The Interaction between Flavonoids and Intestinal Microbes: A Review
Abstract
1. Introduction
2. The Classes of Flavonoids
3. Intestinal Microbiota
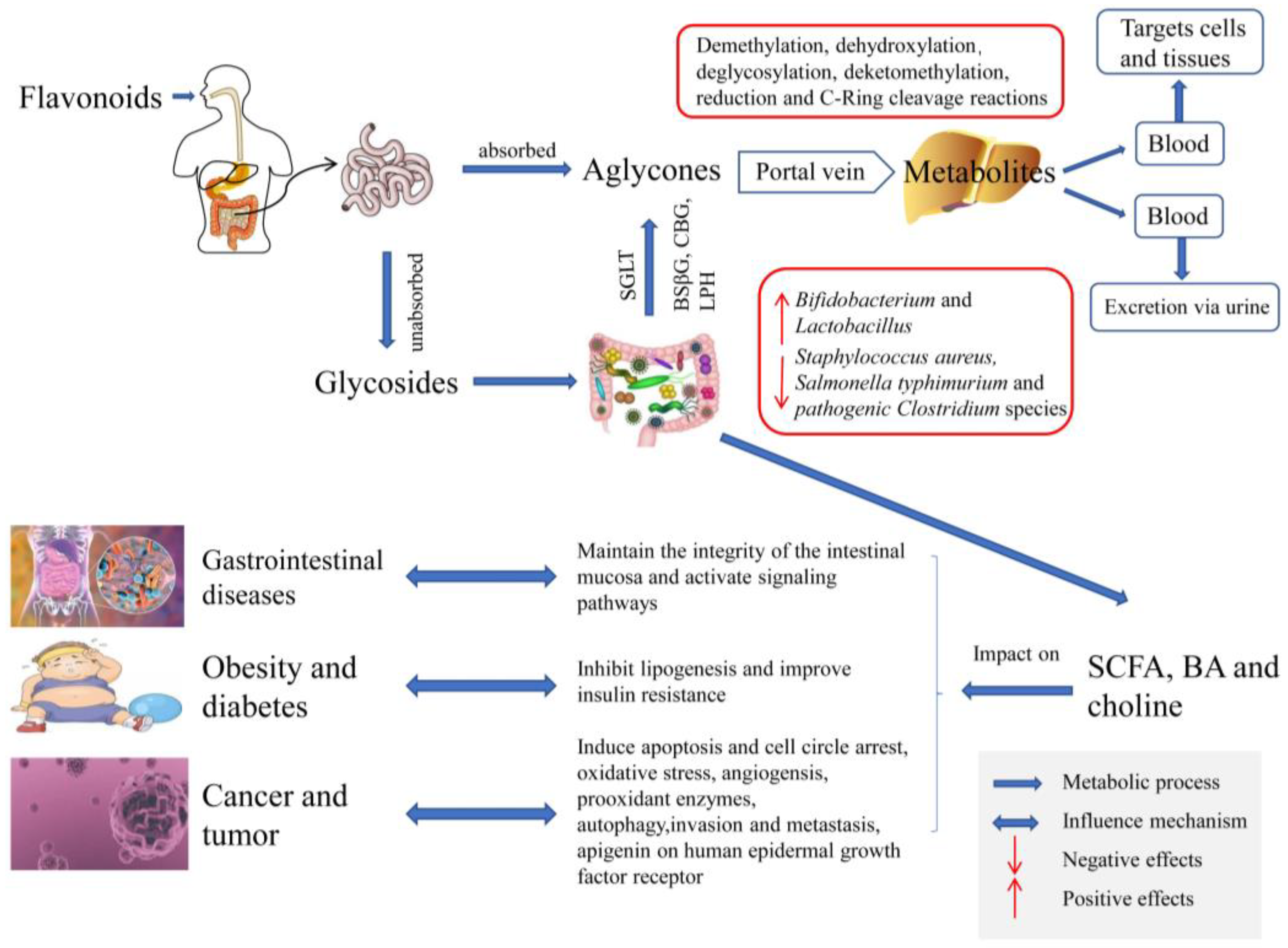
4. Metabolic Effects of Intestinal Microorganisms on Flavonoids
4.1. The Role of Intestinal Microbiota on the Metabolism of Flavonoids
4.1.1. Metabolism of Flavonoids by Gut Microbiota
4.1.2. The Role of Gut Microbiota Metabolism and Biotransformation on Flavonoids
4.2. Mechanism of Biotransformation and Formation of Flavonoids by Intestinal Microorganisms
4.3. The Role of Gut Microbiota in Dictating Bioavailability and Bioactivity of Flavonoids
5. Effects of Flavonoids on Intestinal Microbiota
5.1. Modulation of the Composition and Richness of Intestinal Flora
5.2. Protection of Intestinal Barrier Function
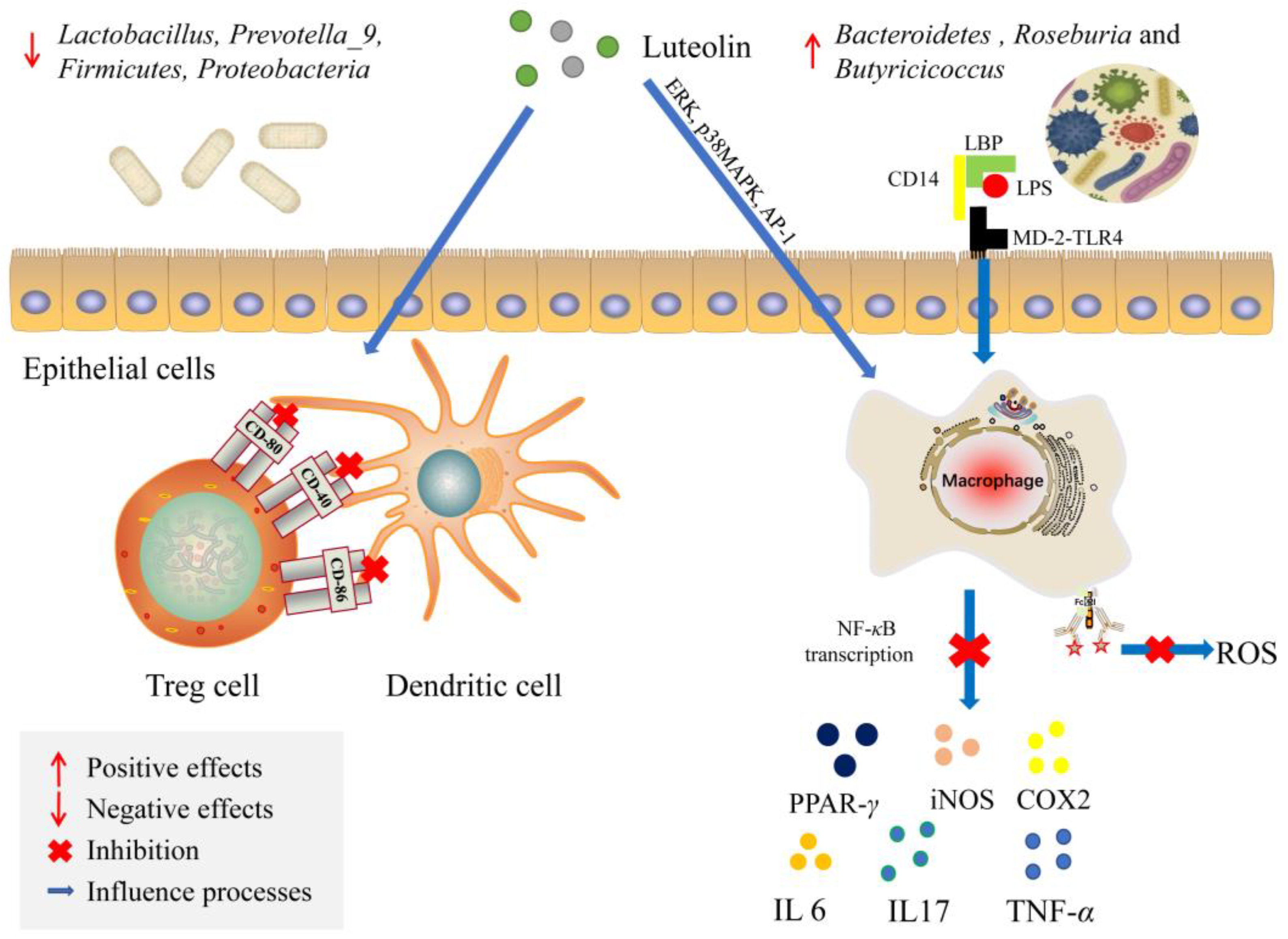
5.3. Influence on Mucosal Immunity System
5.4. Influence on the Gut Microbiota Metabolites
5.5. Affecting the Expression of Signaling Molecules
6. Flavonoids, Gut Microbiota and Health
6.1. Regulation of Flavonoids on Gastrointestinal Diseases
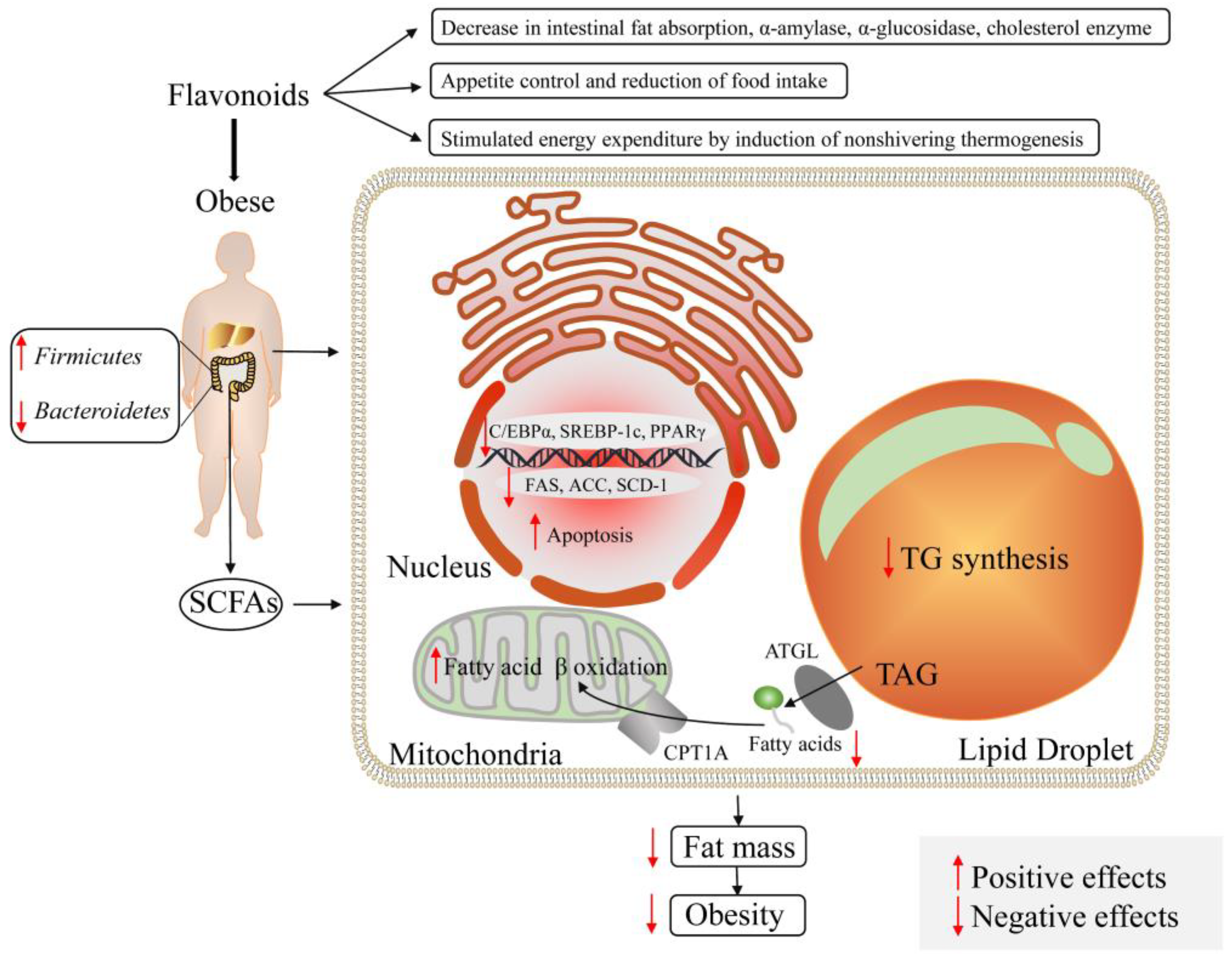
6.2. Modulation of Flavonoids on Obesity and Diabetes
6.3. Impact of Flavonoids on Cancer or Tumor
7. Conclusions
8. Future Perspectives
- (1)
- For specific flavonoids, the effects of different doses on flavonoids need to be studied to determine the main factors associated with the overall association.
- (2)
- The effects of flavonoid intake on intestinal flora and whether it is synergistic or antagonistic in the treatment of diseases can be discussed in the future research.
- (3)
- Target flavonoid delivery methods, include protein peptides, monoclonal antibodies, and living cells, to study the effects of flavonoids on the microflora.
- (4)
- More studies could combine with clinical, cellular, and animal analysis of the safety and efficacy of IBD.
- (5)
- The function of microbial metabolites through multi-omics methods, including metagenomic, metaproteomic and metabolomic deserves to be studied for characterizing the metabolic pathways of small molecules produced by microbial metabolites.
- (6)
- The complex interaction between microbial host and flavonoids also needs to be further explored.
- (7)
- The dose of each flavonoid should be studied to understand the bioavailability of flavonoids, standard extraction and purification methods.
- (8)
- Various pathways have effects on diseases, and it is worth investigating whether these pathways are synergistic or cause side effects.
- (9)
- Effects of treatments on enzymes can be studied to evaluate the anti-disease potential of clinical experiments to clinical use of flavonoids.
- (10)
- By associating the metabolism of flavonoids with organisms, genes and enzymes, the investigation of human intestinal isolates will be conducted to identify individual organisms with specific metabolic abilities. The metabolite action will be detected by means of stable isotope detection, fluorescence in situ hybridization or imaging mass spectrometry.
Author Contributions
Funding
Conflicts of Interest
References
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Valdes, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; Gonzalez, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 7730–7742. [Google Scholar] [CrossRef]
- Iwashina, T. The Structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Xue, B.; Liu, Q.; Ou, S.; Wang, Y.; Peng, X. Different flavonoids can shape unique gut microbiota profile in vitro. J. Food Sci. 2016, 81, H2273–H2279. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, D.W.; Dai, J.G. Advance of synthetic biology of flavonoids. Acta Pharm. Sin. B 2022, 57, 1322–1335. [Google Scholar]
- Miladinović, B.; Branković, S.; Živanović, S.; Kostić, M.; Šavikin, K.; Dorđević, B.; Stojanović, D.; Milutinović, M.; Kitić, N.; Kitić, D. Flavonols composition of Ribes nigrum L. juices and their impact on spasmolytic activity. J. Berry Res. 2021, 11, 171–186. [Google Scholar] [CrossRef]
- Rakers, C.; Schwerdtfeger, S.M.; Mortier, J.; Duwe, S.; Wolff, T.; Wolber, G.; Melzig, M.F. Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 4312–4317. [Google Scholar] [CrossRef]
- Tahir, M.S.; Almezgagi, M.; Zhang, Y.; Bashir, A.; Abdullah, H.M.; Gamah, M.; Wang, X.; Zhu, Q.; Shen, X.; Ma, Q.; et al. Mechanistic new insights of flavonols on neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111253. [Google Scholar] [CrossRef]
- Larkin, T.; Price, W.E.; Astheimer, L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit. Rev. Food Sci. Nutr. 2008, 48, 538–552. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill, E.H.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Li, C.; Sui, Z.; Min, W. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxidative Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef]
- Baranska, A.; Blaszczuk, A.; Polz-Dacewicz, M.; Kanadys, W.; Malm, M.; Janiszewska, M.; Jedrych, M. Effects of soy isoflavones on glycemic control and lipid profile in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2021, 13, 1886. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, M.J.; Ahn, J.; Lee, S.H.; Lee, H.; Kim, J.H.; Park, S.H.; Jang, Y.J.; Ha, T.Y.; Jung, C.H. Nutrikinetics of isoflavone metabolites after fermented soybean product (Cheonggukjang) ingestion in ovariectomized mice. Mol. Nutr. Food Res. 2017, 61, 1700322. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus flavanones: What is their role in cardiovascular protection? J. Agric. Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef]
- Azhar, R.; Sabaha, R.; Sajjad, F.; Nadeem, A.; Amjad, N.; Hafeez, T.; Wahid, S.; Tanweer, A. Effect of citrus flavanones on diabetes: A systematic review. Curr. Diabetes Rev. 2022, 19, e070722206679. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef]
- Trzeciakiewicz, A.; Habauzit, V.; Mercier, S.; Barron, D.; Urpi-Sarda, M.; Manach, C.; Offord, E.; Horcajada, M.N. Molecular mechanism of hesperetin-7-O-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J. Agric. Food Chem. 2010, 58, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, L.; Xu, S. Intakes of citrus fruit and risk of esophageal cancer: A meta-analysis. Medicine 2018, 97, e0018. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary anthocyanins and insulin resistance: When food becomes a medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T.; Lin, H.; Jacques, P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015, 102, 172–181. [Google Scholar] [CrossRef]
- Desjardins, J.; Tanabe, S.; Bergeron, C.; Gafner, S.; Grenier, D. Anthocyanin-rich black currant extract and cyanidin-3-O-glucoside have cytoprotective and anti-inflammatory properties. J. Med. Food. 2012, 15, 1045–1050. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols enhanced production of anti-inflammatory substance(s) by Bifidobacterium adolescentis: Prebiotic actions of galangin, quercetin, and fisetin. Biofactors 2013, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Dias, E.J.S.; Cantanhede Filho, A.J.; Carneiro, F.J.C.; da Rocha, C.Q.; da Silva, L.C.N.; Santos, J.C.B.; Barros, T.F.; Santos, D.M. Antimicrobial activity of extracts from the humiria balsamifera (Aubl). Plants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Guilhon, G.M.S.P.; Zoghbi, M.D.G.; Andrade, E.H.A.; Santos, L.S.; da Silva, J.K.R.; Trovatti Uetanabaro, A.P.; Araujo, I.S. Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh leaves (myrtaceae). Nat. Prod. Res. 2021, 35, 1717–1721. [Google Scholar] [CrossRef]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N.; Kalantaridou, S. Soy isoflavones and breast cancer risk: A meta-analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Lagana, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A review of the properties of anthocyanins and their Influence on factors affecting cardiometabolic and cognitive health. Nutrients 2021, 13, 2831. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, A.; Dzierzanowski, T. Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: An update. Molecules 2021, 26, 4380. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Ledda, A.; Hu, S.; Cesarone, M.R.; Feragalli, B.; Dugall, M. Grape seed procyanidins in pre- and mild hypertension: A registry study. Evid. Based Complement. Altern. Med. 2013, 2013, 313142. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Liu, Y.; Song, Y.; Liu, S.; Zhang, R.; Liang, H.; Xin, H. Effect of procyanidins on lipid metabolism and inflammation in rats exposed to alcohol and iron. Heliyon 2020, 6, e04847. [Google Scholar] [CrossRef]
- Yang, B.; Sun, Y.X.; Lv, C.C.; Zhang, W.; Chen, Y.Z. Procyanidins exhibits neuroprotective activities against cerebral ischemia reperfusion injury by inhibiting TLR4-NLRP3 inflammasome signal pathway. Psychopharmacology 2020, 237, 3283–3293. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.J.; Li, D.X.; Ho, C.T.; Li, J.S.; Wan, X.C. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Rafii, F.; Davis, C.; Park, M.; Heinze, T.M.; Beger, R.D. Variations in metabolism of the soy isoflavonoid daidzein by human intestinal microfloras from different individuals. Arch. Microbiol. 2003, 180, 11–16. [Google Scholar] [CrossRef]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Hendriks, J.J.; Alblas, J.; van der Pol, S.M.; van Tol, E.A.; Dijkstra, C.D.; de Vries, H.E. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J. Exp. Med. 2004, 200, 1667–1672. [Google Scholar] [CrossRef]
- Henkels, K.M.; Frondorf, K.; Gonzalez-Mejia, M.E.; Doseff, A.L.; Gomez-Cambronero, J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett. 2011, 585, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wan, X.H.; Niu, F.J.; Sun, J.J.; Shi, C.X.; Ye, J.M.; Zhou, C.Z. Evaluation of antiviral effect and toxicity of total flavonoids extracted from Robinia pseudoacacia cv. idaho. Biomed. Pharmacother. 2019, 118, 109335. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Sun, H.; You, Z.; Jia, L.; Wang, F. Autism spectrum disorder is associated with gut microbiota disorder in children. BMC Pediatr. 2019, 19, 516. [Google Scholar] [CrossRef]
- Schiller, L.R.; Pardi, D.S.; Sellin, J.H. Chronic diarrhea: Diagnosis and management. Clin. Gastroenterol. Hepatol. 2017, 15, 182–193.e3. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shi, J. Vitamin A supplementation ameliorates ulcerative colitis in gut microbiota–dependent manner. Food Res. Int. 2021, 148, 110568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Wang, X.; Liao, X.; Guo, J.; Hou, W.B.; Wang, X.; Liu, J.P.; Liu, Z.L. An evidence mapping of systematic reviews and meta-analysis on traditional Chinese medicine for ulcerative colitis. BMC Complement. Med. Ther. 2021, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Choi, S.W. Dietary modulation of gut microbiota for the relief of irritable bowel syndrome. Nutr. Res. Pract. 2021, 15, 411–430. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjørsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.Q.; Jiang, Z. Prevalence and predictors of small intestinal bacterial overgrowth in systemic sclerosis: A systematic review and meta-analysis. Clin. Rheumatol. 2021, 40, 3039–3051. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Jiang, Z.; Jiang, Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: A systematic review and meta-analysis. Gut Pathog. 2021, 13, 25. [Google Scholar] [CrossRef]
- Johnson, P. Hepatitis viruses, cirrhosis, and liver cancer. J. Surg. Oncol.Suppl. 1993, 3, 28–33. [Google Scholar] [CrossRef]
- Liu, M.; Tseng, T.C.; Jun, D.W.; Yeh, M.L.; Trinh, H.; Wong, G.L.H.; Chen, C.H.; Peng, C.Y.; Kim, S.E.; Oh, H.; et al. Transition rates to cirrhosis and liver cancer by age, gender, disease and treatment status in Asian chronic hepatitis B patients. Hepatol. Int. 2021, 15, 71–81. [Google Scholar] [CrossRef]
- Beattie, G.; Siriwardena, A. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br. J. Surg. 2000, 87, 250–252. [Google Scholar] [CrossRef]
- Hecker, M.; Mayer, K.; Askevold, I.; Collet, P.; Weigand, M.A.; Krombach, G.A.; Padberg, W.; Hecker, A. Akute Pankreatitis [Acute pancreatitis]. Anaesthesist 2014, 63, 253–263. (In German) [Google Scholar] [CrossRef]
- Wang, Y.H. Current progress of research on intestinal bacterial translocation. Microb. Pathog. 2021, 152, 104652. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Zuo, L.; Zhu, W.; Wang, B.; Li, Q.; Li, J. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br. J. Nutr. 2013, 109, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, H.; Babaya, N.; Noso, S. Beta-cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Leonard, L.M.; Choi, M.S.; Cross, T.L. Maximizing the estrogenic potential of soy isoflavones through the gut microbiome: Implication for cardiometabolic health in postmenopausal women. Nutrients 2022, 14, 553. [Google Scholar] [CrossRef]
- Ma, B.; Liang, J.; Dai, M.; Wang, J.; Luo, J.; Zhang, Z.; Jing, J. Altered gut microbiota in Chinese children with autism spectrum disorders. Front. Cell Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Prim. 2020, 6, 5. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J. Dietary modulation of intestinal microbiota: Future opportunities in experimental autoimmune encephalomyelitis and multiple sclerosis. Front. Microbiol. 2019, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Magyari, M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat. Rev. Neurol. 2021, 17, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Metta, V.; Leta, V.; Mrudula, K.; Prashanth, L.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; Chaudhuri, K.R. Gastrointestinal dysfunction in Parkinson’s disease: Molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J. Neurol. 2022, 269, 1154–1163. [Google Scholar] [CrossRef]
- Cilia, R.; Piatti, M.; Cereda, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cassani, E.; Bonvegna, S.; Ferrarese, C.; Zecchinelli, A.L.; et al. Does gut microbiota influence the course of Parkinson’s disease A 3-year prospective exploratory study in de novo patients. J. Park. Dis. 2021, 11, 159–170. [Google Scholar] [CrossRef]
- Lubomski, M.; Tan, A.H.; Lim, S.Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 2020, 267, 2507–2523. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary favonoid and isofavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Ruiz de la Bastida, A.; Peirotén, Á.; Langa, S.; Arqués, J.L.; Landete, J.M. Heterologous production of equol by lactic acid bacteria strains in culture medium and food. Int. J. Food Microbiol. 2021, 360, 109328. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, X.; Tong, Q.; Zhou, X.; Chen, J.; Xiong, W.; Fang, J.; Wang, W.; Shi, C. Interactions of dihydromyricetin, a flavonoid from vine tea (Ampelopsis grossedentata) with gut microbiota. J. Food Sci. 2018, 83, 1444–1453. [Google Scholar] [CrossRef]
- Koppel, N.; Maini, R.V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017, 356, 2770. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic metabolism of flavonoids by gut microbiota and its impact on gastrointestinal cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Wang, L.; Huang, G.; Hou, R.; Qi, D.; Wu, Q.; Nie, Y.; Zuo, Z.; Ma, R.; Zhou, W.; Ma, Y.; et al. Multi-omics reveals the positive leverage of plant secondary metabolites on the gut microbiota in a non-model mammal. Microbiome 2021, 9, 192. [Google Scholar] [CrossRef]
- Lai, M.Y.; Hsiu, S.L.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2003, 55, 205–209. [Google Scholar] [CrossRef]
- Liu, C.S.; Liang, X.; Wei, X.H.; Chen, F.L.; Tang, Q.F.; Tan, X.M. Comparative metabolism of the eight main bioactive ingredients of gegen qinlian decoction by the intestinal flora of diarrhoeal and healthy piglets. Biomed. Chromatogr. 2019, 33, e4421. [Google Scholar] [CrossRef]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Conversion of rutin to quercetin by acid treatment in relation to biological activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of rutin, a prevalent dietary flavonol, by the human gut microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Roncaglia, L.; De Lucia, M.; Amaretti, A.; Leonardi, A.; Pagnoni, U.M.; Rossi, M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2009, 81, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Hsieh, J.F. The conversion and deglycosylation of isoflavones and anthocyanins in black soymilk process. Food Chem. 2018, 261, 8–14. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Popoff, M.R.; Grimont, P.; Bokkenheuser, V.D. Clostridium orbiscindens sp. nov., a human intestinal bacterium capable of cleaving the flavonoid C-ring. Int. J. Syst. Bacteriol. 1991, 41, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Hidalgo, M.; Sanchez-Moreno, C.; Pelaez, C.; Requena, T.; De Pascual-Teresa, S. Bioconversion of anthocyanin glycosides by bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharm. 2020, 31, 4. [Google Scholar] [CrossRef]
- Zhao, M.; Du, L.; Tao, J.; Qian, D.; Shang, E.X.; Jiang, S.; Guo, J.; Liu, P.; Su, S.L.; Duan, J.A. Determination of metabolites of diosmetin-7-O-glucoside by a newly isolated Escherichia coli from human gut using UPLC-Q-TOF/MS. J. Agric. Food Chem. 2014, 62, 11441–114418. [Google Scholar] [CrossRef]
- Pan, Y.P.; Zhang, Z.H.; Ding, D.M.; Jia, X.B. Advance in studies on biotransformation of flavonoids by intestinal bacteria. J. Chin. Mater. Med. 2013, 38, 3239–3245. (In Chinese) [Google Scholar]
- Jiao, Q.; Xu, L.; Jiang, L.; Jiang, Y.; Zhang, J.; Liu, B. Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MS (n). Xenobiotica 2020, 50, 1311–1322. [Google Scholar] [CrossRef]
- Williamson, G.; Day, A.J.; Plumb, G.W.; Couteau, D. Human metabolic pathways of dietary flavonoids and cinnamates. Biochem. Soc. Trans. 2000, 28, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Burapan, S.; Kim, M.; Han, J. Demethylation of polymethoxyflavones by human gut bacterium, Blautia sp. MRG-PMF1. J. Agric. Food Chem. 2017, 65, 1620–1629. [Google Scholar] [CrossRef]
- Jin, J.S.; Hattori, M. Isolation and characterization of a human intestinal bacterium Eggerthella sp. CAT-1 capable of cleaving the C-ring of (+)-catechin and (-)-epicatechin, followed by pdehydroxylation of the B-ring. Biol. Pharm. Bull. 2012, 35, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006, 102, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, N.; Han, J. Metabolism of kaempferia parviflora polymethoxyflavones by human intestinal bacterium Bautia sp. MRG-PMF1. J. Agric. Food Chem. 2014, 62, 12377–12383. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Yokoyama, S.I.; Niwa, T.; Osawa, T.; Suzuki, T. Characterization of an O-desmethylangolensin-producing bacterium isolated from human feces. Arch. Microbiol. 2010, 192, 15–22. [Google Scholar] [CrossRef]
- Winter, J.; Moore, L.H.; Dowell, V.R., Jr.; Bokkenheuser, V.D. C-ring cleavage of flavonoids by human intestinal bacteria. Appl. Environ. Microbiol. 1989, 55, 1203–1208. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, A.; Morand, C.; Scalbert, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective effect of kaempferol on LPS-induced inflammation and barrier dysfunction in a coculture model of intestinal epithelial cells and intestinal microvascular endothelial cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef]
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M.; et al. The fate of flavonoids after oral administration: A comprehensive overview of its bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escote, X.; Crescenti, A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.X.; Zhang, C.L.; Zeng, X.X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and biotechnological processes to enhance the bioavailability of dietary (poly)phenols in humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
- Liao, Z.L.; Zeng, B.H.; Wei, W.; Li, G.H.; Fei, W.; Li, W.; Zhong, Q.P.; Hong, W.; Xiang, F. Impact of the consumption of tea polyphenols on early atherosclerotic lesion formation and intestinal bifidobacteria in high-fat-fed ApoE/mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef]
- Blair, R.M.; Appt, S.E.; Frank, A.A.; Clarkson, T.B. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J. Nutr. 2003, 133, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterization of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Guadamuro, L.; Azcarate-Peril, M.A.; Tojo, R.; Mayo, B.; Delgado, S. Impact of dietary isoflavone supplementation on the fecal microbiota and its metabolites in postmenopausal women. Int. J. Environ. Res. Public Health 2021, 18, 7939. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Chao, C.; Hao, J.; Li, G.; Yu, G. Function, dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Xu, M.; Qiao, G.; Li, C.; Lin, L.; Zheng, G. Smilax china L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. J. Funct. Foods 2021, 77, 104332. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef]
- Chen, T.; Wu, H.; He, Y.; Pan, W.; Yan, Z.; Liao, Y.; Peng, W.; Gan, L.; Zhang, Y.; Su, W.; et al. Simultaneously quantitative analysis of naringin and its major human gut microbial metabolites naringenin and 3-(4′-Hydroxyphenyl) propanoic acid via stable isotope deuterium-labeling coupled with RRLC-MS/MS method. Molecules 2019, 24, 4287. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Argoty, G.A.; Zhang, L.; Tomasula, P.; Wang, M.; Pontious, S.; Kobori, M.; Xiao, W. Analysis of temporal changes in growth and gene expression for commensal gut microbes in response to the polyphenol naringenin. Microbiol. Insights 2018, 11, 1178636118775100. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Tang, Z.; Shi, X.; Song, Z.; Cao, F.; Wei, P.; Li, M.; Li, X.; Jiang, D.; et al. Improvements in estrogen deficiency-induced hypercholesterolemia by Hypericum perforatum L. extract are associated with gut microbiota and related metabolites in ovariectomized (OVX) rats. Biomed. Pharmacother. 2021, 135, 111131. [Google Scholar] [CrossRef]
- Tao, J.H.; Duan, J.A.; Qian, Y.Y.; Qian, D.W.; Guo, J.M. Investigation of the interactions between Chrysanthemum morifolium flowers extract and intestinal bacteria from human and rat. Biomed. Chromatogr. 2016, 30, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Yang, J.W.; Dou, H.Y.; Li, G.Q.; Li, X.Y.; Shen, L.; Ji, H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Functional interactions between gut microbiota transplantation, quercetin, and high-fat diet determine nonalcoholic fatty liver disease development in germ-free mice. Mol. Nutr. Food Res. 2019, 63, e1800930. [Google Scholar] [CrossRef]
- Song, D.; Ho, C.T.; Zhang, X.; Wu, Z.; Cao, J. Modulatory effect of Cyclocarya paliurus flavonoids on the intestinal microbiota and liver clock genes of circadian rhythm disorder mice model. Food Res. Int. 2020, 138, 109769. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Xu, X.; Dai, M.; Lao, F.; Chen, F.; Hu, X.; Liu, Y.; Wu, J. Effect of glucoraphanin from broccoli seeds on lipid levels and gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2020, 68, 103858. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, 106621. [Google Scholar] [CrossRef] [PubMed]
- Bekiares, N.; Krueger, C.G.; Meudt, J.J.; Shanmuganayagam, D.; Reed, J.D. Effect of sweetened dried cranberry consumption on urinary proteome and fecal microbiome in healthy human subjects. OMICS 2018, 22, 145–153. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, J.; Feng, J.; Ji, J.; Yu, Q.; Li, Y.; Zheng, Y.; Dai, W.; Wu, J.; Guo, C. Crosstalk between PPARs and gut microbiota in NAFLD. Biomed. Pharmacother. 2021, 136, 111255. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef]
- Sharma, R.; Young, C.; Neu, J. Molecular modulation of intestinal epithelial barrier: Contribution of microbiota. J. Biomed. Biotechnol. 2010, 2010, 305879. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H.; Wen, X.; Ho, C.T.; Li, S. Citrus flavonoids and the intestinal barrier: Interactions and effects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 225–251. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Dey, P. Targeting gut barrier dysfunction with phytotherapies: Effective strategy against chronic diseases. Pharmacol. Res. 2020, 161, 105135. [Google Scholar] [CrossRef]
- Polewski, M.A.; Esquivel-Alvarado, D.; Wedde, N.S.; Kruger, C.G.; Reed, J.D. Isolation and characterization of blueberry polyphenolic components and their effects on gut barrier dysfunction. J. Agric. Food Chem. 2020, 68, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Forato Anhê, F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2014, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tripathi, P.; Sharma, J.; Dixit, A. Flavonoids modulate tight junction barrier functions in hyperglycemic human intestinal Caco-2 cells. Nutrition 2020, 78, 110792. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- McCracken, V.J.; Lorenz, R.G. The gastrointestinal ecosystem a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 2001, 3, 1–11. [Google Scholar] [CrossRef]
- Bain, C.C.; Cerovic, V. Interactions of the microbiota with the mucosal immune system. Immunology 2020, 159, 648–652. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Macdonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.Y.; Shi, H.T.; Tan, Y.R.; Shen, S.Y.; Yi, P.F.; Shen, H.Q.; Fu, B.D. Baicalin inhibits APEC-induced lung injury by regulating gut microbiota and SCFA production. Food Funct. 2021, 12, 12621–12633. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, E.C.; Morrish, R.B.; Dillon, M.J.; Leyland, R.; Chahwan, R. Epigenomic modifications mediating antibody maturation. Front. Immunol. 2018, 9, 355. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Zhao, Y.; Wang, X.; Kong, L.C.; Johnston, L.J.; Lu, L.; Ma, X. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit. Rev. Food Sci. Nutr. 2022, 62, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Lin, J.; Liu, Y.E.; Chen, H.F.; Hsu, K.W.; Lin, S.H.; Peng, K.Y.; Lin, K.J.; Hsieh, C.C.; Chen, D.R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Inter. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Wang, J.; Yu, P.; Liu, Q.; Zeng, D.; Song, H.; Kuang, Z. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can. J. Physiol. Pharmacol. 2015, 93, 233–237. [Google Scholar] [CrossRef]
- Dueñas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar] [CrossRef]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. J. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef]
- Pei, L.P.; Song, J.Z.; Liu, W.; Wu, E.; Ling, Y. Effect of water extracts from Cynanchum thesioides (Freyn) K. Schum. on visceral hypersensitivity and gut microbiota profile in maternally separated rats. J. Ethnopharmacol. 2021, 264, 113352. [Google Scholar]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.A.O. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef]
- Eckert, J.K.; Kim, Y.J.; Kim, J.I.; Gurtler, K.; Oh, D.Y.; Sur, S.; Lundvall, L.; Hamann, L.; van der Ploeg, A.; Pickkers, P.; et al. The crystal structure of lipopolysaccharide binding protein reveals the location of a frequent mutation that impairs innate immunity. Immunity 2013, 39, 647–660. [Google Scholar] [CrossRef]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. The gut microbiome and its role in obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Microbiology, L.B. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S. Citrus unshiu peel extract ameliorates hyperglycemia and hepati. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [CrossRef]
- Yan, D.; Fan, P.; Sun, W.; Ding, Q.; Zheng, W.; Xiao, W.; Zhang, B.; Zhang, T.; Zhang, T.; Shi, J.; et al. Anemarrhena asphodeloides modulates gut microbiota and restores pancreatic function in diabetic rats. Biomed. Pharmacother. 2021, 133, 110954. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Wong, S.H.; Zhao, L.; Zhang, X.; Nakatsu, G.; Han, J.; Xu, W.; Xiao, X.; Kwong, T.N.Y.; Tsoi, H.; Wu, W.K.K.; et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 2017, 153, 1621–1633.e6. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Ray Chaudhuri, S. Cancer-associated microbiota: From mechanisms of disease causation to microbiota-centric anti-cancer approaches. Biology 2022, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef]
- Knekt, P.; Jarvinen, R.; Seppanen, R.; Hellovaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjorklund, G.; Lysiuk, R.; Vella, A.; Lenchyk, L.; Upyr, T. Targeting cancer with phytochemicals via their fine tuning of the cell survival signaling pathways. Int. J. Mol. Sci. 2018, 19, 3568. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Franza, L.; Carusi, V.; Nucera, E.; Pandolfi, F. Luteolin, inflammation and cancer: Special emphasis on gut microbiota. Biofactors 2021, 47, 181–189. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, M.S.; Kim, J.Y.; Yu, J.S.; Seo, J.I.; Yoo, H.H.; Kim, D.H. Ginkgo biloba leaf extract suppresses intestinal human breast cancer resistance protein expression in mice: Correlation with gut microbiota. Biomed. Pharmacother. 2021, 140, 111712. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Association of isoflavone biomarkers with risk of chronic disease and mortality: A systematic review and meta-analysis of observational studies. Nutr. Rev. 2017, 75, 616–641. [Google Scholar] [CrossRef]
- Tong, W.G.; Ding, X.Z.; Adrian, T.E. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 296, 942–948. [Google Scholar] [CrossRef]
- Tong, W.G.; Ding, X.Z.; Witt, R.C.; Adrian, T.E. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol. Cancer Ther. 2002, 1, 929–935. [Google Scholar]
- Bednar, W.; Holzmann, K.; Marian, B. Assessing 12(S)-lipoxygenase inhibitory activity using colorectal cancer cells overexpressing the enzyme. Food Chem. Toxicol. 2007, 45, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, J.; Lei, X.; Li, S.; Zhang, Y.; Meng, L.; Xue, R.; Li, Z. Baicalein reduces the invasion of glioma cells via reducing the activity of p38 signaling pathway. PLoS ONE 2014, 9, e90318. [Google Scholar] [CrossRef]
- El-Hosni, M.; Kozik, A.; Cook, M.; Tech, R.L.; Baptist, A.; Schafer, N.; Begley, L.; Huang, Y. Relationships between dietary flavonoid intake, gut microbiota, and Asthma clinical features. J. Allergy. Clin. Immun. 2021, 147, AB49. [Google Scholar] [CrossRef]
- Joseph, T. A synopsis on flavonoids from the roots of Scutellaria baicalensis with some insights on baicalein and its anti-cancer properties. J. Chin. Pharm. Sci. 2019, 28, 217–228. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Nöthlings, U.; Murphy, S.P.; Wilkens, L.R.; Boeing, H.; Schulze, M.B.; Bueno-de-Mesquita, H.B.; Michaud, D.S.; Roddam, A.; Rohrmann, S.; Tjønneland, A.; et al. A food pattern that is predictive of flavonol intake and risk of pancreatic cancer. Am. J. Clin. Nutr. 2008, 88, 1653–1662. [Google Scholar] [CrossRef]
| Types | Bone | Sources of Plants | Main Flavonoids | Functions | References |
|---|---|---|---|---|---|
| Flavonols | 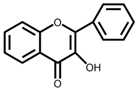 | Dicotyledonous plants, onion, apples, broccoli, tea and red wine | Kaempferol, quercetin, prunetin, rutin | Anti-allergic, anti-cancer, antioxidant anti-inflammatory, the treatment of neurodegenerative diseases and gastrointestinal disorders. etc. | [8,9,10,11] |
| Isoflavones |  | Legumes, Iridaceae, and Rosaceae, cereals, especially rye, oats, wine and tea | Daidzein, genisten, glycitein, biochain A, formononetein | Anti-cancer, antioxidant, anticareinogenic prevention of menopause, improvement of osteoporosis, improvement of diabetes, prevention of cardiovascular disease as well as menopausal symptoms, bone health | [12,13,14,15,16,17] |
| Flavanones |  | Citrus fruit and juice, orange peel, citron, blueberry extract, sweet root, red and blue flowers | Hesperetin, naringenin, eriodictyol, isosakuranetin and their glycosides | Anti-coagulation and hemostasis, anti-tumor activity, anti-cancer, antimutagenic, anti-oxidation, anti-bacterial, anti-inflammatory, prevention of cardiovascular disease and atherosclerosis | [18,19,20,21,22,23] |
| Anthocyanins |  | The cell sap of plant flowers, fruits, stems, leaves and roots | Pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin | Antioxidant, anti-tumor, anti-cancer, anti-inflammatory, inhibition of lipid peroxidation and platelet aggregation, prevention of diabetes, protection of eyesight | [24,25,26,27,28,29] |
| Flavones |  | Angiosperms, mint family (Lamiaceae), cereals, and legumes (millet and sorghum) | Apigenin and luteolin flavone and their glycosides | Antioxidant, lowering cholesterol, inhibition of the activity of drug-metabolizing enzymes | [30,31,32] |
| Diseases Types | Alterations of Intestinal Flora | Reasons | References | |
|---|---|---|---|---|
| Intestinal diseases | Acute and chronic diarrhea | Increase in external pathogens; Decrease in resident bacteria such as Bacteroides, Bifidobacterium, Enterobacter | Irritable stomach syndrome, acute bacterial dysentery post diarrhea, malabsorption syndrome | [60,61] |
| Colitis | Increase in Proteobacteria and Bacteroidetes; Decrease in Firmicutes | Environmental factors, genetic factors, immune factors, microbial infection, etc. Dysfunction of spleen and stomach | [62,63] | |
| Irritable bowel syndrome (IBS) | Increase in Proteus and Bacillus; Decrease in Firmicutes, Bacteroides and Trichospirillum | Changes in diet, stress, and immune function, mucosal secretion, and increase in intestinal mucosal permeability lead to descending microbial diversity and stability | [64,65] | |
| Small intestinal bacterial Overgrowth (SIBO) | Increase in anaerobic bacteria | Intestinal stasis and obstruction of antibacterial mechanism | [66,67] | |
| Hepatitis, cirrhosis, liver cancer | Increase in Enterobacteria, Enterococcus, yeast; Decrease in Bifidobacterium/Staphylococcus, Bifidobacterium | Aerobic gram-negative bacilli proliferate, Gastrointestinal congestion, edema, age, HBV DNA levels, CHB clinical phases, family history of liver cancer and no antiviral treatment | [68,69] | |
| Acute necrotizing pancreatitis | Increase in Escherichia coli; Decrease in Bifidobacterium and Lactobacilli | Obstructive factors, blood circulation disorders, pancreatic trauma, bacterial and viral infections, metabolic diseases | [70,71] | |
| Intestinal bacterial translocation | Increased in Aerobic bacteria such as Enterobacter and Enterococcus; Decrease in intestinal obligate anaerobes such as Bifidobacterium and Lactobacillus | Overgrowth of intestinal bacteria and dysregulation of intestinal flora, reduced host immune function and disruption of the intestinal mucosal barrier | [72,73] | |
| Metabolic diseases | Type 1 diabetes | Increase in Bacteroides, Pseudobacterium ovatus; Decreased in Firmicutes | T cells mediate islet β cell destruction, genetic susceptibilities | [74,75] |
| Type 2 diabetes | Increase in Firmicutes; Decrease in Bacteroides | High-fat diet promotes auxin and insulin secretion, and intestinal microorganisms regulate and enhance the enteric-brain-islet β -cell axis through acetic acid secretion, β -cell dysfunction and insulin resistance | [76,77] | |
| Neurological diseases | Autism spectrum disorder (ASD) | Increase in Bacteroids, paracetobacters, Clostridium, Clostridium faecalis and Keratobacteria; Decrease in Anti-inflammatory genus Bifidobacterium | Genetic and environmental factors | [78,79,80] |
| Multiple sclerosis (MS) | Increase in the relative abundance of Spore and Ruminococcus; Decrease in the relative abundance of Prevotella, p-hydroxybenzobacter, Adlerkreuzia, Collinella, Lactobacillus, symbacteria and Haemophilus | Genetics, infection, environmental factors (e.g., smoking and Vitamin D deficiency) | [81,82,83] | |
| Parkinson’s disease (PD) | Increase in Lactobacillaceae family, Akkermansia sp., Bifidiobacterium sp., Campylobacter, Delft, Haemophilus, Lunaropterus, Neisseria, Actinobacteria, V errucobacteria, and Prevotella, Brucella; Decrease in Blautia sp. and the family Lachnospiraceae | Age, male gender and some environmental factors | [84,85,86,87] | |
| Flavonoids Classes | Conversion Reaction | Enzymes | Species/Strain | Substrate | Products | References |
|---|---|---|---|---|---|---|
| Flavonols | Hydrolysis | β-glucosidase /sulfatase | Escherichia coli | Baicalin | Baicalein | [108,109] |
| Deglycosylation | α-Rhamnosidase | Lachnospiraceae (Lachnoclostridium, and Eisenbergiella), Enterobacteriaceae, Tannerellaceae 63 and Erysipelotrichaceae species | Rutin | Quercetin and quercetin-3-glucoside | [110,111] | |
| Isoflavones | Deglycoslation | β-glucosidase | Bifidobacterium Adolescentis, Bifidobacterium animalis subsp lactis, Bifidobacterium bifidum | Daidzin, daidzein, glycitin and genistin | S-equol, delphinidin and cyanidin | [112,113] |
| Flavanones | Deglycosylation | β-glucosidase | Bacteroides ovatus ATCC 8483T, B. ovatus strain | Rutin | Quercetin | [114] |
| C-ring cleavage | Flavanone reductase | (Clostridium orbiscindens) strains ATCC 49531, 257, 258 and 264 | Quercetin | 3,4-Dihydroxyphenylacetic acid | [115] | |
| Anthocyanins | Deglycosylation | β-glucosidase | Bifidobacterium animalis ssp. Lactis (Bifidobacterium lactis) BB-12, Lactobacillus casei LC-01, L. plantarum IFPL722 | Malvidin-3-O-glucoside | Gallic acid, homogentisic acid, syringic acid | [116] |
| Flavones | Demethylation | Rhamno glucosides, C-glycosyl | Lactobacillus, Bifidobacterium | Tangeretin | Tangeretin-O- glucuronides | [117] |
| Dehydroxylation, deglycosylation, methylation, and acetylation | β-D-glucosidase | Human intestinal bacterium Escherichia sp. 4 | Diosmetin-7-O-glucoside | Diosmetin, acacetin | [118] |
| Ranges of Flavonoids | Active Component | Pathways | Metabolites | Alteration of Gut Microbiota | Health Influence | References |
|---|---|---|---|---|---|---|
| isoflavone | Daidzein | Dihydrodaidzein and detrahydrodaidzein | Equol, O-demethylangolensin and the lignan enterolactone | Increase in Lactobacillus mucosaeEPI2, enterococcus faecium, EPI, Veillonella. sp, train EP, HGH 6 and Julong 732, the taxaPseudoflavonifractor, Dorea, and Lachnospiraceae incertae sedis | Regulation of human obesity, sugar and lipid metabolism, weight loss and lipid lowering effect | [48,96,122,141,142,143,144] |
| Flavanonol | Dihydromyricetin | Reduction, and dihydroxylation | M1, M2, M3 | Increase in Bacteroidia, Betaproteobacteria and Alcaligenaceae; Decrease in Clostridia, Negativicutes, Veillonellaceae, Rikenellaceae, Peptococcaceae, Christensenellaceae and Ruminococcaceae | Treatment of the diseases such as obesity, diabetes and atherosclerosis | [99,145] |
| Favonol | Quercetin | Heme oxygenase-1 (Hmox1, HO-1) dependent Pathway | 3, 4-dihydroxyphenylacetic acid, 3-(3-hydroxyphenyl) propionic acid, protocatechuic acid | Increase in Bacteroidetes/Firmicutes and Bacteroides; Decrease in Proteobacteria, Actinobacteria and segmented filamentous bacteria | Prevention of Inflammatory bowel disease | [146] |
| Flavone | Luteolin | Activator protein (AP)-1 pathway, nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, and signal transducer and activator of transcription (STAT) 3 pathway | Luteolin glucuronides | Increase in Lactobacilli, Bifidobacterial and Bacterodies; Decrease in Staphylococcus aureus, Salmonella typhimurium and Pathogenic Clostridium | Alleviation of inflammation | [147,148,149] |
| Flavonone | Naringin | Reduction and hydrolysis | Naringenin and 3-(4-hydroxyphenyl) propionic acid | Increase in Bifidobacterium catenulatum; Decrease in Enterococcus caccae | Anti-cancer, anti-inflammation, and neuroprotection | [150,151] |
| Hypericum perforatum L. extract | G protein-coupled receptors GPR43 and GPR4 | Primary bile acids such as cholic acids and chenodeoxycholic acid | Increase in Bacteroidetes, Elusimicrobia and Gemmatimonadetes; Decrease in Firmicutes | Menopausal hypercholesterolemia as well as other menopausal symptoms, such as hot flashes and depression | [152] | |
| Flos Chrysanthemi (Luteolin-7-O-glucoside, luteolin, apigenin-7-O-glucoside, diosmetin-7-O-glucoside, quercetin and acacetin) | Hydrolysis, hydroxylation, acetylation, methylation, hydrogenation and deoxidation | A total of 32 metabolites | Increase in Lactobacillus and Bifidobacterium; Decrease in Enterobacter, Enterococcus, Clostridium and Bacteroides | Maintenance of human health and disease prevention such as anti-cancer | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.-H.; Lin, S.-Y.; Chen, L.-L.; Ouyang, K.-H.; Wang, W.-J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. https://doi.org/10.3390/foods12020320
Xiong H-H, Lin S-Y, Chen L-L, Ouyang K-H, Wang W-J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods. 2023; 12(2):320. https://doi.org/10.3390/foods12020320
Chicago/Turabian StyleXiong, Hui-Hui, Su-Yun Lin, Ling-Li Chen, Ke-Hui Ouyang, and Wen-Jun Wang. 2023. "The Interaction between Flavonoids and Intestinal Microbes: A Review" Foods 12, no. 2: 320. https://doi.org/10.3390/foods12020320
APA StyleXiong, H.-H., Lin, S.-Y., Chen, L.-L., Ouyang, K.-H., & Wang, W.-J. (2023). The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods, 12(2), 320. https://doi.org/10.3390/foods12020320







