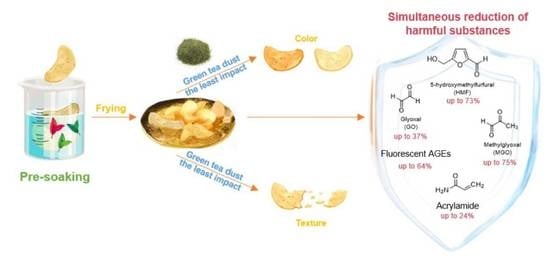
Reduction in Five Harmful Substances in Fried Potato Chips by Pre-Soaking Treatment with Different Tea Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Tea Extracts
2.3. Determination of Total Phenolic Content
2.4. Evaluation of Antioxidant Capacity
2.5. Analysis of Catechins in Different Tea Samples
2.6. Preparation of Potato Crips
2.7. Color and Texture Analysis
2.8. Water Extracts of Potato Chips
2.9. HMF Analysis
2.10. Acrylamide Analysis
2.11. Determination of Glyoxal and Methylglyoxal
2.12. Evaluation of Fluorescent AGEs and Protein Oxidative Products
2.13. Determination of Carbonyl Value
2.14. Statistical Analysis
3. Results and Discussion
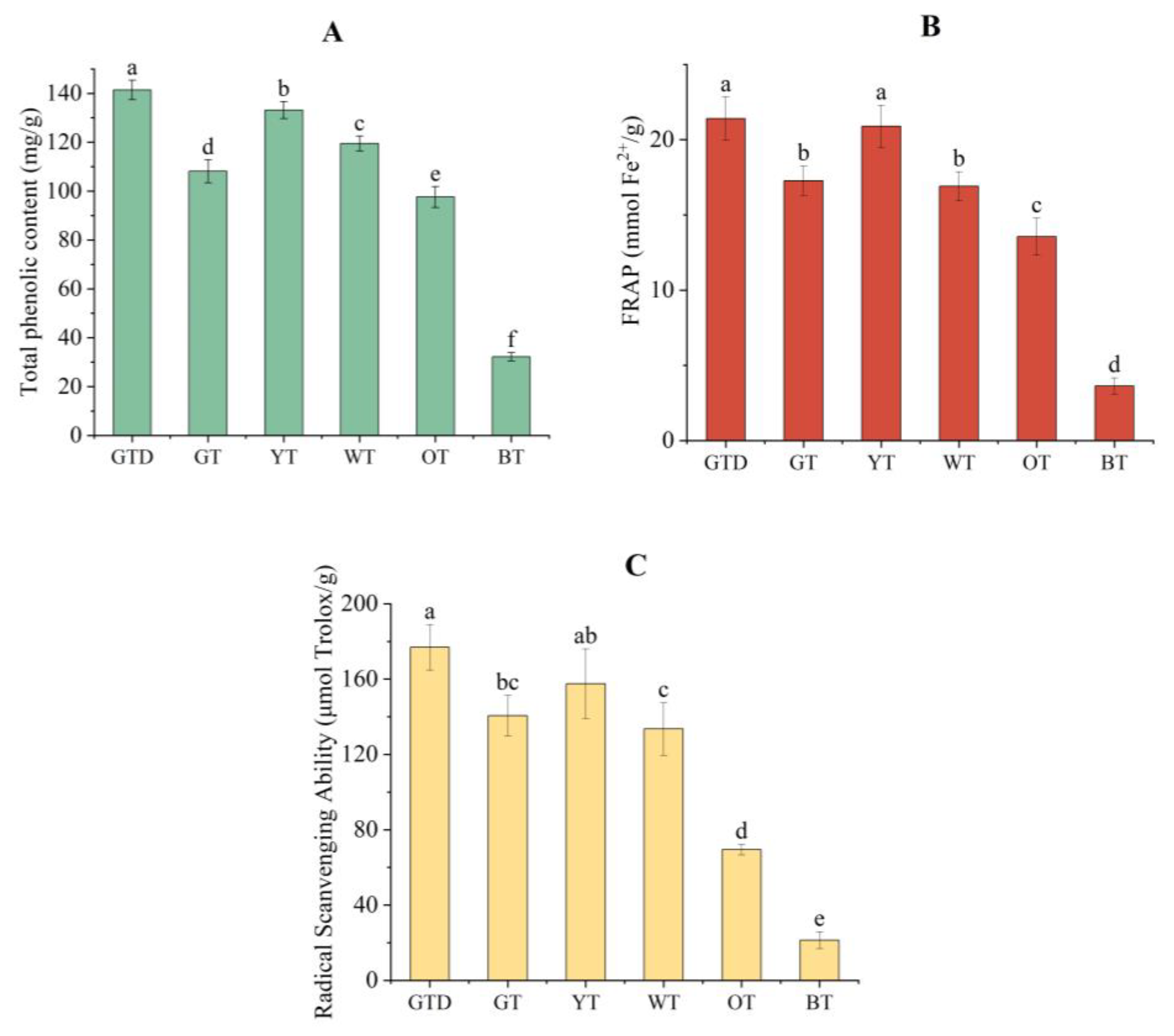
3.1. Total Phenolic Content of Different Tea Samples
3.2. Catechin Profile of the Tea Samples
3.3. Antioxidant Activity of Different Kinds of Tea Samples
3.4. Inhibition of Harmful Substances in Potato Chips by Pre-soaking Treatment with Different Tea Extracts
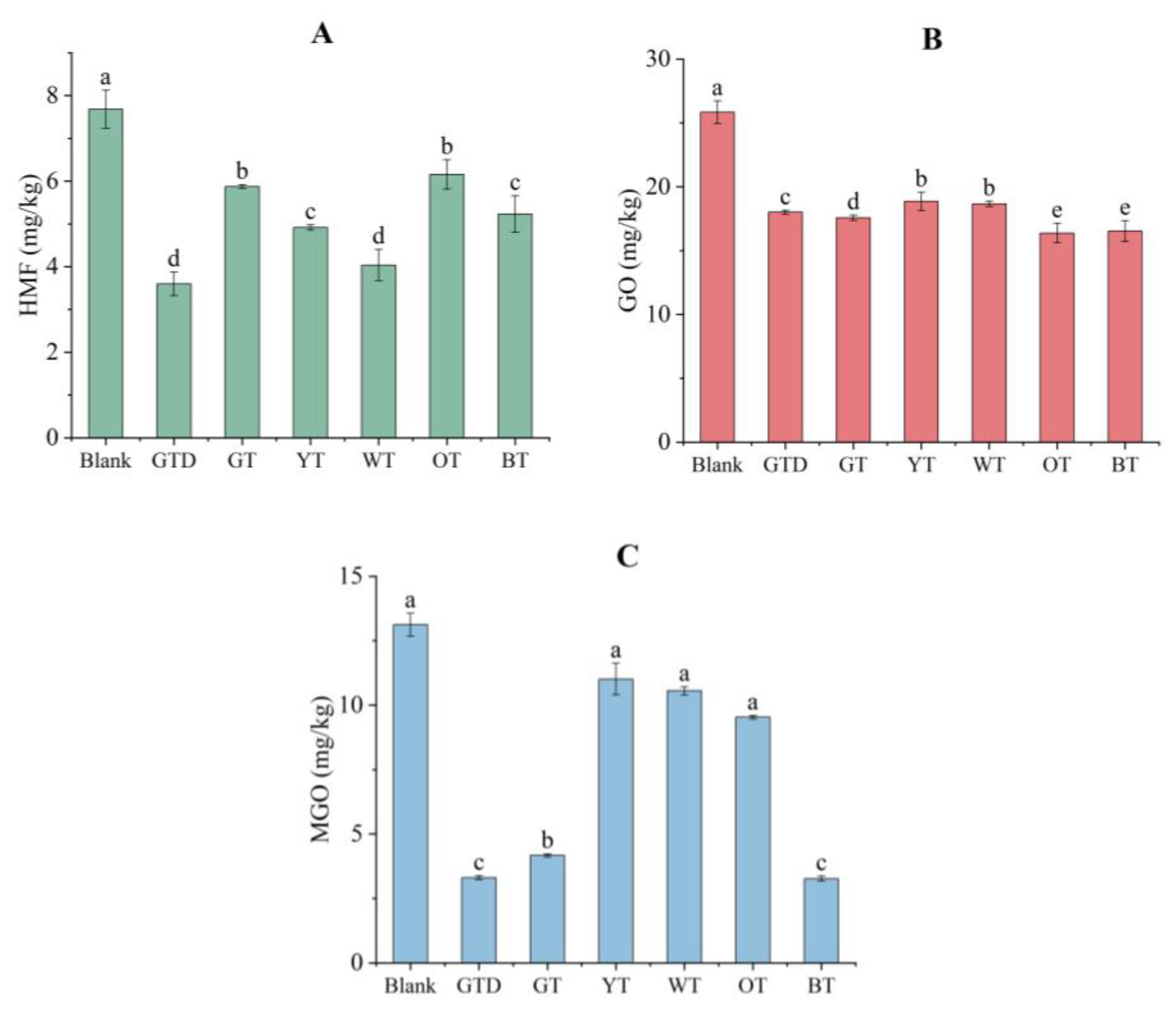
3.4.1. Inhibition of HMF Formation
3.4.2. Inhibition of GO and MGO Formation
3.5. Inhibitory Effects of Different Concentrations of Tea Extracts on the Generation of Diverse Harmful Substances
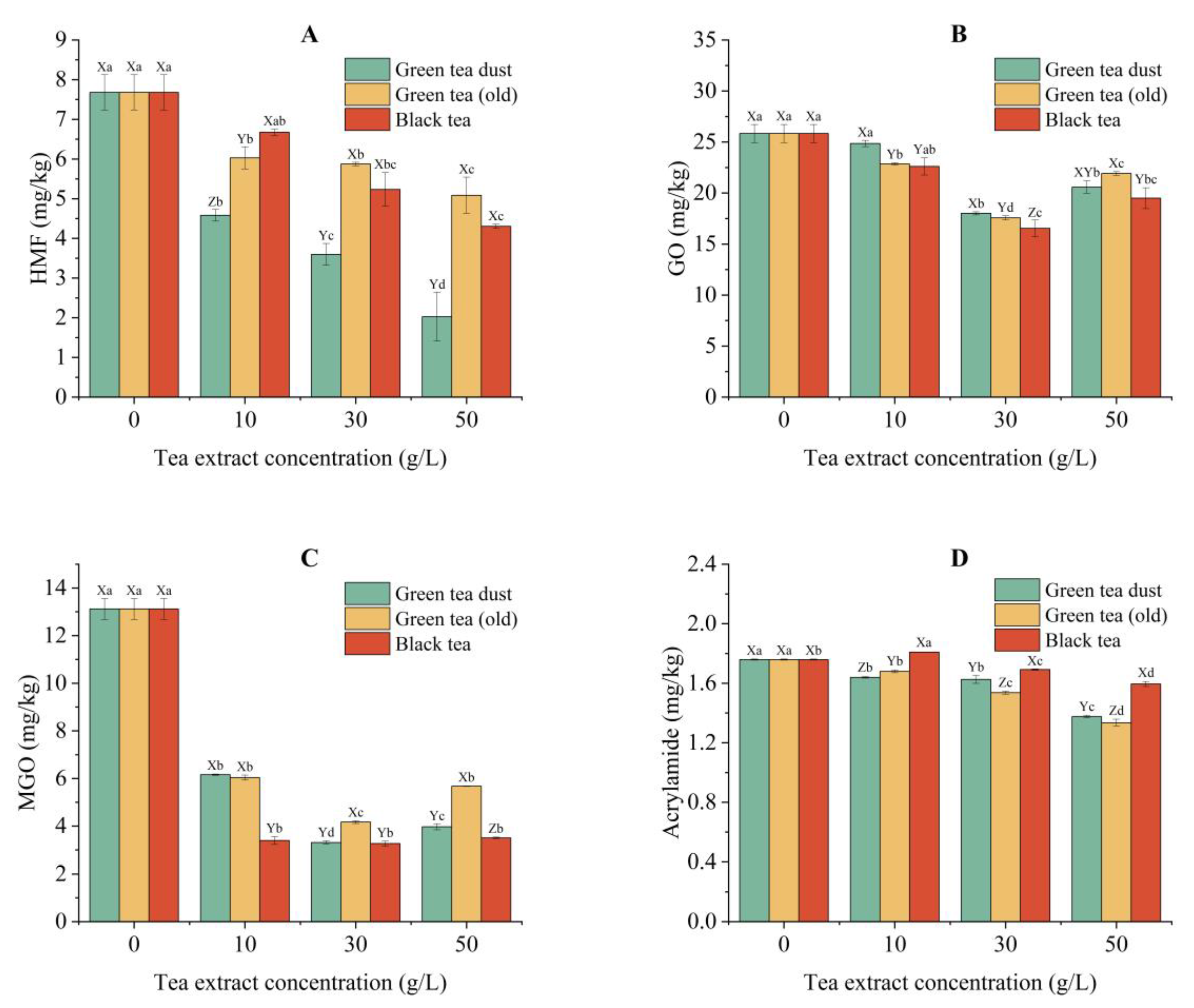
3.5.1. Reduction in Carbonyl Value in the Oil Extract of Potato Chips
3.5.2. Inhibition of HMF
3.5.3. Inhibition of GO and MGO
3.5.4. Inhibition of Acrylamide
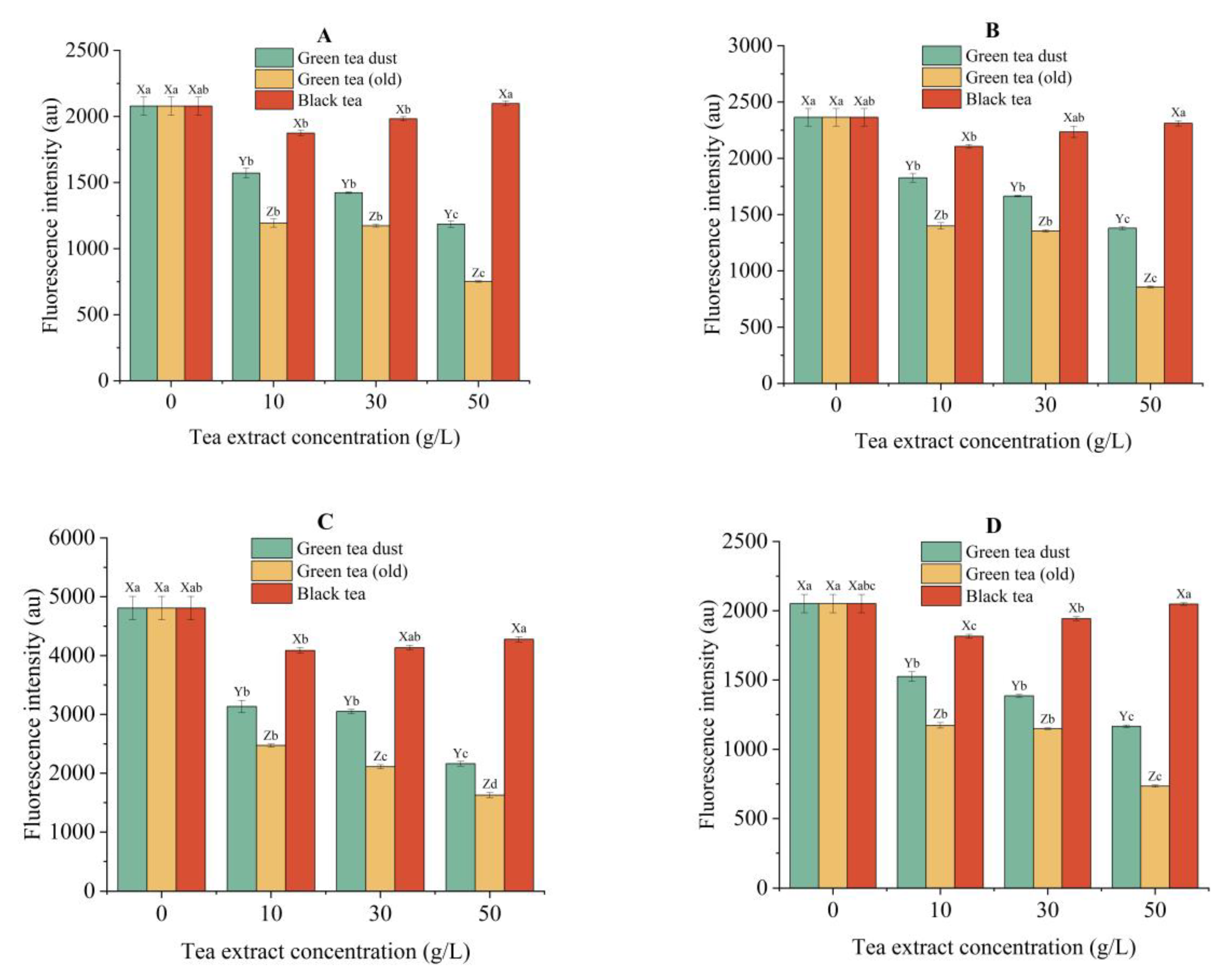
3.5.5. Inhibition of AGEs and Protein Oxidative Products
3.6. Impact of Pre-Soaking Treatment with Tea Extract on Color and Texture of Potato Chips
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liyanage, D.W.K.; Yevtushenko, D.P.; Konschuh, M.; Bizimungu, B.; Lu, Z.X. Processing strategies to decrease acrylamide formation, reducing sugars and free asparagine content in potato chips from three commercial cultivars. Food Control. 2021, 119, 9. [Google Scholar] [CrossRef]
- Ou, J.Y.; Zheng, J.; Huang, J.Q.; Ho, C.T.; Ou, S.Y. Interaction of acrylamide, acrolein, and 5-hydroxymethylfurfural with amino acids and DNA. J. Agric. Food Chem. 2020, 68, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, H.Y.; Ou, J.Y.; Liu, P.Z.; Huang, C.H.; Wang, M.F.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Li, X.D.; Teng, W.D.; Liu, G.M.; Guo, F.Y.; Xing, H.Z.; Zhu, Y.H.; Li, J.W. Allicin promoted reducing effect of garlic powder through acrylamide formation stage. Foods 2022, 11, 2394. [Google Scholar] [CrossRef] [PubMed]
- Ghazouani, T.; Atzei, A.; Talbi, W.; Fenu, M.A.; Tuberoso, C.; Fattouch, S. Occurrence of acrylamide, hydroxymethylfurfural and furaldehyde as process contaminants in traditional breakfast cereals: “Bsissa”. Food Control 2021, 124, 107931. [Google Scholar] [CrossRef]
- Kim, M.; Cho, C.; Lee, C.; Ryu, B.; Kim, S.; Hur, J.; Lee, S.H. Ishige okamurae ameliorates methylglyoxal-induced nephrotoxicity via reducing oxidative stress, RAGE protein expression, and modulating MAPK, Nrf2/ARE signaling pathway in mouse glomerular mesangial cells. Foods 2021, 10, 2000. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. Lwt-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Sobol, Z.; Jakubowski, T.; Surma, M. Effect of potato tuber exposure to UV-C radiation and semi-product soaking in water on acrylamide content in french fries dry matter. Sustainability 2020, 12, 3426. [Google Scholar] [CrossRef]
- Huang, Y.S.; Li, C.; Hu, H.Y.; Wang, Y.T.; Shen, M.Y.; Nie, S.P.; Chen, J.; Zeng, M.M.; Xie, M.Y. Simultaneous determination of acrylamide and 5-hydroxymethylfurfural in heat-processed foods employing enhanced matrix removal-lipid as a new dispersive solid-phase extraction sorbent followed by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2019, 67, 5017–5025. [Google Scholar] [CrossRef]
- Cengiz, S.; Kismiroglu, C.; Cebi, N.; Catak, J.; Yaman, M. Determination of the most potent precursors of advanced glycation end products (AGEs) in chips, crackers, and breakfast cereals by high performance liquid chromatography (HPLC) using precolumn derivatization with 4-nitro-1,2-phenlenediamine. Microchem. J. 2020, 158, 105170. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.L.; Wang, J.; Sun, B.G. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- Martin-Vertedor, D.; Fernandez, A.; Hernandez, A.; Arias-Calderon, R.; Delgado-Adamez, J.; Perez-Nevado, F. Acrylamide reduction after phenols addition to Californian-style black olives. Food Control 2020, 108, 106888. [Google Scholar] [CrossRef]
- Ou, J.Y.; Wang, M.F.; Zheng, J.; Ou, S.Y. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Qi, F.; Shen, P.J.; Hu, R.F.; Xue, T.; Jiang, X.Z.; Qin, L.N.; Chen, Y.Q.; Huang, J.Z. Carotenoids and lipid production from Rhodosporidium toruloides cultured in tea waste hydrolysate. Biotechnol. Biofuels 2020, 13. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kan, Z.P.; Thompson, H.J.; Ling, T.J.; Ho, C.T.; Li, D.X.; Wan, X.C. Impact of six typical processing methods on the chemical composition of tea leaves using a single Camellia sinensis cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.T.; Wang, D.X.; Yu, F.; Zhang, N.; Song, C.K.; Granato, D. Analytical strategy coupled to chemometrics to differentiate Camellia sinensis tea types based on phenolic composition, alkaloids, and amino acids. J. Food Sci. 2020, 85, 3253–3263. [Google Scholar] [CrossRef]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Demirok, E.; Kolsarici, N. Effect of green tea extract and microwave pre-cooking on the formation of acrylamide in fried chicken drumsticks and chicken wings. Food Res. Int. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Fu, Z.J.; Yoo, M.J.Y.; Zhou, W.B.; Zhang, L.; Chen, Y.T.; Lu, J. Effect of (−)-epigallocatechin gallate (EGCG) extracted from green tea in reducing the formation of acrylamide during the bread baking process. Food Chem. 2018, 242, 162–168. [Google Scholar] [CrossRef]
- Torres, J.D.; Dueik, V.; Carre, D.; Bouchon, P. Effect of the addition of soluble dietary fiber and green tea polyphenols on acrylamide formation and in vitro starch digestibility in baked starchy matrices. Molecules 2019, 24, 3674. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Szafrańska, A.; Roszko, M.; Stępniewska, S. Interaction of dough preparation method, green tea extract and baking temperature on the quality of rye bread and acrylamide content. LWT 2022, 154, 112759. [Google Scholar] [CrossRef]
- Capar, T.D.; Inanir, C.; Cimen, F.; Ekici, L.; Yalcin, H. Black garlic fermentation with green tea extract reduced HMF and improved bioactive properties: Optimization study with response surface methodology. J. Food Meas. Charact. 2022, 16, 1340–1353. [Google Scholar] [CrossRef]
- Poojary, M.M.; Zhang, W.; Olesen, S.B.; Rauh, V.; Lund, M.N. Green tea extract decreases arg-derived advanced glycation endproducts but not lys-derived AGEs in UHT milk during 1-year storage. J. Agric. Food Chem. 2020, 68, 14261–14273. [Google Scholar] [CrossRef]
- Budryn, G.; Zyzelewicz, D.; Nebesny, E.; Oracz, J.; Krysiak, W. Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res. Int. 2013, 50, 157–160. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Wang, Y.J.R.; Yang, L.; Peng, X.C.; Zheng, J.; Ou, S.Y. Effect of maize bran feruloylated oligosaccharides on the formation of endogenous contaminants and the appearance and textural properties of biscuits. Food Chem. 2018, 245, 974–980. [Google Scholar] [CrossRef]
- Sobol, Z.; Jakubowski, T.; Nawara, P. The effect of UV-C stimulation of potato tubers and soaking of potato strips inwater on color and analyzed color by CIE L*a*b* (vol 12, 3487, 2020). Sustainability 2020, 12, 7473. [Google Scholar] [CrossRef]
- Yang, N.; Qiu, R.X.; Yang, S.; Zhou, K.N.; Wang, C.T.; Ou, S.Y.; Zheng, J. Influences of stir-frying and baking on flavonoid profile, antioxidant property, and hydroxymethylfurfural formation during preparation of blueberry-filled pastries. Food Chem. 2019, 287, 167–175. [Google Scholar] [CrossRef]
- Li, D.; Xian, F.F.; Ou, J.Y.; Jiang, K.Y.; Zheng, J.; Ou, S.Y.; Liu, F.; Rao, Q.C.; Huang, C.H. Formation and identification of six amino acid-acrylamide adducts and their cytotoxicity toward gastrointestinal cell lines. Front. Nutr. 2022, 9, 902040. [Google Scholar] [CrossRef]
- Ou, S.Y.; Shi, J.J.; Huang, C.H.; Zhang, G.W.; Teng, J.W.; Jiang, Y.; Yang, B.R. Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater. 2010, 182, 863–868. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.J.; Brighenti, F.; Crozier, A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; D’Almeida, C.T.D.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Chen, Y.L.; Jiang, Y.M.; Duan, J.; Shi, J.; Xue, S.; Kakuda, Y. Variation in catechin contents in relation to quality of ‘Huang Zhi Xiang’ Oolong tea (Camellia sinensis) at various growing altitudes and seasons. Food Chem. 2010, 119, 648–652. [Google Scholar] [CrossRef]
- Liang, Y.R.; Ma, W.Y.; Lu, J.L.; Wu, Y. Comparison of chemical compositions of Ilex latifolia thumb and Camellia sinensis L. Food Chem. 2001, 75, 339–343. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.Y.; Zhou, J.A.; He, W.; Zeng, J.M.; Jiang, Y.W.; Cheng, H. Catechin contents in tea (Camellia sinensis) as affected by cultivar and environment and their relation to chlorophyll contents. Food Chem. 2011, 125, 44–48. [Google Scholar] [CrossRef]
- Jia, W.B.; Zhao, Y.Q.; Liao, S.Y.; Li, P.W.; Zou, Y.; Chen, S.X.; Chen, W.; He, C.L.; Du, X.; Zhu, M.Z.; et al. Dynamic changes in the diversity and function of bacterial community during black tea processing. Food Res. Int. 2022, 161, 111856. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.B.; Jiang, X.H. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kozukue, N. Stability of green tea catechins in commercial tea leaves during storage for 6 months. J. Food Sci. 2009, 74, H47–H51. [Google Scholar] [CrossRef]
- Vinci, G.; D’Ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The influence of green and black tea infusion parameters on total polyphenol content and antioxidant activity by ABTS and DPPH assays. Beverages 2022, 8, 18. [Google Scholar] [CrossRef]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.W.; Heber, D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr. Cancer-Int. J. 2003, 45, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.J.; Zhang, H.; Wu, G.C.; Zhang, H.; Wang, L.; Qian, H.F.; Qi, X.G. Reduction of 5-hydroxymethylfurfural formation by flavan-3-ols in Maillard reaction models and fried potato chips. J. Sci. Food Agric. 2018, 98, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, Y.T.; Hsieh, H.J.; Chen, K.T.; Chen, Y.A.; Wu, J.T.; Tsai, M.S.; Lin, J.A.; Hsieh, C.W. Exploring epigallocatechin gallate impregnation to inhibit 5-hydroxymethylfurfural formation and the effect on antioxidant ability of black garlic. Lwt-Food Sci. Technol. 2020, 117, 108628. [Google Scholar] [CrossRef]
- Tu, A.T.; Lin, J.A.; Lee, C.H.; Chen, Y.A.; Wu, J.T.; Tsai, M.S.; Cheng, K.C.; Hsieh, C.W. Reduction of 3-Deoxyglucosone by epigallocatechin gallate results partially from an addition reaction: The possible mechanism of decreased 5-hydroxymethylfurfural in epigallocatechin gallate-treated black garlic. Molecules 2021, 26, 4746. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Z.; Zou, Y.Y.; Huang, C.H.; Lan, P.; Zheng, J.; Ou, S.Y. Formation of a hydroxymethylfurfural-cysteine adduct and its absorption and cytotoxicity in caco-2 cells. J. Agric. Food Chem. 2017, 65, 9903–9909. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, G.W.; Zheng, J.; Zhou, H.; Wang, Y.; Huang, C.H.; Hu, W.Z.; Ou, S.Y. Formation and identification of two hydroxmethylfurfural-glycine adducts and their cytotoxicity and absorption in caco-2 cells. J. Agric. Food Chem. 2020, 68, 384–389. [Google Scholar] [CrossRef]
- Wang, G.; Liu, P.Z.; He, J.; Yin, Z.; Yang, S.; Zhang, G.W.; Ou, S.Y.; Yang, X.Q.; Zheng, J. Identification of a 5-hydroxymethylfurfural-lysine schiff base and its cytotoxicity in three cell lines. J. Agric. Food Chem. 2019, 67, 10214–10221. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.N.; Ho, C.T.; Li, S.M. Management of Maillard reaction-derived reactive carbonyl species and advanced glycation end products by tea and tea polyphenols. Food Sci. Hum. Wellness 2022, 11, 557–567. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, L.; Wan, X.C.; Zhan, J.F.; Ho, C.T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- Hu, J.M.; Jiang, K.Y.; Huang, C.H.; Zheng, J.; Zhou, H.; Ou, J.Y.; Ou, S.Y. Glycine and serine markedly eliminate methylglyoxal in the presence of formaldehyde via the formation of imidazole salts. Food Chem. 2022, 369, 130952. [Google Scholar] [CrossRef]
- Maasen, K.; Scheijen, J.; Opperhuizen, A.; Stehouwer, C.D.A.; Van Greevenbroek, M.M.; Schalkwijk, C.G. Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem. 2021, 339, 128063. [Google Scholar] [CrossRef]
- Tavakoli, J.; Emadi, T.; Hashemi, S.M.B.; Khaneghah, A.M.; Munekata, P.E.S.; Lorenzo, J.M.; Brncic, M.; Barba, F.J. Chemical properties and oxidative stability of Arjan (Amygdalus reuteri) kernel oil as emerging edible oil. Food Res. Int. 2018, 107, 378–384. [Google Scholar] [CrossRef]
- Zheng, J.; Ou, J.; Ou, S. Alpha-dicarbonyl compounds. In Chemical Hazards in Thermally-Processed Foods; Springer: Singapore, 2019; pp. 19–46. [Google Scholar] [CrossRef]
- Liu, Y.B.; Wang, P.P.; Chen, F.; Yuan, Y.; Zhu, Y.C.; Yan, H.Y.; Hu, X.S. Role of plant polyphenols in acrylamide formation and elimination. Food Chem. 2015, 186, 46–53. [Google Scholar] [CrossRef]
- Jiao, Y.; He, J.L.; Li, F.L.; Tao, G.J.; Zhang, S.; Zhang, S.K.; Qin, F.; Zeng, M.M.; Chen, J. N-epsilon-(carboxymethyl)lysine and N-epsilon-(carboxyethyl)lysine in tea and the factors affecting their formation. Food Chem. 2017, 232, 683–688. [Google Scholar] [CrossRef]
- Jiao, Y.; He, J.L.; He, Z.Y.; Gao, D.M.; Qin, F.; Xie, M.Y.; Zeng, M.M.; Chen, J. Formation of N-epsilon-(carboxymethyl)lysine and N-epsilon-(carboxyethyl)lysine during black tea processing. Food Res. Int. 2019, 121, 738–745. [Google Scholar] [CrossRef]
- Trujillo-Agudelo, S.; Osorio, A.; Gomez, F.; Contreras-Calderon, J.; Mesias-Garcia, M.; Delgado-Andrade, C.; Morales, F.; Vega-Castro, O. Evaluation of the application of an edible coating and different frying temperatures on acrylamide and fat content in potato chips. J. Food Process. Eng. 2020, 43, e13198. [Google Scholar] [CrossRef]
- Graham-Acquaah, S.; Ayernor, G.S.; Bediako-Amoa, B.; Saalia, F.S.; Afoakwa, E.O.; Abbey, L. Effect of blanching and frying on textural profile and appearance of yam (Dioscorea rotundata) french fries. J. Food Process. Preserv. 2015, 39, 19–29. [Google Scholar] [CrossRef]

| Compound | Green Tea Dust | Green Tea (Old) | Yellow Tea | White Tea | Oolong Tea | Black Tea |
|---|---|---|---|---|---|---|
| C | 0.68 ± 0.11 bcK | 1.18 ± 0.05 cJ | 2.41 ± 0.07 dI | 0.79 ± 0.01 bcK | 2.63 ± 0.09 eH | ND |
| EC | 2.50 ± 0.26 bcIJ | 9.38 ± 0.49 bH | 11.88 ± 0.24 cH | 4.05 ± 0.24 bI | 13.00 ± 0.20 cH | 0.11 ± 0.02 abJ |
| CG | 0.12 ± 0.01 cH | 0.10 ± 0.01 dH | 0.10 ± 0.01 fH | 0.09 ± 0.01 dH | 0.09 ± 0.01 gH | ND |
| ECG | 21.85 ± 2.79 abcHIJKL | 9.91 ± 0.26 bJ | 17.39 ± 0.27 bH | 14.79 ± 0.42 aI | 4.04 ± 0.12 dK | 0.10 ± 0.01 bL |
| EGC | 10.50 ± 0.91 bcK | 39.95 ± 1.17 aK | 44.03 ± 0.62 aH | 7.29 ± 0.64 bcdL | 21.79 ± 0.18 aJ | 0.16 ± 0.02 abM |
| GC | 1.03 ± 0.12 bcJ | 2.00 ± 0.22 cdI | 2.64 ± 0.29 defH | 0.64 ± 0.10 cdJ | 2.28 ± 0.22 defgHI | ND |
| EGCG | 48.58 ± 0.20 aH | 33.95 ± 0.52 aJ | 45.89 ± 0.23 aI | 22.28 ± 1.23 aK | 15.42 ± 0.32 bK | 0.18 ± 0.01 aL |
| GCG | 0.83 ± 0.01 bHI | 0.86 ± 0.02 cHI | 0.83 ± 0.02 eHI | 0.81 ± 0.01 bcH | 0.79 ± 0.02 fI | ND |
| Total catechin content | 86.09 ± 3.57 J | 97.33 ± 2.55 I | 125.18 ± 1.24 H | 50.75 ± 2.19 L | 60.05 ± 0.90 K | 0.58 ± 0.05 M |
| Parameters | Sample | 0 g/L | 10 g/L | 30 g/L | 50 g/L | |
|---|---|---|---|---|---|---|
| Color | L* | Green tea dust | 60.30 ± 2.20 a | 57.46 ± 0.73 aY | 50.68 ± 1.50 bY | 50.07 ± 2.33 bY |
| Green tea (old) | 60.30 ± 2.20 a | 60.24 ± 0.97 aX | 62.67 ± 1.27 aX | 55.50 ± 1.39 bX | ||
| Black tea | 60.30 ± 2.20 a | 49.85 ± 1.46 bZ | 47.33 ± 1.35 bcZ | 44.60 ± 1.00 cZ | ||
| a* | Green tea dust | 13.40 ± 0.80 a | 11.00 ± 0.67 aY | 12.06 ± 0.15 aXY | 12.33 ± 0.05 aXY | |
| Green tea (old) | 13.40 ± 0.80 a | 8.84 ± 0.45 bZ | 9.92 ± 0.20 abY | 12.30 ± 0.29 aY | ||
| Black tea | 13.40 ± 0.80 b | 14.00 ± 0.67 abX | 15.27 ± 1.03 aX | 15.15 ± 0.48 aX | ||
| b* | Green tea dust | 36.00 ± 2.60 a | 33.87 ± 1.11 abX | 30.60 ± 1.92 bXY | 31.20 ± 1.07 bX | |
| Green tea (old) | 36.00 ± 2.60 a | 33.07 ± 0.96 abX | 32.13 ± 1.11 bX | 31.30 ± 1.27 bX | ||
| Black tea | 36.00 ± 2.60 a | 33.93 ± 0.78 aX | 27.15 ± 1.13 bY | 28.30 ± 2.21 bX | ||
| Fracturability (g) | Green tea dust | 75.00 ± 9.90 b | 122.00 ± 6.38 aY | 85.33 ± 7.61 bX | 76.67 ± 2.49 bX | |
| Green tea (old) | 75.00 ± 9.90 b | 106.00 ± 4.32 aZ | 85.25 ± 4.66 bX | 80.33 ± 7.70 bX | ||
| Black tea | 75.00 ± 9.90 b | 211.00 ± 7.87 aX | 78.50 ± 8.65 bX | 83.00 ± 2.68 bX | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, H.; Wu, Z.; Duan, T.; Liu, P.; Ou, S.; El-Nezami, H.; Zheng, J. Reduction in Five Harmful Substances in Fried Potato Chips by Pre-Soaking Treatment with Different Tea Extracts. Foods 2023, 12, 321. https://doi.org/10.3390/foods12020321
Wang W, Wang H, Wu Z, Duan T, Liu P, Ou S, El-Nezami H, Zheng J. Reduction in Five Harmful Substances in Fried Potato Chips by Pre-Soaking Treatment with Different Tea Extracts. Foods. 2023; 12(2):321. https://doi.org/10.3390/foods12020321
Chicago/Turabian StyleWang, Weitao, Huaixu Wang, Zhongjun Wu, Tingting Duan, Pengzhan Liu, Shiyi Ou, Hani El-Nezami, and Jie Zheng. 2023. "Reduction in Five Harmful Substances in Fried Potato Chips by Pre-Soaking Treatment with Different Tea Extracts" Foods 12, no. 2: 321. https://doi.org/10.3390/foods12020321
APA StyleWang, W., Wang, H., Wu, Z., Duan, T., Liu, P., Ou, S., El-Nezami, H., & Zheng, J. (2023). Reduction in Five Harmful Substances in Fried Potato Chips by Pre-Soaking Treatment with Different Tea Extracts. Foods, 12(2), 321. https://doi.org/10.3390/foods12020321