Abstract
Abiotic stresses rewire plant central metabolism to maintain metabolic and energy homeostasis. Metabolites involved in the plant central metabolic network serve as a hub for regulating carbon and energy metabolism under various stress conditions. In this review, we introduce recent metabolomics techniques used to investigate the dynamics of metabolic responses to abiotic stresses and analyze the trend of publications in this field. We provide an updated overview of the changing patterns in central metabolic pathways related to the metabolic responses to common stresses, including flooding, drought, cold, heat, and salinity. We extensively review the common and unique metabolic changes in central metabolism in response to major abiotic stresses. Finally, we discuss the challenges and some emerging insights in the future application of metabolomics to study plant responses to abiotic stresses.
Keywords:
plant central metabolism; abiotic stresses; metabolomics; flooding; drought; salt; heat; freezing 1. Introduction
Plants are constantly exposed to a plethora of stresses under natural conditions. Stress in plants can be described as anything that can cause a change from ideal growth and developmental conditions [1]. Stresses can be classified as abiotic or biotic, where abiotic stresses are caused by nonliving factors in the surrounding environment, such as extremes in temperature, drought, flooding, and high salinity [2]. Abiotic stresses are unavoidable to plants due to their inability to move [3]. Global warming and climate change result in increases in the frequency and intensity of abiotic stresses, such as heatwaves, cold snaps, droughts, and floods [4,5,6,7]. These abiotic stresses are the primary causes for the reduction in crop yield and quality and may threaten food security [8,9]. The economic losses caused by abiotic stresses are estimated to be around USD 14–19 million yearly, worldwide [10]. Therefore, five abiotic stresses, including drought, flooding, salinity, cold, and heat, which strongly impact crop yield and the food industry are discussed in this review.
Heat stress has become a global concern that adversely affects crop yield worldwide because of global warming, with steadily increasing ambient temperatures over the past 40 years with frequently occurring heat waves [11,12]. Global warming leads to climate change and could exacerbate drought stress. Drought stress occurs due to various environments, such as temperature dynamics, light intensity, and low rainfall, and is the leading abiotic stress that hampers crop productivity and threatens food security worldwide [13]. Soil flooding is one of the most important abiotic stresses in wetland and high-rainfall areas in crops and woody tree species [14,15]. It is estimated that 10% of the global land area is affected by soil flooding or severe soil drainage constraints [16]. These climate disasters can cause harsh soil conditions, for example, high soil salinity, extreme pH, and high level of environmental pollutants, such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), herbicides, and pesticides [7]. Salinity stress affects about one-third of the irrigated land on earth by uneven rainfall, coastal lands flooded with seawater, and poor quality of irrigation water as a result of groundwater depletion and degradation of high-salt rocks [17]. Cold stress impacts the reproductive development of chilling sensitive, tropical, and subtropical crops, and is judged to be the major abiotic stress for seedlings [18]. The yield reduction in food crops caused by abiotic stresses worldwide is considered a major challenge in agronomy [19]. Thus, understanding plant responses to various abiotic stress events is central in plant research.
Plant metabolism responds sensitively and dynamically to various abiotic stresses. Metabolic responses to stresses can be very rapid, making metabolic changes an important feature of plant stress responses [20]. The metabolic perturbations under abiotic stresses can be caused by inhibition of metabolic enzymes or lack of specific substrates or cofactors [21]. The plant metabolic network has to be reprogrammed under stresses so that essential metabolic homeostasis is maintained and protective metabolites are produced to enhance stress tolerance [21]. Exogenous application of metabolites, such as amino acids, sugars, and specialized metabolites (secondary metabolites), has proven to effectively increase stress tolerance in various crop plants [17,22,23,24]. Evolving metabolomics approaches have shed light on the regulation of central metabolism and specialized metabolism under abiotic stresses [1,25,26,27,28,29]. The response of genes and specialized metabolites to abiotic stresses varies among species and have been extensively reviewed recently [30,31,32,33,34,35,36,37,38]. Common stress-induced specialized metabolites include flavonoids, terpenes, phenols, and alkaloids, synthesized in certain species, organs, tissues, and cells [31,32,33]. However, a comprehensive review of plant central metabolic changes in response to different abiotic stresses is needed. Many excellent reviews summarize various metabolic responses under a specific stress, we aim to systematically examine several key abiotic stresses to reveal the reprogramming of plant central metabolism with a network-wide perspective.
Plant central metabolism functions as a hub to quickly adjust metabolic demands in response to various abiotic stresses. The reconfiguration of metabolic fluxes in central metabolism upon abiotic stresses is highly conserved in plant species [21]. It is essential to understand the regulation of central metabolites if we are to rationally modify plant systems to maximize plant resilience to various abiotic stresses. In the following sections, we briefly introduce the recent metabolomics techniques used to investigate plant metabolism. We review the key metabolic responses to the major abiotic stresses, such as flooding, drought, cold, heat, and salinity. We focus on discussing the changes in metabolites involved in central metabolic pathways, such as the Calvin–Benson cycle (CBC), photorespiration, glycolysis, the tricarboxylic acid (TCA) cycle, and the metabolism of sugars, sugar alcohols, and amino acids. Recent metabolomics studies that identify common and unique signatures of central metabolites in response to the five major abiotic stresses are discussed. This review highlights the recent advances in understanding the metabolic reprogramming in plant responses to abiotic stresses with an emphasis on central metabolism.
2. Using Metabolomics as a Tool to Study Plant Abiotic Stress Responses
Metabolomics is an emerging field in the post-genomic era that enables scientists to better understand an organism’s physiological state and response to stimuli. Metabolomics is the comprehensive, quantitative, and qualitative analysis of the metabolome, the complete set of small molecules in a biological system [39]. As the final product of cellular regulatory processes, metabolites provide a more direct representation of the phenotype than genes and proteins whose functions are affected by epigenetic regulation and post-translational modifications [1]. The application of metabolomics has become increasingly common in studying plant responses to abiotic stresses. Searching for research articles in the Web of Science core collection revealed that research using metabolomics or metabolic profiling to study plant abiotic stress responses has progressively increased over the past two decades (Figure 1). There was a drastic increase in publications from 2018 to 2021, with many of them focusing on drought, salinity, and cold responses (Figure 1).
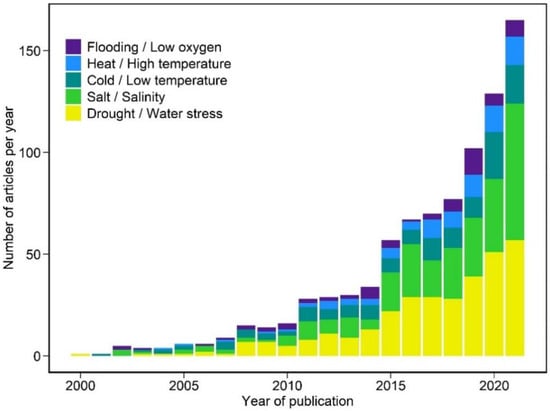
Figure 1.
Number of publications per year from a Web of Science core collection search for research articles on metabolomics or metabolic profiling applied to plant response to major abiotic stresses from 2000 to 2021.
Metabolomic analysis can be classified as either non-targeted or targeted. The non-targeted analysis focuses on the pattern-based classification of as many metabolites as possible in the system with unbiased and global screening (Figure 2A). Non-targeted metabolomics is more commonly used for discovery-based questions, such as the characterization of the most dramatic metabolic changes, when comparing stress treatments and the control [40]. Non-targeted metabolomics has advantages of high unbiased coverage, but faces challenges in complex data-processing processes, relative quantification, and identification of unknowns. In contrast, targeted metabolomic analysis focuses on the identification, quantification, and interpretation of specific responses, and is more often employed to address questions in a hypothesis-driven manner [17,41]. Compared to non-targeted metabolomics, targeted metabolomics focuses on known metabolites with simple data processing, absolute quantification, but low coverage. Both non-targeted and targeted metabolomics analyses have been commonly used in characterizing plant responses to abiotic stresses.
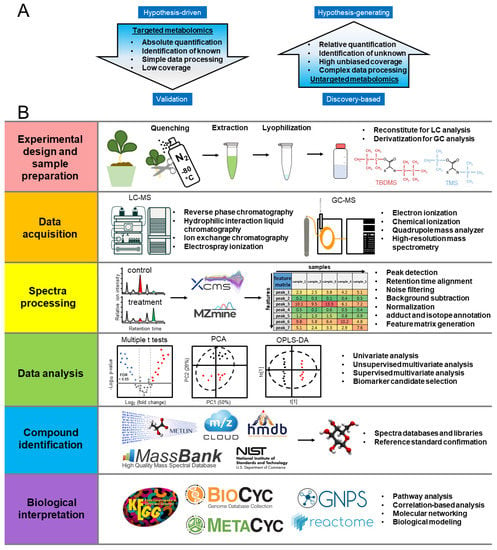
Figure 2.
(A) Targeted and non-targeted metabolomics. (B) Protocol workflow of plant metabolomics studies. Abbreviations: GC-MS: gas chromatography-mass spectrometry; LC-MS: liquid chromatography-mass spectrometry; PCA: principal component analysis; OPLS-DA: orthogonal projections to latent structures discriminant analysis.
Evolving metabolomics approaches provide a new opportunity to capture the metabolic changes under abiotic stresses. The most widely used analytical technologies in metabolomics research include nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) [42]. The NMR-based metabolomic analysis is non-destructive and powerful in providing structural information about the metabolites [43]. Although MS-based metabolomic analysis is destructive, it has gained popularity because of its high sensitivity in metabolite detection [42]. MS approaches are often coupled with chromatographic separation techniques, such as gas chromatography (GC) and liquid chromatography (LC) [42]. The separation of the complex biological samples with a mixture of metabolites before ion detection aids in distinguishing isobaric compounds that have a similar mass. Alternatively, metabolites may be directly measured by the direct-infusion mass spectrometry (DIMS) approach without prior chromatographic separation [44]. DIMS has been extended to rapid, high-throughput fingerprinting strategies using high-resolution mass spectrometers, such as Fourier Transform Ion Cyclotron Resonance (FT-ICR) mass spectrometers [45]. However, no single analytical platform can cover the entire metabolome due to the broad range of chemical properties of metabolites and wide variation in their cellular abundances.
The metabolomics workflow incorporates six steps, as shown in Figure 2B, including experimental design and sample preparation, data acquisition, spectra preprocessing, data analysis, compound identification, and biological interpretation [46]. First, experimental design should define the biological questions to be addressed with appropriate quality controls, such as reagent blanks and sample pools [46]. After sample collection and extraction, the data are collected from different analytical instruments. The choice of instruments should be based on the chemical properties of metabolites of interest. LC-MS can analyze a wide variety of metabolites from polar to non-polar by the selection of columns, such as reversed-phase, hydrophobic interaction, or ion exchange columns [47]. The electrospray ionization (ESI) commonly utilized in LC-MS can be operated in both positive and negative modes to increase the metabolite coverage [48]. However, LC-MS generates adducts that can complicate analyses, and it has less consistent retention indices and spectra libraries than GC-MS [39]. GC-MS is suitable for volatile compounds that can be ionized by electron ionization (EI) or chemical ionization (CI) modes. Trimethylsilyl (TMS) and tert-butyldimethylsilyl (TBDMS) derivatization are suitable for the GC-MS analysis of a wide variety of central metabolites, including amino acids, organic acids, and sugars [49]. Compared to LC-MS, GC-MS has higher reproducibility with larger spectral libraries for metabolite annotation [48].
After data collection, the spectra can be preprocessed for peak detection, retention time alignment, noise filtering, background subtraction, normalization, and annotation of adducts and isotopes. After spectra processing, the feature matrix of peak relative abundance can be generated for further statistical analysis. A variety of statistical approaches can then be performed by univariate, unsupervised multivariate, and supervised multivariate analysis [50,51]. Univariate statistics, such as the t-test and analysis of variance (ANOVA), can test hypotheses on each metabolite of interest and measure significance, which is commonly used in targeted metabolomics [52]. In comparison, multivariate statistics, such as unsupervised multivariate techniques, including principal component analysis (PCA), and supervised multivariate techniques, such as partial least squares discriminant analysis (PLS-DA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA), are widely used in untargeted metabolomics for global matrix dataset. Unsupervised multivariate techniques are particularly useful for sample clustering to show groups of observations, trends, and outliers [53]. Once the trend is identified, supervised techniques, such as OPLS-DA, can be used for greater discrimination power. The variable importance in projection (VIP) scores for OPLS-DA is commonly used for the selection of biomarker candidates [46]. After data analysis, the compounds of interest can be putatively identified by spectra databases and then confirmed by reference standards.
Finally, the biological interpretation of those confidently identified metabolites can be linked back to metabolic networks by pathway analysis, correlation-based analysis, molecular networking, and biological modeling to produce insight into their biological functions [46,54]. Metabolomics and its integration with other omics platforms has been actively developed in recent years, including pathway databases and viewers, and molecular networking tools, such as KEGG (http://www.genome.ad.jp/kegg/, accessed date: 15 April 2022) [55,56], BioCyc (http://biocyc.org/, accessed date: 15 April 2022) [57], MetaCyc (http://metacyc.org/, accessed date: 15 April 2022) [58], Reactome (https://reactome.org/, accessed date: 15 April 2022) [59], and GNPS (https://gnps.ucsd.edu/, accessed date: 15 April 2022) [54].
3. Plant Central Metabolism as a Hub to Respond to Abiotic Stress
3.1. Flooding Stress
The major damage to plants from soil flooding is oxygen deprivation, which negatively affects mitochondrial respiration [14]. When the oxidative phosphorylation of the mitochondrial respiration is impaired under anaerobic conditions, respiratory adenosine triphosphate (ATP) production drops substantially [60]. To cope with the energy crisis, plants increase the glycolytic flux to produce more ATP via a faster depletion of sugar reservoirs [14]. In such stress conditions, plants must generate sufficient ATP to maintain cellular functions and regenerate oxidized NAD+ to maintain the glycolytic flux. Pyruvate accumulated from glycolysis can be channeled through fermentation pathways to restore the pool of NAD+ required for glycolysis [60].
Ethanol fermentation and lactate fermentation are the two fermentation pathways in plants that use pyruvate as the substrate. In ethanol fermentation, pyruvate is decarboxylated to acetaldehyde via pyruvate decarboxylase (PDC) and then reduced to ethanol via alcohol dehydrogenase (ADH) with concomitant oxidation of NADH to NAD+ [61]. Due to the substantially lower energy yield of ethanol fermentation (2 mol ATP per mol glucose consumed), as compared to mitochondrial respiration (36–38 mol ATP per mol glucose consumed), ethanol fermentation must proceed at higher rates to meet the energy demand of cellular functions [62]. Accumulation of the volatile and phytotoxic ethanol and acetaldehyde has been measured in various tree and grass species exposed to flooding [63,64,65]. In flooding tolerant trees, a large amount of ethanol produced from ethanol fermentation in flooded roots could be transported to leaves via the transpiration stream, where it is sequentially oxidized to acetaldehyde and acetate via ADH and aldehyde dehydrogenase in leaves [65,66]. Acetate is converted into acetyl-CoA via acetate-activating enzymes and re-enters central metabolism, which recovers carbon that would otherwise be lost as ethanol in hypoxic tissues [67]. In lactate fermentation, pyruvate is reduced to lactate by lactate dehydrogenase with concomitant oxidation of NADH [68]. Because lactate is a weak acid, its accumulation could cause cellular acidification, potentially leading to the inactivation of enzymes and cell damage [69].
In addition to the adjustment in carbon metabolism via ethanol and lactate fermentation, oxygen deprivation also greatly affects nitrogen metabolism in plant cells [70]. Alanine is one of the most dramatically accumulated amino acids upon oxygen deficiency [71]. The major route for anaerobic accumulation of alanine is via alanine aminotransferase (AlaAT), which favors the conversion of pyruvate and glutamate to alanine and 2-oxoglutarate under hypoxia [72]. How do plants regenerate glutamate as the substrate for AlaAT under hypoxia? The reductive amination of 2-oxoglutarate via the NADH-dependent glutamate synthase (NADH-GOGAT) may be responsible for the newly synthesized glutamate under hypoxia [73]. The increased NADH-GOGAT activity also regenerates NAD+ needed for maintaining the glycolytic flux upon oxygen deficiency [70]. Another route for anaerobic accumulation of alanine is via a process known as γ-aminobutyric acid (GABA) shunt, where glutamate-derived GABA is converted to succinic semialdehyde, concomitantly converting pyruvate to alanine [74]. The accumulation of alanine and GABA has been proposed as an adaptive mechanism under hypoxia to safeguard the carbon that would be otherwise lost during ethanol fermentation and save the ATP that would be used otherwise for assimilating glutamine and asparagine via ATP-consuming enzymes [74]. Changes in many other amino acids, such as aspartate, glutamate, and tyrosine, have been observed in several species under flooding stress [75,76,77,78,79]. In addition, photorespiratory intermediates, such as serine, glycine, glycolate, and glycerate, increased in roots of Medicago truncatula under waterlogging, suggesting a higher photorespiration rate, probably due to the lower stomatal conductance [76].
The TCA cycle operates in noncyclic mode upon oxygen deficiency [73]. Anaerobic accumulation of alanine is accompanied by the production of 2-oxoglutarate, which can enter mitochondria to form succinate via 2-oxoglutarate dehydrogenase and succinate CoA ligase, generating additional ATP to alleviate the energy shortage due to the oxygen limitation. The mitochondrial NAD+ required to oxidize 2-oxoglutarate is generated by reducing oxaloacetate to malate via malate dehydrogenase [75]. Because the TCA cycle enzyme succinate dehydrogenase (SDH) requires oxygen, the accumulation of succinate is typical during hypoxia conditions induced by flooding [73]. Changes in other TCA cycle intermediates, such as citrate, malate, and fumarate, have occurred in several species under flooding stress [75,76,77,78,79].
3.2. Drought Stress
The low water availability in drought-stressed plants limits photosynthesis and restricts plant growth and development [80]. The decline in net CO2 assimilation under a water limitation is due to the decreased CO2 diffusion from the atmosphere to the sites of carboxylation within chloroplasts, which is caused by stomatal closure and probably also the increased mesophyll diffusional resistance [80]. The diffusional resistances of CO2 under water deficits are thought to restrict photosynthesis more directly than the metabolic limitations under water stress [81]. As photosynthesis is the major sink for photosynthetic electrons, water-stressed leaves with decreased photosynthesis are subjected to excess energy, leading to ROS formation that can impair ATP synthesis [82,83]. There is evidence that the activity of ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) decreases under water stress [84], which could be related to decreased ATP and Rubisco activase activity [82]. As CO2 availability is decreased, photorespiratory flux relatively increases in leaves of C3 plants under water deficit, contributing to electron sinks and resulting in high rates of H2O2 production [85]. The imbalance between the supply and demand of ATP or NADPH may be the main factor driving the metabolic pool-size changes induced by drought stress [86,87].
Osmotic adjustment, the accumulation of solutes, is one of the main strategies plants use to maintain positive turgor pressure in water-limited environments [88]. The osmolytes that are accumulated following drought stress are chemically diverse, including soluble sugars (e.g., glucose, fructose, sucrose, and trehalose); the raffinose family oligosaccharides (RFOs, e.g., raffinose, galactinol, and myo-inositol); amino acids (e.g., proline and GABA); quaternary ammonium compounds (e.g., glycine betaine); and polyamines (e.g., putrescine and spermidine) [27,89]. Many of these osmolytes are also involved in other abiotic stresses, such as salinity, cold, and flooding [90]. Soluble sugars are not only important for osmoregulation and the balance between the supply and utilization of carbon and energy in water-stressed plants; they also function as signaling molecules governing many changes in physiology and development [91]. Multiple time-course experiments revealed that sugars, such as RFOs, glucose, and fructose, generally accumulate earlier and more rapidly than many other metabolites in response to drought stress [92,93].
The accumulation of amino acids, such as proline and GABA, occurs later than sugars in response to drought [89,93]. Increased pools of amino acids require more nitrogen assimilation, which is inhibited when ATP is limited in the stressed plants. An alternative source of ammonium would be via glutamate dehydrogenase (GDH), which reversibly catalyzes the formation of glutamate by the amination of 2-oxoglutarate produced from the TCA cycle [94]. The GDH may become important for ammonium assimilation when plants are ATP-limited under drought stress, evidenced by the increased GDH activity in drought-stressed plants with the concomitant rise in proline levels [95]. The increase in branched-chain amino acids (BCAAs), such as leucine, isoleucine, and valine, is commonly observed in many plant species under drought stress [96,97]. The accumulation of BCAAs is probably associated with the high demand for the catabolism of BCAAs to fuel the alternative pathways of mitochondrial respiration during drought stress [97].
3.3. Cold Stress
Cold stress impairs plant development, reduces plant growth and development, and causes crop economic loss. Cold stress can lead to various plant symptoms, including poor germination, stunted seedlings, yellowing of leaves, reduced leaf expansion and wilting, and severe membrane damage caused by acute dehydration associated with the formation of ice crystals [18]. The molecular basis and regulatory mechanisms for plant cold stress responses have been widely studied, including Ca2+ fluxes, inositol phosphates, mitogen activated protein (MAP)-kinase-mediated cascades, Ca-dependent protein kinases, and many transcription factors. Inducer of CBF Expression-1 (ICE1) and the C-repeat-binding factors (CBFs) are best-characterized transcripts that control an important regulon of target genes that include many of the downstream core genes [98,99]. About 10–15% of all the cold-regulated genes are activated by transcriptional activators C-repeat-binding factors/dehydration responsive element-binding factors (CBF1/DREB1b, CBF2/DREB1c, CBF3/DREB1a) [100,101].
Cold stress regulates GABA shunt and the accumulation of proline, raffinose, and galactinol [102,103]. Cold stress-induced transcripts for genes encoding enzymes involved in the induction of callose, fermentation, phospholipid, starch, sugar, flavonoid, protein amino acids, GABA, and terpenoid biosynthesis, and the repression of photorespiration, folic acid, betaine, sulfate assimilation, ethylene, fatty acid, gluconeogenesis, amino acids, brassinosteroids, and chlorophyll biosynthesis [102]. Metabolomic responses to cold stress have been widely studied in Arabidopsis thaliana traditionally and have recently expanded to crop, grass, and medicinal plants [104,105,106]. Cold stress was found to cause more changes to metabolite levels than heat stress [102,103]. Cold stress leads to an increase in a diverse range of metabolites, including proline, GABA, soluble sugars (e.g., glucose, fructose, inositol, galactinol, raffinose, sucrose, and trehalose), ascorbate, putrescine, citrulline, TCA-cycle intermediates, polyamines, and lipids [103,107,108,109,110,111]. Plants under cold stress showed an increase in the proportion of unsaturated fatty acids to stabilize the membranes and maintain membrane fluidity against freeze injury [102,103,112,113,114].
3.4. Heat Stress
Heat stress can disrupt plant physiology by reducing membrane stability and inhibiting respiration and photosynthesis [115,116]. Heat and cold stresses shared many common responses, including the induction of osmolytes that function to reduce cellular dehydration, compatible solutes that are important to stabilize enzymes and membranes, chelating agents that can neutralize metals and inorganic ions, and energy sources [102,109,117].
Plants under heat shock and prolonged warming showed different responses. In response to heat shock, plants produce heat-shock proteins (HSPs) that function as molecular chaperons to defend against heat stress [118]. The heat-shock response is regulated by the transcription factor HSFs family. Part of heat-shock-affected genes was controlled by two major HSF genes, HsfA1a and HsfA1b [119]. HSFA1a/1b regulated genes encoding enzymes involved in signaling, transport processes, and the biosynthesis of osmolytes.
Several metabolomics studies have revealed the impacts of heat shock on plant central metabolism, including amino acids, organic acids, amines, and carbohydrates. Amino acids derived from oxaloacetate and pyruvate (asparagine, leucine, isoleucine, threonine, alanine, and valine), oxaloacetate precursors (fumarate and malate), amine-containing metabolites (β-alanine and GABA), and carbohydrates (maltose, sucrose, trehalose, galactinol, myo-inositol, raffinose, and monosaccharide cell-wall precursors) were reported to increase in response to heat shock [3,58,103,120]. The increase in free-amino acids during heat stress was associated with the breakdown of proteins [58,120]. The increase in the TCA-cycle intermediates under heat stress suggests that higher amounts of Coenzyme A may be important for increased biosynthetic and energy needs [103]. The induction of the raffinose biosynthesis pathway and accumulation of galactinol and raffinose during heat shock were mediated by galactinol synthase-1 (GolS1) controlled by HSFs [119]. In contrast to the short-term heat shock, plants exposed to prolonged warming enhance the glycolysis pathway but inhibit the TCA cycle [121]. Wheat (Triticum aestivum), under prolonged warming, showed an increase in tryptophan [122]. Cytokinins (CKs), fatty acid metabolism, flavonoid, terpenoid biosynthesis, and secondary metabolite biosynthesis were identified as the most important pathways involved in prolonged warming response [122].
3.5. Salinity Stress
Salinity stress negatively impacts plants’ water and nutrients uptake, growth and development, photosynthesis, and protein biosynthesis [123]. Salinity stress may induce both osmotic and ion stresses [124]. A previous study showed that the high-voltage electrical discharge treatment could improve the germination and early growth of wheat in drought and salinity conditions [125]. The main difference between osmotic adjustment induced by salinity and drought stresses is the total amount of water available. In addition to low water potential, the concentration of harmful ions, such as Na+, Cl−, or SO42−, increased associated with salinity stress, causing specific ion toxicity effects [126]. NaCl is the most abundant salt in plants under salinity stress. A high concentration of Na+ and/or Cl− in cells inhibits photosynthesis [127]. The transport systems, such as K+–Na+ transporter (HKT1), Na+–H+ antiporter SOS1 (salt overly sensitive 1) AtNHX1, and calcium-regulated transporters SOS2/SOS3, are important in regulating Na+ compartmentation during salinity stress [128,129,130,131,132].
Metabolomics has been extensively used to characterize the salinity responses of various plant species. Central metabolites, including sugars, polyols, and amino acids, play important roles in osmotic adjustment, cell turgor pressure maintenance, signaling molecules, carbon storage, and free-radical scavenging [17]. A variety of plants under salt stress were reported to accumulate osmolytes as soluble sugars (sucrose, trehalose, and raffinose) and sugar alcohols (sorbitol, galactinol, and mannitol) [133,134,135,136,137,138]. Amino acids, such as proline, can also function as osmolytes to protect plants under salt stress in many varieties [38,139,140,141]. For example, Tibetan wild barley (Hordeum spontaneum) and cultivated barley (H. vulgare) under salt stress were reported with changes in amino acids, including proline, alanine, aspartate, glutamate, threonine, and valine, with genotype-dependent manners [142]. Eight amino acids and amines, including 4-hydroxy-proline, asparagine, alanine, arginine, phenylalanine, citrulline, glutamine, and proline, were reported to be significantly increased in multiple barley varieties under salt stress [138]. Both Thellungiella halophila and Arabidopsis thaliana under salinity stresses showed an increase in proline and sugars. Triticum durum Desf. Exposed to salinity stress showed an accumulation in proline, GABA, threonine, leucine, glutamic acid, glycine, mannose, and fructose, and the depletion of organic acids, including TCA-cycle intermediates [143]. Rice (Oryza sativa) pretreated with chemical priming reagent hydrogen sulfide (H2S) showed better growth and development under salt stress with elevated levels of ascorbic acid, glutathione, redox states, and the enhanced activities of ROS- and methylglyoxal-detoxifying enzymes [17].
The biomarkers for salt-tolerant varieties vary between species. Three halophytes, Sesuvium portulacastrum, Spartina maritima, and Salicornia brachiate, were compared under salinity stress [144]. Proline increased in Sesuvium portulacastrum and Spartina maritima, while glycine betaine and polyols increased in Spartina maritima and Salicornia brachiate [144]. Salinity-resistant Lotus japonicus seedlings showed an increase in threonine, serine, ononitol, glucuronic acids, and gulonic acids, and decreased asparagine and glutamine [145]. Salt-tolerant cultivar barley (Hordeum vulgare) showed increased proline, carbohydrates, hexose phosphates, and TCA-cycle intermediates [142,146]. Salt-tolerant rice (Oryza sativa) showed increased concentrations of amino acids, serotonin, and gentisic acid, and decreased concentrations of TCA intermediates [147]. Salinity-resistant transgenic tobacco (Nicotiana) plants showed an increase in proline, glutathione, and trehalose, and a decrease in fructose [148]. Omeprazole-treated tomato (Solanum lycopersicum) with improved salinity tolerance showed increased polyamine conjugates, alkaloids, sesquiterpene lactones, and abscisic acid, and a decrease in auxins and cytokinin, and gibberellic acid [149]. Sugar-beet (Beta vulgaris subsp. vulgaris) seedlings under salinity stress showed an increase in malic acid and 2-oxoglutaric acid in the short-term treatment and an increase in betaine and melatonin in the long-term treatment [150]. Hulless barley (Hordeum distichon) under salinity stress showed increased tryptophan, glutamic acid, phenylalanine, cinnamic acid, inosine 5-monophosphate, and abscisic acid [151].
4. Common and Unique Metabolic Changes in Central Metabolites under Abiotic Stresses
Plants exhibit diverse metabolic responses to different abiotic stresses. We reviewed the published literature for metabolomics studies on plant abiotic stress responses, including cold, heat, drought, flooding, and salinity stresses, and summarized key metabolites in major metabolic pathways in central metabolism that are affected by each stress (Figure 3, Table 1). Among the summarized metabolites, 52 were affected by cold stresses, 55 by drought, 46 by flooding, 42 by heat, and 58 by salinity. Some stress-related metabolites showed common responses to multiple abiotic stresses, and some metabolites only responded to specific stress. Interestingly, 23 metabolites showed common stress responses to all stresses reviewed in this study, including cold, heat, drought, flooding, and salinity.
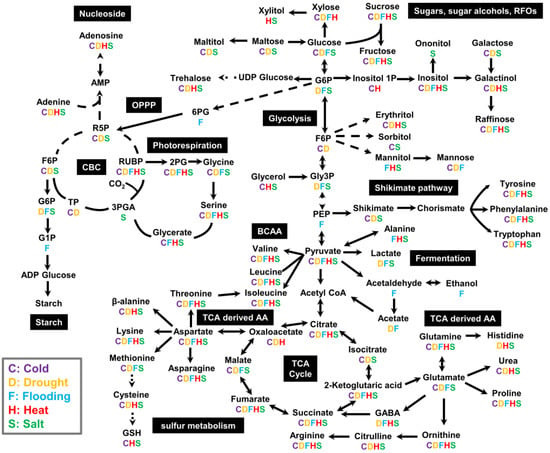
Figure 3.
Summary scheme showing the significantly changed central metabolites under cold, drought, flooding, heat, and salinity stress. The letter below each metabolite indicates that the metabolite is significantly changed under the specific stress. Depicted data are extracted from published studies shown in Table 1. Abbreviations: 2PG: 2-phosphoglycolate; 3PGA: 3-phosphoglyceric acid; 6PG: 6-Phosphogluconate; ADP Glucose: adenosine diphosphate glucose; AMP: Adenosine monophosphate; CBC: the Calvin–Benson–Bassham cycle; F6P: fructose-6-phosphate; G1P: glucose-1-phosphate; G6P: glucose-6-phosphate; GABA: gamma-aminobutyric acid; Gly3P: Glycerol 3-phosphate; GSH: reduced glutathione; OPPP: the oxidative pentose phosphate pathway; PEP: phosphoenolpyruvate; R5P: ribose-5-phosphate; RUBP: ribulose-1,5-bisophosphate; TCA: the tricarboxylic acid cycle; UDP Glucose: uridine diphosphate glucose.

Table 1.
List of significantly changed central metabolites under cold, drought, flooding, heat, and salinity stress from published studies.
As leaf photosynthesis and photorespiration are highly sensitive to environmental changes, many intermediates in the CBC and photorespiratory pathway respond to various abiotic stresses (Figure 3). When the absorbed light energy exceeds its consumption, oxidative damage occurs and causes alterations in plant central metabolism under most abiotic stresses. The common trends for metabolic changes in response to oxidative damage include the prevention of ROS formation, maintenance of essential metabolite biosynthesis, and accumulation of protective compounds, such as compatible solutes or osmolytes to protect plants from oxidative damage [195,196]. Various abiotic stresses trigger the accumulation of compatible solutes, such as sucrose, trehalose, raffinose, mannitol, sorbitol, inositol, and proline (Figure 3). These molecules have properties of high solubility in water, non-toxicity at high concentrations, and reduction in protein–solvent interactions at low water activities [109,197]. Introducing biosynthetic pathways for these compatible solutes can increase crop abiotic stress tolerance, which has been of interest to metabolic engineering [197,198].
The shikimate pathway is activated under various abiotic stresses, which leads to the accumulation of aromatic amino acids, such as tyrosine, phenylalanine, and tryptophan (Figure 3). These aromatic amino acids are the precursors for the biosynthesis of flavonoids, alkaloids, and phytoalexins with antioxidant properties [199]. Sulfur-containing amino acids and sulfur-containing metabolites, such as methionine, cysteine, and glutathione, are also induced by various abiotic stresses (Figure 3). These metabolites have important roles in plant antioxidant systems [200,201,202]. The synthesis of cysteine and its product glutathione are modulated by the ratio between reduced and oxidized glutathione [203,204].
Oxidative damage induced by various abiotic stresses can lead to the redistribution of glycolytic carbon flux into the oxidative pentose phosphate pathway (OPPP) and perturbation of the TCA cycle, resulting in the accumulation of sugar phosphates related to glycolysis and OPPP and decreases in TCA-cycle intermediates and TCA-cycle-derived amino acids [205,206]. Common stress responses have been observed in glutamate (derived from 2-oxoglutarate) and aspartate (derived from oxaloacetate), both of which are important substrates for other TCA-cycle-derived amino acids. In addition, the nonessential amino acid citrulline (derived from glutamate) also functions as an antioxidant and efficient hydroxyl radical scavenger [207,208].
GABA is rapidly accumulated under various abiotic stresses [209,210,211]. The main function of GABA includes being a signaling molecule, osmoregulator, and regulation of cytosolic pH [209]. BCAAs (valine, leucine, and isoleucine) and other amino acids sharing synthetic pathways with BCAA (lysine, threonine, and methionine) generally have similar patterns with GABA that are accumulated in response to various abiotic stresses [178,212]. The accumulation of BCAAs are either from the activation of the biosynthetic pathway or protein degradation [178,212]. BCAAs are critical for normal plant growth and can function as compatible solutes, alternative electron donors for the mitochondrial electron transport chain, and substrates for protective secondary metabolites, such as cyanogenic glycosides, glucosinolates, and acyl-sugars [178,212,213].
Changes in ethanol fermentation intermediates, such as ethanol, acetaldehyde, and acetate, are more specific to the flooding stress (Figure 3). The ability to recover the carbon that would be lost as ethanol by converting it into acetate and then acetyl-CoA to support the flooded plant’s carbon metabolism could be related to plant flooding tolerance [66]. Acetate is also recently identified as a key metabolite that mediates a novel drought-survival mechanism [189]. The metabolic switch from glycolysis to acetate biosynthesis via pyruvate decarboxylase and aldehyde dehydrogenase stimulates the jasmonate signaling pathway for plant drought tolerance [189]. Furthermore, engineering increased the expression of the acetate biosynthesis pathway, and the exogenous application of acetic acid enhanced plant survival under drought stress [24,214,215].
5. Challenges and Perspectives in Deciphering Plant Metabolic Responses to Abiotic Stresses in Time and Space
A major challenge in characterizing plant metabolism under stress is the compartmentalization at the level of cells and organelles. Distinct tissue-specific metabolic responses to abiotic stresses have been characterized in many plant species [76,216,217]. In contrast, our knowledge of cell-specific and organelle-specific metabolic responses has been hampered by the limitations of current analytical methods to determine the cellular and subcellular location of metabolites. Although aqueous fractionation methods have been used to analyze cell-specific and organelle-specific metabolites [218,219], it is laborious and requires extensive optimizations to reduce the risk of contamination, low coverage, and missing compartments [220]. The other challenge in capturing the plant metabolome during stresses is the quenching step that stops the metabolism faster than the turnover of metabolites to preserve the metabolic profile at the point of the stress treatment applied. Because turnover times of many metabolites are in the order of seconds or less [221], it is essential to ensure the fast harvest and quenching of samples, which can be difficult to apply in field conditions.
Another major challenge in characterizing plant metabolism under stress is the identification of unknown metabolites due to complex and dynamic plant metabolism, comprehensive detection methods in databases, instruments, and platforms. The plant kingdom is estimated to contain between 200,000 and 1,000,000 metabolites [21]. Due to this great chemical diversity and the wide range in concentrations, to date, there is no single instrument platform for the comprehensive examination of the whole metabolome [222]. Recently, liquid chromatography-high resolution-mass spectrometry (LC-HR-MS) has become a leading technology for metabolomics because it provides a more universal view of the metabolism [1,47]. However, it is accompanied by major challenges in metabolite annotation [223].
The identification of unknowns by LC-MS is complicated and challenging because of no uniform databases and platforms. The three-level identification includes MS1 spectral identification, MS2 spectral identification, and authentic standard identification. First, putative identification can be performed by query of MS1 compounds and spectral databases, such as METLIN (https://metlin.scripps.edu, accessed date 15 April 2022) [224], ChemSpider (http://www.chemspider.com, accessed date 15 April 2022), Human Metabolome DataBase (HMDB) (https://hmdb.ca, accessed date 15 April 2022), KEGG (https://www.genome.jp/kegg, accessed date 15 April 2022) [55], MassBank (https://massbank.eu/MassBank, accessed date 15 April 2022) [225], mzCloud (https://www.mzcloud.org, accessed date 15 April 2022), and GNPS (https://gnps.ucsd.edu, accessed date 15 April 2022) [54]. However, even the high-mass accuracy measurement with less than 1 ppm error is not sufficient to always fully determine the elemental composition [226]. After putative identification by MS1, further information, such as MS2 fragmentation data, is needed to perform the MS2 spectral database search. Nonetheless, the spectra databases for MS2 searching are currently considered incomplete because the fragmentation patterns are greatly affected by instrument, collision energy, and ionization source. The scores for MS2 searching can often result in false positive and false negative results [227]. Therefore, to confidently identify the metabolite, authentic standards are always needed [46].
While metabolomic profiling has become increasingly common in plant-stress physiology studies, the measurement of metabolite abundances alone is insufficient to inform the activity of the metabolic pathways involved and the time-dependent flux changes in response to abiotic stresses. Stable isotope-assisted metabolomics enables global assessment of fluxes through plant metabolic networks [228]. Typically, an isotope-labeled precursor is introduced into plant tissues, and the redistribution of the label from the precursor Into downstream metabolites is detected by MS or NMR [229]. The labeling data can be coupled to mathematical modeling to construct flux maps, representing a quantitative description of metabolic phenotypes [230]. Transient labeling-based flux approaches, such as isotopically nonstationary metabolic flux analysis (INST-MFA), have been applied in several plant species to examine intracellular fluxes through central carbon metabolism [188,231,232]. Future applications of metabolic flux analysis in the context of abiotic stresses hold the key to elucidating the temporal dynamics of the central metabolic network under stress conditions.
Emerging advances in mass spectrometry imaging (MSI) offer a unique capability to simultaneously capture the spatial distributions of metabolites and macromolecules at the molecular level. MSI has been successfully applied to map the spatial localization of various plant metabolites, providing a mechanistic understanding of plant metabolism [233]. The most widely used MSI technique is matrix-assisted laser desorption ionization (MALDI) [234]. MALDI operates under a vacuum and has been demonstrated to visualize many lipids at a high spatial resolution of ~5 µm in maize leaves and roots [235,236]. MALDI-MSI was also used to reveal the detailed spatial distribution of lipids in barley roots in response to salinity stress [237]. In addition to MALDI, ambient ionization technique, such as electrospray laser desorption ionization, was used to study the spatial distribution of carbohydrates, organic acids, amino acids, and flavonoids in Coleus leaves in response to the change of illumination [238]. In combination with stable isotope labeling, MSI has great potential to map spatially resolved metabolic fluxes, which has been applied to mammalian tissues [239]. With improved spatial resolution and sensitivity to quantify the isotopic labeling of less abundant metabolites, stable isotope-assisted MSI holds the promise of unraveling spatially resolved metabolic flux reprogramming under abiotic stresses.
Author Contributions
Conceptualization, Y.X. and X.F.; writing, Y.X. and X.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for Plant Stress Response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Sunkar, R. Plant Stress Tolerance. Methods Mol. Biol. 2010, 639, 291–297. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-Driven Risks to the Climate Mitigation Potential of Forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef] [PubMed]
- von der Gathen, P.; Kivi, R.; Wohltmann, I.; Salawitch, R.J.; Rex, M. Climate Change Favours Large Seasonal Loss of Arctic Ozone. Nat. Commun. 2021, 12, 3886. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting Long-Term Dynamics of Soil Salinity and Sodicity on a Global Scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 Availability and Inactivation of Rubisco Limit Photosynthesis in Cotton Plants under Heat and Drought Stress in the Field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and Combined Effects of Transient Drought and Heat Stress on Carbon Assimilation and Seed Filling in Chickpea. Proc. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. Climate Change 2014 Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107415379. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and Crop Yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-Y.; Peng, S.-X.; Yang, S.; Chen, Y.-H.; Zhang, J.-Y.; Mo, W.-P.; Zhu, J.-Y.; Ye, Y.-X.; Huang, X.-M. Effects of Flooding on Grafted Annona Plants of Different Scion/Rootstock Combinations. Agric. Sci. 2012, 3, 249–256. [Google Scholar] [CrossRef]
- Setter, T.L.; Waters, I. Review of Prospects for Germplasm Improvement for Waterlogging Tolerance in Wheat, Barley and Oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.S.P. Enhancing Salt Tolerance of Plants: From Metabolic Reprogramming to Exogenous Chemical Treatments and Molecular Approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature Stress and Redox Homeostasis in Agricultural Crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M.N.V. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9781461406341. [Google Scholar]
- Caldana, C.; Degenkolbe, T.; Cuadros-Inostroza, A.; Klie, S.; Sulpice, R.; Leisse, A.; Steinhauser, D.; Fernie, A.R.; Willmitzer, L.; Hannah, M.A. High-Density Kinetic Analysis of the Metabolomic and Transcriptomic Response of Arabidopsis to Eight Environmental Conditions. Plant J. 2011, 67, 869–884. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The Use of Metabolomics to Dissect Plant Responses to Abiotic Stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Lei, S.; Rossi, S.; Huang, B. Metabolic and Physiological Regulation of Aspartic Acid-Mediated Enhancement of Heat Stress Tolerance in Perennial Ryegrass. Plants 2022, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mostofa, M.G.; Keya, S.S.; Rahman, A.; Das, A.K.; Islam, R.; Abdelrahman, M.; Bhuiyan, S.U.; Naznin, T.; Ansary, M.U.; et al. Acetic Acid Improves Drought Acclimation in Soybean: An Integrative Response of Photosynthesis, Osmoregulation, Mineral Uptake and Antioxidant Defense. Physiol. Plant. 2021, 172, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Á. Role of Metabolites in Abiotic Stress Tolerance. Plant Life Chang. Environ. Responses Manag. 2020, 30, 755–774. [Google Scholar] [CrossRef]
- Sytar, O.; Mbarki, S.; Zivcak, M.; Brestic, M. The Involvement of Different Secondary Metabolites in Salinity Tolerance of Crops. Salin. Responses Toler. Plants 2018, 2, 21–48. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in Plant Stress Physiology. Adv. Biochem. Eng. Biotechnol. 2018, 164, 187–236. [Google Scholar] [CrossRef]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions Between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant Metabolomics in Biotic and Abiotic Stress: A Critical Overview. Phytochem. Rev. 2021, 21, 503–524. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Zehra, A.; Choudhary, S.; Naeem, M.; Masroor Khan, M.A.; Khan, A.; Aftab, T.; Tariq Aftab, C.; Masroor, M.A. A Review of Medicinal and Aromatic Plants and Their Secondary Metabolites Status under Abiotic Stress. J. Med. Plants Stud. 2019, 7, 99–106. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant Secondary Metabolites Synthesis and Their Regulations under Biotic and Abiotic Constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of Biostimulation of Plants Under Abiotic Stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef] [PubMed]
- Alves de Freitas Guedes, F.; Menezes-Silva, P.E.; DaMatta, F.M.; Alves-Ferreira, M. Using Transcriptomics to Assess Plant Stress Memory. Theor. Exp. Plant Physiol. 2019, 31, 47–58. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, C.; Zhu, S.; Wang, W.; Xu, J.; Zhao, X. Characterizing the Metabolites Related to Rice Salt Tolerance with Introgression Lines Exhibiting Contrasting Performances in Response to Saline Conditions. Plant Growth Regul. 2020, 92, 157–167. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass Spectrometry-Based Metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G.; Kind, T.; Niehaus, T.D.; Broadbelt, L.J.; Hanson, A.D.; Fiehn, O.; Tyo, K.E.J.; Henry, C.S.; et al. Innovation: Metabolomics: The Apogee of the Omics Trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Dudley, E.; Yousef, M.; Wang, Y.; Griffiths, W.J. Targeted Metabolomics and Mass Spectrometry. Adv. Protein Chem. Struct. Biol. 2010, 80, 45–83. [Google Scholar] [CrossRef]
- Dunn, W.B.; Bailey, N.J.C.; Johnson, H.E. Measuring the Metabolome: Current Analytical Technologies. Analyst 2005, 130, 606–625. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The Future of NMR-Based Metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, J.I.; Hayes, A.; Mohammed, S.; Gaskell, S.J.; Oliver, S.G. An Optimized Protocol for Metabolome Analysis in Yeast Using Direct Infusion Electrospray Mass Spectrometry. Phytochemistry 2003, 62, 929–937. [Google Scholar] [CrossRef]
- Ghaste, M.; Mistrik, R.; Shulaev, V. Applications of Fourier Transform Ion Cyclotron Resonance (FT-ICR) and Orbitrap Based High Resolution Mass Spectrometry in Metabolomics and Lipidomics. Int. J. Mol. Sci. 2016, 17, 816. [Google Scholar] [CrossRef] [PubMed]
- Guitton, Y.; Tremblay-Franco, M.; Le Corguillé, G.; Martin, J.F.; Pétéra, M.; Roger-Mele, P.; Delabrière, A.; Goulitquer, S.; Monsoor, M.; Duperier, C.; et al. Create, Run, Share, Publish, and Reference Your LC–MS, FIA–MS, GC–MS, and NMR Data Analysis Workflows with the Workflow4Metabolomics 3.0 Galaxy Online Infrastructure for Metabolomics. Int. J. Biochem. Cell Biol. 2017, 93, 89–101. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Allwood, J.W.; Ellis, D.I.; Goodacre, R. Metabolomic Technologies and Their Application to the Study of Plants and Plant-Host Interactions. Physiol. Plant. 2008, 132, 117–135. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: The Combination of Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2017, 7, 232–235. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Han, Y.; Wang, P.; Sun, H.; Song, G.; Dong, T.; Yuan, Y.; Yuan, X.; Zhang, M.; et al. Urine Metabolomics Analysis for Biomarker Discovery and Detection of Jaundice Syndrome in Patients With Liver Disease. Mol. Cell. Proteom. 2012, 11, 370–380. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A Guideline to Univariate Statistical Analysis for LC/MS-Based Untargeted Metabolomics-Derived Data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Paley, S.M.; Karp, P.D. The Pathway Tools Cellular Overview Diagram and Omics Viewer. Nucleic Acids Res. 2006, 34, 3771–3778. [Google Scholar] [CrossRef]
- Caspi, R.; Foerster, H.; Fulcher, C.A.; Hopkinson, R.; Ingraham, J.; Kaipa, P.; Krummenacker, M.; Paley, S.; Pick, J.; Rhee, S.Y.; et al. MetaCyc: A Multiorganism Database of Metabolic Pathways and Enzymes. Nucleic Acids Res. 2006, 34, D511–D516. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Tope, G.; Gillespie, M.; Vastrik, I.; D’Eustachio, P.; Schmidt, E.; de Bono, B.; Jassal, B.; Gopinath, G.R.; Wu, G.R.; Matthews, L.; et al. Reactome: A Knowledgebase of Biological Pathways. Nucleic Acids Res. 2005, 33, D428–D432. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.C.J.; van Dongen, J.T. Making Sense of Low Oxygen Sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef]
- Tadege, M.; Dupuis, I.; Kuhlemeier, C. Ethanolic Fermentation: New Functions for an Old Pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 2319. [Google Scholar] [CrossRef]
- Boamfa, E.I.; Ram, P.C.; Jackson, M.B.; Reuss, J.; Harren, F.J.M. Dynamic Aspects of Alcoholic Fermentation of Rice Seedlings in Response to Anaerobiosis and to Complete Submergence: Relationship to Submergence Tolerance. Ann. Bot. 2003, 91, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Rottenberger, S.; Kleiss, B.; Kuhn, U.; Wolf, A.; Piedade, M.T.F.; Junk, W.; Kesselmeier, J. The Effect of Flooding on the Exchange of the Volatile C 2-Compounds Ethanol, Acetaldehyde and Acetic Acid between Leaves of Amazonian Floodplain Tree Species and the Atmosphere. Biogeosciences 2008, 5, 1085–1100. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of Flooding on C Metabolism of Flood-Tolerant (Quercus Robur) and Non-Tolerant (Fagus sylvatica) Tree Species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Papadopoulou, E.; Rennenberg, H. Interaction of Flooding with Carbon Metabolism of Forest Trees. Plant Biol. 2004, 6, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yang, H.; Pangestu, F.; Nikolau, B.J. Failure to Maintain Acetate Homeostasis by Acetate-Activating Enzymes Impacts Plant Development. Plant Physiol. 2020, 182, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Rivoal, J.; Hanson, A.D. Metabolic Control of Anaerobic Glycolysis: Overexpression of Lactate Dehydrogenase in Transgenic Tomato Roots Supports the Davies-Roberts Hypothesis and Points to a Critical Role for Lactate Secretion. Plant Physiol. 1994, 106, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.D.; Grego, S.; Kenworthy, P. The Control of the Production of Lactate and Ethanol by Higher Plants. Planta 1974, 118, 297–310. [Google Scholar] [CrossRef]
- Limami, A.M. Adaptations of Nitrogen Metabolism to Oxygen Deprivation in Plants. Plant Cell Monogr. 2014, 21, 209–221. [Google Scholar] [CrossRef]
- Streeter, J.G.; Thompson, J.F. Anaerobic Accumulation of $γ$-Aminobutyric Acid and Alanine in Radish Leaves (Raphanus sativus, L.). Plant Physiol. 1972, 49, 572–578. [Google Scholar] [CrossRef]
- Ricoult, C.; Echeverria, L.O.; Cliquet, J.B.; Limami, A.M. Characterization of Alanine Aminotransferase (AlaAT) Multigene Family and Hypoxic Response in Young Seedlings of the Model Legume Medicago Truncatula. J. Exp. Bot. 2006, 57, 3079–3089. [Google Scholar] [CrossRef]
- António, C.; Päpke, C.; Rocha, M.; Diab, H.; Limami, A.M.; Obata, T.; Fernie, A.R.; van Dongen, J.T. Regulation of Primary Metabolism in Response to Low Oxygen Availability as Revealed by Carbon and Nitrogen Isotope Redistribution. Plant Physiol. 2016, 170, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Limami, A.M.; Glévarec, G.; Ricoult, C.; Cliquet, J.B.; Planchet, E. Concerted Modulation of Alanine and Glutamate Metabolism in Young Medicago Truncatula Seedlings under Hypoxic Stress. J. Exp. Bot. 2008, 59, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the Tricarboxylic Acid Cycle Are Linked by Alanine Aminotransferase during Hypoxia Induced by Waterlogging of Lotus Japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Lothier, J.; Diab, H.; Cukier, C.; Limami, A.M.; Tcherkez, G. Metabolic Responses to Waterlogging Differ between Roots and Shoots and Reflect Phloem Transport Alteration in Medicago Truncatula. Plants 2020, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamamoto, R.; Hiraga, S.; Nakayama, N.; Okazaki, K.; Takahashi, H.; Uchimiya, H.; Komatsu, S. Evaluation of Metabolite Alteration under Flooding Stress in Soybeans. Jpn. Agric. Res. Q. 2012, 46, 237–248. [Google Scholar] [CrossRef]
- Barding, G.A.; Béni, S.; Fukao, T.; Bailey-Serres, J.; Larive, C.K. Comparison of GC-MS and NMR for Metabolite Profiling of Rice Subjected to Submergence Stress. J. Proteome Res. 2013, 12, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Davanture, M.; Zivy, M.; Lamade, E.; Tcherkez, G. Metabolic Responses to Potassium Availability and Waterlogging Reshape Respiration and Carbon Use Efficiency in Oil Palm. New Phytol. 2019, 223, 310–322. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of Decreased Photosynthetic Rate and Metabolic Capacity in Water-Deficient Leaf Cells: A Critical Evaluation of Mechanisms and Integration of Processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased Rubisco Activity during Water Stress Is Not Induced by Decreased Relative Water Content but Related to Conditions of Low Stomatal Conductance and Chloroplast CO2 Concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and Oxidative Load in the Leaves of C3 Plants: A Predominant Role for Photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to Photosynthesis in Water-Stressed Leaves: Stomata vs. Metabolism and the Role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor Maintenance by Osmotic Adjustment: 40 Years of Progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and Molecular Approaches Reveal Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Suprasanna, P.; Nikalje, G.C.; Rai, A.N. Osmolyte Accumulation and Implications in Plant Abiotic Stress Tolerance. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: New Delhi, India, 2015; pp. 1–12. [Google Scholar] [CrossRef]
- Hanson, J.; Smeekens, S. Sugar Perception and Signaling—An Update. Curr. Opin. Plant Biol. 2009, 12, 562–567. [Google Scholar] [CrossRef]
- Rabara, R.C.; Tripathi, P.; Reese, R.N.; Rushton, D.L.; Alexander, D.; Timko, M.P.; Shen, Q.J.; Rushton, P.J. Tobacco Drought Stress Responses Reveal New Targets for Solanaceae Crop Improvement. BMC Genom. 2015, 16, 484. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the Vascular Brassinosteroid Receptor BRL3 Confers Drought Resistance without Penalizing Plant Growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hessini, K.; Kronzucker, H.J.; Abdelly, C.; Cruz, C. Drought Stress Obliterates the Preference for Ammonium as an N Source in the C4 Plant Spartina Alterniflora. J. Plant Physiol. 2017, 213, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and Phenotypic Responses of Greenhouse-Grown Maize Hybrids to Experimentally Controlled Drought Stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The Influence of Alternative Pathways of Respiration That Utilize Branched-Chain Amino Acids Following Water Shortage in Arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 Overexpression Induces COR Genes and Enhances Freezing Tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.H.; Hong, X.; Agarwal, M.; Zhu, J.K. ICE1: A Regulator of Cold-Induced Transcriptome and Freezing Tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular Responses to Dehydration and Low Temperature: Differences and Cross-Talk between Two Stress Signaling Pathways. Curr. Opin. Plant Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Hannah, M.A. Natural Genetic Variation of Freezing Tolerance in Arabidopsis. Plant Physiol. 2006, 142, 98–112. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and Metabolite Profiling during Cold Acclimation of Arabidopsis Reveals an Intricate Relationship of Cold-Regulated Gene Expression with Modifications in Metabolite Content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Sun, C.X.; Gao, X.X.; Li, M.Q.; Fu, J.Q.; Zhang, Y.L. Plastic Responses in the Metabolome and Functional Traits of Maize Plants to Temperature Variations. Plant Biol. 2016, 18, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Fontaine, J.X.; Molinié, R.; Pelloux, J.; Mesnard, F.; Gillet, F.; Fliniaux, O. NMR-Based Metabolomics to Study the Cold-Acclimation Strategy of Two Miscanthus Genotypes. Phytochem. Anal. 2017, 28, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; Delangiz, N.; Lajayer, B.A.; Saghafi, D.; Maggi, F. Review and Future Prospects on the Mechanisms Related to Cold Stress Resistance and Tolerance in Medicinal Plants. Acta Ecol. Sin. 2021, 41, 120–129. [Google Scholar] [CrossRef]
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M.F. A Prominent Role for the CBF Cold Response Pathway in Configuring the Low-Temperature Metabolome of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 15243–15248. [Google Scholar] [CrossRef] [PubMed]
- Wienkoop, S.; Morgenthal, K.; Wolschin, F.; Scholz, M.; Selbig, J.; Weckwerth, W. Integration of Metabolomic and Proteomic Phenotypes: Analysis of Data Covariance Dissects Starch and RFO Metabolism from Low and High Temperature Compensation Response in Arabidopsis Thaliana. Mol Cell Proteomics 2008, 7, 1725–1736. [Google Scholar] [CrossRef]
- Guy, C.L. Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Korn, M.; Gärtner, T.; Erban, A.; Kopka, J.; Selbig, J.; Hincha, D.K. Predicting Arabidopsis Freezing Tolerance and Heterosis in Freezing Tolerance from Metabolite Composition. Mol. Plant 2010, 3, 224–235. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Tartari, A.; Cattivelli, L.; Forlani, G. Metabolism of γ-Aminobutyric Acid during Cold Acclimation and Freezing and Its Relationship to Frost Tolerance in Barley and Wheat. J. Exp. Bot. 2006, 57, 3755–3766. [Google Scholar] [CrossRef]
- Williams, J.P.; Khan, M.U.; Mitchell, K.; Johnson, G. The Effect of Temperature on the Level and Biosynthesis of Unsaturated Fatty Acids in Diacylglycerols of Brassica Napus Leaves. Plant Physiol. 1988, 87, 904–910. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, Salinity and Drought Stresses: An Overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Bohn, M.; Lüthje, S.; Sperling, P.; Heinz, E.; Dörffling, K. Plasma Membrane Lipid Alterations Induced by Cold Acclimation and Abscisic Acid Treatment of Winter Wheat Seedlings Differing in Frost Resistance. J. Plant Physiol. 2007, 164, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Hemantaranjan, A. Heat Stress Responses and Thermotolerance. Adv. Plants Agric. Res. 2014, 1, 62–70. [Google Scholar] [CrossRef]
- Végh, B.; Marček, T.; Karsai, I.; Janda, T. Darkó Heat Acclimation of Photosynthesis in Wheat Genotypes of Different Origin. South Afr. J. Bot. 2018, 117, 184–192. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of Temperature Stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Nover, L.; Bharti, K.; Döring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the Heat Stress Transcription Factor World: How Many Heat Stress Transcription Factors Do We Need? Cell Stress Chaperones 2001, 6, 177. [Google Scholar] [CrossRef]
- Panikulangara, T.J.; Eggers-Schumacher, G.; Wunderlich, M.; Stransky, H.; Schöffl, F. Galactinol Synthase1. A Novel Heat Shock Factor Target Gene Responsible for Heat-Induced Synthesis of Raffinose Family Oligosaccharides in Arabidopsis. Plant Physiol. 2004, 136, 3148–3158. [Google Scholar] [CrossRef]
- Zhang, P.; Foerster, H.; Tissier, C.P.; Mueller, L.; Paley, S.; Karp, P.D.; Rhee, S.Y. MetaCyc and AraCyc. Metabolic Pathway Databases for Plant Research. Plant Physiol. 2005, 138, 27–37. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential Physiological, Transcriptomic and Metabolomic Responses of Arabidopsis Leaves under Prolonged Warming and Heat Shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef]
- Thomason, K.; Babar, M.A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; MacDonald, G. Comparative Physiological and Metabolomics Analysis of Wheat (Triticum aestivum L.) Following Post-Anthesis Heat Stress. PLoS ONE 2018, 13, e0197919. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Bressan, R.A.; Nelson, D.E.; Iraki, N.M.; LaRosa, P.C.; Singh, N.K.; Hasegawa, P.M.; Carpita, N.C. Reduced Cell Expansion and Changes in Cell Walls of Plant Cells Adapted to NaCl. In Environmental Injury to Plants; Academic Press: Cambridge, MA, USA, 1990; pp. 137–171. [Google Scholar]
- Marček, T.; Kovač, T.; Jukić, K.; Lončarić, A.; Ižaković, M. Application of High Voltage Electrical Discharge Treatment to Improve Wheat Germination and Early Growth under Drought and Salinity Conditions. Plants 2021, 10, 2137. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Läuchli, A.; Polito, V.S. Displacement of Ca2+ by Na+ from the Plasmalemma of Root Cells. Plant Physiol. 1985, 79, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Binzel, M.L.; Hess, F.D.; Bressan, R.A.; Hasegawa, P.M. Intracellular Compartmentation of Ions in Salt Adapted Tobacco Cells. Plant Physiol. 1988, 86, 607–614. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis Thaliana Salt Tolerance Gene SOS1 Encodes a Putative Na+/H+ Antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Läuchli, A. Spatial Distribution of Solutes, K, Na, Ca and Their Deposition Rates in the Growth Zone of Primary Cotton Roots: Effects of NaCl and CaCl2. Planta 1994, 194, 34–41. [Google Scholar] [CrossRef]
- Quintero, F.J.; Blatt, M.R.; Pardo, J.M. Functional Conservation between Yeast and Plant Endosomal Na+/H+ Antiporters. FEBS Lett. 2000, 471, 224–228. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. An Arabidopsis Mutant That Requires Increased Calcium for Potassium Nutrition and Salt Tolerance. Proc. Natl. Acad. Sci. USA 1997, 94, 14960–14964. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt Tolerance Conferred by Overexpression of a Vacuolar Na+/H+ Antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Zhu, H.; Paul, M.; Zu, Y.; Tang, Z. Exogenous Trehalose Largely Alleviates Ionic Unbalance, ROS Burst, and PCD Occurrence Induced by High Salinity in Arabidopsis Seedlings. Front. Plant Sci. 2014, 5, 570. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Dias, D.A.; Hill, C.B.; Jayasinghe, N.S.; Atieno, J.; Sutton, T.; Roessner, U. Quantitative Profiling of Polar Primary Metabolites of Two Chickpea Cultivars with Contrasting Responses to Salinity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1000, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bendaly, A.; Messedi, D.; Smaoui, A.; Ksouri, R.; Bouchereau, A.; Abdelly, C. Physiological and Leaf Metabolome Changes in the Xerohalophyte Species Atriplex Halimus Induced by Salinity. Plant Physiol. Biochem. 2016, 103, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.F.; Duro, N.; da Costa, M.; Florian, A.; Ramalho, J.C.; Ribeiro-Barros, A.I.; Fernie, A.R.; António, C. GC-TOF-MS Analysis Reveals Salt Stress-Responsive Primary Metabolites in Casuarina Glauca Tissues. Metabolomics 2017, 13, 95. [Google Scholar] [CrossRef]
- Cao, D.; Lutz, A.; Hill, C.B.; Callahan, D.L.; Roessner, U. A Quantitative Profiling Method of Phytohormones and Other Metabolites Applied to Barley Roots Subjected to Salinity Stress. Front. Plant Sci. 2017, 7, 2070. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, X.; Han, L.; Hou, L.; Li, B. Effects of Exogenous Spermidine on Root Metabolism of Cucumber Seedlings under Salt Stress by GC-MS. Agronomy 2020, 10, 459. [Google Scholar] [CrossRef]
- Pang, Q.; Zhang, A.; Zang, W.; Wei, L.; Yan, X. Integrated Proteomics and Metabolomics for Dissecting the Mechanism of Global Responses to Salt and Alkali Stress in Suaeda Corniculata. Plant Soil 2016, 402, 379–394. [Google Scholar] [CrossRef]
- Sobhanian, H.; Motamed, N.; Jazii, F.R.; Nakamura, T.; Komatsu, S. Salt Stress Induced Differential Proteome and Metabolome Response in the Shoots of Aeluropus Lagopoides (Poaceae), a Halophyte C4 Plant. J. Proteome Res. 2010, 9, 2882–2897. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue Metabolic Responses to Salt Stress in Wild and Cultivated Barley. PLoS ONE 2013, 8, e55431. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of Metabolic and Mineral Changes in Response to Salt Stress in Durum Wheat (Triticum turgidum ssp. durum) Genotypes, Which Differ in Salinity Tolerance. Plant Physiol. Biochem. 2018, 133, 57–70. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic Insights into the Mechanisms Underlying Tolerance to Salinity in Different Halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Lippold, F.; Redestig, H.; Hannah, M.A.; Erban, A.; Krämer, U.; Kopka, J.; Udvardi, M.K. Integrative Functional Genomics of Salt Acclimatization in the Model Legume Lotus Japonicus. Plant J. 2008, 53, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Widodo; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic Responses to Salt Stress of Barley (Hordeum vulgare L.) Cultivars, Sahara and Clipper, Which Differ in Salinity Tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; De, B. Metabolomics Analysis of Rice Responses to Salinity Stress Revealed Elevation of Serotonin, and Gentisic Acid Levels in Leaves of Tolerant Varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Li, P.; Ma, S.; Indu Rupassara, S.; Bohnert, H.J. Salinity Stress Adaptation Competence in the Extremophile Thellungiella Halophila in Comparison with Its Relative Arabidopsis Thaliana. Plant J. 2005, 44, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Raimondi, G.; Lucini, L.; Carillo, P.; Kyriacou, M.C.; Colla, G.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; De Pascale, S. Physiological and Metabolic Responses Triggered by Omeprazole Improve Tomato Plant Tolerance to NaCl Stress. Front. Plant Sci. 2018, 9, 249. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B.; Liu, D.; Zou, C.; Wu, P.; Wang, Z.; Wang, Y.; Li, C. Transcriptomic and Metabolomic Analyses Reveal Mechanisms of Adaptation to Salinity in Which Carbon and Nitrogen Metabolism Is Altered in Sugar Beet Roots. BMC Plant Biol. 2020, 20, 138. [Google Scholar] [CrossRef]
- Muchate, N.S.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. NaCl Induced Salt Adaptive Changes and Enhanced Accumulation of 20-Hydroxyecdysone in the in Vitro Shoot Cultures of Spinacia oleracea (L.). Sci. Rep. 2019, 9, 138. [Google Scholar] [CrossRef]
- Xu, Y.; Freund, D.M.; Hegeman, A.D.; Cohen, J.D. Metabolic Signatures of Arabidopsis Thaliana Abiotic Stress Responses Elucidate Patterns in Stress Priming, Acclimation, and Recovery. Stress Biol. 2022. [Google Scholar] [CrossRef]
- Lu, Y.; Lam, H.; Pi, E.; Zhan, Q.; Tsai, S.; Wang, C.; Kwan, Y.; Ngai, S. Comparative Metabolomics in Glycine Max and Glycine Soja under Salt Stress to Reveal the Phenotypes of Their Offspring. J. Agric. Food Chem. 2013, 61, 8711–8721. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.A.; Stanley, P.D.; Holmes, E. Application of NMR-Based Metabolomics to the Investigation of Salt Stress in Maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Du, Y.; Chen, S.; Tang, H. Dynamic Metabonomic Responses of Tobacco (Nicotiana tabacum) Plants to Salt Stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Salicylic Acid Alleviates NaCl-Induced Changes in the Metabolism of Matricaria Chamomilla Plants. Ecotoxicology 2009, 18, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Yang, X.; Zhu, J.; Liu, Z.; Ni, Y.; Wu, H.; Zhang, H.; Liu, T. Salinity-Induced Metabolic Profile Changes in Nitraria Tangutorum Bobr. Suspension Cells. Plant Cell. Tissue Organ Cult. 2015, 122, 239–248. [Google Scholar] [CrossRef]
- Espinoza, C.; Degenkolbe, T.; Caldana, C.; Zuther, E.; Leisse, A.; Willmitzer, L.; Hincha, D.K.; Hannah, M.A. Interaction with Diurnal and Circadian Regulation Results in Dynamic Metabolic and Transcriptional Changes during Cold Acclimation in Arabidopsis. PLoS ONE 2010, 5, e14101. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Köhl, K.I.; Hincha, D.K.; Zuther, E. Dissecting Rice Polyamine Metabolism under Controlled Long-Term Drought Stress. PLoS ONE 2013, 8, e60325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Involvement of Polyamines in the Drought Resistance of Rice. J. Exp. Bot. 2007, 58, 1545–1555. [Google Scholar] [CrossRef]
- Skirycz, A.; de Bodt, S.; Obata, T.; de Clercq, I.; Claeys, H.; de Rycke, R.; Andriankaja, M.; van Aken, O.; van Breusegem, F.; Fernie, A.R.; et al. Developmental Stage Specificity and the Role of Mitochondrial Metabolism in the Response of Arabidopsis Leaves to Prolonged Mild Osmotic Stress. Plant Physiol. 2010, 152, 226–244. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-Targeted Metabolomics and Scavenging Activity of Reactive Oxygen Species Reveal the Potential of Salicornia Brachiata as a Functional Food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.R.; Marriott, A.S.; Bergström, E.; et al. Molecular Mechanisms of Desiccation Tolerance in the Resurrection Glacial Relic Haberlea Rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought Responses of Leaf Tissues from Wheat Cultivars of Differing Drought Tolerance at the Metabolite Level. Proc. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite Adjustments in Drought Tolerant and Sensitive Soybean Genotypes in Response to Water Stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and Salinity Stress in Grapevines: Early and Late Changes in Transcript and Metabolite Profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ford, K.L.; Roessner, U.; Natera, S.; Cassin, A.M.; Patterson, J.H.; Bacic, A. Rice Suspension Cultured Cells Are Evaluated as a Model System to Study Salt Responsive Networks in Plants Using a Combined Proteomic and Metabolomic Profiling Approach. Proteomics 2013, 13, 2046–2062. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.B.; Jha, D.; Bacic, A.; Tester, M.; Roessner, U. Characterization of Ion Contents and Metabolic Responses to Salt Stress of Different Arabidopsis AtHKT1;1 Genotypes and Their Parental Strains. Mol. Plant 2013, 6, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Nagler, M.; Nukarinen, E.; Weckwerth, W.; Nägele, T. Integrative Molecular Profiling Indicates a Central Role of Transitory Starch Breakdown in Establishing a Stable C/N Homeostasis during Cold Acclimation in Two Natural Accessions of Arabidopsis Thaliana. BMC Plant Biol. 2015, 15, 284. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-Regulated Global Responses to Dehydration in Arabidopsis by Metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Semel, Y.; Schauer, N.; Roessner, U.; Zamir, D.; Fernie, A.R. Metabolite Analysis for the Comparison of Irrigated and Non-Irrigated Field Grown Tomato of Varying Genotype. Metabolomics 2007, 3, 289–295. [Google Scholar] [CrossRef]
- Sicher, R.C.; Barnaby, J.Y. Impact of Carbon Dioxide Enrichment on the Responses of Maize Leaf Transcripts and Metabolites to Water Stress. Physiol. Plant. 2012, 144, 238–253. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A Reassessment of the Function of the So-Called Compatible Solutes in the Halophytic Plumbaginaceae Limonium Latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef]
- Komatsu, S.; Yamamoto, A.; Nakamura, T.; Nouri, M.Z.; Nanjo, Y.; Nishizawa, K.; Furukawa, K. Comprehensive Analysis of Mitochondria in Roots and Hypocotyls of Soybean under Flooding Stress Using Proteomics and Metabolomics Techniques. J. Proteome Res. 2011, 10, 3993–4004. [Google Scholar] [CrossRef]
- Komatsu, S.; Nakamura, T.; Sugimoto, Y.; Sakamoto, K. Proteomic and Metabolomic Analyses of Soybean Root Tips Under Flooding Stress. Protein Pept. Lett. 2014, 21, 865–884. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.H.; Bouchereau, A.; Berardocco, S.; Seal, C.E.; Flowers, T.J.; Zörb, C. Metabolic and Physiological Adjustment of Suaeda Maritima to Combined Salinity and Hypoxia. Ann. Bot. 2017, 119, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Patel, M.K.; Mishra, A.; Jha, B. Physio-Biochemical Composition and Untargeted Metabolomics of Cumin (Cuminum cyminum L.) Make It Promising Functional Food and Help in Mitigating Salinity Stress. PLoS ONE 2015, 10, e0144469. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of Threonine, Methionine and Isoleucine Metabolism in Plants: Accumulation and Transcriptional Regulation under Abiotic Stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Erxleben, A.; Gessler, A.; Vervliet-Scheebaum, M.; Reski, R. Metabolite Profiling of the Moss Physcomitrella Patens Reveals Evolutionary Conservation of Osmoprotective Substances. Plant Cell Rep. 2012, 31, 427–436. [Google Scholar] [CrossRef]
- Rathore, N.; Thakur, D.; Kumar, D.; Chawla, A.; Kumar, S. Time-Series Eco-Metabolomics Reveals Extensive Reshuffling in Metabolome during Transition from Cold Acclimation to de-Acclimation in an Alpine Shrub. Physiol. Plant. 2021, 173, 1824–1840. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Pieckenstain, F.L.; Escaray, F.; Erban, A.; Kraemer, U.; Udvardi, M.K.; Kopka, J. Comparative Ionomics and Metabolomics in Extremophile and Glycophytic Lotus Species under Salt Stress Challenge the Metabolic Pre-Adaptation Hypothesis. Plant Cell Environ. 2011, 34, 605–617. [Google Scholar] [CrossRef]
- Qi, X.; Xu, W.; Zhang, J.; Guo, R.; Zhao, M.; Hu, L.; Wang, H.; Dong, H.; Li, Y. Physiological Characteristics and Metabolomics of Transgenic Wheat Containing the Maize C4 Phosphoenolpyruvate Carboxylase (PEPC) Gene under High Temperature Stress. Protoplasma 2017, 254, 1017–1030. [Google Scholar] [CrossRef]
- Kim, J.K.; Bamba, T.; Harada, K.; Fukusaki, E.; Kobayashi, A. Time-Course Metabolic Profiling in Arabidopsis Thaliana Cell Cultures after Salt Stress Treatment. J. Exp. Bot. 2007, 58, 415–424. [Google Scholar] [CrossRef]
- Brosché, M.; Vinocur, B.; Alatalo, E.R.; Lamminmäki, A.; Teichmann, T.; Ottow, E.A.; Djilianov, D.; Afif, D.; Bogeat-Triboulot, M.B.; Altman, A.; et al. Gene Expression and Metabolite Profiling of Populus Euphratica Growing in the Negev Desert. Genome Biol. 2005, 6, R101. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible Solute Accumulation and Stress-Mitigating Effects in Barley Genotypes Contrasting in Their Salt Tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Takahara, K.; Hirabayashi, T.; Matsumura, H.; Fujisawa, S.; Terauchi, R.; Uchimiya, H.; Kawai-Yamada, M. Metabolome Analysis of Response to Oxidative Stress in Rice Suspension Cells Overexpressing Cell Death Suppressor Bax Inhibitor-1. Plant Cell Physiol. 2010, 51, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Hasnain, G.; Logue, T.; Lynch, M.; Wu, S.; Guan, J.C.; Alseekh, S.; Fernie, A.R.; Hanson, A.D.; McCarty, D.R. A Core Metabolome Response of Maize Leaves Subjected to Long-Duration Abiotic Stresses. Metabolites 2021, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wieloch, T.; Kaste, J.A.M.; Shachar-Hill, Y.; Sharkey, T.D. Reimport of Carbon from Cytosolic and Vacuolar Sugar Pools into the Calvin–Benson Cycle Explains Photosynthesis Labeling Anomalies. Proc. Natl. Acad. Sci. USA 2022, 119, e2121531119. [Google Scholar] [CrossRef]
- Kim, J.M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Habu, Y.; Ogawa, D.; Sakamoto, T.; Matsunaga, S.; et al. Acetate-Mediated Novel Survival Strategy against Drought in Plants. Nat. Plants 2017, 3, 17097. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Henning, L.M.M.; Döpp, S.A.; Nepomuceno, A.; Moraes, L.A.C.; Marcolino-Gomes, J.; Richter, C.; Schwalbe, H.; Colnago, L.A. Flooded Soybean Metabolomic Analysis Reveals Important Primary and Secondary Metabolites Involved in the Hypoxia Stress Response and Tolerance. Environ. Exp. Bot. 2018, 153, 176–187. [Google Scholar] [CrossRef]
- Mustroph, A.; Boamfa, E.I.; Laarhoven, L.J.J.; Harren, F.J.M.; Pörs, Y.; Grimm, B. Organ Specific Analysis of the Anaerobic Primary Metabolism in Rice and Wheat Seedlings II: Light Exposure Reduces Needs for Fermentation and Extends Survival during Anaerobiosis. Planta 2006, 225, 139–152. [Google Scholar] [CrossRef]
- Jain, M.; Aggarwal, S.; Nagar, P.; Tiwari, R.; Mustafiz, A. A D-Lactate Dehydrogenase from Rice Is Involved in Conferring Tolerance to Multiple Abiotic Stresses by Maintaining Cellular Homeostasis. Sci. Rep. 2020, 10, 12835. [Google Scholar] [CrossRef]
- Antonio, C.; Pinheiro, C.; Chaves, M.M.; Ricardo, C.P.; Ortuño, M.F.; Thomas-Oates, J. Analysis of Carbohydrates in Lupinus Albus Stems on Imposition of Water Deficit, Using Porous Graphitic Carbon Liquid Chromatography-Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 2008, 1187, 111–118. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, H.; Zhang, J.; Liu, P.; Chen, X.; Li, Z.; Xu, Y.; Lu, P.; Cao, P. Integrated Transcriptomics and Metabolomics Analysis to Characterize Cold Stress Responses in Nicotiana Tabacum. BMC Genom. 2017, 18, 496. [Google Scholar] [CrossRef]
- Savchenko, T.; Tikhonov, K. Oxidative Stress-Induced Alteration of Plant Centralmetabolism. Life 2021, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Darko, E.; Végh, B.; Khalil, R.; Marček, T.; Szalai, G.; Pál, M.; Janda, T. Metabolic Responses of Wheat Seedlings to Osmotic Stress Induced by Various Osmolytes under Iso-Osmotic Conditions. PLoS ONE 2019, 14, e0226151. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.H.; Murata, N. Glycinebetaine Protects Plants against Abiotic Stress: Mechanisms and Biotechnological Applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shachar-Hill, Y. Insights into Metabolic Efficiency from Flux Analysis. J. Exp. Bot. 2012, 63, 2343–2351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur Metabolism and Stress Defense Responses in Plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Colovic, M.B.; Vasic, V.M.; Djuric, D.M.; Krstic, D.Z. Sulphur-Containing Amino Acids: Protective Role Against Free Radicals and Heavy Metals. Curr. Med. Chem. 2018, 25, 324–335. [Google Scholar] [CrossRef]
- Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential Role of Sulfur-Containing Antioxidant Systems in Highly Oxidative Environments. Molecules 2014, 19, 19376–19389. [Google Scholar] [CrossRef]
- Bick, J.A.; Setterdahl, A.T.; Knaff, D.B.; Chen, Y.; Pitcher, L.H.; Zilinskas, B.A.; Leustek, T. Regulation of the Plant-Type 5′-Adenylyl Sulfate Reductase by Oxidative Stress. Biochemistry 2001, 40, 9040–9048. [Google Scholar] [CrossRef]
- Queval, G.; Thominet, D.; Vanacker, H.; Miginiac-Maslow, M.; Gakire, B.; Noctor, G. H2O2-Activated up-Regulation of Glutathione in Arabidopsis Involves Induction of Genes Encoding Enzymes Involved in Cysteine Synthesis in the Chloroplast. Mol. Plant 2009, 2, 344–356. [Google Scholar] [CrossRef]
- Baxter, C.J.; Redestig, H.; Schauer, N.; Repsilber, D.; Patil, K.R.; Nielsen, J.; Selbig, J.; Liu, J.; Fernie, A.R.; Sweetlove, L.J. The Metabolic Response of Heterotrophic Arabidopsis Cells to Oxidative Stress. Plant Physiol. 2007, 143, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Schwarzländer, M.; Obata, T.; Sirikantaramas, S.; Burow, M.; Olsen, C.E.; Tohge, T.; Fricker, M.D.; Møller, B.L.; Fernie, A.R.; et al. The Metabolic Response of Arabidopsis Roots to Oxidative Stress Is Distinct from That of Heterotrophic Cells in Culture and Highlights a Complex Relationship between the Levels of Transcripts, Metabolites, and Flux. Mol. Plant 2009, 2, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a Novel Compatible Solute in Drought-Tolerant Wild Watermelon Leaves, Is an Efficient Hydroxyl Radical Scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of Citrulline in Watermelon Rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in Plants: Just a Metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and Functions of Gamma-Aminobutyric Acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress. CRC Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein Degradation—An Alternative Respiratory Substrate for Stressed Plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef]
- Binder, S. Branched-Chain Amino Acid Metabolism in Arabidopsis Thaliana. Arab. Book 2010, 8, e0137. [Google Scholar] [CrossRef]
- Utsumi, Y.; Utsumi, C.; Tanaka, M.; Van Ha, C.; Takahashi, S.; Matsui, A.; Matsunaga, T.M.; Matsunaga, S.; Kanno, Y.; Seo, M.; et al. Acetic Acid Treatment Enhances Drought Avoidance in Cassava (Manihot esculenta Crantz). Front. Plant Sci. 2019, 10, 521. [Google Scholar] [CrossRef]
- Rasheed, S.; Bashir, K.; Kim, J.M.; Ando, M.; Tanaka, M.; Seki, M. The Modulation of Acetic Acid Pathway Genes in Arabidopsis Improves Survival under Drought Stress. Sci. Rep. 2018, 8, 7831. [Google Scholar] [CrossRef] [PubMed]
- Lawas, L.M.F.; Li, X.; Erban, A.; Kopka, J.; Jagadish, S.V.K.; Zuther, E.; Hincha, D.K. Metabolic Responses of Rice Cultivars with Different Tolerance to Combined Drought and Heat Stress under Field Conditions. Gigascience 2019, 8, giz050. [Google Scholar] [CrossRef] [PubMed]
- Skodra, C.; Michailidis, M.; Dasenaki, M.; Ganopoulos, I.; Thomaidis, N.S.; Tanou, G.; Molassiotis, A. Unraveling Salt-Responsive Tissue-Specific Metabolic Pathways in Olive Tree. Physiol. Plant. 2021, 173, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Szecowka, M.; Heise, R.; Tohge, T.; Nunes-Nesi, A.; Vosloh, D.; Huege, J.; Feil, R.; Lunn, J.; Nikoloski, Z.; Stitt, M.; et al. Metabolic Fluxes in an Illuminated Arabidopsis Rosette. Plant Cell 2013, 25, 694–714. [Google Scholar] [CrossRef]
- Arrivault, S.; Obata, T.; Szecówka, M.; Mengin, V.; Guenther, M.; Hoehne, M.; Fernie, A.R.; Stitt, M. Metabolite Pools and Carbon Flow during C4 Photosynthesis in Maize: 13CO 2 Labeling Kinetics and Cell Type Fractionation. J. Exp. Bot. 2017, 68, 283–298. [Google Scholar] [CrossRef]
- Dietz, K.J. Subcellular Metabolomics: The Choice of Method Depends on the Aim of the Study. J. Exp. Bot. 2017, 68, 5695–5698. [Google Scholar] [CrossRef]
- Arrivault, S.; Guenther, M.; Ivakov, A.; Feil, R.; Vosloh, D.; Van Dongen, J.T.; Sulpice, R.; Stitt, M. Use of Reverse-Phase Liquid Chromatography, Linked to Tandem Mass Spectrometry, to Profile the Calvin Cycle and Other Metabolic Intermediates in Arabidopsis Rosettes at Different Carbon Dioxide Concentrations. Plant J. 2009, 59, 826–839. [Google Scholar] [CrossRef]
- Bino, R.J.; Hall, R.D.; Fiehn, O.; Kopka, J.; Saito, K.; Draper, J.; Nikolau, B.J.; Mendes, P.; Roessner-Tunali, U.; Beale, M.H.; et al. Potential of Metabolomics as a Functional Genomics Tool. Trends Plant Sci. 2004, 9, 418–425. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Benton, H.P.; Siuzdak, G. Bioinformatics: The next Frontier of Metabolomics. Anal. Chem. 2015, 87, 147–156. [Google Scholar] [CrossRef]
- Smith, A.; O’maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A Public Repository for Sharing Mass Spectral Data for Life Sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Fiehn, O. Metabolomic Database Annotations via Query of Elemental Compositions: Mass Accuracy Is Insufficient Even at Less than 1 Ppm. BMC Bioinform. 2006, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.K. Quantifying Plant Phenotypes with Isotopic Labeling & Metabolic Flux Analysis. Curr. Opin. Biotechnol. 2016, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, R.G.; Shachar-Hill, Y. Measuring Multiple Fluxes through Plant Metabolic Networks. Plant J. 2006, 45, 490–511. [Google Scholar] [CrossRef] [PubMed]
- Libourel, I.G.L.; Shachar-Hill, Y. Metabolic Flux Analysis in Plants: From Intelligent Design to Rational Engineering. Annu. Rev. Plant Biol. 2008, 59, 625–650. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fu, X.; Sharkey, T.D.; Shachar-Hill, Y.; Walker, B.J. The Metabolic Origins of Non-Photorespiratory CO2 Release during Photosynthesis: A Metabolic Flux Analysis. Plant Physiol. 2021, 186, 297–314. [Google Scholar] [CrossRef]
- Ma, F.; Jazmin, L.J.; Young, J.D.; Allen, D.K. Isotopically Nonstationary 13C Flux Analysis of Changes in Arabidopsis Thaliana Leaf Metabolism Due to High Light Acclimation. Proc. Natl. Acad. Sci. USA 2014, 111, 16967–16972. [Google Scholar] [CrossRef]
- Lee, Y.J.; Perdian, D.C.; Song, Z.; Yeung, E.S.; Nikolau, B.J. Use of Mass Spectrometry for Imaging Metabolites in Plants. Plant J. 2012, 70, 81–95. [Google Scholar] [CrossRef]
- Boughton, B.A.; Thinagaran, D.; Sarabia, D.; Bacic, A.; Roessner, U. Mass Spectrometry Imaging for Plant Biology: A Review. Phytochem. Rev. 2016, 15, 445–488. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Dueñas, M.E.; Lee, Y.J. Five Micron High Resolution MALDI Mass Spectrometry Imaging with Simple, Interchangeable, Multi-Resolution Optical System. J. Am. Soc. Mass Spectrom. 2017, 28, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.E.; Klein, A.T.; Alexander, L.E.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. High Spatial Resolution Mass Spectrometry Imaging Reveals the Genetically Programmed, Developmental Modification of the Distribution of Thylakoid Membrane Lipids among Individual Cells of Maize Leaf. Plant J. 2017, 89, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, L.D.; Boughton, B.A.; Rupasinghe, T.; van de Meene, A.M.L.; Callahan, D.L.; Hill, C.B.; Roessner, U. High-Mass-Resolution MALDI Mass Spectrometry Imaging Reveals Detailed Spatial Distribution of Metabolites and Lipids in Roots of Barley Seedlings in Response to Salinity Stress. Metabolomics 2018, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- McVey, P.A.; Alexander, L.E.; Fu, X.; Xie, B.; Galayda, K.J.; Nikolau, B.J.; Houk, R.S. Light-Dependent Changes in the Spatial Localization of Metabolites in Solenostemon Scutellarioides (Coleus henna) Visualized by Matrix-Free Atmospheric Pressure Electrospray Laser Desorption Ionization Mass Spectrometry Imaging. Front. Plant Sci. 2018, 9, 1348. [Google Scholar] [CrossRef]
- Louie, K.B.; Bowen, B.P.; McAlhany, S.; Huang, Y.; Price, J.C.; Mao, J.H.; Hellerstein, M.; Northen, T.R. Mass Spectrometry Imaging for in Situ Kinetic Histochemistry. Sci. Rep. 2013, 3, 1656. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).