2.1. Spectral Properties and Research of Sensor TQA
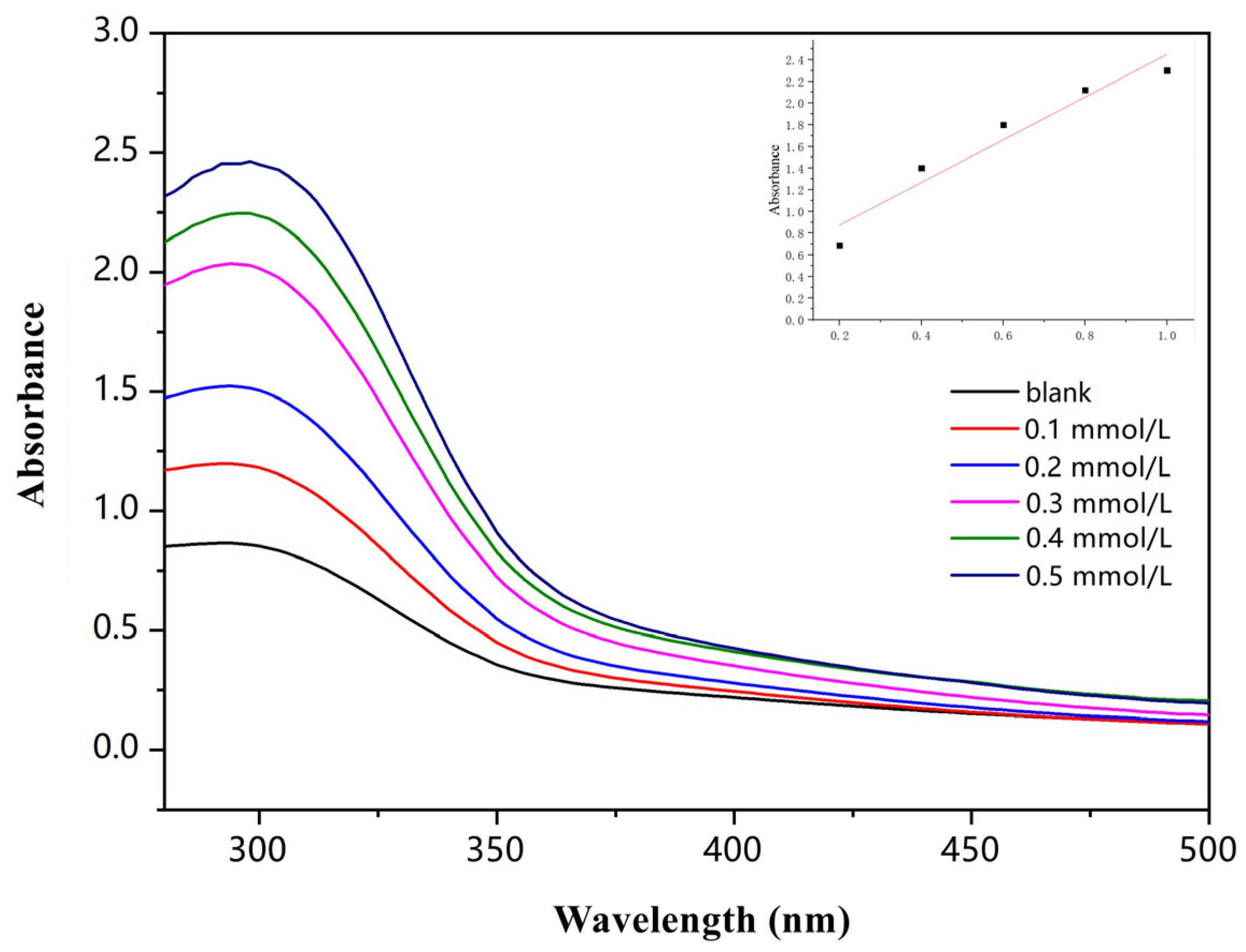
The UV–visible absorption spectrum of Sensor TQA for different concentrations of Fe
3+ is illustrated in
Figure 1. Sensor TQA was prepared in a 0.5 mmol/L DMF: water (1:1,
v:
v) solution. The maximum absorption peak of blank Sensor TQA appeared at 301 nm. Under the same testing environment, 0.1 mmol/L, 0.2 mmol/L, 0.3 mmol/L, 0.4 mmol/L, 0.5 mmol/L of Fe
3+ were added to the solution of Sensor TQA. As the Fe
3+ concentration increased, absorbance at 301 nm gradually increased, and the linear fitting of different concentrations and absorbance at 301 nm were carried out; therefore, in subsequent tests, 301 nm was selected as the excitation wavelength for fluorescence emission spectroscopy testing.
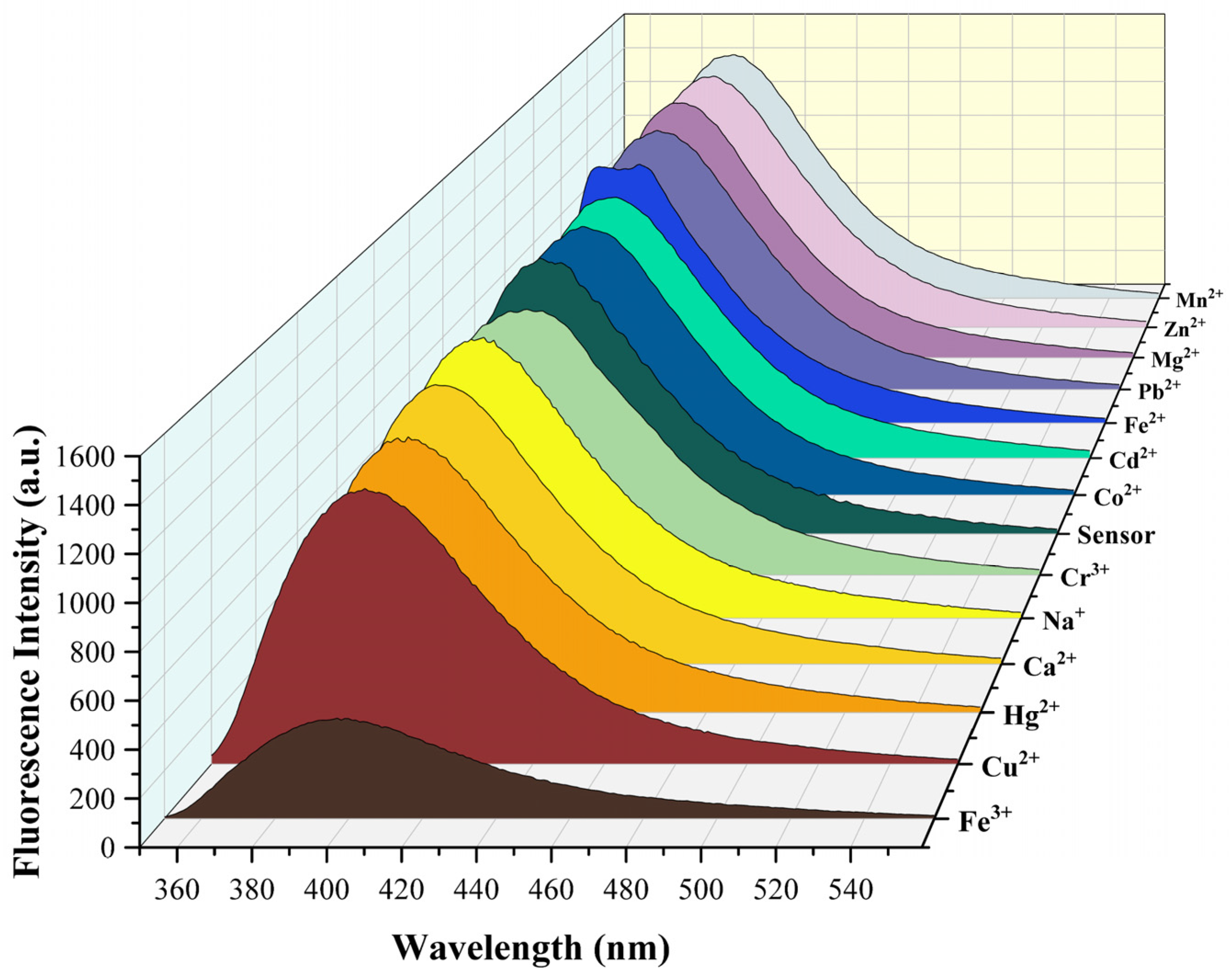
Next, in order to test the selectivity of Sensor TQA, the prepared metal ion solutions were dissolved in a HEPES-NaOH buffer solution (pH = 7.4). Sensor TQA solution was prepared in
N,
N-Dimethylformamide (DMF). The solvent condition in the experiment is that the ratio of organic phase to aqueous phase is 1:1. Solutions of common metal ions (Na
+, Mg
2+, Zn
2+, Cd
2+, Ca
2+, Mn
2+, Pb
2+, Cu
2+, Hg
2+, Cr
3+, Fe
3+, Fe
2+, Co
2+, pH = 7.4) with equal volume and concentration were added to the solution of Sensor TQA (DMF: water = 5:5,
v:
v). The fluorescence responses of Sensor TQA (0.5 mmol/L) to different metal ions (0.5 mmol/L) are illustrated in
Figure 2 (λ
ex = 301 nm). The maximum emission peak of blank Sensor TQA in the fluorescence spectrum appeared at 397 nm, and only Fe
3+ significantly reduced the fluorescence intensity of Sensor TQA. However, the addition of other metal ions at the same concentration did not change the fluorescence intensity of Sensor TQA; therefore, it can be concluded that Sensor TQA has good selectivity for Fe
3+. Subsequently, to further demonstrate the detection capability of Sensor TQA for Fe
3+, tests were conducted in a more complex system. In addition, in order to verify whether common anions affect the fluorescence emission of Sensor TQA, common anions were added to the Sensor TQA solution under the same test conditions. As shown in
Figure S7, the anions did not affect the fluorescence emission of the sensor.
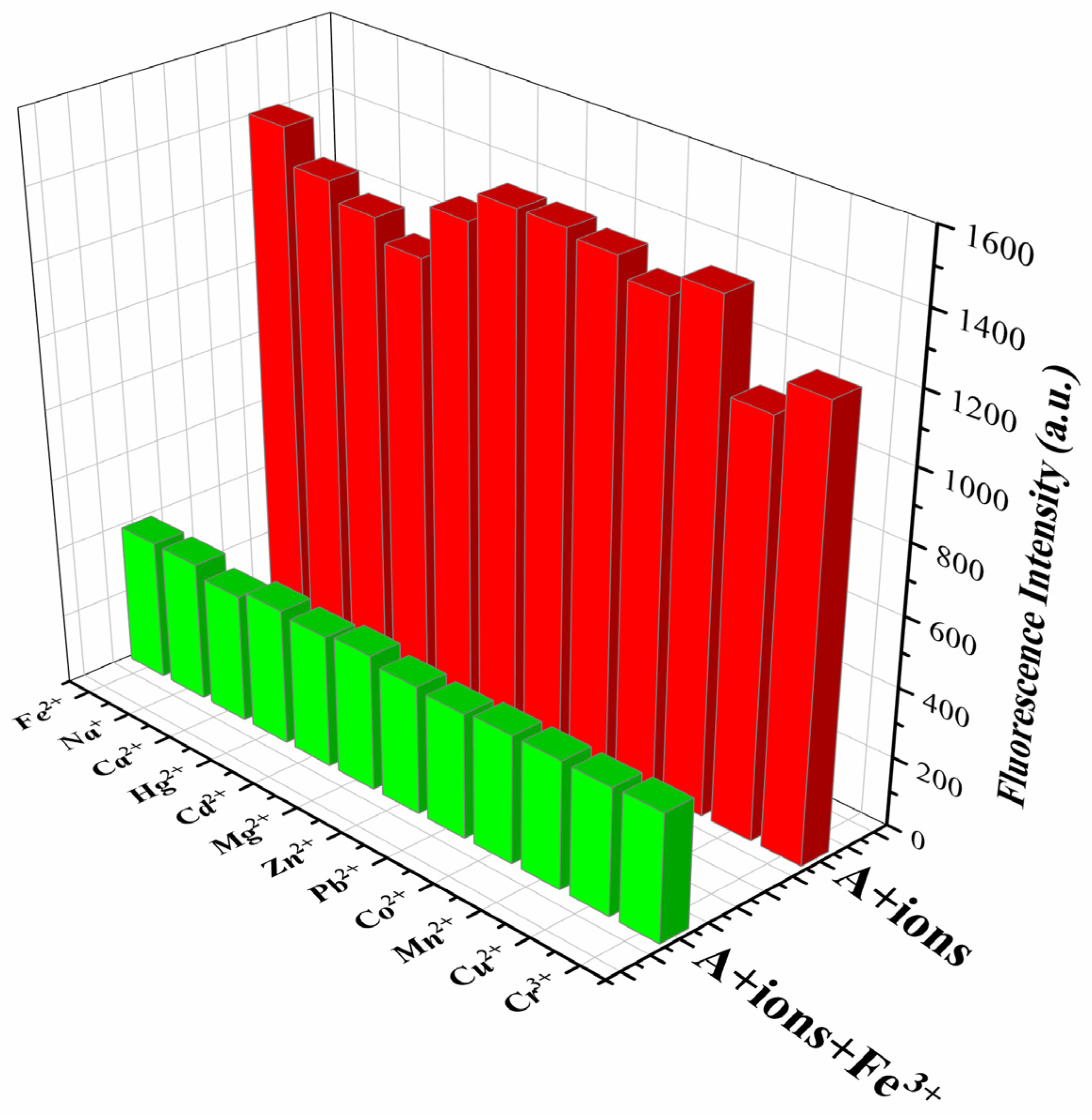
The ability to effectively detect ions under complex conditions is an important criterion for evaluating the performance of fluorescence sensors. Therefore, a series of common metal cations were selected for competitive experiments on Sensor TQA. Before the experiment, the solution of Sensor TQA (0.5 mmol/L) was induced to undergo fluorescence quenching by equal volume and concentration of the Fe
3+ solution (pH = 7.4). Then, 10 equivalents of other cations were added (Na
+, Mg
2+, Fe
2+, Zn
2+,Cd
2+, Ca
2+, Mn
2+, Pb
2+, Cu
2+, Hg
2+, Cr
3+, Co
2+). The emission spectrum was recorded with a fluorescence spectrophotometer. The maximum fluorescence intensity at 397 nm was selected as the data for the competitive experiment. The experimental results are shown in
Figure 3. The green bar represents the competitive experimental data; in addition, the results of selective experiments (red bar) are also used as a reference. It can be seen that the introduction of 10 equivalents of other cations (5 mmol/L) did not significantly affect Fe
3+ recognition based on fluorescence quenching. This indicates that Sensor TQA is able to produce a targeted fluorescence intensity response to Fe
3+ under complex conditions, which also proves that Sensor TQA has good selectivity and anti-interference ability, and it provides a certain guarantee for detection under actual complex conditions. Therefore, Sensor TQA can be considered as a potentially valuable qualitative analysis tool for Fe
3+.
In order to further determine the effect of Fe
3+ on the fluorescence emission spectrum of Sensor TQA, the fluorescence spectrum response of fluorescent molecular Sensor TQA to Fe
3+ for different concentration conditions (0.05 mmol/L~0.5 mmol/L) was studied. In
Figure 4, it is easy to perceive that the fluorescence emission of the blank solution of Sensor TQA is very strong. As the concentration of Fe
3+ added to the solution continues to increase, the fluorescence quenching effect of Sensor TQA becomes more and more obvious in the emission spectrum. When the Fe
3+ concentration increases to 0.5 mmol/L, the fluorescence intensity of Sensor TQA is quenched by about 70%, indicating that Sensor TQA is completely bound to iron ions and has high sensitivity at this time. In order to determine whether Sensor TQA can carry out a quantitative analysis of Fe
3+, the fluorescence intensity of Sensor TQA solution at 397 nm was selected and plotted against the corresponding Fe
3+ concentration data. The fluorescence intensity of Sensor TQA solution showed a linear weakening trend with the increasing concentration of Fe
3+, and a good linear relationship was observed. After linear fitting, the linear equation y = −494.91x + 0.34 (R
2 = 0.9936) was obtained, indicating that Sensor TQA is able to perform quantitative detection of Fe
3+.
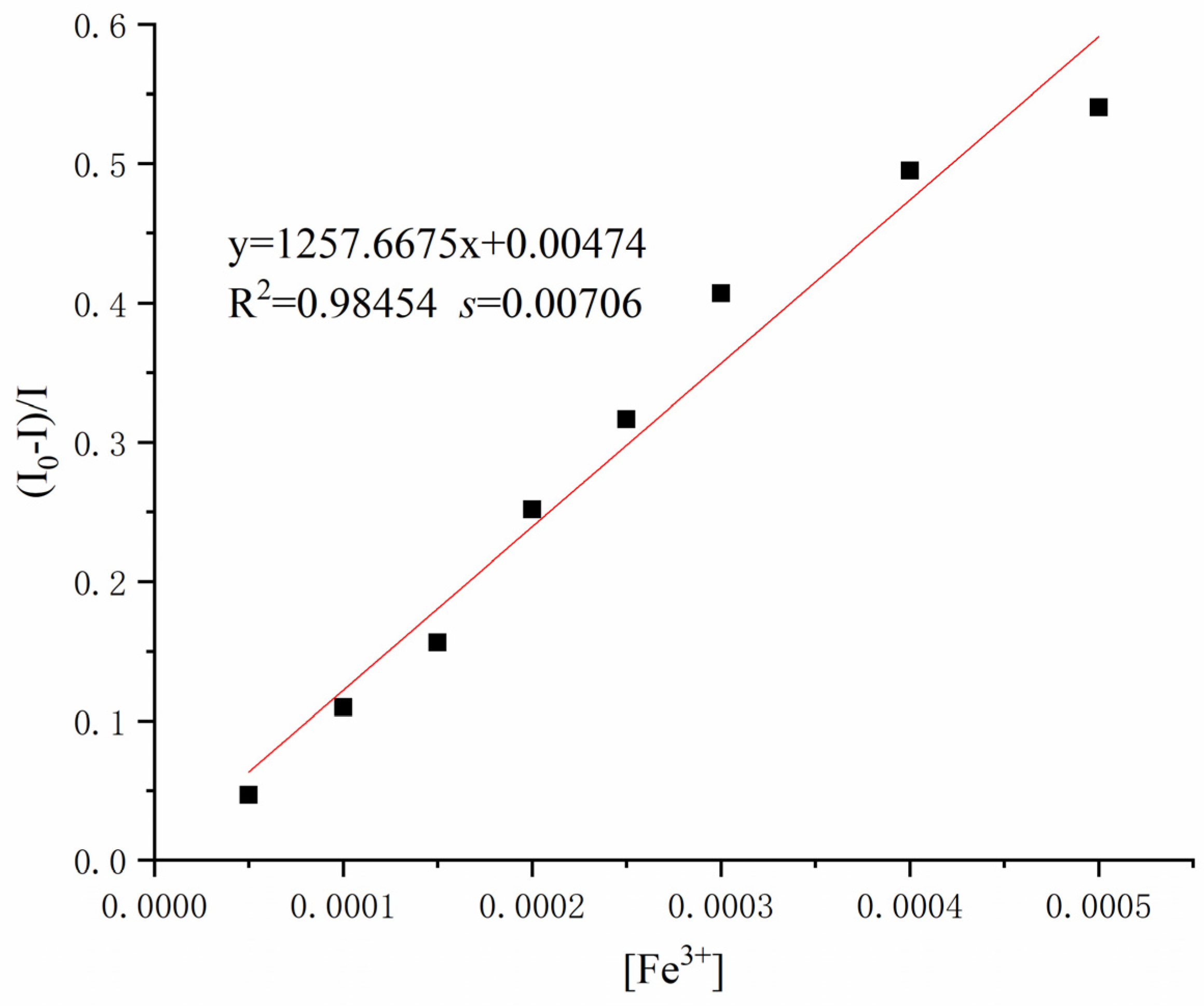
To further study the detection ability of Sensor TQA for Fe
3+, the Stern–Volmer equation was used to estimate the detection limit, where (
I0 −
I)/
I is plotted on the
Y-axis vs. [Fe
3+] on the
x-axis, and
I0 is the fluorescence intensity of Sensor TQA, while
I is the fluorescence intensity of Sensor TQA–Fe
3+. As illustrated in
Figure 5, after linear fitting, the linear equation y = 1257.6675x + 0.00474 (R
2 = 0.98454,
s = 0.00706) was obtained. According to the detection limit in Equation (1),
s is the standard deviation of replication measurements, and
m is the slope of the calibration curve [
22]. The detection limit of Sensor TQA for Fe
3+ is calculated to be 0.16841 μM.
The fluorescence quantum yields of Sensor TQA and Sensor TQA–Fe
3+ were determined according to Equation (2), where F
U and F
S denote the integral fluorescence intensity of the determinand and standard substance; A
U and A
S denote the relevant maximum absorbance of the determinand and standard substance, respectively (quinine sulphate was used as a reference quantum yield standard, λ
ex = 410 nm, quantum yield = 0.54 in 0.1 M H
2SO
4). The quantum yields for Sensor TQA and Sensor TQA–Fe
3+ calculated from the equation in aqueous ethanol were 0.053 and 0.31, respectively.
To understand the complexation ability and binding ratio of Sensor TQA molecule with Fe
3+, the modified Stern–Volmer Equation (3) was used to calculate the complexation constant and complexation ratio of Sensor TQA to Fe
3+ [
23]. In the equation,
KSV is the Stern–Volmer constant;
n is the number of binding sites; and
Q is the concentration of Fe
3+. By taking the logarithm value of the data in
Figure 6, the linear equation of the modified Stern–Volmer equation is obtained; it can be calculated that
KSV is 2.767 × 10
3 M
−1, and
n is 1.11. Therefore, the complexation constant of Sensor TQA with Fe
3+ is 2.767 × 10
3 M
−1, and the complexation ratio of Sensor TQA with Fe
3+ is 1:1.
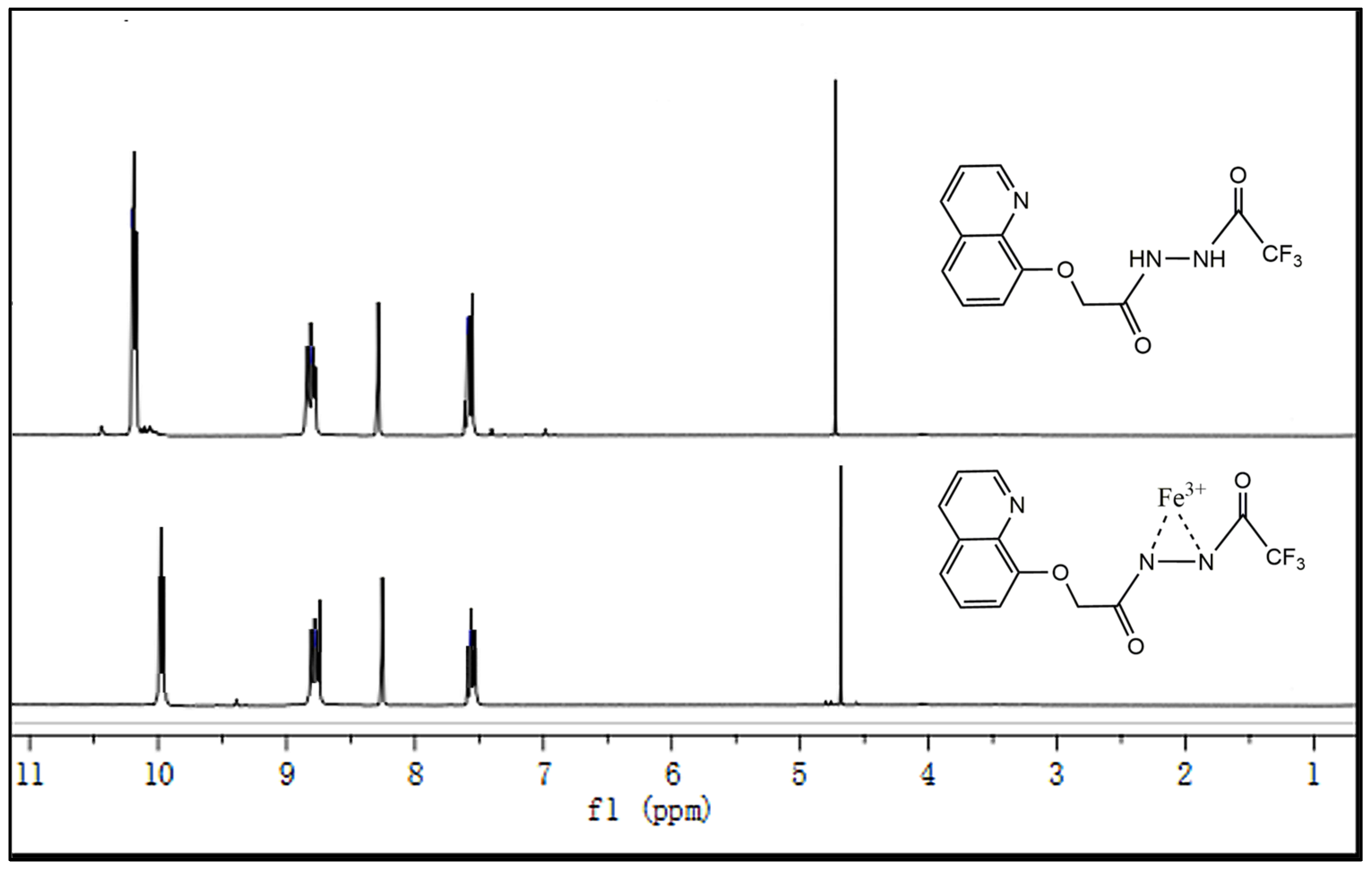
To obtain further details of the co-ordination between Sensor TQA and Fe
3+, we carried out
1H NMR titration experiments in DMSO-
d6 (containing 10% D
2O). As shown in
Figure 6, upon the addition of Fe
3+ (10 μmol/L) dissolved in D
2O/DMSO-
d6 (1:9,
v:
v), the chemical shift in the protons of Sensor TQA (10 μmol/L) in DMSO-
d6 (containing 10% D
2O) changed, especially in the two NH groups, which verified that the two nitrogen atoms are involved in co-ordination with Fe
3+.
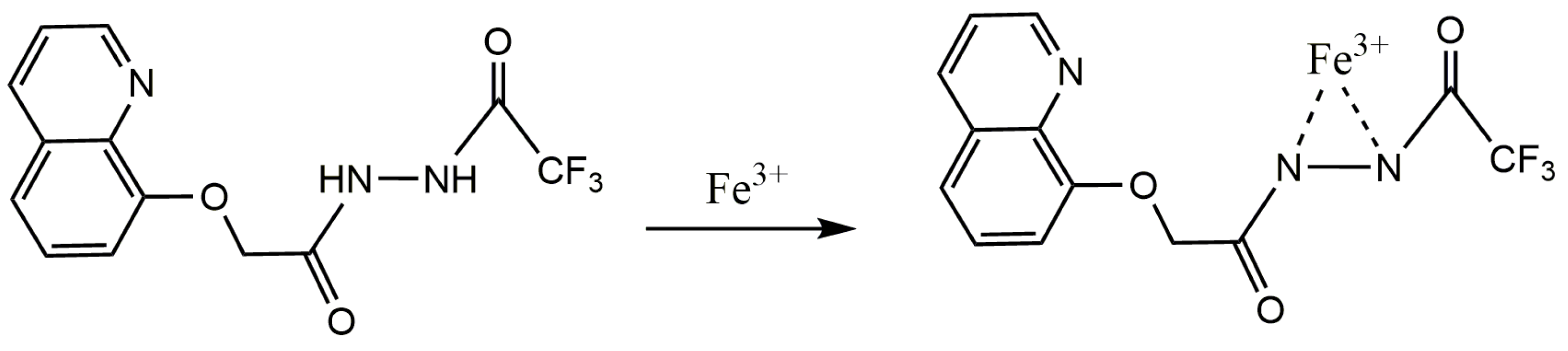
Based on the experimental results of
1H NMR titration and the 1:1 binding ratio between Sensor TQA and Fe
3+, the possible binding model between Sensor TQA and Fe
3+ is further speculated, as shown in
Figure 7. Based on the electronic configuration of Fe
3+, the detection mechanism of Sensor TQA may be attributed to the spin forbidden by the n–π transition that occurs when Sensor TQA binds to Fe
3+ during excitation. The lone-pair electrons of the N atom used for co-ordination undergo non-radiative relaxation, suppressing the original electron transition process and further generating the effect of PET, resulting in significant fluorescence quenching of Sensor TQA.
2.2. Theoretical Calculation Research
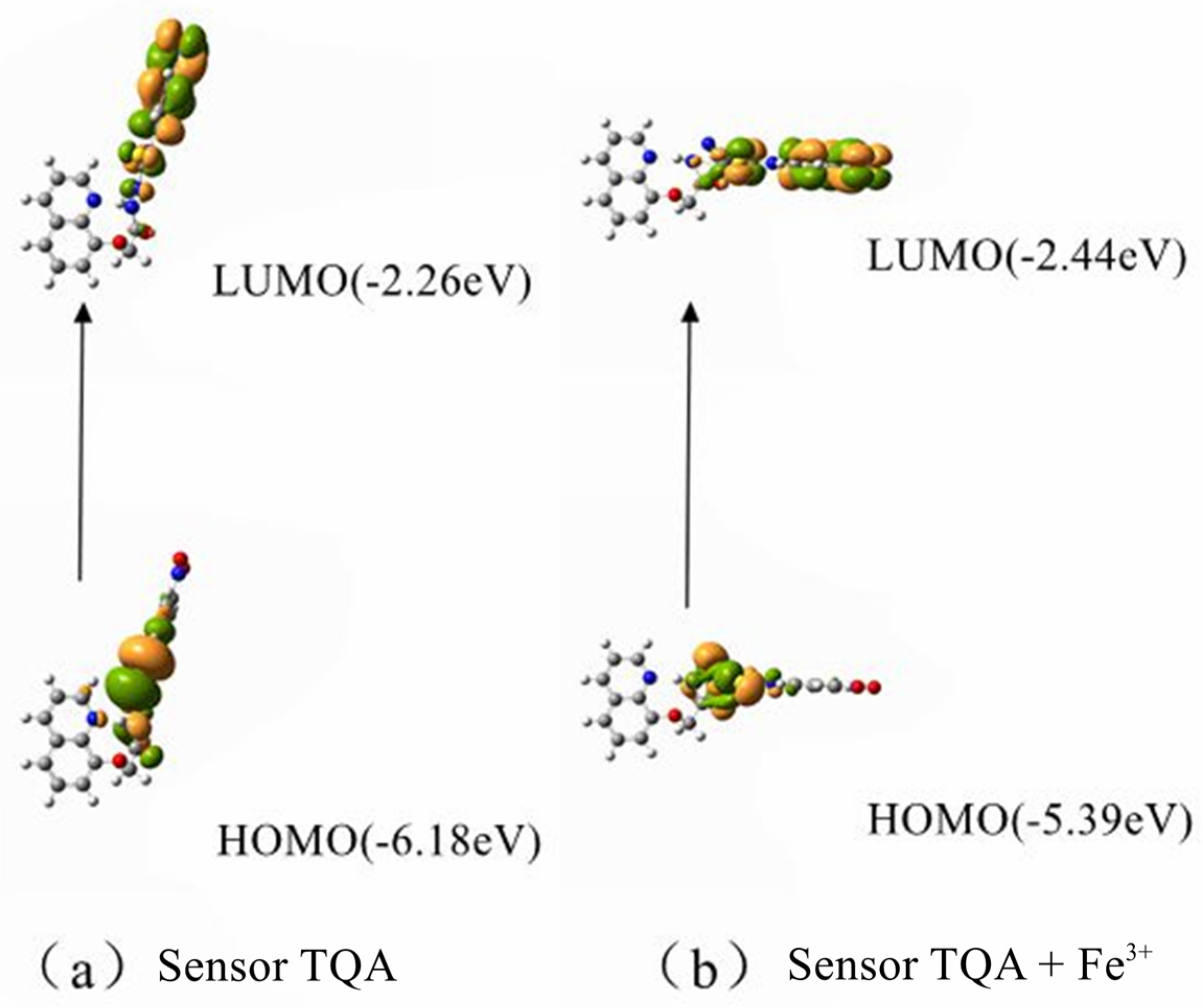
To better analyze the spatial electron cloud arrangement and sensing mechanism before and after the interaction between Sensor TQA and Fe
3+, based on quantum chemistry, Gaussian software (Version c01) was used to systematically calculate the spatial electron cloud arrangement of Sensor TQA. Functional theory (PBE0) was selected, and Gaussian 09 software (Version c01) was used for calculations with 3–21G as the basis set. As shown in
Figure 8, the HOMO and LUMO of Sensor TQA are mainly distributed on the trifluoroacetyl group, and the arrangement of HOMO and LUMO also indicates that electrons are capable of transitioning well to the excited state. The synthesized Sensor TQA has a good conjugated structure and is rich in electrons, which are able to efficiently bind with Fe
3+. The emission peak of the fluorescent molecule is generated by S
0, corresponding to HOMO → LUMO. The fluorescence emission peak of the product molecule is larger than the emission peak wavelength of the sensor molecule, and the large difference in fluorescence signal is conducive to fluorescence detection and practical applications. All theoretical calculation results are in good agreement with actual experimental test data, which also verifies the scientific validity of the experimental results from a theoretical perspective.
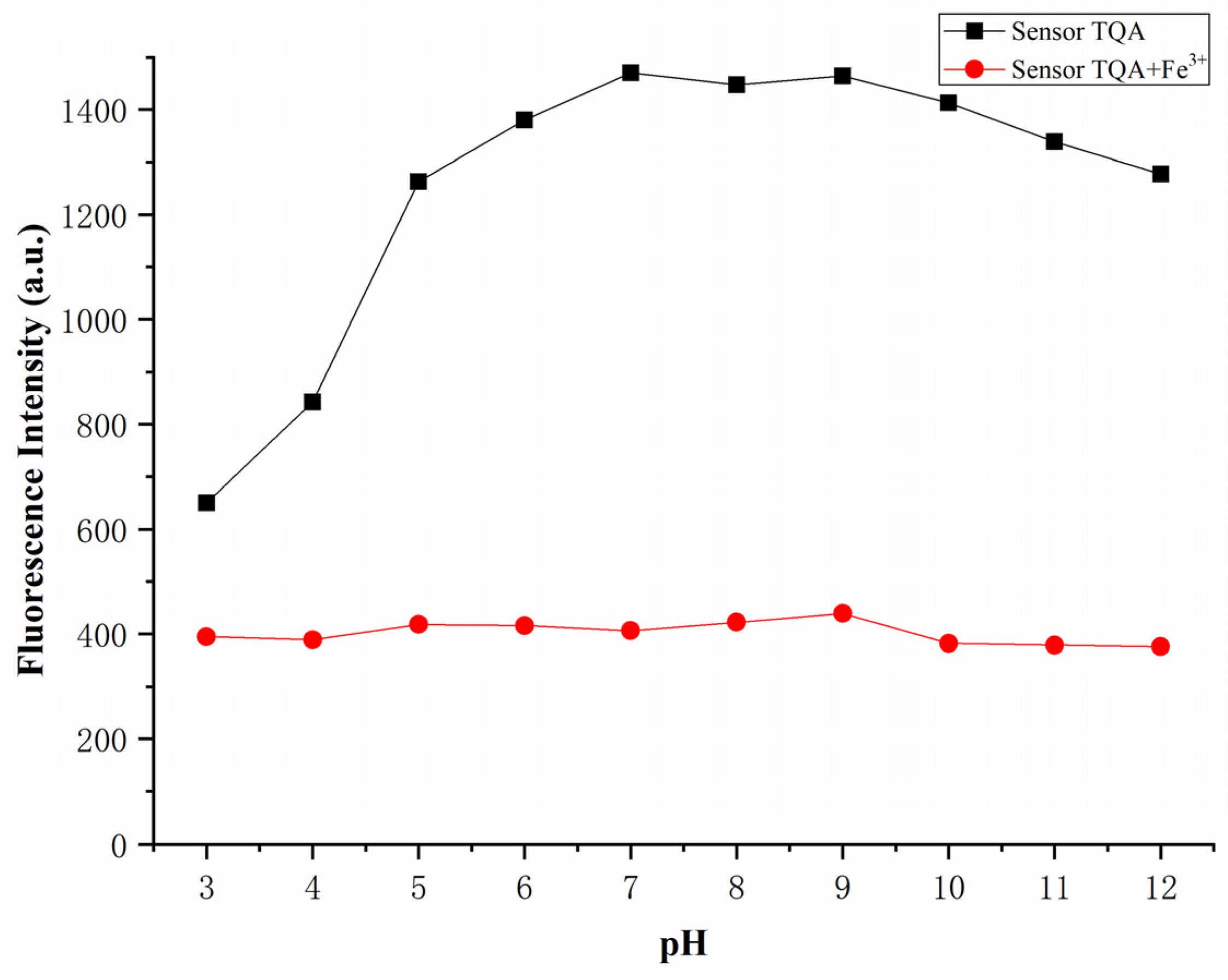
The effects of pH on the fluorescence intensities of Sensor TQA and Sensor TQA–Fe
3+ were tested to verify their properties under extreme acid-base conditions. In order to maintain the pH under different test conditions, the following buffer systems were added, respectively: HCl (pH = 3–4), NaAc-HAc (pH = 5–6), HEPES-NaOH (pH = 7), NH
3-NH
4Cl (pH = 8–11), NaOH (pH = 12). As shown in
Figure 9, the independent Sensor TQA could maintain fluorescence emission at pH = 5–12, while the high acidic conditions had an obvious impact on fluorescence intensity. This may be due to the fluorescence quenching caused by high hydrogen ion concentration. Nevertheless, the pH had little effect on Sensor TQA–Fe
3+; therefore, we believe that Sensor TQA could effectively detect Fe
3+ within a wide pH range.
2.3. Research on Biological Applications of Fluorescence Sensor TQA
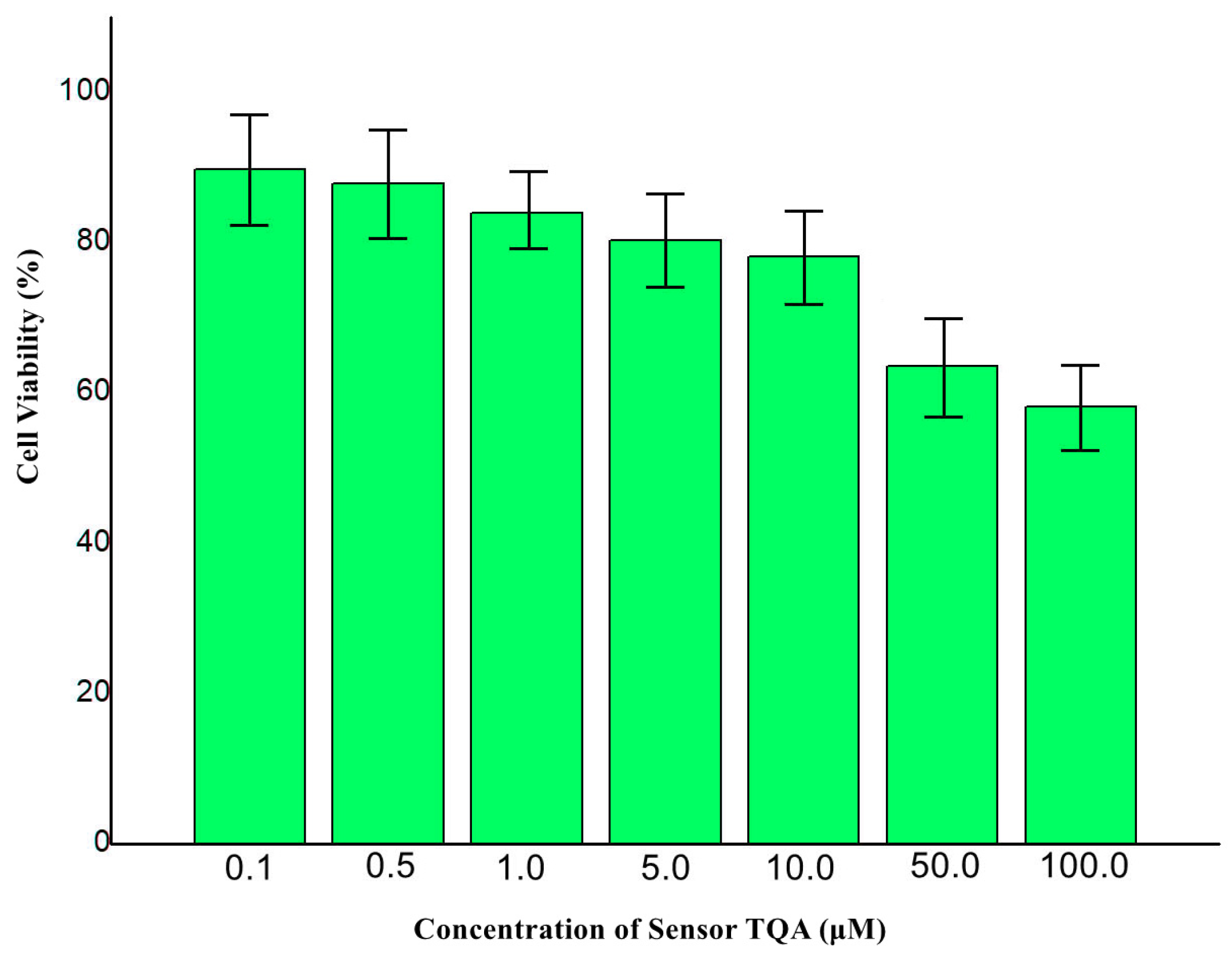
To research the biological application of Sensor TQA, a cytotoxicity test was adopted for live liver cancer cells. A concentration-dependent cell viability assay was conducted under 0.1 μM, 0.5 μM, 1.0 μM, 5.0 μM, 10.0 μM, 100.0 μM Sensor TQA. As illustrated in
Figure 10, cell viabilities could maintain a high level under the concentrations of 0.1 µM–10.0 µM. However, when the concentration reached 50 µM, cell viability decreased to below 80%, and the IC
50 value was ascertained as 201.6 μM, the same level as many reported fluorescent sensors [
24,
25,
26]. Therefore, we believe that the TQA sensor still has certain cytotoxicity. The reason for this may be that the sensor TQA, under high concentration conditions, causes damage to the cell membrane or interferes with cell metabolism, leading to a decrease in cell viability.
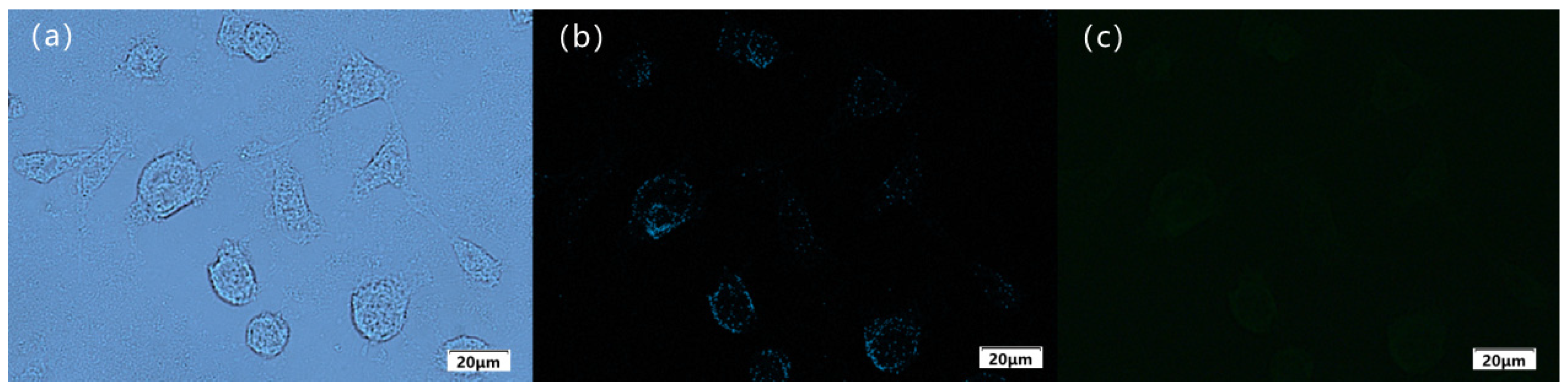
To further investigate the biocompatibility of Sensor TQA and its practical application in the field of biological tracing, live liver cancer cells (HepG2) were selected to further test the Fe
3+ detection ability of Sensor TQA. First, pre-cultured liver cancer cells were removed from the incubator. A micropipette was used to aspirate the cell culture medium. Cells were rinsed with phosphate-buffered solution (PBS) at pH = 7.4; the process was repeated three times to ensure removal of the culture medium. A volume of 30 µM of Sensor TQA (dissolved in DMSO) was added to the cell culture plate. Cells were cultivated at room temperature for 30 min until Sensor TQA completely entered the liver cancer cells. Rinsing was continued with phosphate-buffered solution (PBS) and repeated three times. Finally, cells were transferred to a fluorescence microscope for observation and imaging. As shown in
Figure 11a,b, the contour of the cultured cells is good and exhibits obvious fluorescence inside the cell under a fluorescence microscope, indicating that Sensor TQA is able to penetrate the cell membrane and be absorbed by the cells. Afterward, the prepared Fe
3+ (30 µM) solution was further added to the cells and cultured for 30 min for fluorescence imaging. As shown in
Figure 11c, the fluorescence intensity of the cells significantly decreased, with almost no fluorescence. In the fluorescence imaging experiment, it can be found that Sensor TQA is able to penetrate the cell membrane and enter the cell to recognize Fe
3+, indicating that Sensor TQA has good biocompatibility and potential applications in the field of biological tracing.
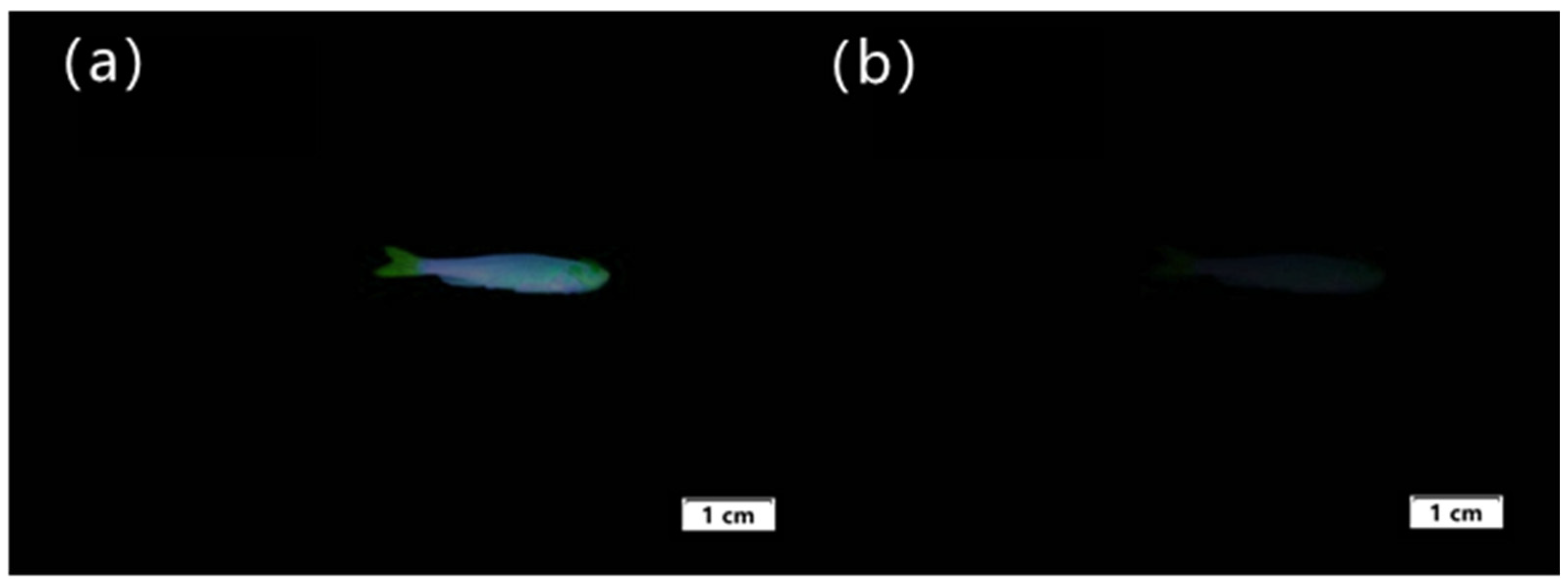
After verifying that Sensor TQA is capable of performing fluorescence imaging in cells, we continued to study its biosensing performance and selected zebrafish for the animal specimen experiments. Zebrafish is a common tropical fish with strong vitality and a similarity of up to 87% with human genes. It is approximately 2–4 cm in length and is usually used for biological imaging experiments. Therefore, we further validated the biocompatibility of Sensor TQA using zebrafish. At room temperature, zebrafish were cultured in sterile water for 24 h. After changing the water three times, the cultured zebrafish were added to an aqueous solution containing Sensor TQA (30 µM) and cultured for 1 h. The zebrafish were then removed and washed three times with HEPES-NaOH buffer solution. Subsequently, fluorescence imaging experiments were conducted. In
Figure 12a, it can be found that zebrafish absorbed Sensor TQA and exhibited significant fluorescence emission. The morphology of zebrafish can be clearly observed. Afterward, Fe
3+ was added to the culture medium to further culture the zebrafish for 1 h. After washing the zebrafish three times with the HEPES-NaOH buffer solution, the image of zebrafish under UV light shown in
Figure 12b was obtained. It is difficult to observe fluorescence emission from zebrafish, and only the outline of zebrafish is roughly observed. The experimental results showed that Fe
3+ added to the culture medium reacted chemically with Sensor TQA-covered zebrafish, resulting in a significant decrease in fluorescence intensity. This indirectly proves the biocompatibility of Sensor TQA, which can be used in the field of practical biological imaging and has certain application value.