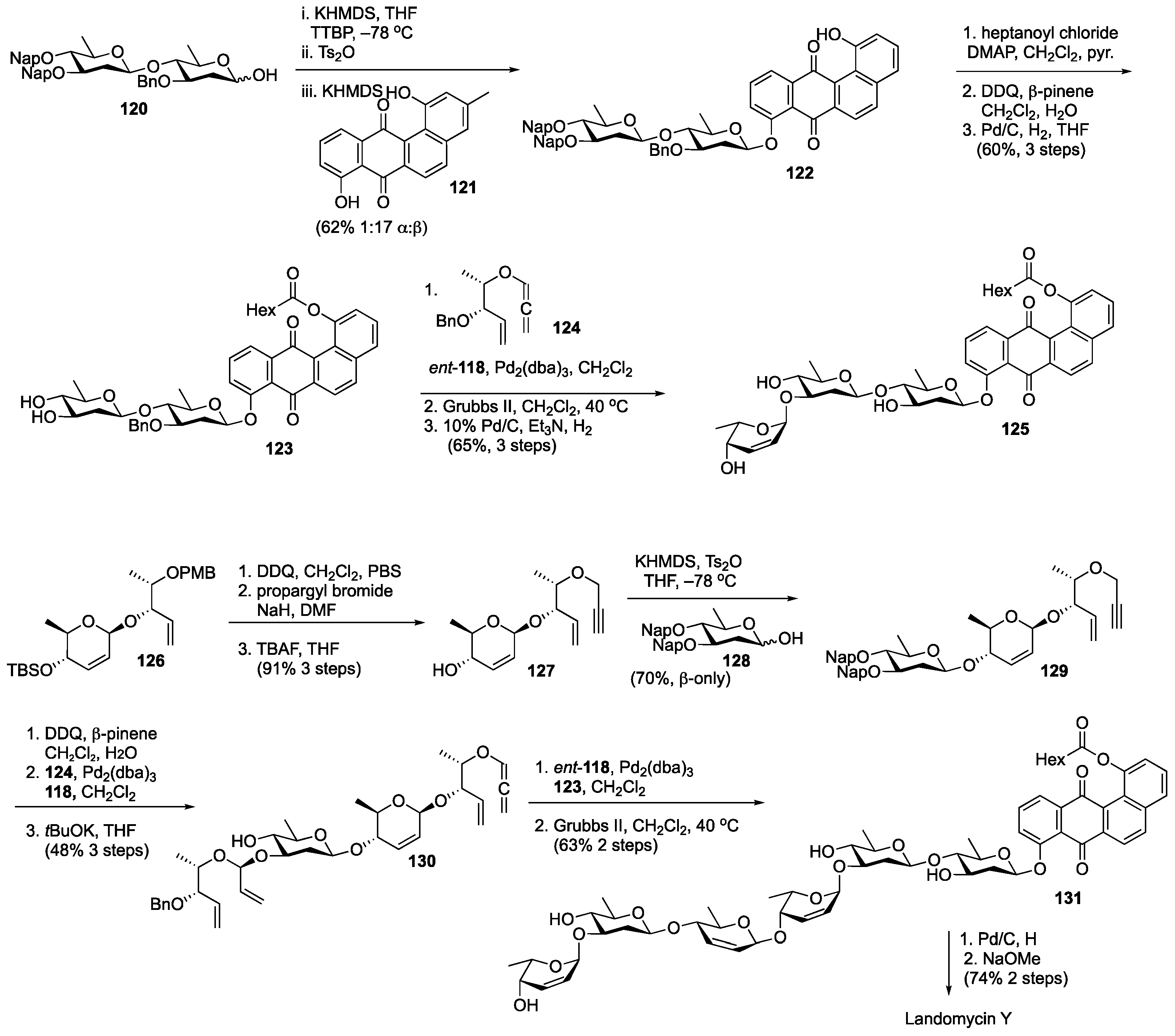
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennett, C.S. Stereoselective Approaches to β-Linked 2-Deoxy Sugars. Molecules 2025, 30, 1578. https://doi.org/10.3390/molecules30071578
Bennett CS. Stereoselective Approaches to β-Linked 2-Deoxy Sugars. Molecules. 2025; 30(7):1578. https://doi.org/10.3390/molecules30071578
Chicago/Turabian StyleBennett, Clay S. 2025. "Stereoselective Approaches to β-Linked 2-Deoxy Sugars" Molecules 30, no. 7: 1578. https://doi.org/10.3390/molecules30071578
APA StyleBennett, C. S. (2025). Stereoselective Approaches to β-Linked 2-Deoxy Sugars. Molecules, 30(7), 1578. https://doi.org/10.3390/molecules30071578





