Recovering Iron Concentrate from Low-Grade Siderite Tailings Based on the Process Mineralogy Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Process Mineralogy Characteristics
2.3. Flash Suspension Roasting-Magnetic Separation Procedure
2.4. Recovery of Concentrate
3. Results and Discussion
3.1. The Iron Phase Compositions
3.2. Particle Size Distribution Characteristics of Major Iron Minerals
3.3. Liberation Degree and Intergrowth Characteristics of Major Iron Minerals
3.4. Application in Flash Suspension Roasting-Magnetic Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florén, H.; Frishammar, J.; Löf, A.; Ericsson, M. Raw materials management in iron and steelmaking firms. Miner. Econ. 2019, 32, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Cen, P.; Bian, X.; Liu, Z.; Gu, M.; Wu, W.; Li, B. Extraction of rare earths from bastnaesite concentrates: A critical review and perspective for the future. Miner. Eng. 2021, 171, 107081. [Google Scholar] [CrossRef]
- Fijorek, K.; Jurkowska, A.; Jonek-Kowalska, I. Financial contagion between the financial and the mining industries–Empirical evidence based on the symmetric and asymmetric CoVaR approach. Resour. Policy 2021, 70, 101965. [Google Scholar] [CrossRef]
- Mishra, D.P.; Swain, S.K. Global trends in reserves, production and utilization of iron ore and its sustainability with special emphasis to India. J. Mines Met. Fuels 2020, 68, 11–18. [Google Scholar]
- Roy, S.K.; Nayak, D.; Rath, S.S. A review on the enrichment of iron values of low-grade Iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- Yu, J.; Han, Y.; Li, Y.; Gao, P. Recent advances in magnetization roasting of refractory iron ores: A technological review in the past decade. Miner. Process. Extr. Metall. Rev. 2020, 41, 349–359. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, P.; Han, Y.; Guo, W. Fluidized bed roasting technology in iron ores dressing in China: A review on equipment development and application prospect. J. Miner. Metall. B 2019, 55, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, X.; Han, Y.; Li, Y. A new approach for recovering iron from iron ore tailings using suspension magnetization roasting: A pilot-scale study. Powder Technol. 2020, 361, 571–580. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Han, Y.; Li, Y. Green magnetization roasting technology for refractory iron ore using siderite as a reductant. J. Clean. Prod. 2019, 206, 40–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Producing magnetite concentrate via self-magnetization roasting in N2 atmosphere: Phase and structure transformation, and extraction kinetics. J. Ind. Eng. Chem. 2021, 104, 571–581. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Gao, P.; Li, Y. Thermal Decomposition Kinetics of Siderite Ore during Magnetization Roasting. Min. Metall. Explor. 2021, 38, 1497–1508. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y. Pyrolysis behavior of a green and clean reductant for suspension magnetization roasting. J. Clean. Prod. 2020, 268, 122173. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Li, Y.; Sun, Y. Effect of heating rate on pyrolysis behavior and kinetic characteristics of siderite. Minerals 2017, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Sun, Y.; Han, Y. Research on Kinetics of FeO Magnetization Reaction in the Thermal Decomposition of Siderite. Met. Mine 2019, 48, 50. [Google Scholar]
- Feng, Z.; Yu, Y.; Liu, G.; Chen, W. Thermal decomposition kinetics of siderite in nitrogen. J. Wuhan Univ. Technol. 2009, 31, 11–14. [Google Scholar]
- Yuan, S.; Zhou, W.; Han, Y.; Li, Y. Efficient enrichment of low-grade refractory rhodochrosite by preconcentration-neutral suspension roasting-magnetic separation process. Powder Technol. 2020, 361, 529–539. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.; Han, Y.; Li, Y. Individual enrichment of manganese and iron from complex refractory ferromanganese ore by suspension magnetization roasting and magnetic separation. Powder Technol. 2020, 373, 689–701. [Google Scholar] [CrossRef]
- Liu, J.; Nie, Q.; Han, Y. Effect of particle size of suspension roasting of oolitic hematite. J. China Univ. Min. Technol. 2018, 2, 415–420. (In Chinese) [Google Scholar]
- Gao, P.; An, Y.; Li, G.; Han, Y. Effect of particle size on reduction kinetics of hematite ore in suspension roaster. Physicochem. Probl. Miner. Processing 2020, 56, 449–459. [Google Scholar] [CrossRef]
- Jiao, Y.; Qiu, K.H.; Zhang, P.C.; Li, J.F.; Zhang, W.T.; Chen, X.F. Process mineralogy of Dalucao rare earth ore and design of beneficiation process based on AMICS. Rare Met. 2020, 39, 959–966. [Google Scholar] [CrossRef]
- Hu, W.; Tian, K.; Zhang, Z.; Guo, J.; Liu, X.; Yu, H.; Wang, H. Flotation and Tailing Discarding of Copper Cobalt Sulfide Ores Based on the Process Mineralogy Characteristics. Minerals 2021, 11, 1078. [Google Scholar] [CrossRef]
- Xu, W.; Shi, B.; Tian, Y.; Chen, Y.; Li, S.; Cheng, Q.; Mei, G. Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite fromGuizhou, China. Minerals 2021, 11, 1249. [Google Scholar] [CrossRef]
- Qu, S. Process mineralogy of an iron ore from Daxigou. Min. Met. Eng. 2019, 39, 70–72. [Google Scholar]
- Gu, Y. Automated scanning electron microscope based mineral liberation analysis an introduction to JKMRC/FEI mineral liberation analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-Based Mineral Liberation Analysis. Inter. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Sandmann, D. Use of mineral liberation analysis (MLA) in the characterization of lithium-bearing micas. J. Miner. Mater. Character. Eng. 2013, 1, 285. [Google Scholar] [CrossRef] [Green Version]
- Schulz, B.; Merker, G.; Gutzmer, J. Automated SEM mineral liberation analysis (MLA) with generically labelled EDX spectra in the mineral processing of rare earth element ores. Minerals 2019, 9, 527. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Zhou, W.; Sun, Y.; Han, Y.; Li, Y. Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore. Minerals 2021, 11, 247. [Google Scholar] [CrossRef]
- Guo, X.; Ren, W.; Zhang, M.; Dai, S.; Zhao, T.; Zhu, J. Effects of grinding fineness on magnetism and agglomeration of magnetite. J. Cent. South Univ. Sci. Technol. 2020, 51, 2373–2378. (In Chinese) [Google Scholar]
- Li, L.; Sheng, S.; Yuan, Z. Loss mechanism of fine-grained ilmenite in magnetic separation. China Mine 2018, 27, 138–144. (In Chinese) [Google Scholar]
- Zhang, X.; Han, Y.; Li, Y.; Tang, Z. New Process of Comprehensive Utilization for Daxigou Refractory Siderite Ore. J. Northeast. Univ. Nat. Sci. 2018, 39, 248. [Google Scholar]
- Li, Y.; Sun, T.; Zou, A.; Xu, C. Effect of coal levels during direct reduction roasting of high phosphorus oolitic hematite ore in a tunnel kiln. Int. J. Min. Sci. Technol. 2012, 22, 323–328. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Sun, Y.; Li, Y. Innovative utilization of refractory iron ore via suspension magnetization roasting: A pilot-scale study. Powder Technol. 2019, 352, 16–24. [Google Scholar] [CrossRef]
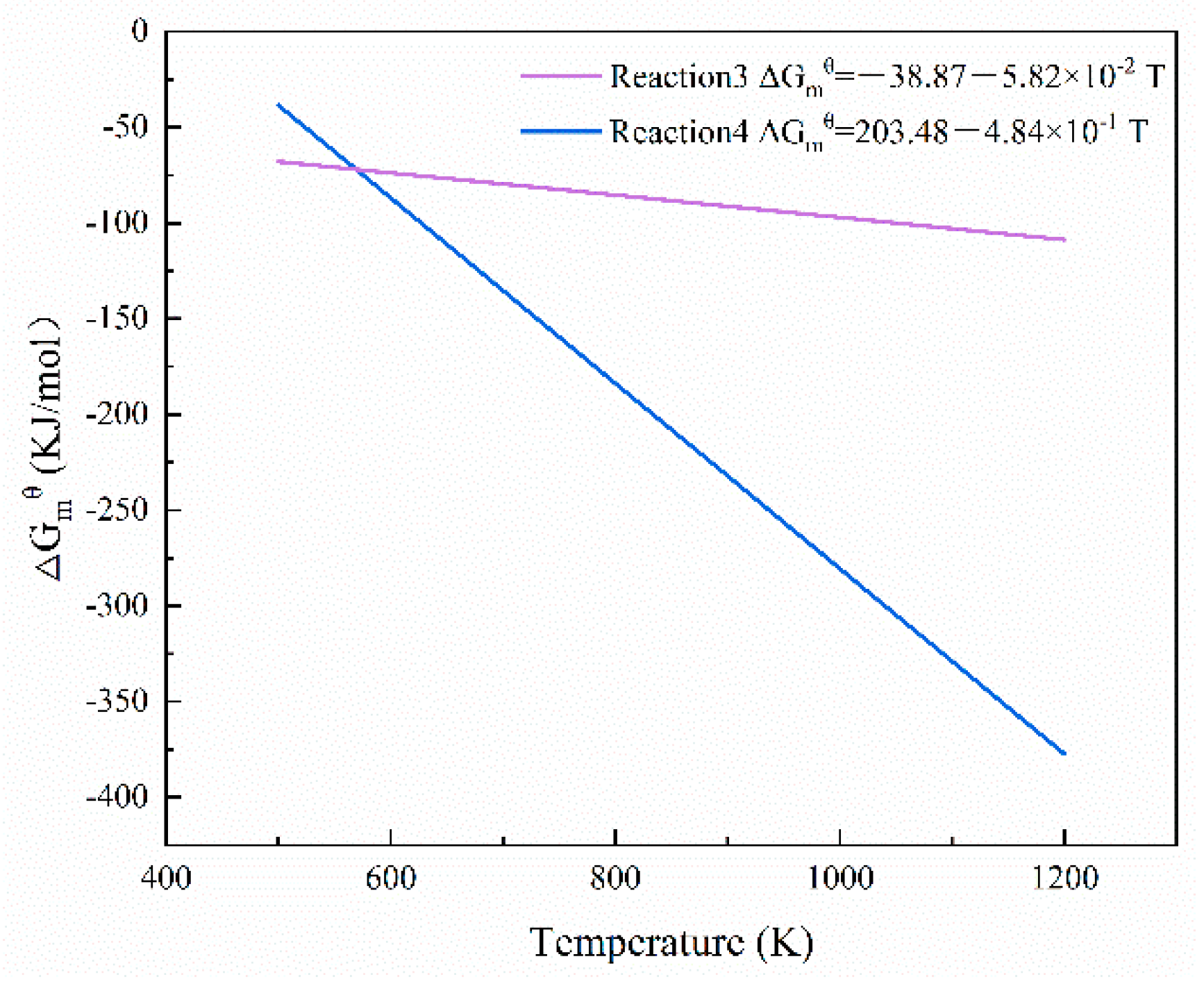
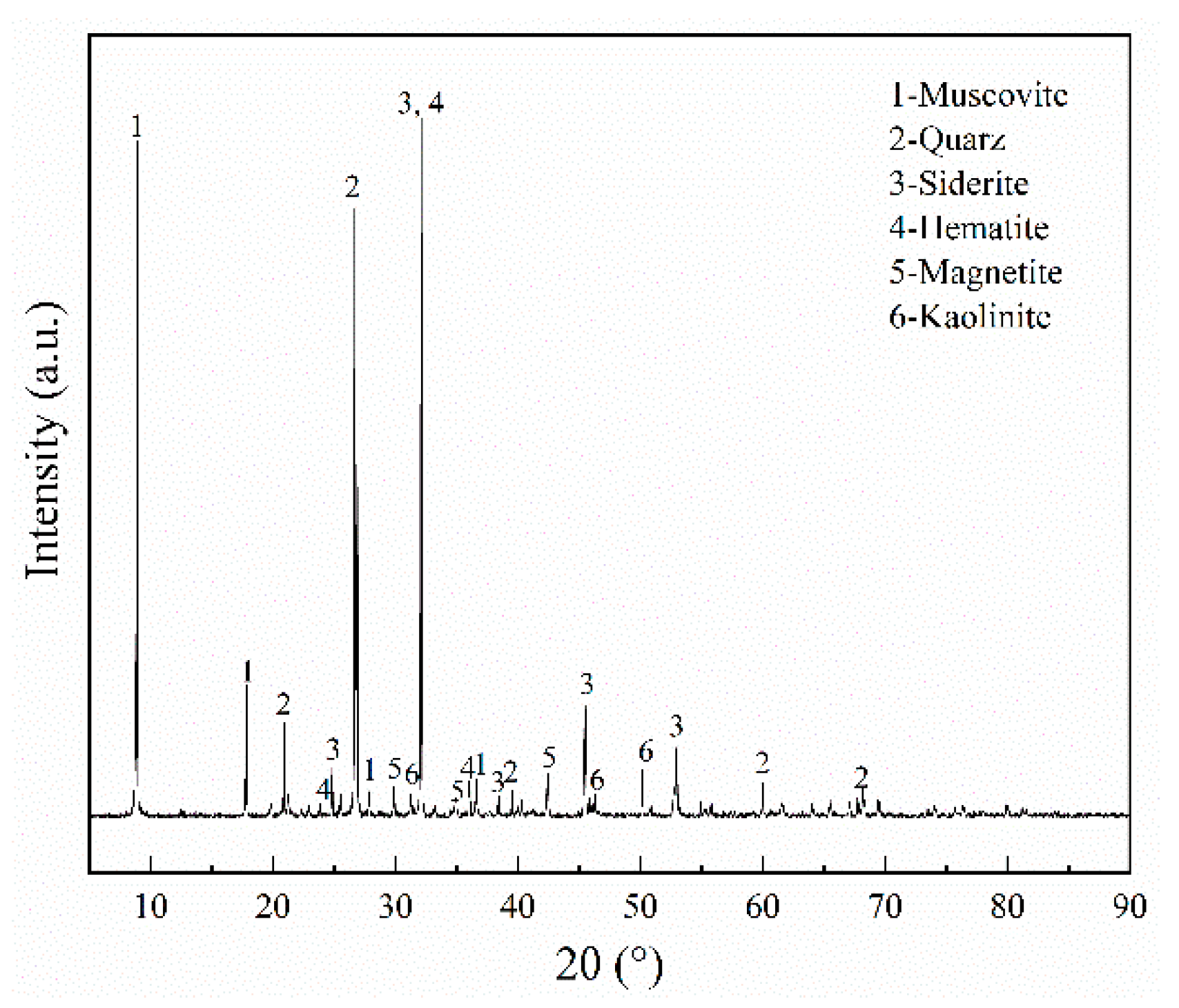
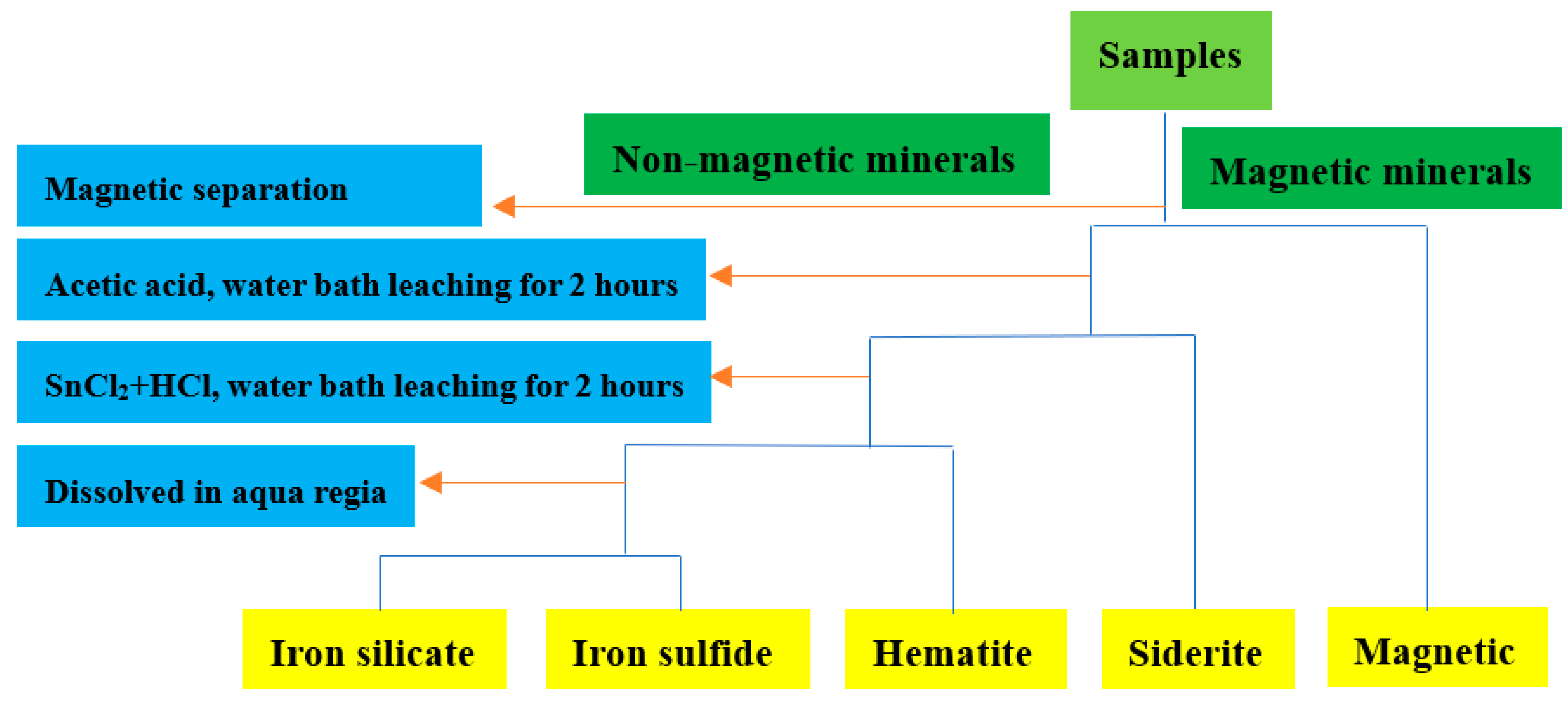
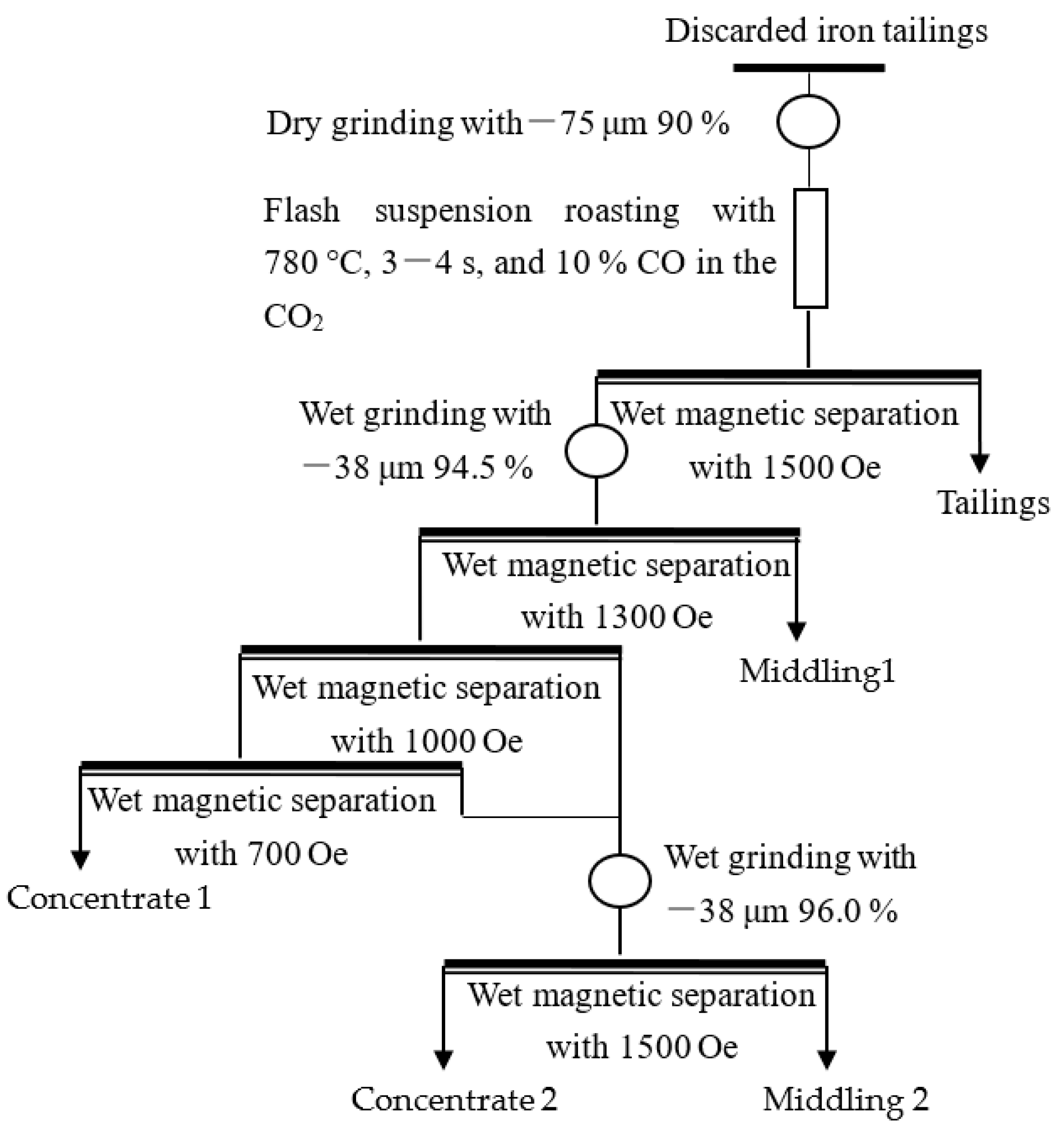

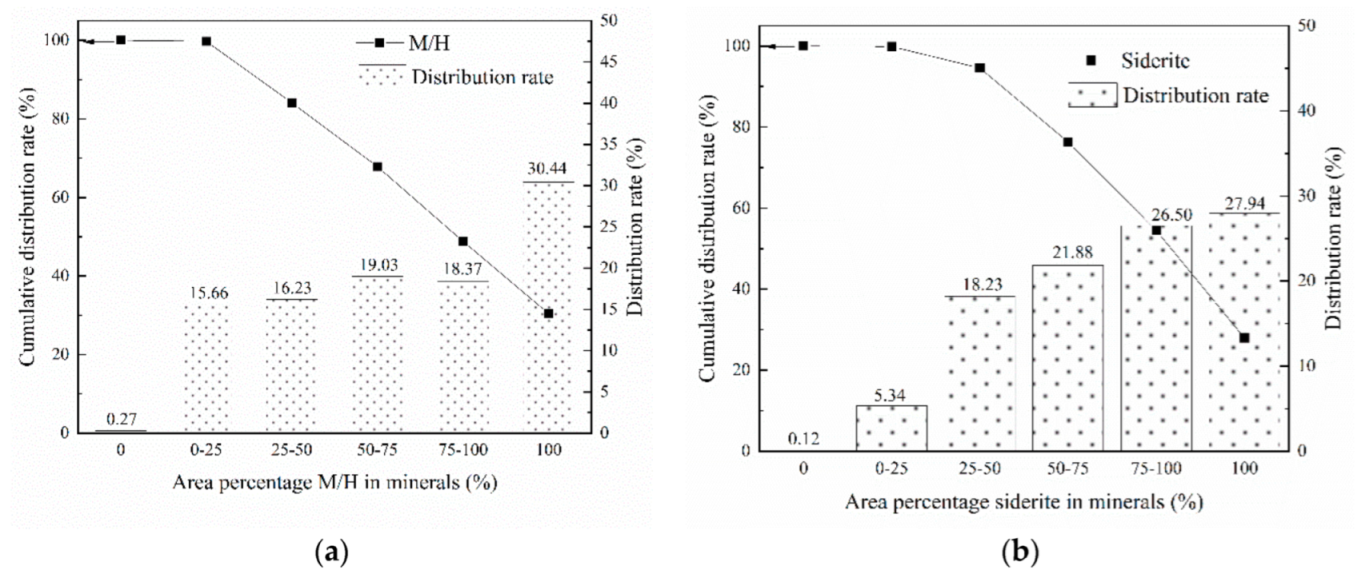
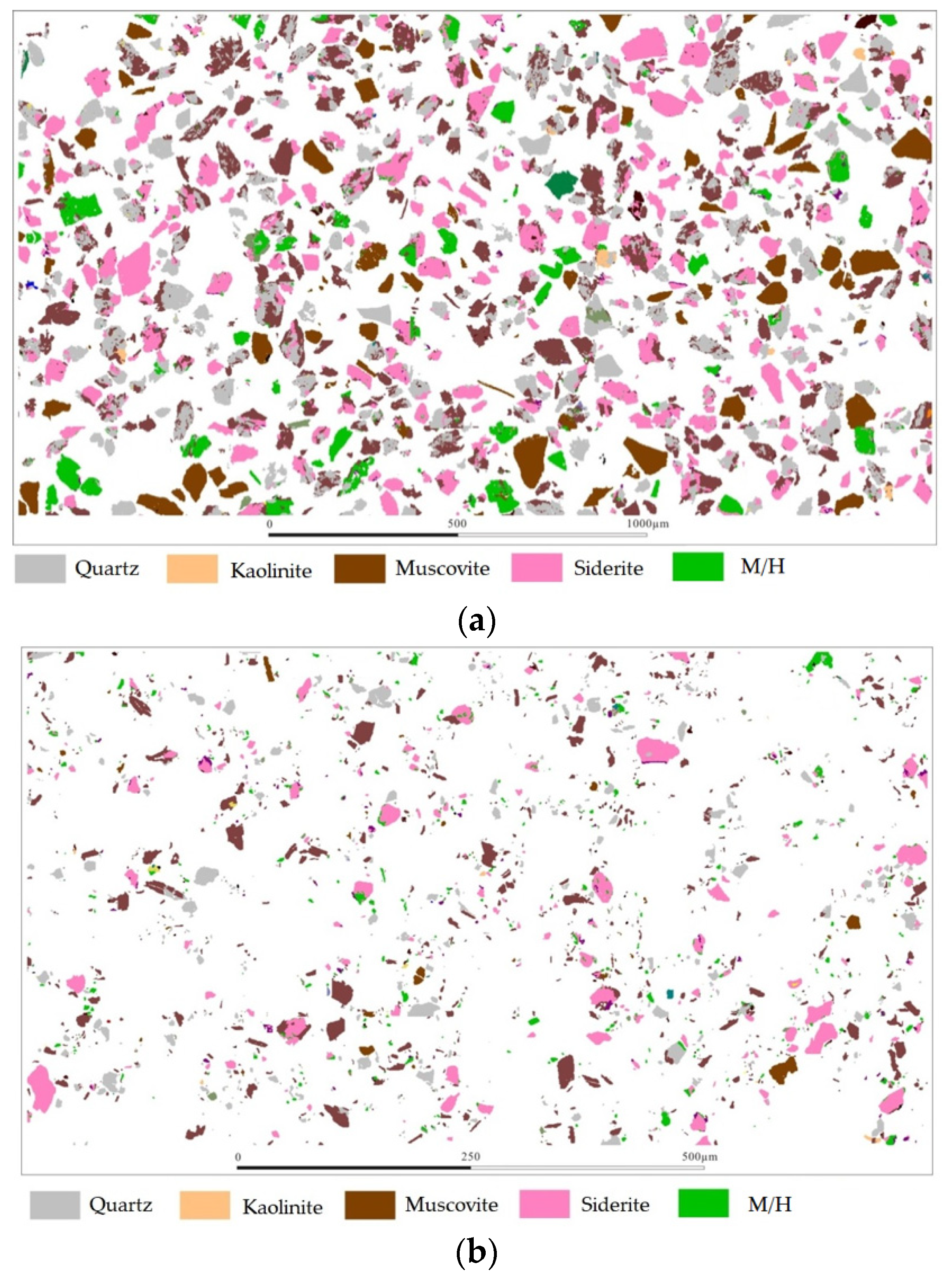
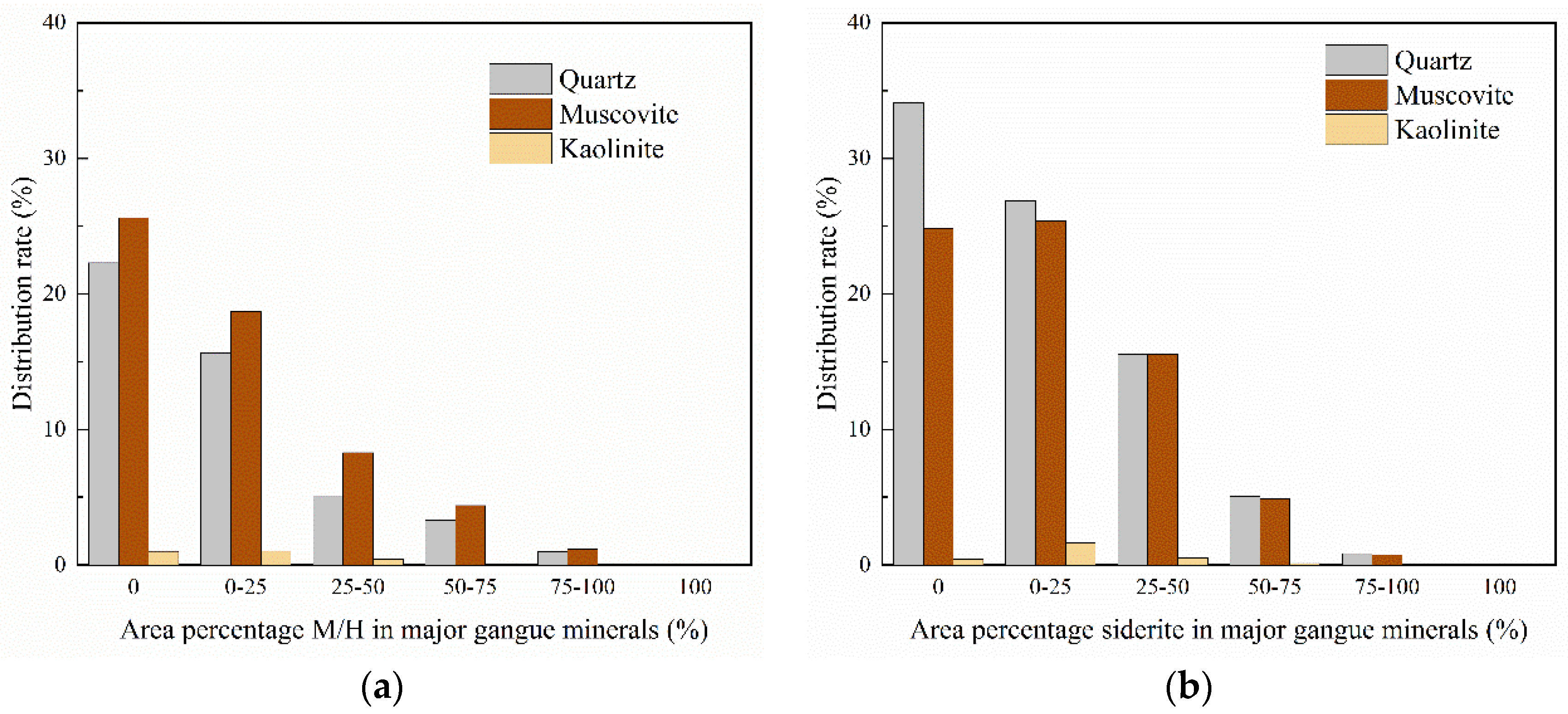
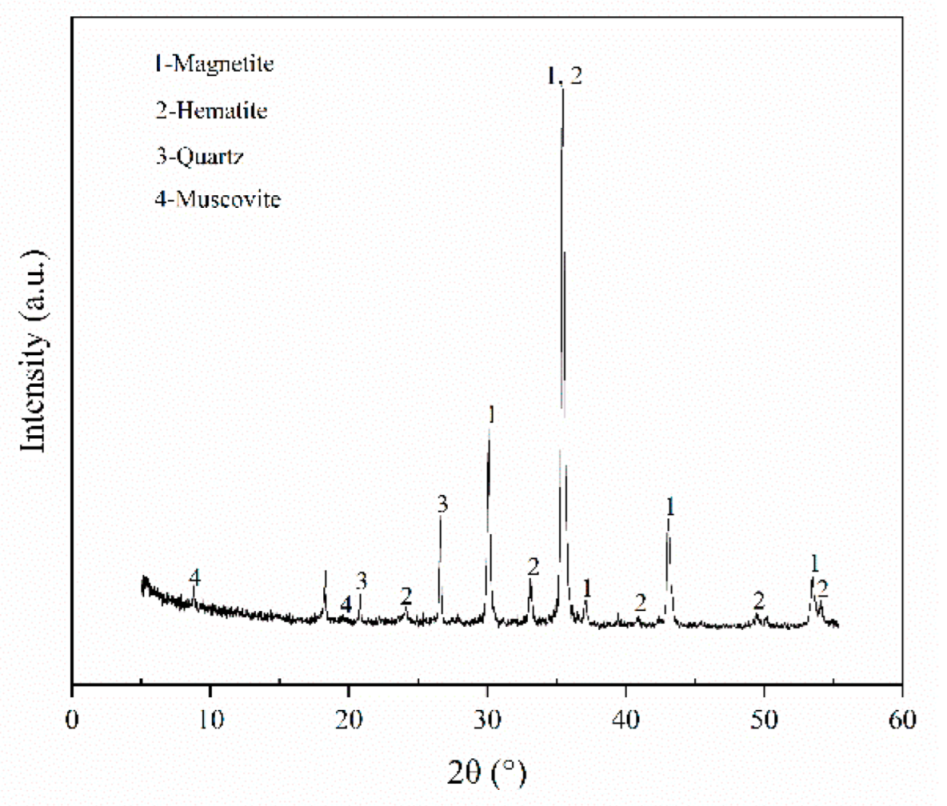
| Component | TFe | Al2O3 | SiO2 | CaO | MgO | K2O | P | S | MnO | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 20.25 | 15.22 | 41.45 | 0.44 | 1.30 | 1.30 | 0.01 | 0.25 | 0.80 | 0.45 |
| Fe Phase | In Carbonate | In Hematite | In Magnetic Iron | In Silicate | TFe |
|---|---|---|---|---|---|
| Content (%) | 12.85 | 5.28 | 1.60 | 0.85 | 20.58 |
| Distribution rate (%) | 62.44 | 25.66 | 7.77 | 4.13 | 100.00 |
| Particle Size/μm | Siderite (%) | M/H (%) | ||
|---|---|---|---|---|
| Content | Cumulative Distribution | Content | Cumulative Distribution | |
| +75 | 4.82 | 14.63 | 4.93 | 7.25 |
| −75~+38 | 14.56 | 85.37 | 11.72 | 92.75 |
| −38~+19 | 30.9 | 7.77 | ||
| −19~+9.6 | 23.28 | 12.55 | ||
| −9.6~+4.8 | 12.81 | 26.72 | ||
| −4.8~+2.4 | 3.52 | 28.72 | ||
| −2.4~+1.2 | 0.27 | 5.18 | ||
| −1.2 | 0.02 | 0.1 | ||
| Name | Yield (%) | TFe (%) | ||
|---|---|---|---|---|
| Grade | Recovery | Cumulative Grade | ||
| Concentrate 1 | 28.60 | 60.10 | 73.35 | |
| Concentrate 2 | 3.03 | 60.15 | 7.78 | 25.58 |
| Middling 2 | 7.04 | 10.85 | 3.26 | |
| Middling1 | 12.82 | 9.44 | 5.16 | |
| Tailings | 48.51 | 5.05 | 10.45 | |
| Roasted ore | 100.00 | 23.43 | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Yi, P.; Luukkanen, S.; Qu, J.; Zhang, C.; Yang, S.; Bu, X. Recovering Iron Concentrate from Low-Grade Siderite Tailings Based on the Process Mineralogy Characteristics. Minerals 2022, 12, 676. https://doi.org/10.3390/min12060676
Wan H, Yi P, Luukkanen S, Qu J, Zhang C, Yang S, Bu X. Recovering Iron Concentrate from Low-Grade Siderite Tailings Based on the Process Mineralogy Characteristics. Minerals. 2022; 12(6):676. https://doi.org/10.3390/min12060676
Chicago/Turabian StyleWan, He, Peng Yi, Saija Luukkanen, Juanping Qu, Chonghui Zhang, Shenghong Yang, and Xianzhong Bu. 2022. "Recovering Iron Concentrate from Low-Grade Siderite Tailings Based on the Process Mineralogy Characteristics" Minerals 12, no. 6: 676. https://doi.org/10.3390/min12060676
APA StyleWan, H., Yi, P., Luukkanen, S., Qu, J., Zhang, C., Yang, S., & Bu, X. (2022). Recovering Iron Concentrate from Low-Grade Siderite Tailings Based on the Process Mineralogy Characteristics. Minerals, 12(6), 676. https://doi.org/10.3390/min12060676






