The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel
Abstract
1. Introduction
2. Results
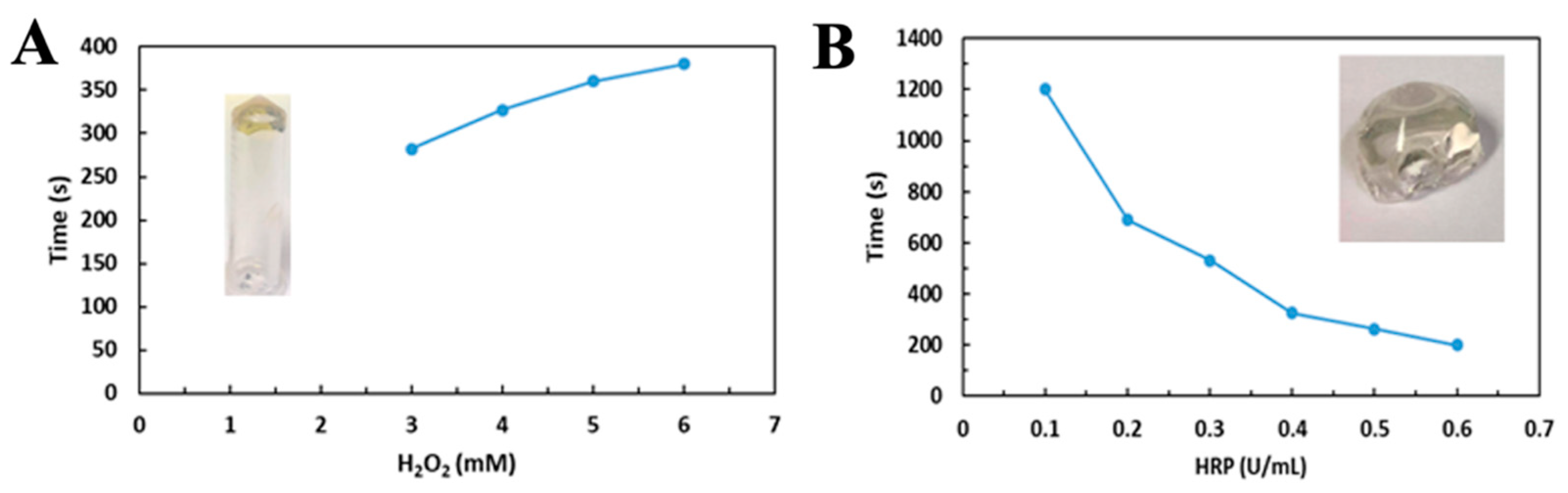
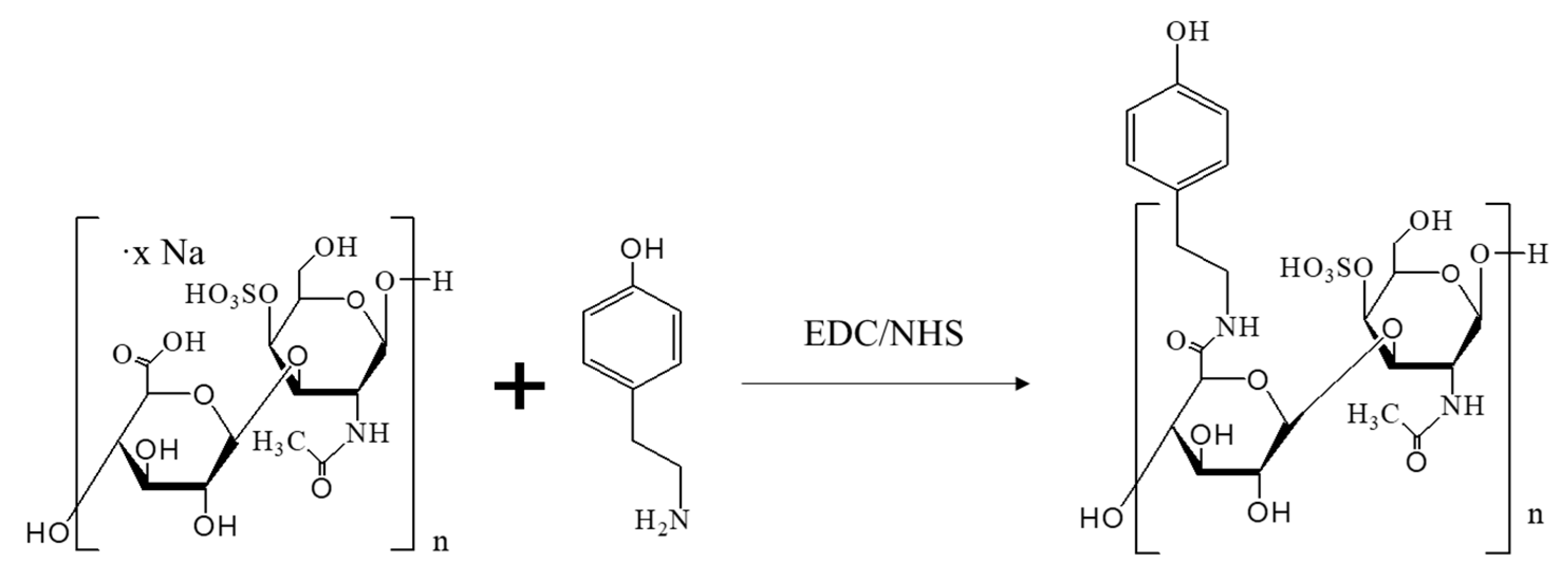
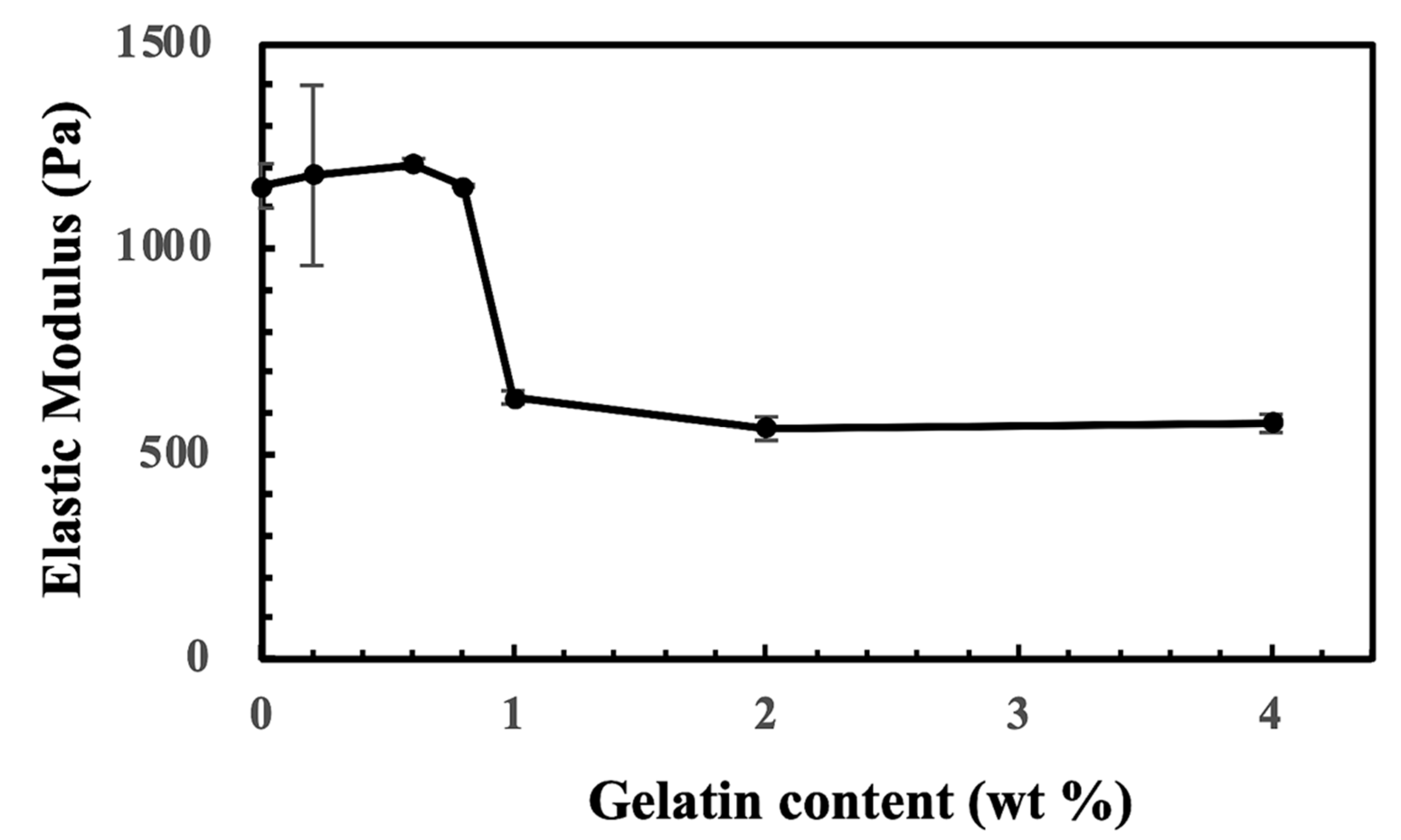
2.1. The Generation of CS-Tyr/Gel Hydrogel
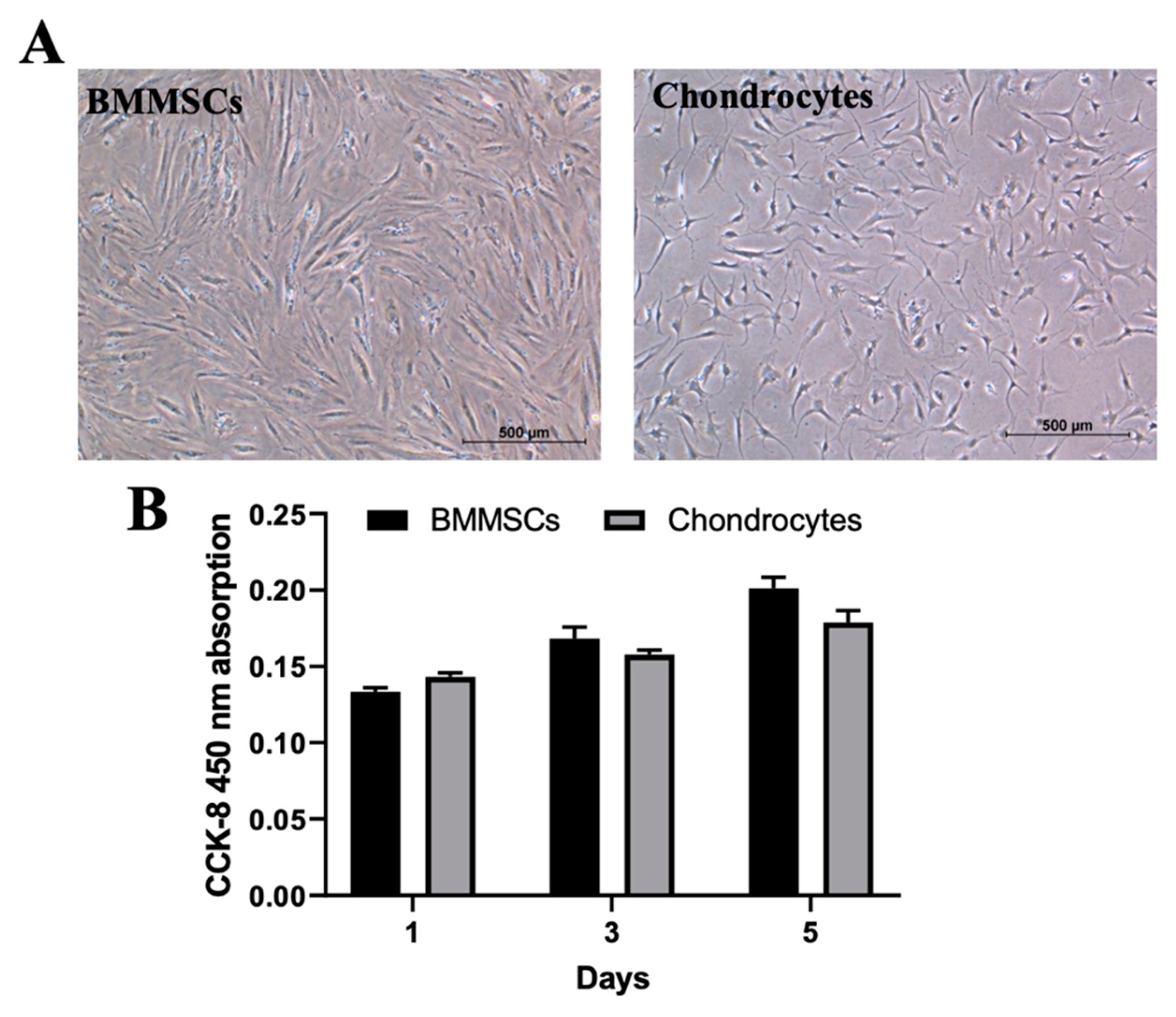
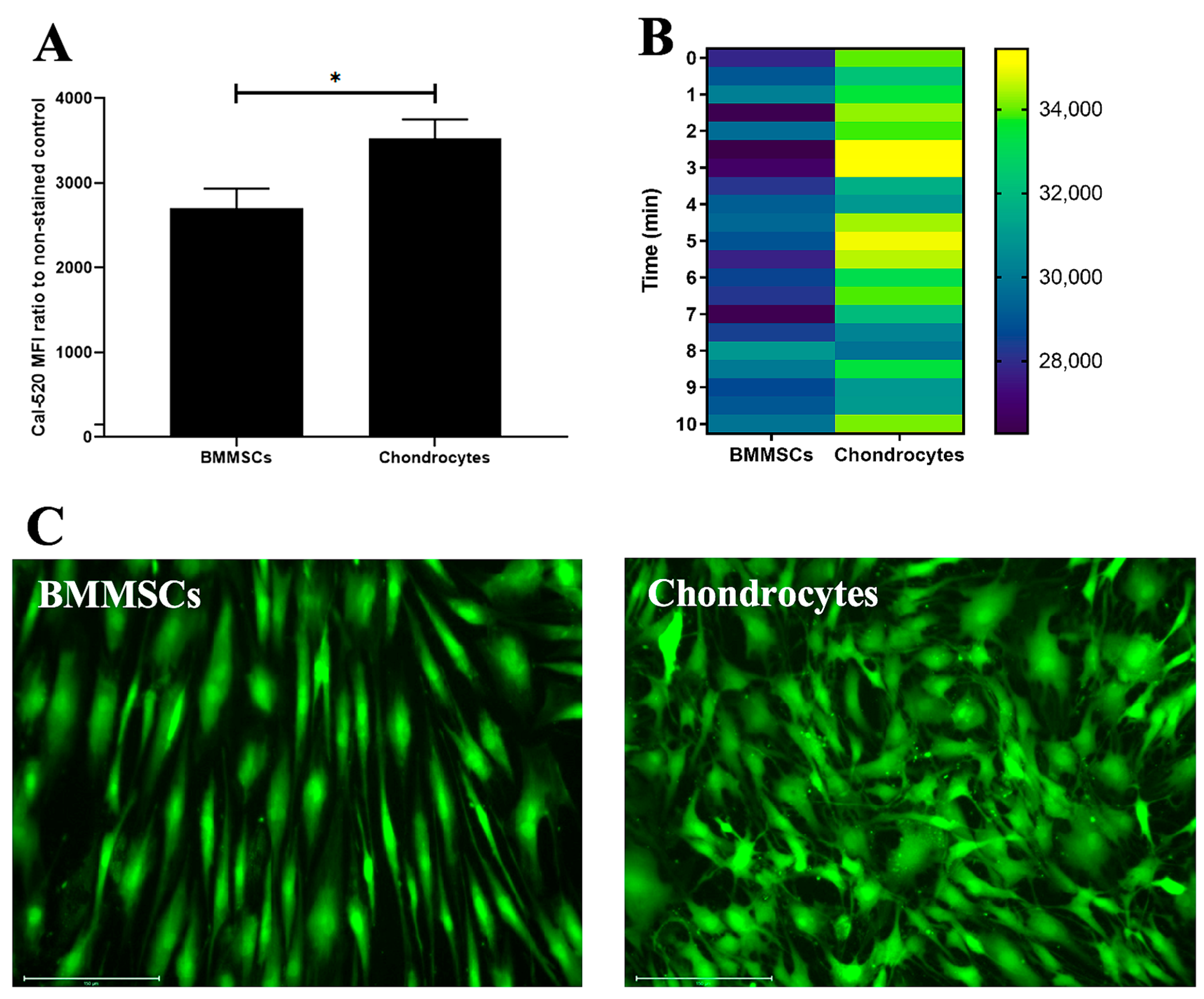
2.2. The Characterization of Human BMMSCs and Chondrocytes
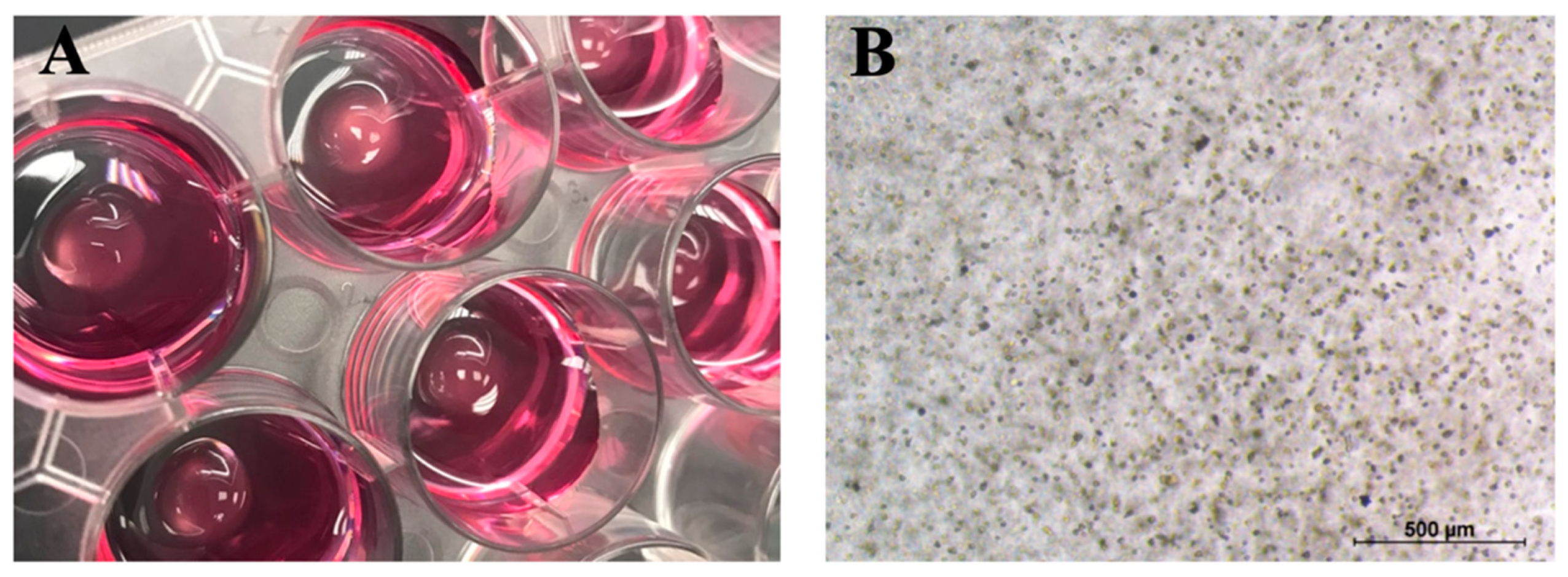
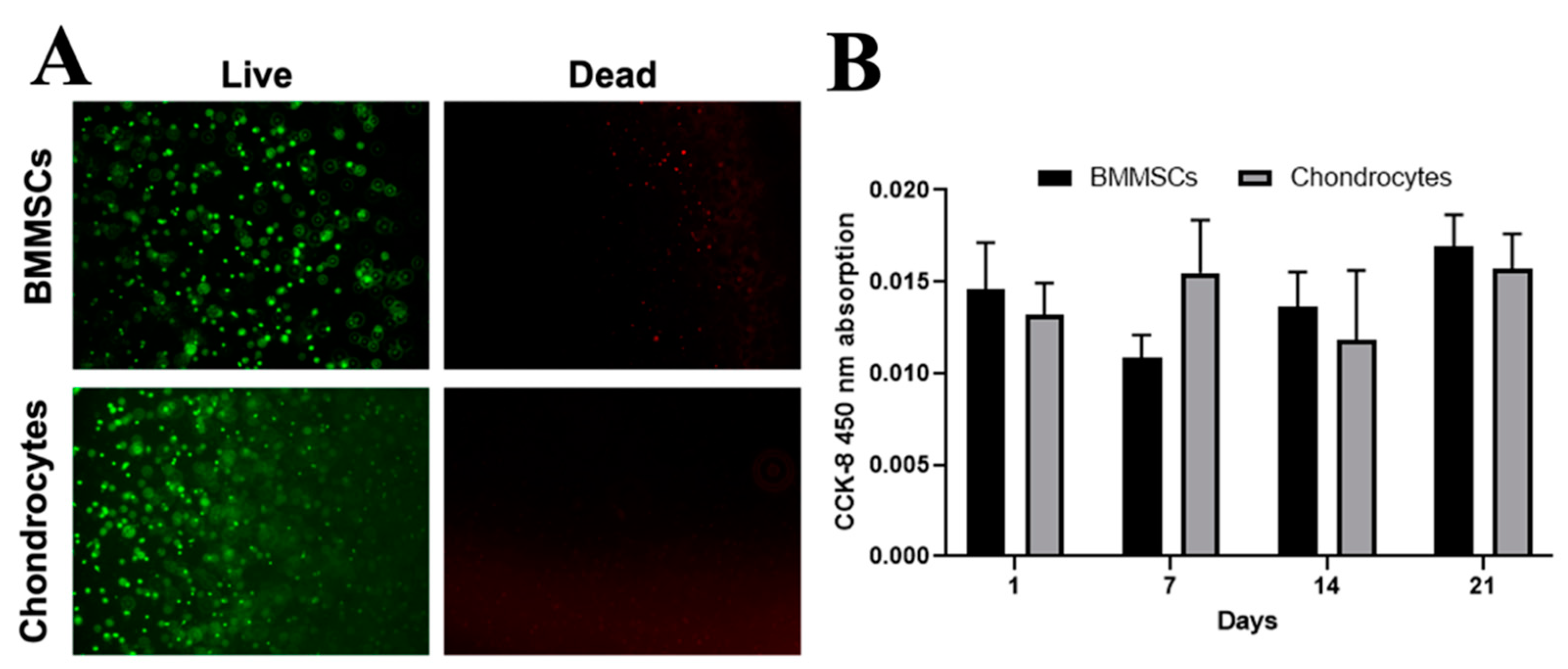
2.3. The Encapsulation of BMMSCs and Chondrocytes into CS-Tyr/Gel Hydrogel and Viability Measurement
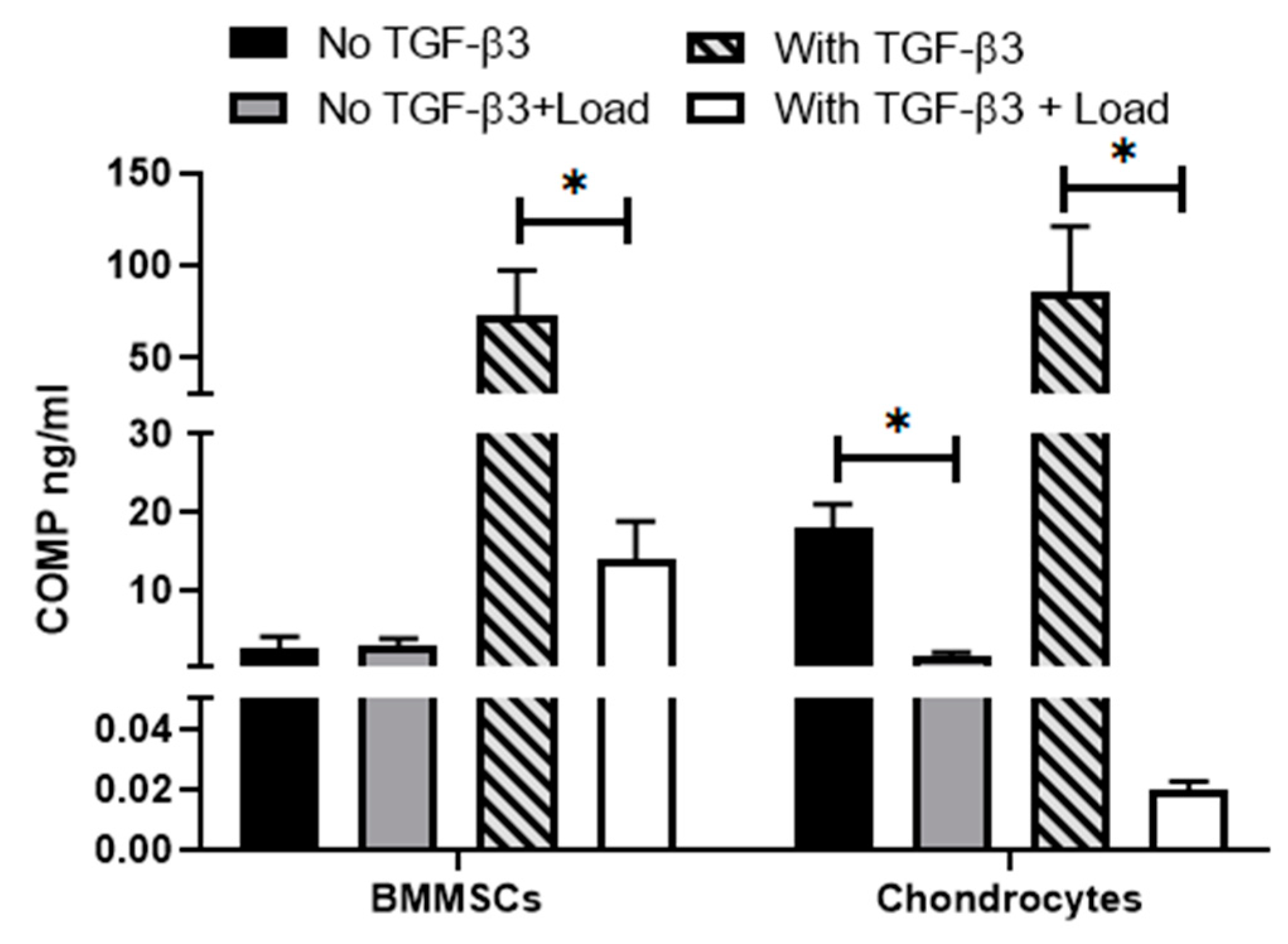
2.4. The Effect of Mechanical Load on Cell Viability and Release of Cartilage Oligomeric Matrix Protein (COMP) in Hydrogel/Cell Composites
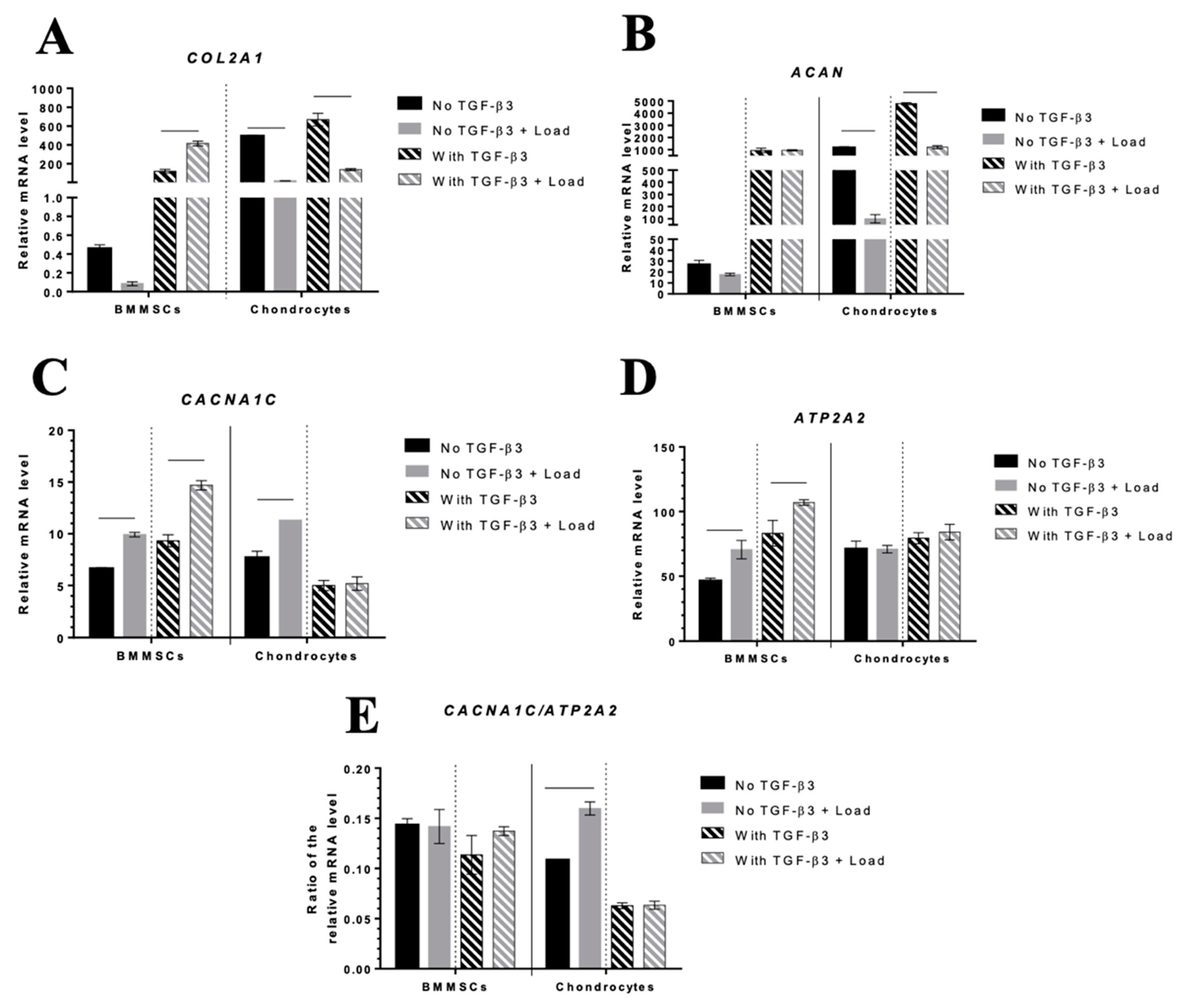
2.5. The Effect of Mechanical Load on Chondrogenic Differentiation Markers and iCa2+-Regulating Channels in Hydrogel/Cell Composites
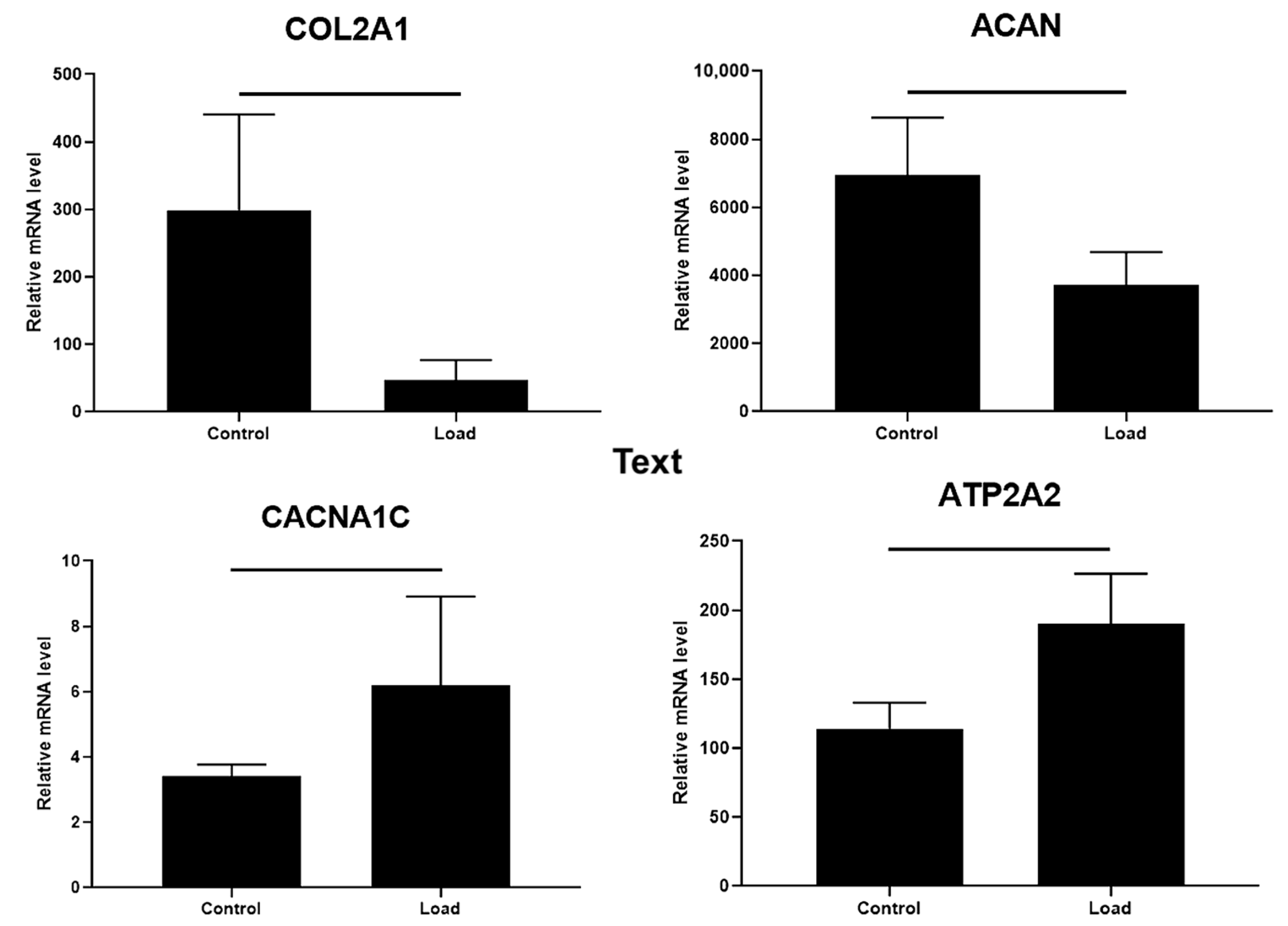
2.6. The Effect of Mechanical Load on Chondrogenic Differentiation Markers and iCa2+-Regulating Channels in OA Cartilage Explants
3. Discussion
4. Methods and Materials
4.1. CS-Tyr Synthesis
4.2. Formation and Investigation of CS-Tyr/Gel Hydrogel
4.3. Cell Isolation and Culture
4.4. The Viability and Metabolic Activity of Cells Encapsulated into CS-Tyr Hydrogel
4.5. Analysis of Intracellular Calcium Level
4.6. Chondrogenic Differentiation of Cells Encapsulated into CS-Tyr/Gel with/without the Mechanical Loading
4.7. Isolation and Mechanical Compression of Human Cartilage Explants
4.8. Secretion of Cartilage Oligomeric Matrix Protein (COMP)
4.9. RNA Extraction from the CS-Tyr/Gel/Cells and Cartilage Explants
4.10. RT-qPCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAN | aggrecan gene |
| ATP2A2 | Serca2 pump gene |
| BMMSCs | bone marrow mesenchymal stem cells |
| BSA | bovine serum albumin |
| Ca2+ | calcium ions |
| CACNA1C | L-type Ca2+ channel subunit CaV1.2 |
| CCK-8 | cell counting kit 8 |
| COL1A1 | collagen type I gene |
| COL2A1 | collagen type II gene |
| COMP | cartilage oligomeric matrix protein |
| CPs | chondroprogenitors |
| CS | chondroitin sulfate |
| CS-Tyr | chondroitin sulfate tyramine hydrogels |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ECM | extracellular matrix |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| ER | endoplasmic reticulum |
| FBS | fetal bovine serum |
| FGF2 | fibroblast growth factor-2 |
| Gtn-HPA | crosslinked gelatin-hydroxyphenylpropionic acid |
| HBSS | Hanks’ buffered saline solution |
| iCa2+ | intracellular Ca2+; |
| iPSC | induced pluripotent stem cells |
| LDH | lactate dehydrogenase |
| MDCs | mesodermal articular-derived chondrocytes |
| MES | 2-(N-morpholino)ethanesulfonic acid buffer |
| MFI | median fluorescence intensity |
| MSCs | mesenchymal stem cells |
| NCDCs | nasal crest-derived chondrocytes |
| NHS | N-hydroxysuccinimide |
| OA | osteoarthritis |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| RT | room temperature (24 °C) |
| SD | standard deviation |
| Tyr | tyramine |
| TGF-β3 | transforming growth factor β3 |
| TSP-5 | thrombospondin-5 (COMP) |
| VOCC | voltage-operated calcium channels |
References
- Sanjurjo-Rodríguez, C.; Castro-Viñuelas, R.; Hermida-Gómez, T.; Fuentes-Boquete, I.M.; de Toro, F.J.; Blanco, F.J.; Díaz-Prado, S.M. Human Cartilage Engineering in an In Vitro Repair Model Using Collagen Scaffolds and Mesenchymal Stromal Cells. Int. J. Med. Sci. 2017, 14, 1257–1262. [Google Scholar] [CrossRef]
- Guo, T.; Yu, L.; Lim, C.G.; Goodley, A.S.; Xiao, X.; Placone, J.K.; Ferlin, K.M.; Nguyen, B.N.B.; Hsieh, A.H.; Fisher, J.P. Effect of Dynamic Culture and Periodic Compression on Human Mesenchymal Stem Cell Proliferation and Chondrogenesis. Ann. Biomed. Eng. 2016, 44, 2103–2113. [Google Scholar] [CrossRef]
- Lee, C.; Grad, S.; Wimmer, M.; Alini, M. The Influence of Mechanical Stimuli on Articular Cartilage Tissue Engineering. In Topics in Tissue Engineering; Expertissues: Barco, Portugal, 2006; Volume 2, pp. 1–32. [Google Scholar]
- Uzieliene, I.; Bironaite, D.; Bernotas, P.; Sobolev, A.; Bernotiene, E. Mechanotransducive Biomimetic Systems for Chondrogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021, 22, 9690. [Google Scholar] [CrossRef]
- De Bari, C.; Roelofs, A.J. Stem Cell-Based Therapeutic Strategies for Cartilage Defects and Osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef]
- Pizzute, T.; Lynch, K.; Pei, M. Impact of Tissue-Specific Stem Cells on Lineage-Specific Differentiation: A Focus on the Musculoskeletal System. Stem Cell Rev. Rep. 2015, 11, 119–132. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The Control of Chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, Chondrocyte Differentiation, and Articular Cartilage Metabolism in Health and Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells for Cartilage Regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Kemp, K.C.; Hows, J.; Donaldson, C. Bone Marrow-Derived Mesenchymal Stem Cells. Leuk. Lymphoma 2005, 46, 1531–1544. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Ashrafizadeh, M.; Pardakhty, A.; Uzieliene, I.; Denkovskij, J.; Bernotiene, E.; Janssen, L.; Lorite, G.S.; Saarakkala, S.; Mobasheri, A. Nanotechnological Strategies for Osteoarthritis Diagnosis, Monitoring, Clinical Management, and Regenerative Medicine: Recent Advances and Future Opportunities. Curr. Rheumatol. Rep. 2020, 22, 12. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A Biomimetic Extracellular Matrix for Cartilage Tissue Engineering Centered on Photocurable Gelatin, Hyaluronic Acid and Chondroitin Sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mulloy, B. Glycosaminoglycans and Proteoglycans. Pharmaceuticals 2018, 11, 27. [Google Scholar] [CrossRef]
- Salinas, E.Y.; Hu, J.C.; Athanasiou, K. A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng. Part B Rev. 2018, 24, 345–358. [Google Scholar] [CrossRef]
- O’Conor, C.J.; Case, N.; Guilak, F. Mechanical Regulation of Chondrogenesis. Stem Cell Res. Ther. 2013, 4, 61. [Google Scholar] [CrossRef]
- He, Y.; Yocum, L.; Alexander, P.G.; Jurczak, M.J.; Lin, H. Urolithin A Protects Chondrocytes From Mechanical Overloading-Induced Injuries. Front. Pharmacol. 2021, 12, 1515. [Google Scholar] [CrossRef]
- El-Ayoubi, R.; DeGrandpré, C.; DiRaddo, R.; Yousefi, A.-M.; Lavigne, P. Design and Dynamic Culture of 3D-Scaffolds for Cartilage Tissue Engineering. J. Biomater. Appl. 2011, 25, 429–444. [Google Scholar] [CrossRef]
- Li, K.; Zhang, C.; Qiu, L.; Gao, L.; Zhang, X. Advances in Application of Mechanical Stimuli in Bioreactors for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 399–411. [Google Scholar] [CrossRef]
- Mauck, R.L.; Nicoll, S.B.; Seyhan, S.L.; Ateshian, G.A.; Hung, C.T. Synergistic Action of Growth Factors and Dynamic Loading for Articular Cartilage Tissue Engineering. Tissue Eng. 2003, 9, 597–611. [Google Scholar] [CrossRef]
- Chuang, E.-Y.; Chiang, C.-W.; Wong, P.-C.; Chen, C.-H. Hydrogels for the Application of Articular Cartilage Tissue Engineering: A Review of Hydrogels. Adv. Mater. Sci. Eng. 2018, 2018, 4368910. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Chondrogenic Differentiation of Bovine Bone Marrow Mesenchymal Stem Cells (MSCs) in Different Hydrogels: Influence of Collagen Type II Extracellular Matrix on MSC Chondrogenesis. Biotechnol. Bioeng. 2006, 93, 1152–1163. [Google Scholar] [CrossRef]
- Freyria, A.-M.; Ronzière, M.-C.; Cortial, D.; Galois, L.; Hartmann, D.; Herbage, D.; Mallein-Gerin, F. Comparative Phenotypic Analysis of Articular Chondrocytes Cultured within Type I or Type II Collagen Scaffolds. Tissue Eng. Part A 2009, 15, 1233–1245. [Google Scholar] [CrossRef]
- Skotak, M.; Noriega, S.; Larsen, G.; Subramanian, A. Electrospun Cross-Linked Gelatin Fibers with Controlled Diameter: The Effect of Matrix Stiffness on Proliferative and Biosynthetic Activity of Chondrocytes Cultured in Vitro. J. Biomed. Mater. Res. A 2010, 95, 828–836. [Google Scholar] [CrossRef]
- Wang, L.-S.; Du, C.; Toh, W.S.; Wan, A.C.A.; Gao, S.J.; Kurisawa, M. Modulation of Chondrocyte Functions and Stiffness-Dependent Cartilage Repair Using an Injectable Enzymatically Crosslinked Hydrogel with Tunable Mechanical Properties. Biomaterials 2014, 35, 2207–2217. [Google Scholar] [CrossRef]
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin Sulfate Based Niches for Chondrogenic Differentiation of Mesenchymal Stem Cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bryant, S.J. The Role of Chondroitin Sulfate in Regulating Hypertrophy during MSC Chondrogenesis in a Cartilage Mimetic Hydrogel under Dynamic Loading. Biomaterials 2019, 190, 51–62. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ohya, S.; Giles, W.R. Editorial: Regulatory Mechanisms of Ca2+ Activated Ion Channels and Their Impact on Physiological/Pathophysiological Function. Front. Physiol. 2022, 13, 627. [Google Scholar] [CrossRef]
- Yang, S.; Berggren, P. β-Cell CaV Channel Regulation in Physiology and Pathophysiology. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E16–E28. [Google Scholar] [CrossRef]
- Li, G.-R.; Sun, H.; Deng, X.; Lau, C.-P. Characterization of Ionic Currents in Human Mesenchymal Stem Cells from Bone Marrow. Stem Cells 2005, 23, 371–382. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA Control of Cell Death and Survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-Dependent Calcium Channels in Chondrocytes: Roles in Health and Disease. Curr. Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef]
- Steward, A.; Kelly, D.; Wagner, D. The Role of Calcium Signalling in the Chondrogenic Response of Mesenchymal Stem Cells to Hydrostatic Pressure. Eur. Cells Mater. 2014, 28, 358–371. [Google Scholar] [CrossRef]
- Lewis, R.S. Calcium Signaling Mechanisms in T Lymphocytes. Annu. Rev. Immunol. 2001, 19, 497–521. [Google Scholar] [CrossRef]
- Censi, R.; Dubbini, A.; Matricardi, P. Bioactive Hydrogel Scaffolds-Advances in Cartilage Regeneration through Controlled Drug Delivery. Curr. Pharm. Des. 2015, 21, 1545–1555. [Google Scholar] [CrossRef]
- Fontaine, K.R.; Haaz, S.; Heo, M. Projected Prevalence of US Adults with Self-Reported Doctor-Diagnosed Arthritis, 2005 to 2050. Clin. Rheumatol. 2007, 26, 772–774. [Google Scholar] [CrossRef]
- Urlić, I.; Ivković, A. Cell Sources for Cartilage Repair—Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef]
- Xu, F.; Xu, L.; Wang, Q.; Ye, Z.; Zhou, Y.; Tan, W.-S. 3D Dynamic Culture of Rabbit Articular Chondrocytes Encapsulated in Alginate Gel Beads Using Spinner Flasks for Cartilage Tissue Regeneration. Biomed. Res. Int. 2014, 2014, 539789. [Google Scholar] [CrossRef]
- Son, Y.-B.; Jeong, Y.I.; Jeong, Y.W.; Hossein, M.S.; Olsson, P.O.; Tinson, A.; Singh, K.K.; Lee, S.-Y.; Hwang, W.S. Cell Source-Dependent In Vitro Chondrogenic Differentiation Potential of Mesenchymal Stem Cell Established from Bone Marrow and Synovial Fluid of Camelus Dromedarius. Animals 2021, 11, 1918. [Google Scholar] [CrossRef]
- Studer, D.; CaValli, E.; Formica, F.A.; Kuhn, G.A.; Salzmann, G.; Mumme, M.; Steinwachs, M.R.; Laurent-Applegate, L.A.; Maniura-Weber, K.; Zenobi-Wong, M. Human Chondroprogenitors in Alginate-Collagen Hybrid Scaffolds Produce Stable Cartilage in Vivo. J. Tissue Eng. Regen. Med. 2017, 11, 3014–3026. [Google Scholar] [CrossRef]
- Dicks, A.; Wu, C.-L.; Steward, N.; Adkar, S.S.; Gersbach, C.A.; Guilak, F. Prospective Isolation of Chondroprogenitors from Human IPSCs Based on Cell Surface Markers Identified Using a CRISPR-Cas9-Generated Reporter. Stem Cell Res. Ther. 2020, 11, 66. [Google Scholar] [CrossRef]
- Djouad, F.; Bouffi, C.; Ghannam, S.; Noël, D.; Jorgensen, C. Mesenchymal Stem Cells: Innovative Therapeutic Tools for Rheumatic Diseases. Nat. Rev. Rheumatol. 2009, 5, 392–399. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, T.; Zhu, B.; Le, Y.; Liu, J.; Qin, Z.; Chen, H.; Zhong, G.; Zheng, L.; Zhao, J.; et al. In Vitro Culture Expansion Impairs Chondrogenic Differentiation and the Therapeutic Effect of Mesenchymal Stem Cells by Regulating the Unfolded Protein Response. J. Biol. Eng. 2018, 12, 26. [Google Scholar] [CrossRef]
- Cao, W.; Lin, W.; Cai, H.; Chen, Y.; Man, Y.; Liang, J.; Wang, Q.; Sun, Y.; Fan, Y.; Zhang, X. Dynamic Mechanical Loading Facilitated Chondrogenic Differentiation of Rabbit BMSCs in Collagen Scaffolds. Regen. Biomater. 2019, 6, 99–106. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Wang, Z.; Li, L.; Teng, H.; Jiang, Q. Effects of Mechanical Compression on Chondrogenesis of Human Synovium-Derived Mesenchymal Stem Cells in Agarose Hydrogel. Front. Bioeng. Biotechnol. 2021, 9, 697281. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Tang, L.; El-din, T.M.G.; Payandeh, J.; Martinez, G.Q.; Heard, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural Basis for Ca 2 1 Selectivity of a Voltage-Gated Calcium Channel. Nature 2014, 505, 56–61. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotiene, E.; Rakauskiene, G.; Denkovskij, J.; Bagdonas, E.; Mackiewicz, Z.; Porvaneckas, N.; Kvederas, G.; Mobasheri, A. The Antihypertensive Drug Nifedipine Modulates the Metabolism of Chondrocytes and Human Bone Marrow-Derived Mesenchymal Stem Cells. Front. Endocrinol. 2019, 10, 756. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Sun, Y.; Li, G.; Bian, L. Effect of Cartilaginous Matrix Components on the Chondrogenesis and Hypertrophy of Mesenchymal Stem Cells in Hyaluronic Acid Hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2292–2300. [Google Scholar] [CrossRef]
- Fitzgerald, J.B.; Jin, M.; Dean, D.; Wood, D.J.; Zheng, M.H.; Grodzinsky, A.J. Mechanical Compression of Cartilage Explants Induces Multiple Time-Dependent Gene Expression Patterns and Involves Intracellular Calcium and Cyclic AMP*. J. Biol. Chem. 2004, 279, 19502–19511. [Google Scholar] [CrossRef]
- Pang, X.; Lin, L.; Tang, B. Unraveling the Role of Calcium Ions in the Mechanical Properties of Individual Collagen Fibrils. Sci. Rep. 2017, 7, 46042. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The Regulation of Reactive Oxygen Species Production during Programmed Cell Death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, F.; Minor, D.L. Progress in the Structural Understanding of Voltage-Gated Calcium Channel (CaV) Function and Modulation. Channels 2010, 4, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.; Colsoul, B.; Nilius, B. The Role of Transient Receptor Potential Cation Channels in Ca2+ Signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.; Reddi, A.H.; Di Cesare, P.E. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights 2009, 4, 33–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Cao, Y.; Wang, J.; Liu, M.; Pei, R. Injectable Hydrogels from Enzyme-Catalyzed Crosslinking as BMSCs-Laden Scaffold for Bone Repair and Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 841–849. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzieliene, I.; Bironaite, D.; Bagdonas, E.; Pachaleva, J.; Sobolev, A.; Tsai, W.-B.; Kvederas, G.; Bernotiene, E. The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. Int. J. Mol. Sci. 2023, 24, 2915. https://doi.org/10.3390/ijms24032915
Uzieliene I, Bironaite D, Bagdonas E, Pachaleva J, Sobolev A, Tsai W-B, Kvederas G, Bernotiene E. The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. International Journal of Molecular Sciences. 2023; 24(3):2915. https://doi.org/10.3390/ijms24032915
Chicago/Turabian StyleUzieliene, Ilona, Daiva Bironaite, Edvardas Bagdonas, Jolita Pachaleva, Arkadij Sobolev, Wei-Bor Tsai, Giedrius Kvederas, and Eiva Bernotiene. 2023. "The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel" International Journal of Molecular Sciences 24, no. 3: 2915. https://doi.org/10.3390/ijms24032915
APA StyleUzieliene, I., Bironaite, D., Bagdonas, E., Pachaleva, J., Sobolev, A., Tsai, W.-B., Kvederas, G., & Bernotiene, E. (2023). The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. International Journal of Molecular Sciences, 24(3), 2915. https://doi.org/10.3390/ijms24032915







