Drying Kinetic of Jaboticaba Berries and Natural Fermentation for Anthocyanin-Rich Fruit Vinegar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vacuum Oven Drying of Jaboticaba Berries
2.2. Thin-Layer Drying Kinetic of Jaboticaba Berries
2.3. Natural Fermentation of Jaboticaba Vinegar
2.4. Physiochemical Analysis of Jaboticaba Vinegar
2.5. Phenolic Composition of Vinegar
2.6. Antioxidant Capacity of Vinegar
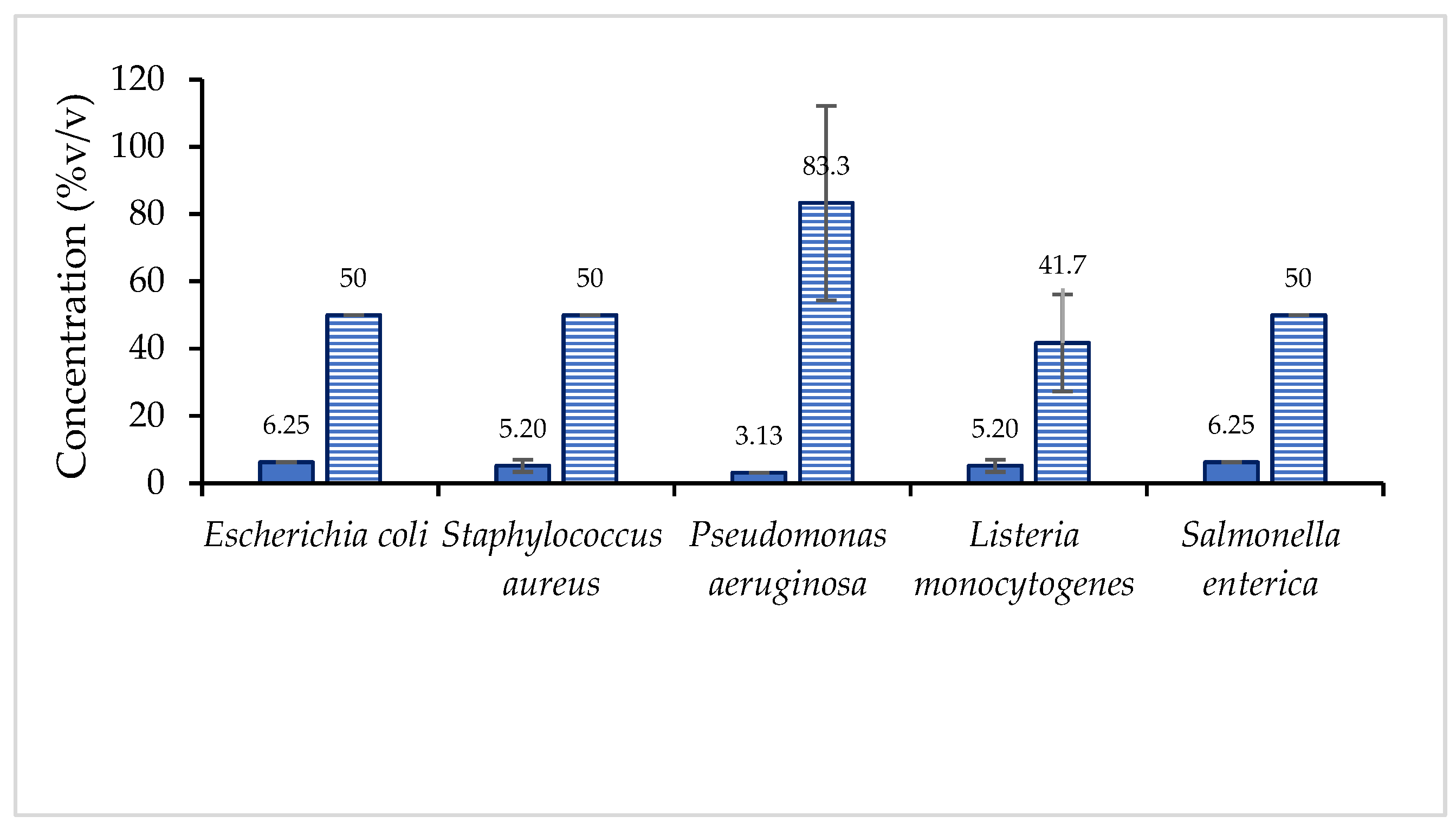
2.7. Antimicrobial Capacity of Vinegar
2.8. Headspace GC-MS
2.9. UHPLC-TWIMS-QTOFMS
3. Results and Discussion
3.1. Effects of Osmotic Dehydration Pretreatment
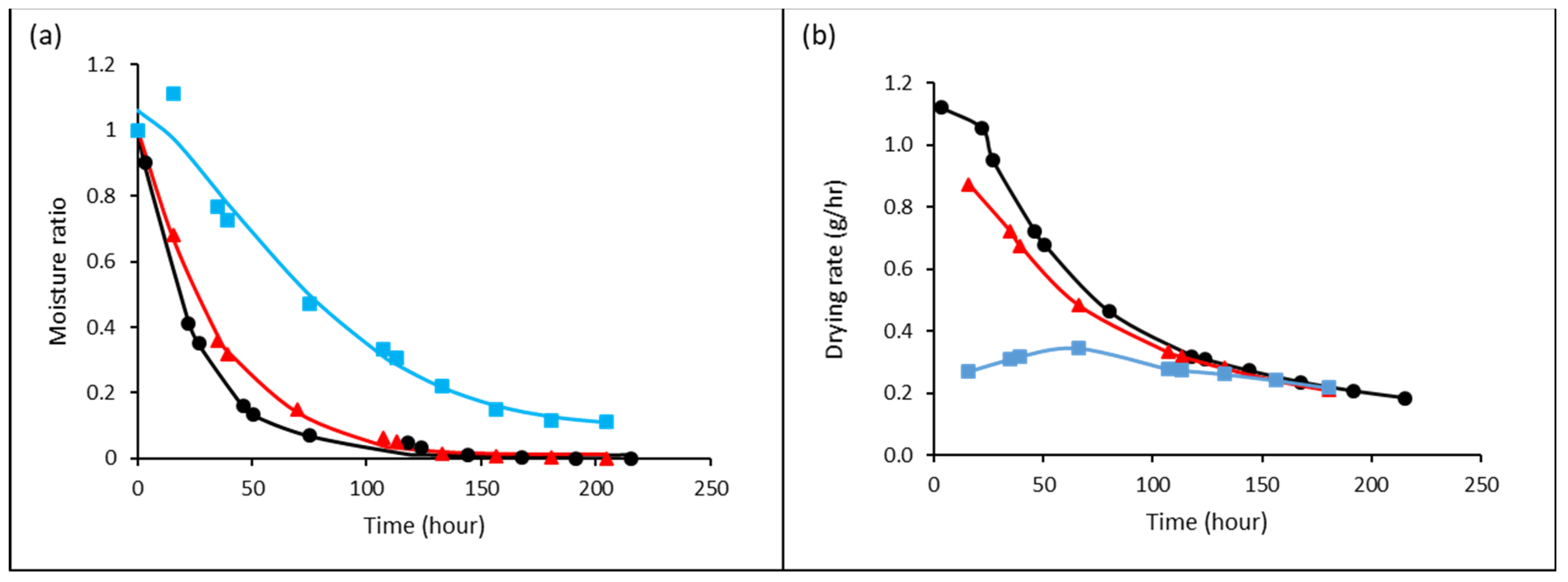
3.2. Thin-Layer Drying Kinetic of Jaboticaba Berries
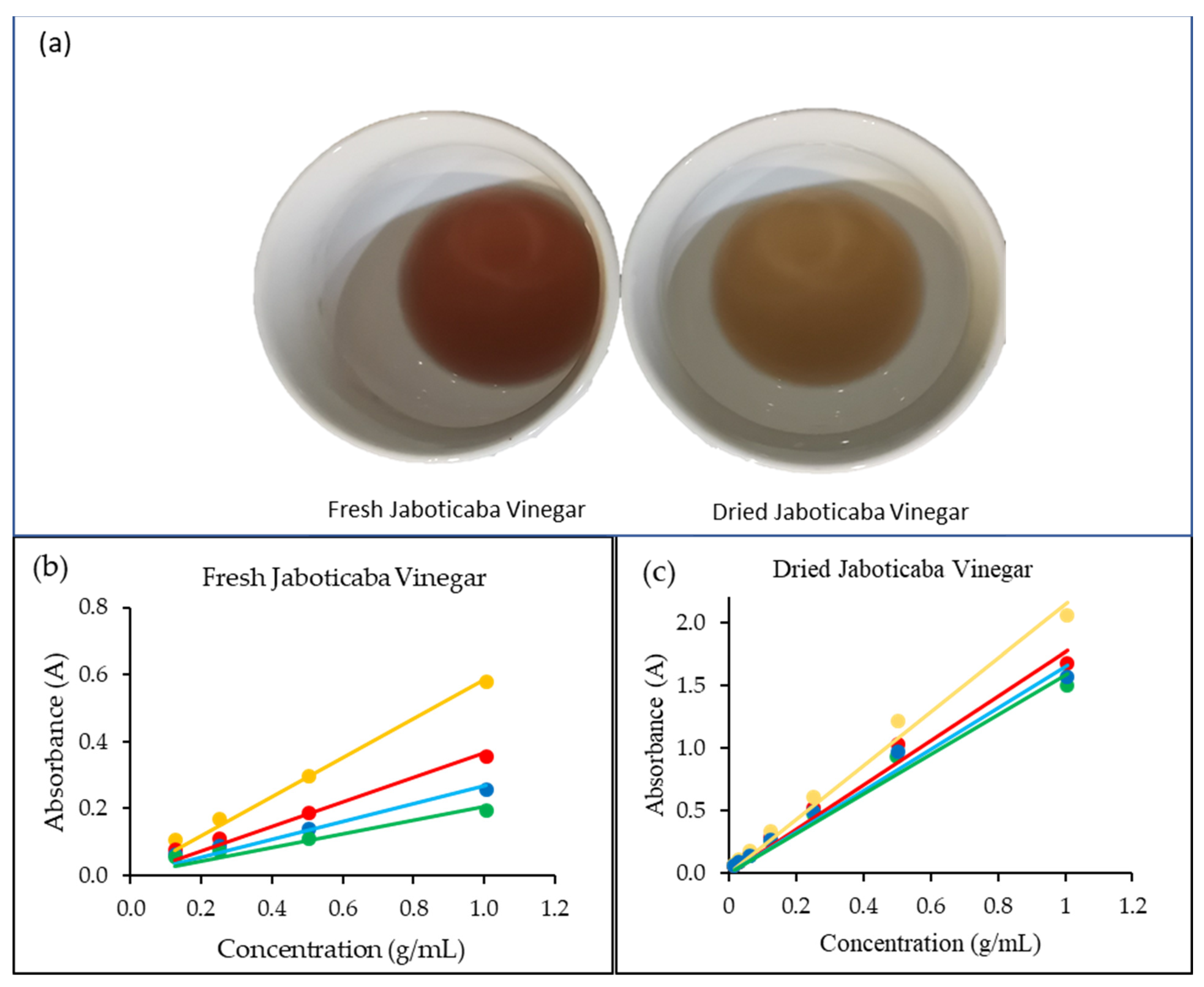
3.3. Physiochemical Properties of Jaboticaba Vinegar
3.4. Phytochemical Content and Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamenković, Z.; Pavkov, I.; Radojčin, M.; Horecki, A.T.; Kešelj, K.; Kovačević, D.B.; Putnik, P. Convective drying of fresh and frozen raspberries and change of their physical and nutritive properties. Foods 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Radojčin, M.; Babić, M.; Babić, L.; Pavkov, I.; Bukurov, M.; Bikić, S.; Mitrevski, V. Effects of osmotic pretreatment on quality and physical properties of dried quinces (Cydonia oblonga). J. Food Nutr. Res. 2015, 54, 142–154. [Google Scholar]
- Furtado, T.D.R.; Muniz, J.A.; Silva, E.M.; Fernandes, J.G. Drying kinetics of jabuticaba pulp by regression models. Rev. Bras. Frutic. 2019, 42, e-097. [Google Scholar] [CrossRef]
- Tai, N.V.; Linh, M.N.; Thuy, N.M. Modeling of thin layer drying characteristics of “xiem” banana peel cultivated at U Minh district, Ca Mau Province, Vietnam. Food Res. 2021, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Bolaji, O.; Adepoju, P.A.; Adelana, E.O.; Adesina, B. Drying kinetics and thin layer modeling of ogi produced from six maize varieties at varying soaking period and drying temperature. Food Res. 2021, 5, 431–440. [Google Scholar] [CrossRef]
- Mugodo, K.; Workneh, T.S. The kinetics of thin-layer drying and modelling for mango slices and the influence of differing hot-air drying methods on quality. Heliyon 2021, 7, e07182. [Google Scholar] [CrossRef]
- Afolabi, T.J.; Agarry, S.E. Thin layer drying kinetics and modelling of Okra (Abelmoschus esculentus (L.) Moench) slices under natural and forced convective air drying. Food Sci. Qual. Manag. 2014, 28, 35–50. [Google Scholar]
- Da Silva Brito, T.G.; da Silva, A.P.S.A.; da Cunha, R.X.; da Fonseca, C.S.M.; da Silva Araújo, T.F.; de Lima Campos, J.K.; Nascimento, W.M.; de Araujo, H.D.A.; Silva, J.P.R.E.; Tavares, J.F.; et al. Anti-inflammatory, hypoglycemic, hypolipidemic, and analgesic activities of Plinia cauliflora (Mart.) Kausel (Brazilian grape) epicarp. J. Ethnopharmacol. 2021, 268, 113611. [Google Scholar] [CrossRef]
- Leite-Legatti, A.V.; Batista, A.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; Carvalho-Silva, L.B.; Ruiz, A.L.T.G.; et al. Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Z.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zoche, E.P.; de Figueredo, O.; Pazuch, C.M.; Colla, E.; da Silva-Buzanello, R.A. Achievement of jabuticaba vinegar by spontaneous fermentation. Braz. J. Food Res. 2015, 6, 80–91. [Google Scholar] [CrossRef]
- Dias, D.R.; Silva, M.S.; de Souza, A.C.; Magalhães-Guedes, K.T.; de Rezende Ribeiro, F.S.; Schwan, R.F. Vinegar production from jabuticaba (Myrciaria jaboticaba) fruit using immobilized acetic acid bacteria. Food Technol. Biotechnol. 2016, 54, 351. [Google Scholar] [CrossRef] [PubMed]
- Vavřiník, A.; Štusková, K.; Baroň, M.; Sochor, J. The production of wine vinegar using different types of acetic acid bacteria. Potravin. Slovak. J. Food Sci. 2022, 16, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. Overview of vinegar production. Palarch’s J. Archaeol. Egypt/Egyptol. 2020, 17, 4027–4037. [Google Scholar]
- IFU Analysis. Measurement of the Colour of Clear and Hazy Juices; No. 80; International Federation of Fruit Juice Producers: Paris, France, 2010; 5p. [Google Scholar]
- Alezandro, M.R.; Dubé, P.; Desjardins, Y.; Lajolo, F.M.; Genovese, M.I. Comparative study of chemical and phenolic compositions of two species of jaboticaba: Myrciaria jaboticaba (Vell.) Berg and Myrciaria cauliflora (Mart.) O. Berg. Food Res. Int. 2013, 54, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Kung, Y.C.; Chua, L.S.; Yam, M.F.; Soo, J. Alkaline hydrolysis and discrimination of propolis at different pH values using high throughput 2D IR spectroscopy and LC-MS/MS. Bioresour. Technol. Rep. 2022, 18, 101120. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Chew, C.Y.; Chua, L.S.; Soontorngun, N.; Lee, C.T. Discovering potential bioactive compounds from Tualang honey. Agric. Nat. Resour. 2018, 52, 361–365. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Mayor, L.; Sereno, A.M. Modelling shrinkage during convective drying of food materials: A review. J. Food Eng. 2004, 61, 373–386. [Google Scholar] [CrossRef]
- Touil, A.; Chemkhi, S.; Zagrouba, F. Moisture diffusivity and shrinkage of fruit and cladode of Opuntia ficus-indica during infrared drying. J. Food Process. 2014, 2014, 175402. [Google Scholar] [CrossRef] [Green Version]
- Rasooli Sharabiani, V.; Kaveh, M.; Abdi, R.; Szymanek, M.; Tanaś, W. Estimation of moisture ratio for apple drying by convective and microwave methods using artificial neural network modeling. Sci. Rep. 2021, 11, 9155. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, J.; Zhang, Z.; Deng, X. Influence of microwave vacuum drying on the effective moisture diffusivity of seedless white grapes. Food Sci. Technol. 2022, 42, e37020. [Google Scholar] [CrossRef]
- Mujumdar, A.S.; Devahastin, S. Fundamental Principles of Drying. In Guide to Industrial Drying; Mujumdar, A., Ed.; Taylor & Francis Group: Hyderabad, India, 2008. [Google Scholar]
- Ndiaye, K.; Kane, C.; Cisse, O.B.K.; Ayessou, N.; Diop, C.M. Monitoring of anthocyanins and colour in electrochemically processed Hibiscus sabdariffa juice. Food Nutr. Sci. 2021, 12, 1073–1087. [Google Scholar]
- Bourgeois, J.F.; Mccoll, I.; Barja, F. Formic acid, acetic acid and methanol: Their relevance to the verification of the authenticity of vinegar. Arch. Des. Sci. 2006, 59, 107–112. [Google Scholar]
- Xiao, Z.; Lu, J.R. Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S. The antioxidant activity of prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef] [Green Version]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar. J. Food Sci. 2014, 79, 757–764. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/1750-3841.12434 (accessed on 1 December 2022). [CrossRef]
- Harris, C.M.; Williams, S.K. The antimicrobial properties of a vinegar-based ingredient on Salmonella Typhimurium and psychrotrophs inoculated in ground chicken breast meat and stored at 3 ± 1 °C for 7 days. J. Appl. Poult. Res. 2019, 28, 118–123. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef] [PubMed]
| Pretreatment | Mass Loss by Pretreatment (%) | Total Mass Loss (%) |
|---|---|---|
| Non-pretreatment | 0 | 76.6 ± 1.1 |
| 70% sugar solution | 26.8 ± 0.6 | 89.8 ± 1.3 |
| 10% salt solution | 8.2 ± 1.1 * | 61.4 ± 1.2 |
| Thin-Layer Drying Model | Without Pretreatment | Pretreatment with 70% Sugar Solution | Pretreatment with 10% Salt Solution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | R2 | Adjusted R2 | RMSE | χ2 | Constant | R2 | Adjusted R2 | RMSE | χ2 | Constant | R2 | Adjusted R2 | RMSE | χ2 | |
| Newton | k = 0.0391 | 0.9978 | 0.9978 | 0.0159 | 0.001 | k = 0.0277 | 0.9976 | 0.9976 | 0.016 | 0.0010 | k = 0.0195 | 0.9478 | 0.9478 | 0.0100 | 0.0179 |
| MR = exp(−kt) | |||||||||||||||
| Handerson and Pabis | k = 0.0397 | 0.9980 | 0.9978 | 0.0152 | 0.0009 | k = 0.0281 | 0.9978 | 0.9974 | 0.0155 | 0.0010 | k = 0.0114 | 0.9619 | 0.9565 | 0.0707 | 0.0102 |
| MR = a.exp(−kt) | a = 1.0126 | a = 1.0117 | a = 1.1328 | ||||||||||||
| Logarithmic | k = 0.0408 | 0.9985 | 0.9981 | 0.0132 | 0.0007 | k = 0.0281 | 0.9978 | 0.997 | 0.0155 | 0.0010 | k = 0.0114 | 0.9619 | 0.9492 | 0.0707 | 0.0102 |
| MR= a.exp(−kt) + b | a = 1.0034 | a = 1.0117 | a = 1.1328 | ||||||||||||
| b = 0.0111 | b = 0 | b = 0 | |||||||||||||
| Two term | k1 = 0.0397 | 0.9979 | 0.9975 | 0.0152 | 0.0009 | k1 = 0.0281 | 0.9978 | 0.997 | 0.0155 | 0.0010 | k1 = 0.0114 | 0.9619 | 0.939 | 0.0707 | 0.0102 |
| a.exp(-k1t) + b.exp (-k2t) | k2 = 0.0397 | k2 = 1.3437 | k2 = 0 | ||||||||||||
| a = 0.5063 | a = 1.0117 | a = 1.1328 | |||||||||||||
| b = 0.5063 | b = 0 | b = 0 | |||||||||||||
| Page | k = 0.0347 | 0.9979 | 0.9977 | 0.0155 | 0.001 | k = 0.0214 | 0.9981 | 0.9978 | 0.0142 | 0.0008 | k = 0.0013 | 0.97 | 0.9657 | 0.0627 | 0.0083 |
| MR = exp(−ktn) | n = 1.0360 | n = 1.0729 | n = 1.4373 | ||||||||||||
| Midilli et al. | k = 0.0355 | 0.9984 | 0.9978 | 0.0137 | 0.0007 | k = 0.0192 | 0.9982 | 0.9972 | 0.0141 | 0.0007 | k = 0.0018 | 0.9775 | 0.964 | 0.0543 | 0.0061 |
| MR = a exp(−ktn) + bt | n = 1.0339 | n = 1.1084 | n = 1.4121 | ||||||||||||
| a = 1.0088 | a = 1.0044 | a = 1.0623 | |||||||||||||
| b = 0.00006 | b = 0.00006 | b = 0.0004 | |||||||||||||
| Parameter | Maceration Solution | Fresh Fruit Vinegar | Dried Fruit Vinegar |
|---|---|---|---|
| pH | 4.5 ± 0.1 | 3.0 ± 0.1 | 3.1 ± 0.1 |
| Conductivity (mS/cm) * | 2.35 ± 0.30 | 3.15 ± 0.25 | 4.25 ± 0.33 |
| Acidity (% acetic acid) | 0 | 3.1 ± 0.1 | 3.0 ± 0.1 |
| Brown index * | 1.762 ± 0.037 | 1.794 ± 0.025 | 1.170 ± 0.030 |
| Blue index * | 0.691 ± 0.073 | 0.668 ± 0.012 | 0.937 ± 0.003 |
| Parameter | Fresh Berry Vinegar | Dried Berry Vinegar |
|---|---|---|
| Solid (%) | 1.8 ± 0.2 | 1.6 ± 0.1 |
| Sugar (°Bx) | 3.0 ± 0.1 | 1.1 ± 0.2 |
| Total phenolic content (mg GA/g) * | 39.49 ± 0.48 | 54.71 ± 3.16 |
| Total flavonoid content (mg Q/g) * | 6.49 ± 0.13 | 1.91 ± 0.15 |
| Total anthocyanin content (mg C3G/g) * | 2.71 ± 0.37 | 0.24 ± 0.13 |
| IC50 DPPH (Trolox, 0.005 mg/mL) * | 0.94 ± 0.23 | 3.34 ± 0.19 |
| IC50 ABTS (Trolox, 0.114 mg/mL) | 1.59 ± 0.29 | 1.47 ± 0.40 |
| ORAC (µmol T/g) * | 26.93 ± 2.56 | 6.64 ± 1.29 |
| FRAP (f.mol T/kg) | 0.252 ± 0.063 | 0.205 ± 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, L.S.; Abd Wahab, N.S. Drying Kinetic of Jaboticaba Berries and Natural Fermentation for Anthocyanin-Rich Fruit Vinegar. Foods 2023, 12, 65. https://doi.org/10.3390/foods12010065
Chua LS, Abd Wahab NS. Drying Kinetic of Jaboticaba Berries and Natural Fermentation for Anthocyanin-Rich Fruit Vinegar. Foods. 2023; 12(1):65. https://doi.org/10.3390/foods12010065
Chicago/Turabian StyleChua, Lee Suan, and Nurul Syafiqah Abd Wahab. 2023. "Drying Kinetic of Jaboticaba Berries and Natural Fermentation for Anthocyanin-Rich Fruit Vinegar" Foods 12, no. 1: 65. https://doi.org/10.3390/foods12010065






