Pyrolysis Combined with the Dry Reforming of Waste Plastics as a Potential Method for Resource Recovery—A Review of Process Parameters and Catalysts
Abstract
:1. Introduction
2. Pyrolysis
3. Dry Reforming
4. Pyrolysis-Catalytic Dry Reforming
4.1. Influence of Process Parameters
4.1.1. Feedstock Composition
4.1.2. Temperature
4.1.3. Catalysts
4.1.4. Catalyst-to-Plastic-Feedstock Ratio
4.1.5. Steam Addition
4.2. The Strategies and Challenge on Carbon Disposal
5. Concluding Remarks and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2021; Plastics Europe: Brussels, Belgium, 2021. [Google Scholar]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- European Parliament; The Council of the European Union. Official Journal of the European Union L 150: Legislation. Off. J. Eur. Union 2018, 2018, 26–42. [Google Scholar]
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128196571. [Google Scholar]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Al Khourdajie, A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Moe, E.; Røttereng, J.-K.S. The post-carbon society: Rethinking the international governance of negative emissions. Energy Res. Soc. Sci. 2018, 44, 199–208. [Google Scholar] [CrossRef]
- Yamada, H.; Mori, H.; Tagawa, T. CO2 reforming of waste plastics. J. Ind. Eng. Chem. 2010, 16, 7–9. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Pyrolysis-Catalytic-Dry Reforming of Waste Plastics and Mixed Waste Plastics for Syngas Production. Energy Fuels 2016, 30, 3198–3204. [Google Scholar] [CrossRef]
- Al-Asadi, M.; Miskolczi, N. High temperature pyrolysis of municipal plastic waste using Me/Ni/ZSM-5 catalysts: The effect of metal/nickel ratio. Energies 2020, 13, 1284. [Google Scholar] [CrossRef] [Green Version]
- Younis, A.; Gennequin, C.; Aad, E.A.; Estephane, J.; Aouad, S. Valorization of plastics in the presence of Ru-Ni/Al2O3 catalysts to produce syngas. In Proceedings of the 2021 12th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 26–28 October 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Pyrolysis-catalytic dry (CO2) reforming of waste plastics for syngas production: Influence of process parameters. Fuel 2017, 193, 7–14. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Catalytic dry reforming of waste plastics from different waste treatment plants for production of synthesis gases. Waste Manag. 2016, 58, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.M.; Williams, P.T. Manipulating the H2/CO ratio from dry reforming of simulated mixed waste plastics by the addition of steam. Fuel Process. Technol. 2017, 156, 331–338. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Al-Asadi, M.; Miskolczi, N.; Gombor, L. Dry reforming of waste polymers in horizontal reactor to syngas production. Chem. Eng. Trans. 2019, 76, 1429–1434. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Piñar, A.; Ligero, A.; Blázquez, G.; Calero, M. Characterization and use of char produced from pyrolysis of post-consumer mixed plastic waste. Water 2021, 13, 1188. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Alvarez, J.; Artetxe, M.; Arregi, A.; Bilbao, J.; Olazar, M. A sequential process for hydrogen production based on continuous HDPE fast pyrolysis and in-line steam reforming. Chem. Eng. J. 2016, 296, 191–198. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Chapter 2 Thermal decomposition of polymers. In Analytical Pyrolysis of Synthetic Organic Polymers; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ray, S.; Cooney, R.P. Thermal Degradation of Polymer and Polymer Composites, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780323524735. [Google Scholar]
- Rasaq, W.A.; Golonka, M.; Scholz, M.; Białowiec, A. Opportunities and challenges of high-pressure fast pyrolysis of biomass: A review. Energies 2021, 14, 5426. [Google Scholar] [CrossRef]
- Suresh, A.; Alagusundaram, A.; Kumar, P.S.; Vo, D.V.N.; Christopher, F.C.; Balaji, B.; Viswanathan, V.; Sankar, S. Microwave pyrolysis of coal, biomass and plastic waste: A review. Environ. Chem. Lett. 2021, 19, 3609–3629. [Google Scholar] [CrossRef]
- Dhahak, A.; Grimmer, C.; Neumann, A.; Rüger, C.; Sklorz, M.; Streibel, T.; Zimmermann, R.; Mauviel, G.; Burkle-Vitzthum, V. Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manag. 2020, 106, 226–239. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. The Influence of Temperature and Heating Rate on the Slow Pyrolysis of Biomass. Renew. Energy 1996, 1481, 6–7. [Google Scholar] [CrossRef]
- Phan, A.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. Characterisation of slow pyrolysis products from segregated wastes for energy production. J. Anal. Appl. Pyrolysis 2008, 81, 65–71. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. The effect of slow pyrolysis on the conversion of packaging waste plastics (PE and PP) into fuel. Waste Manag. 2018, 79, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Grieco, E.M.; Baldi, G. Pyrolysis of polyethylene mixed with paper and wood: Interaction effects on tar, char and gas yields. Waste Manag. 2012, 32, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Thermogravimetric analysis and carbon stability of chars produced from slow pyrolysis and hydrothermal carbonization of manure waste. J. Anal. Appl. Pyrolysis 2019, 140, 434–443. [Google Scholar] [CrossRef] [Green Version]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Tokmurzin, D.; Kuspangaliyeva, B.; Aimbetov, B.; Abylkhani, B.; Inglezakis, V.; Anthony, E.J.; Sarbassov, Y. Characterization of solid char produced from pyrolysis of the organic fraction of municipal solid waste, high volatile coal and their blends. Energy 2020, 191, 116562. [Google Scholar] [CrossRef]
- Waheed, Q.M.K.; Nahil, M.A.; Williams, P.T. Pyrolysis of waste biomass: Investigation of fast pyrolysis and slow pyrolysis process conditions on product yield and gas composition. J. Energy Inst. 2013, 86, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.J.; Williams, P.T. Fast pyrolysis of halogenated plastics recovered from waste computers. Energy Fuels 2006, 20, 1536–1549. [Google Scholar] [CrossRef] [Green Version]
- Kannan, P.; Al Shoaibi, A.; Srinivasakannan, C. Temperature effects on the yield of gaseous olefins from waste polyethylene via flash pyrolysis. Energy Fuels 2014, 28, 3363–3366. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: Product yield analysis and their characterization. J. Energy Inst. 2019, 92, 1647–1657. [Google Scholar] [CrossRef]
- Ibrahim, H.A. Introductory chapter: Pyrolysis. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2020; pp. 1–12. [Google Scholar]
- Hang, J.; Haoxi, B.; Ying, L.; Rui, W. Catalytic Fast Pyrolysis of Poly (Ethylene Terephthalate) (PET) with Zeolite and Nickel Chloride. Polymers 2020, 12, 705. [Google Scholar]
- Lim, S.; Kim, Y.M. Catalytic pyrolysis of waste polyethylene terephthalate over waste concrete. Appl. Chem. Eng. 2019, 30, 707–711. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; McMahon, A.; Phan, A.N. Benzoic acid recovery via waste poly(ethylene terephthalate) (PET) catalytic pyrolysis using sulphated zirconia catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhang, Z.; Zhang, Z.; Sun, J.; Wang, Q.; Pittman, C.U. Catalytic fast pyrolysis of a wood-plastic composite with metal oxides as catalysts. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Klaimy, S.; Lamonier, J.F.; Casetta, M.; Heymans, S.; Duquesne, S. Recycling of plastic waste using flash pyrolysis–Effect of mixture composition. Polym. Degrad. Stab. 2021, 187, 109540. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F. Pyrolysis of synthetic polymers and plastic wastes. Kinetic study. Fuel Process. Technol. 2008, 89, 678–686. [Google Scholar] [CrossRef]
- Williams, P.T.; Williams, E.A. Interaction of plastics in mixed-plastics pyrolysis. Energy Fuels 1999, 13, 188–196. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Berrueco, C.; Mastral, E.J.; Esperanza, E.; Ceamanos, J. Production of waxes and tars from the continuous pyrolysis of high density polyethylene. Influence of operation variables. Energy Fuels 2002, 16, 1148–1153. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostrup-Nielsen, J.R.; Bak Hansen, J.H. CO2-reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Sullivan, M.M.; Bhan, A. Effects of oxygen coverage on rates and selectivity of propane-CO2 reactions on molybdenum carbide. J. Catal. 2018, 357, 195–205. [Google Scholar] [CrossRef]
- Yan, B.; Yang, X.; Yao, S.; Wan, J.; Myint, M.N.Z.; Gomez, E.; Xie, Z.; Kattel, S.; Xu, W.; Chen, J.G. Dry Reforming of Ethane and Butane with CO2 over PtNi/CeO2 Bimetallic Catalysts. ACS Catal. 2016, 6, 7283–7292. [Google Scholar] [CrossRef]
- Song, X.; Guo, Z. Technologies for direct production of flexible H2/CO synthesis gas. Energy Convers. Manag. 2006, 47, 560–569. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Lotina, A.; Daza, L. Influence of the operating parameters over dry reforming of methane to syngas. Int. J. Hydrog. Energy 2014, 39, 4089–4094. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Shah, Y.T.; Gardner, T.H. Dry reforming of hydrocarbon feedstocks. Catal. Rev. Sci. Eng. 2014, 56, 476–536. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Silva, C.G.; Passos, F.B.; da Silva, V.T. Influence of the support on the activity of a supported nickel-promoted molybdenum carbide catalyst for dry reforming of methane. J. Catal. 2019, 375, 507–518. [Google Scholar] [CrossRef]
- José-Alonso, D.S.; Illán-Gómez, M.J.; Román-Martínez, M.C. Low metal content Co and Ni alumina supported catalysts for the CO2 reforming of methane. Int. J. Hydrog. Energy 2013, 38, 2230–2239. [Google Scholar] [CrossRef]
- Saad, J.M.; Nahil, M.A.; Wu, C.; Williams, P.T. Influence of nickel-based catalysts on syngas production from carbon dioxide reforming of waste high density polyethylene. Fuel Process. Technol. 2015, 138, 156–163. [Google Scholar] [CrossRef]
- Saad, J.M.; Nahil, M.A.; Williams, P.T. Influence of process conditions on syngas production from the thermal processing of waste high density polyethylene. J. Anal. Appl. Pyrolysis 2015, 113, 35–40. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño Sánchez, P. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Luo, G.; Chandler, D.S.; Anjos, L.C.A.; Eng, R.J.; Jia, P.; Resende, F.L.P. Pyrolysis of whole wood chips and rods in a novel ablative reactor. Fuel 2017, 194, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Williams, P.T. Pyrolysis-gasification of plastics, mixed plastics and real-world plastic waste with and without Ni-Mg-Al catalyst. Fuel 2010, 89, 3022–3032. [Google Scholar] [CrossRef]
- Al-asadi, M.; Miskolczi, N. Hydrogen rich products from waste HDPE/LDPE/PP/PET over Me/Ni-ZSM-5 catalysts combined with dolomite. J. Energy Inst. 2021, 96, 251–259. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef]
- Wampler, T.P. Thermometric behavior of polyolefins. J. Anal. Appl. Pyrolysis 1989, 15, 187–195. [Google Scholar] [CrossRef]
- Day, M. Influence of temperature and environment on the thermal decomposition of poly(ethylene terephthalate) fibres with and without the flame retardant tris(2,3-dibromopropyl) phosphate. J. Anal. Appl. Pyrolysis 1984, 7, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Mastral, F.J.; Esperanza, E.; Garciía, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Alvarez, J.; Kumagai, S.; Wu, C.; Yoshioka, T.; Bilbao, J.; Olazar, M.; Williams, P.T. Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification. Int. J. Hydrog. Energy 2014, 39, 10883–10891. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeon, S.G.; Kim, K.H.; Noh, N.S.; Shin, D.H.; Park, J.; Seo, Y.; Yee, J.J.; Kim, G.T. Thermal and Catalytic Degradation of Waste High-density Polyethylene (HDPE) Using Spent FCC Catalyst. Korean J. Chem. Eng. 2003, 20, 693–697. [Google Scholar] [CrossRef]
- Sehested, J. Sintering of nickel steam-reforming catalysts. J. Catal. 2003, 217, 417–426. [Google Scholar] [CrossRef]
- Fekhar, B.; Gombor, L.; Miskolczi, N. Pyrolysis of chlorine contaminated municipal plastic waste: In-situ upgrading of pyrolysis oils by Ni/ZSM-5, Ni/SAPO-11, red mud and Ca(OH)2 containing catalysts. J. Energy Inst. 2019, 92, 1270–1283. [Google Scholar] [CrossRef]
- Borsodi, N.; Miskolczi, N.; Angyal, A.; Bartha, L. Hydrocarbons obtained by pyrolysis of contaminated waste plastics. In Proceedings of the 45th International Petroleum Conference, Bratislava, Slovakia, 13 June 2011; pp. 1–9. [Google Scholar]
- Palmay, P.; Puente, C.; Barzallo, D.; Bruno, J.C. Determination of the Thermodynamic Parameters of the Pyrolysis Process of Post-Consumption Thermoplastics by Non-Isothermal Thermogravimetric Analysis. Polymers 2021, 13, 4379. [Google Scholar] [CrossRef]
- Er-rbib, H.; Bouallou, C.; Werkoff, F. Dry reforming of methane—Review of feasibility studies. Chem. Eng. Trans. 2012, 29, 163–168. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. From hydrocarbon to hydrogen-carbon to hydrogen economy. Int. J. Hydrog. Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Goodman, D.W.; Company, C.; Centre, B.T. Methane Decomposition: Production of Hydrogen and Carbon Filaments. Catalysis 2007, 19, 164–183. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Hu, Q.; Nahil, M.A.; Chen, H.; Williams, P.T. Hydrogen production from catalytic reforming of the aqueous fraction of pyrolysis bio-oil with modified Ni-Al catalysts. Int. J. Hydrog. Energy 2014, 39, 14642–14652. [Google Scholar] [CrossRef] [Green Version]
- Aghamohammadi, S.; Haghighi, M.; Maleki, M.; Rahemi, N. Sequential impregnation vs. sol-gel synthesized Ni/Al2O3-CeO2 nanocatalyst for dry reforming of methane: Effect of synthesis method and support promotion. Mol. Catal. 2017, 431, 39–48. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Wei, F.; Zhu, C.; Wei, X.Y. Extension of catalyst lifetime by doping of Ce in Ni-loaded acid-washed Shengli lignite char for biomass catalytic gasification. Catal. Sci. Technol. 2017, 7, 5741–5749. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, W.; Li, Z.; Wang, J.; Ma, J. Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts. Int. J. Hydrog. Energy 2009, 34, 6646–6654. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Jafari, M.F.; Khorram, S. Non-thermal plasma assisted synthesis and physicochemical characterizations of Co and Cu doped Ni/Al2O3 nanocatalysts used for dry reforming of methane. Int. J. Hydrog. Energy 2013, 38, 16048–16061. [Google Scholar] [CrossRef]
- Czaplicka, N.; Rogala, A.; Wysocka, I. Metal (Mo, W, Ti) carbide catalysts: Synthesis and application as alternative catalysts for dry reforming of hydrocarbons—A review. Int. J. Mol. Sci. 2021, 22, 2337. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Du, X.; France, L.J.; Kuznetsov, V.L.; Xiao, T.; Edwards, P.P.; AlMegren, H.; Bagabas, A. Dry reforming of methane over ZrO2-supported Co–Mo carbide catalyst. Appl. Petrochem. Res. 2014, 4, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Yamaguchi, A.; Cho, Y.; Anjaneyulu, O.; Fujita, T.; Abe, H.; Miyauchi, M. Metal Carbide as A Light-Harvesting and Anticoking Catalysis Support for Dry Reforming of Methane. Glob. Chall. 2020, 4, 1900067. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhang, A.J.; Zhu, A.M.; Xu, Y.; Au, C.T.; Shi, C. A carbide catalyst effective for the dry reforming of methane at atmospheric pressure. ACS Symp. Ser. 2010, 1056, 181–196. [Google Scholar] [CrossRef]
- Brungs, A.J.; York, A.P.E.; Claridge, J.B.; Márquez-Alvarez, C.; Green, M.L.H. Dry reforming of methane to synthesis gas over supported molybdenum carbide catalysts. Catal. Lett. 2000, 70, 117–122. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-Pérez, L.; Gu, S.; Reina, T.R. Transition metal carbides (TMCS) catalysts for gas phase CO2 upgrading reactions: A comprehensive overview. Catalysts 2020, 10, 955. [Google Scholar] [CrossRef]
- Mounfield, W.P.; Harale, A.; Román-Leshkov, Y. Impact of morphological effects on the activity and stability of tungsten carbide catalysts for dry methane reforming. Energy Fuels 2019, 33, 5544–5550. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Yao, Z.; Yan, S.; Kang, X. Finding of a new cycle route in Ni/Mo2C catalyzed CH4-CO2reforming. Catal. Sci. Technol. 2021, 11, 479–483. [Google Scholar] [CrossRef]
- Lalsare, A.D.; Leonard, B.; Robinson, B.; Sivri, A.C.; Vukmanovich, R.; Dumitrescu, C.; Rogers, W.; Hu, J. Self-regenerable carbon nanofiber supported Fe–Mo2C catalyst for CH4-CO2 assisted reforming of biomass to hydrogen rich syngas. Appl. Catal. B Environ. 2021, 282, 119537. [Google Scholar] [CrossRef]
- Shao, H.; Kugler, E.L.; Ma, W.; Dadyburjor, D.B. Effect of temperature on structure and performance of in-house cobalt-tungsten carbide catalyst for dry reforming of methane. Ind. Eng. Chem. Res. 2005, 44, 4914–4921. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhang, X.; Qiu, J.; Yu, L.; Shi, C. Ni modified WC x catalysts for methane dry reforming. In Advances in CO2 Capture, Sequestration, and Conversion; American Chemical Society: Washington, DC, USA, 2015; pp. 171–189. [Google Scholar]
- Porosoff, M.D.; Myint, M.N.Z.; Kattel, S.; Xie, Z.; Gomez, E.; Liu, P.; Chen, J.G. Identifying Different Types of Catalysts for CO2 Reduction by Ethane through Dry Reforming and Oxidative Dehydrogenation. Angew. Chem. Int. Ed. 2015, 54, 15501–15505. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, F.; Németh, R.; Oszkó, A. The oxidative dehydrogenation of propane with CO2 over supported Mo2C catalyst. Stud. Surf. Sci. Catal. 2001, 136, 339–344. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Marakatti, V.S.; Sloof, W.G.; Delgado, J.J.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Rothenberg, G.; Shiju, N.R. Butane Dry Reforming Catalyzed by Cobalt Oxide Supported on Ti2AlC MAX Phase. ChemSusChem 2020, 13, 6401–6408. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: A review. Renew. Sustain. Energy Rev. 2020, 119, 109600. [Google Scholar] [CrossRef]
- Ferella, F.; Stoehr, J.; De Michelis, I.; Hornung, A. Zirconia and alumina based catalysts for steam reforming of naphthalene. Fuel 2013, 105, 614–629. [Google Scholar] [CrossRef]
- Barbarias, I.; Artetxe, M.; Lopez, G.; Arregi, A.; Santamaria, L.; Bilbao, J.; Olazar, M. Catalyst performance in the HDPE pyrolysis-reforming under reaction-regeneration cycles. Catalysts 2019, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Arandia, A.; Remiro, A.; García, V.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Oxidative steam reforming of raw bio-oil over supported and bulk Ni catalysts for hydrogen production. Catalysts 2018, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Koike, M.; Wang, L.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Regenerability of hydrotalcite-derived nickel-iron alloy nanoparticles for syngas production from biomass tar. ChemSusChem 2014, 7, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nakagawa, Y.; Tomishige, K. Development of Ni-based catalysts for steam reforming of tar derived from biomass pyrolysis. Cuihua Xuebao/Chin. J. Catal. 2012, 33, 583–594. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Effects of gasification temperature and catalyst ratio on hydrogen production from catalytic steam pyrolysis-gasification of polypropylene. Energy Fuels 2008, 22, 4125–4132. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis-gasification of post-consumer municipal solid plastic waste for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 949–957. [Google Scholar] [CrossRef]
- Wu, S.L.; Kuo, J.H.; Wey, M.Y. Thermal degradation of waste plastics in a two-stage pyrolysis-catalysis reactor over core-shell type catalyst. J. Anal. Appl. Pyrolysis 2019, 142, 104641. [Google Scholar] [CrossRef]
- Wysocka, I.; Mielewczyk-Gryń, A.; Łapiński, M.; Cieślik, B.; Rogala, A. Effect of small quantities of potassium promoter and steam on the catalytic properties of nickel catalysts in dry/combined methane reforming. Int. J. Hydrog. Energy 2021, 46, 3847–3864. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H.; Claridge, J.B. Methane oxyforming for synthesis gas production. Catal. Rev. Sci. Eng. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Guilhaume, N.; Bianchi, D.; Wandawa, R.A.; Yin, W.; Schuurman, Y. Study of CO2 and H2O adsorption competition in the combined dry/steam reforming of biogas. Catal. Today 2021, 375, 282–289. [Google Scholar] [CrossRef]
- Nileshkumar, K.D.; Patel, T.M.; Rathod, G.P. Effect of Blend Ratio of Plastic Pyrolysis Oil and Diesel Fuel on the Performance of Single Cylinder CI Engine. Int. J. Sci. Technol. Eng. 2015, 1, 195–203. [Google Scholar]
- Rehan, M.; Nizami, A.S.; Shahzad, K.; Ouda, O.K.M.; Ismail, I.M.I.; Almeelbi, T.; Iqbal, T.; Demirbas, A. Pyrolytic liquid fuel: A source of renewable electricity generation in Makkah. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 2598–2603. [Google Scholar] [CrossRef]
- Saptoadi, H.; Pratama, N.N. Utilization of Plastics Waste Oil as Partial Substitute for Kerosene in Pressurized Cookstoves. Int. J. Environ. Sci. Dev. 2015, 6, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Ravenni, G.; Cafaggi, G.; Sárossy, Z.; Rohde Nielsen, K.T.; Ahrenfeldt, J.; Henriksen, U.B. Waste chars from wood gasification and wastewater sludge pyrolysis compared to commercial activated carbon for the removal of cationic and anionic dyes from aqueous solution. Bioresour. Technol. Rep. 2020, 10, 100421. [Google Scholar] [CrossRef]
- Doumer, M.E.; Arízaga, G.G.C.; Da Silva, D.A.; Yamamoto, C.I.; Novotny, E.H.; Santos, J.M.; Dos Santos, L.O.; Wisniewski, A.; De Andrade, J.B.; Mangrich, A.S. Slow pyrolysis of different Brazilian waste biomasses as sources of soil conditioners and energy, and for environmental protection. J. Anal. Appl. Pyrolysis 2015, 113, 434–443. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef]
- Garg, K.K.; Pandey, S.; Kumar, A.; Rana, A.; Sahoo, N.G.; Singh, R.K. Graphene nanosheets derived from waste plastic for cost-effective thermoelectric applications. Results Mater. 2022, 13, 100260. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Behaviour of waste polypropylene pyrolysis char-based epoxy composite materials. Environ. Sci. Pollut. Res. 2020, 27, 3871–3884. [Google Scholar] [CrossRef] [PubMed]
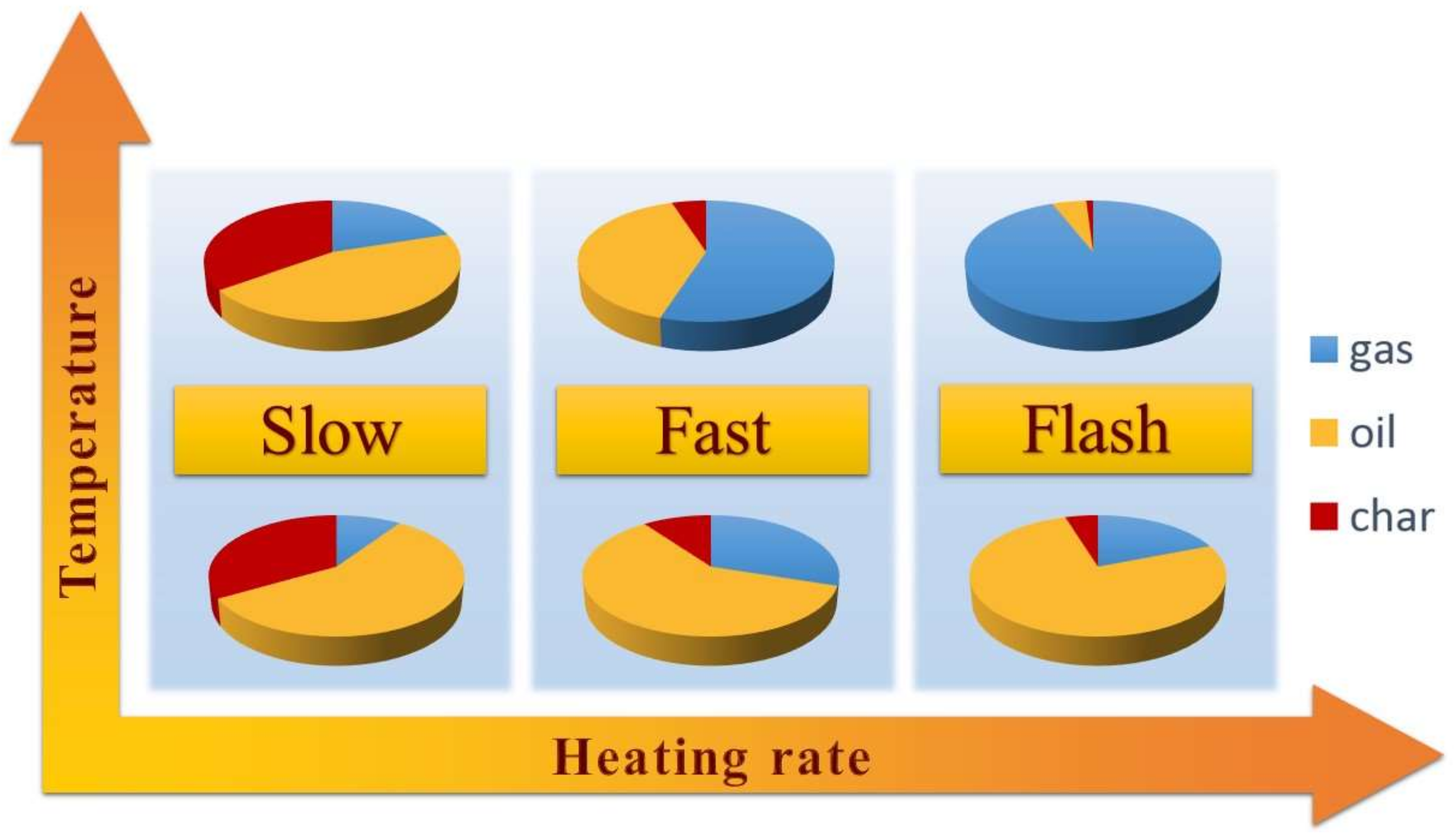
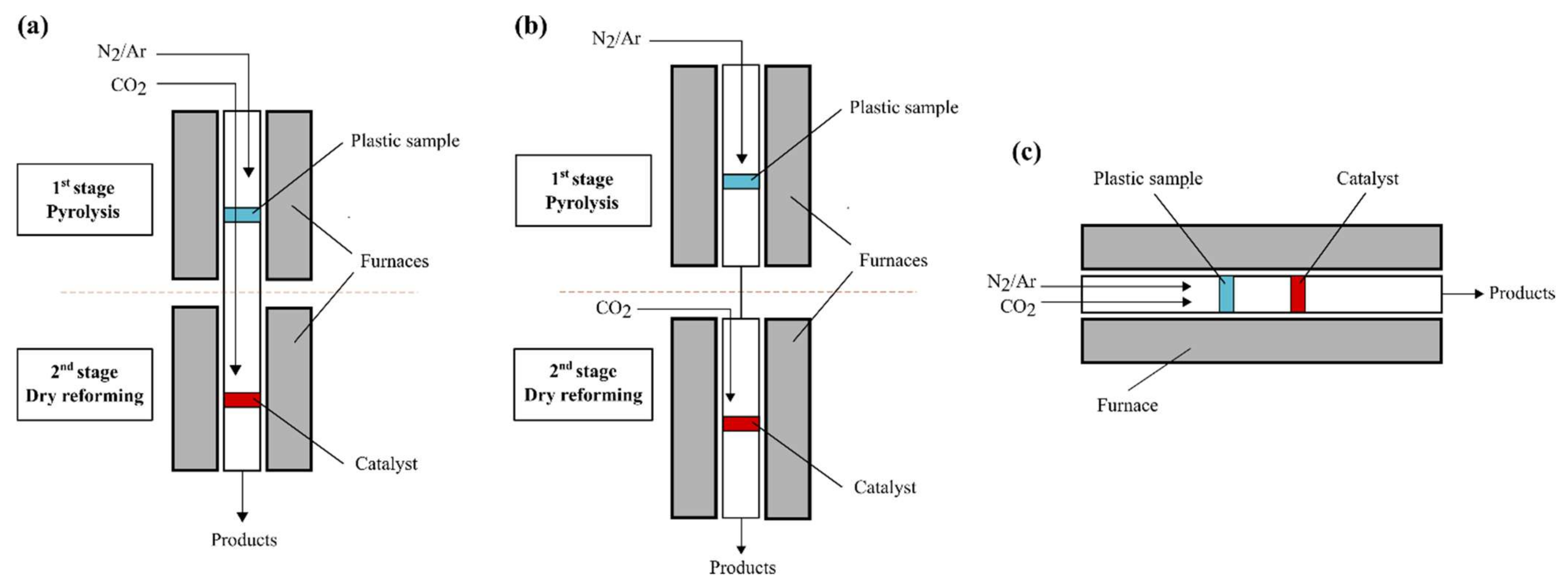
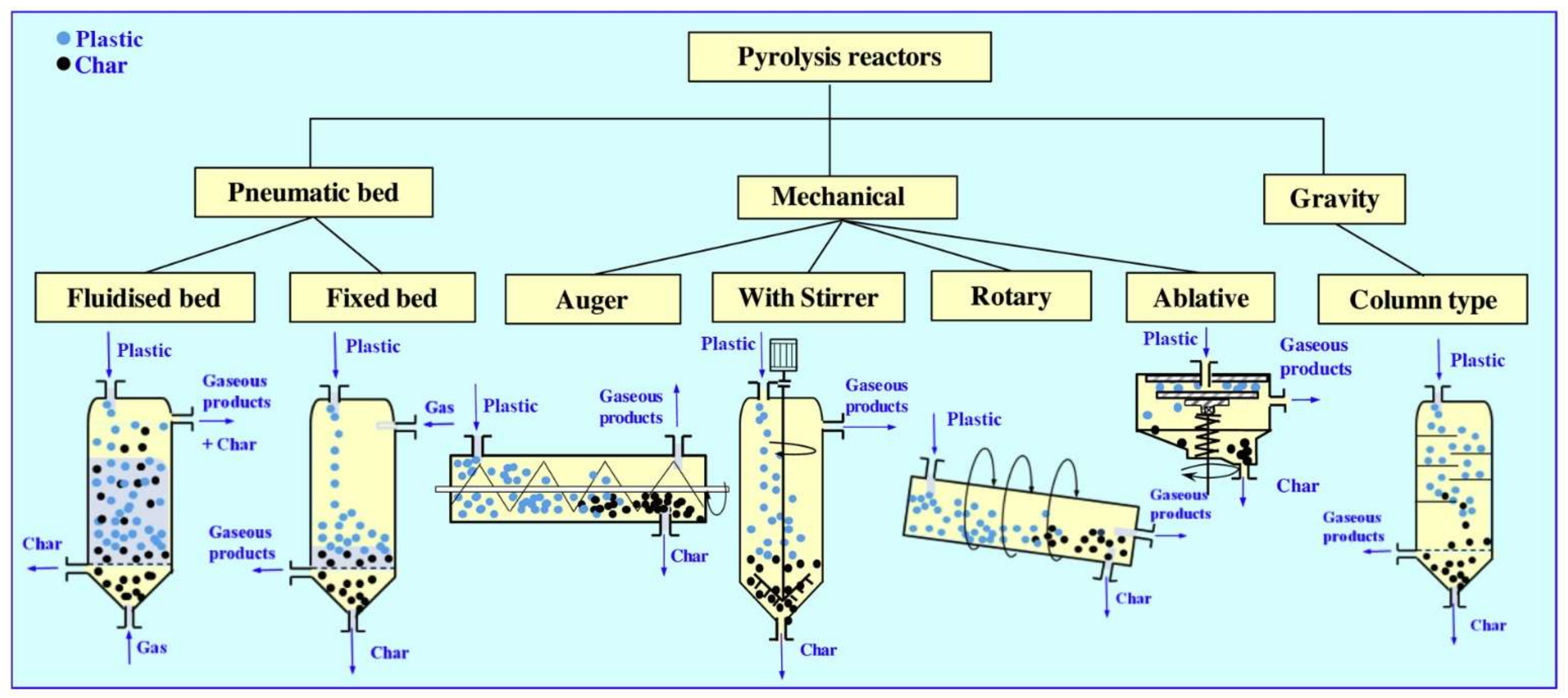
| Pyrolysis Type | Process Duration | Temperature [K] | Heating Rate [K/min] |
|---|---|---|---|
| Conventional/slow | 10 min–10 h | 500–900 | 5–10 |
| Fast | 10–20 min | 700–900 | 50–100 |
| Flash/ultra-fast | <10 min | 1000–1300 | >100 |
| Plastic Feedstock | Pyrolysis Type | Temperature [K] | Heating Rate | Catalyst | Cat.:Plastic Mass Ratio | Product Distribution | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| [wt %] | |||||||||
| [K/min] | Gas | Oil | Char | ||||||
| Mixed plastic waste 1 | Slow | 773 | 10 | - | - | 14.2 | 75.8 | 10.0 | [36] |
| Mixed plastic waste 1 | Slow | 773 | 20 | - | - | 10.5 | 82.0 | 8.5 | [36] |
| Mixed plastic waste 1 | Flash | 773 | Isothermal | - | - | 91.0 | 7.0 | 2.0 | [36] |
| ABS | Fast | 773 | Isothermal | - | - | 5.2 | 91.0 | 3.8 | [34] |
| PVC | Fast | 773 | Isothermal | - | - | 44.3 | 35.9 | 19.8 | [34] |
| PE | Flash | 1273 | Isothermal | - | - | >99 | n.d. | n.d. | [35] |
| PP | Flash | 873 | Isothermal | - | - | 61 | 39 | 0 | [43] |
| PE | Flash | 873 | Isothermal | - | - | 67 | 33 | 0 | [43] |
| PS | Flash | 873 | Isothermal | - | - | 65 | 35 | 0 | [43] |
| PET | Flash | 873 | Isothermal | - | - | 48 | 40 | 12 | [43] |
| PS | Slow | 1073 | 5 | - | - | 2.28 | 95.77 | 1.95 | [44] |
| PS | Slow | 1073 | 10 | - | - | 3.40 | 95.79 | 1.81 | [44] |
| PS | Slow | 1073 | 15 | - | - | 5.65 | 92.75 | 1.60 | [44] |
| PS | Slow | 1073 | 20 | - | - | 6.31 | 92.65 | 1.04 | [44] |
| PE | Slow | 1073 | 5 | - | - | 18.17 | 81.65 | 0.18 | [44] |
| PE | Slow | 1073 | 10 | - | - | 18.57 | 81.33 | 0.10 | [44] |
| PE | Slow | 1073 | 15 | - | - | 27.36 | 72.63 | 0.01 | [44] |
| PE | Slow | 1073 | 20 | - | - | 38.76 | 61.24 | 0.00 | [44] |
| ABS | Slow | 1073 | 5 | - | - | 2.89 | 95.99 | 1.12 | [44] |
| ABS | Slow | 1073 | 10 | - | - | 5.91 | 92.66 | 1.43 | [44] |
| ABS | Slow | 1073 | 15 | - | - | 8.06 | 90.47 | 1.47 | [44] |
| ABS | Slow | 1073 | 20 | - | - | 8.86 | 89.57 | 1.57 | [44] |
| PET | Slow | 1073 | 5 | - | - | 51.61 | 39.02 | 9.37 | [44] |
| PET | Slow | 1073 | 10 | - | - | 56.32 | 35.40 | 8.28 | [44] |
| PET | Slow | 1073 | 15 | - | - | 64.54 | 29.71 | 5.75 | [44] |
| PET | Slow | 1073 | 20 | - | - | 65.21 | 29.16 | 5.63 | [44] |
| PP | Slow | 1073 | 5 | - | - | 16.55 | 83.34 | 0.11 | [44] |
| PP | Slow | 1073 | 10 | - | - | 17.20 | 82.67 | 0.13 | [44] |
| PP | Slow | 1073 | 15 | - | - | 17.88 | 82.02 | 0.10 | [44] |
| PP | Slow | 1073 | 20 | - | - | 31.84 | 68.06 | 0.10 | [44] |
| PE | Slow | 773 | 10 | - | - | n.d. | 95% | n.d. | [42] |
| PE | Slow | 773; TC = 673 | 10 | ZSM-5 | n.d. | 5 | 84 | 11 | [42] |
| PE | Slow | 773; TC = 723 | 10 | ZSM-5 | n.d. | 10 | 82 | 8 | [42] |
| PE | Slow | 773; TC = 773 | 10 | ZSM-5 | n.d. | 18 | 76 | 6 | [42] |
| PE | Slow | 773; TC = 823 | 10 | ZSM-5 | n.d. | 22 | 73 | 5 | [42] |
| PE | Slow | 773; TC = 873 | 10 | ZSM-5 | n.d. | 28 | 67 | 5 | [42] |
| PE | Slow | 773; TC = 673 | 10 | Y -zeolite | n.d. | 5 | 84 | 11 | [42] |
| PE | Slow | 773; TC = 723 | 10 | Y -zeolite | n.d. | 10 | 79 | 11 | [42] |
| PE | Slow | 773; TC = 773 | 10 | Y -zeolite | n.d. | 11 | 77 | 12 | [42] |
| PE | Slow | 773; TC = 823 | 10 | Y -zeolite | n.d. | 11 | 76 | 13 | [42] |
| PE | Slow | 773; TC = 873 | 10 | Y -zeolite | n.d. | 19 | 70 | 11 | [42] |
| PET | Fast | 723 | Isothermal | - | - | 20 | 59 | 21 | [38] |
| PET | Fast | 873 | Isothermal | - | - | 19 | 67 | 14 | [38] |
| PET | Fast | 723 | Isothermal | ZSM-5 | 2:1 | 55 | 21 | 24 | [38] |
| PET | Fast | 723 | Isothermal | ZSM-5 | 4:1 | 63 | 12 | 25 | [38] |
| PET | Fast | 723 | Isothermal | ZSM-5 | 6:1 | 69 | 9 | 22 | [38] |
| PET | Fast | 873 | Isothermal | ZSM-5 | 2:1 | 51 | 29 | 20 | [38] |
| PET | Fast | 873 | Isothermal | ZSM-5 | 4:1 | 60 | 26 | 14 | [38] |
| PET | Fast | 873 | Isothermal | ZSM-5 | 6:1 | 66 | 14 | 10 | [38] |
| PET | Fast | 723 | Isothermal | NiCl2 | 1:2 | 27 | 65 | 8 | [38] |
| PET | Fast | 723 | Isothermal | NiCl2 | 1:1 | 18 | 77 | 5 | [38] |
| PET | Fast | 873 | Isothermal | NiCl2 | 1:2 | 42 | 55 | 3 | [38] |
| PET | Fast | 873 | Isothermal | NiCl2 | 1:1 | 29 | 69 | 2 | [38] |
| HDPE | Slow | 973 | 25 | - | - | 18.0 | 79.7 | 0.0 | [45] |
| LDPE | Slow | 973 | 25 | - | - | 15.1 | 84.3 | 0.0 | [45] |
| PP | Slow | 973 | 25 | - | - | 15.3 | 84.4 | 0.2 | [45] |
| PS | Slow | 973 | 25 | - | - | 3.4 | 83.8 | 3.5 | [45] |
| PVC | Slow | 973 | 25 | - | - | 2.5 | 84.62 | 13.8 | [45] |
| PET | Slow | 973 | 25 | - | - | 38.7 | 41.3 | 15.6 | [45] |
| LDPE | Slow | 773 | 6 | - | - | 9.1 | 90.9 | 0.0 | [28] |
| LDPE | Fast | 773 | 60 | - | - | 13.7 | 86.2 | 0.0 | [28] |
| LDPE | Slow | 673 | 1 | - | - | 16.88 | 82.68 | 0.44 | [27] |
| HDPE | Slow | 673 | 1 | - | - | 16.78 | 82.66 | 0.56 | [27] |
| PP | Slow | 673 | 1 | - | - | 16.53 | 83.16 | 0.31 | [27] |
| LDPE, HDPE, PP mixture 2 | Slow | 673 | 1 | - | - | 17.04 | 82.25 | 0.71 | [27] |
| Packaging plastic waste 3 | Slow | 673 | 1 | - | - | 21.36 | 74.98 | 3.66 | [27] |
| HDPE | Slow | 773 | 5 | - | - | 7 | 93 | 0 | [46] |
| PP | Slow | 773 | 5 | - | - | 5 | 95 | 0 | [46] |
| PS | Slow | 773 | 5 | - | - | 2 | 71 | 27 | [46] |
| PET | Slow | 773 | 5 | - | - | 32 | 15 | 53 | [46] |
| HDPE | Flash | 923 | Isothermal | - | - | 21.1 | 78.9 | 0.0 | [47] |
| HDPE | Flash | 1003 | Isothermal | - | - | 79.3 | 20.7 | 0.0 | [47] |
| HDPE | Flash | 1123 | Isothermal | - | - | 83.8 | 16.2 | 0.0 | [47] |
| Feedstock | Reactor Configuration | Operating Conditions | Catalyst | Cat.:Plastic Mass Ratio | Product Yield | H2/CO Molar Ratio | Ref. |
|---|---|---|---|---|---|---|---|
| PE | Two-stage fixed bed reactor system | TPyr = 720 K | Pd/Al2O3 | 11.2 | YH2 = 35.4% | n.d. | [9] |
| TDR = 910 K | YCO = 24.5% 1 | ||||||
| LDPE | Two-stage fixed bed reactor system | TPyr = 773 K | Ni–Co–Al | 0.5 | Ysyngas = 154.7 mmol g−1plastic | 0.6 | [10] |
| HDPE | Ysyngas = 149.4 mmol g−1plastic | 0.5 | |||||
| PP | Ysyngas = 136.0 mmol g−1plastic | 0.5 | |||||
| PS | TDR = 1073 K | Ysyngas = 126.3 mmol g−1plastic | 0.3 | ||||
| PET | Ysyngas = 63.0 mmol g−1plastic | 0.2 | |||||
| SWP 2 | Ysyngas = 148.6 mmol g−1plastic | 0.5 | |||||
| SWP 2 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni–Co–Al2O3 | 0.5 | Ysyngas = 116.2 mmol g−1plastic | 0.55 | [13] |
| TDR = 873 K | |||||||
| TPyr = 773 K | Ysyngas = 144.0 mmol g−1plastic | 0.48 | |||||
| TDR = 973 K | |||||||
| TPyr = 773 K | Ysyngas = 148.6 mmol g−1plastic | 0.49 | |||||
| TDR = 1073 K | |||||||
| TPyr = 773 K | Ysyngas = 125.8 mmol g−1plastic | 0.66 | |||||
| TDR = 1173 K | |||||||
| SWP 2 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni–Co–Al2O3 | 0.25 | Ysyngas = 141.3 mmol g−1plastic | 0.48 | [13] |
| 0.5 | Ysyngas = 148.6 mmol g−1plastic | 0.49 | |||||
| TDR = 1073 K | 1 | Ysyngas = 143.9 mmol g−1plastic | 0.49 | ||||
| 1.5 | Ysyngas = 139.9 mmol g−1plastic | 0.51 | |||||
| SWP 2 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni–Co/Al2O3 | 0.5 | Ysyngas = 96 mmol g−1plastic | 0.72 | [15] |
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:0 | |||||||
| TPyr = 773 K | Ysyngas = 107 mmol g−1plastic | 0.88 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:0.5 | |||||||
| TPyr = 773 K | Ysyngas = 136 mmol g−1plastic | 0.93 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:1 | |||||||
| TPyr = 773 K | Ysyngas = 159 mmol g−1plastic | 0.85 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:1.5 | |||||||
| TPyr = 773 K | Ysyngas = 156 mmol g−1plastic | 0.83 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:2 | |||||||
| SWP 2 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni–Mg/Al2O3 | 0.5 | Ysyngas = 108 mmol g−1plastic | 0.69 | [15] |
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:0 | |||||||
| TPyr = 773 K | Ysyngas = 113 mmol g−1plastic | 0.60 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:0.5 | |||||||
| TPyr = 773 K | Ysyngas = 147mmol g−1plastic | 0.75 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:1 | |||||||
| TPyr = 773 K | Ysyngas = 144 mmol g−1plastic | 1.09 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:2 | |||||||
| TPyr = 773 K | Ysyngas = 132 mmol g−1plastic | 1.41 | |||||
| TDR = 1073 K | |||||||
| Steam addition: | |||||||
| CO2:steam = 4:3 | |||||||
| HDPE | Two-stage fixed bed reactor system | TPyr = 773 K | Ni–Mg/Al2O3 | 0.5 | Ysyngas = 132 mmol g−1plastic | 0.5 | [66] |
| TDR = 1073 K | |||||||
| HDPE | Two-stage fixed bed reactor system | TPyr = 773 K | Ni-Al | 0.5 | Ysyngas = 138.81 mmol g−1plastic | 0.47 | [65] |
| Ni–Cu–Al | Ysyngas = 130.56 mmol g−1plastic | 0.51 | |||||
| TDR = 1073 K | Ni–Mg–Al | Ysyngas = 146.96 mmol g−1plastic | 0.49 | ||||
| Ni–Co–Al | Ysyngas = 149.42 mmol g−1plastic | 0.47 | |||||
| PP | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 170 mmol g−1plastic | 0.41 | [12] |
| TDR = 1073 K | Ru–Ni/Al2O3 | Ysyngas = 160 mmol g−1plastic | 0.35 | ||||
| Mixture of real waste plastics 3 | One-stage horizontal tubular reactor | T = 823 K | Ni/ZSM-5 | 0.5 | Ysyngas = 60.2 mmol g−1plastic | 1.80 | [17] |
| Ca/Ni/ZSM-5 | Ysyngas = 58.0 mmol g−1plastic | 2.03 | |||||
| Ce/Ni/ZSM-5 | Ysyngas = 61.1 mmol g−1plastic | 1.83 | |||||
| La/Ni/ZSM-5 | Ysyngas = 53.7 mmol g−1plastic | 2.23 | |||||
| Mg/Ni/ZSM-5 | Ysyngas = 51.9 mmol g−1plastic | 2.18 | |||||
| Mn/Ni/ZSM-5 | Ysyngas = 57.4 mmol g−1plastic | 2.12 | |||||
| Mixture of real waste plastics 3 | One-stage horizontal tubular reactor | T = 1123 K | Ni/ZSM-5 | 0.5 | Ysyngas = 112.6 mmol g−1plastic | 1.81 | [17] |
| Ca/Ni/ZSM-5 | Ysyngas = 112.2 mmol g−1plastic | 2.03 | |||||
| Ce/Ni/ZSM-5 | Ysyngas = 132.0 mmol g−1plastic | 1.89 | |||||
| La/Ni/ZSM-5 | Ysyngas = 119.2 mmol g−1plastic | 2.23 | |||||
| Mg/Ni/ZSM-5 | Ysyngas = 103.8 mmol g−1plastic | 2.19 | |||||
| Mn/Ni/ZSM-5 | Ysyngas = 100.3 mmol g−1plastic | 2.09 | |||||
| Mixture of real waste plastics 3 | One-stage horizontal tubular reactor | T = 823 K; | Ni/ZSM-5 | 0.5 | Ysyngas = 12.7 mmol g−1plastic | 1.70 | [72] |
| Ca/Ni/ZSM-5 | Ysyngas = 11.7 mmol g−1plastic | 1.93 | |||||
| Ce/Ni/ZSM-5 | Ysyngas = 15.1 mmol g−1plastic | 2.08 | |||||
| In situ CO2 generation using dolomite | La/Ni/ZSM-5 | Ysyngas = 13.8 mmol g−1plastic | 2.07 | ||||
| Mg/Ni/ZSM-5 | Ysyngas = 10.5 mmol g−1plastic | 2.00 | |||||
| Mn/Ni/ZSM-5 | Ysyngas = 9.8 mmol g−1plastic | 1.80 | |||||
| Mixture of real waste plastics 3 | One-stage horizontal tubular reactor | T = 1123 K; | Ni/ZSM-5 | 0.5 | Ysyngas = 95.2 mmol g−1plastic | 1.83 | [72] |
| Ca/Ni/ZSM-5 | Ysyngas = 96.3 mmol g−1plastic | 2.01 | |||||
| Ce/Ni/ZSM-5 | Ysyngas = 115.6 mmol g−1plastic | 1.78 | |||||
| In situ CO2 generation using dolomite | La/Ni/ZSM-5 | Ysyngas = 111.8 mmol g−1plastic | 2.11 | ||||
| Mg/Ni/ZSM-5 | Ysyngas = 90.8 mmol g−1plastic | 1.99 | |||||
| Mn/Ni/ZSM-5 | Ysyngas = 106.3 mmol g−1plastic | 1.98 | |||||
| Mixed plastics from household packaging 4 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 146.32 mmol g−1plastic | 0.46 | [14] |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 156.45 mmol g−1plastic | 0.48 | ||||
| Mixed plastics from building construction 5 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 143.85 mmol g−1plastic | 0.50 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 141.47 mmol g−1plastic | 0.47 | ||||
| Mixed plastics from agriculture 6 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 153.67 mmol g−1plastic | 0.48 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 121.26 mmol g−1plastic | 0.54 | ||||
| Mixed plastics from freezer and refrigerator equipment | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 72.51 mmol g−1plastic | 0.46 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 72.12 mmol g−1plastic | 0.42 | ||||
| Mixed plastic from cathode ray tube | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 79.84 mmol g−1plastic | 0.50 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 92.63 mmol g−1plastic | 0.46 | ||||
| Mixed plastics from electrical and electronic equipment | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 85.49 mmol g−1plastic | 0.34 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 87.26 mmol g−1plastic | 0.37 | ||||
| Refuse-derived fuel | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 41.24 mmol g−1plastic | 0.32 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 41.49 mmol g−1plastic | 0.34 | ||||
| SWP 2 | Two-stage fixed bed reactor system | TPyr = 773 K | Ni/Al2O3 | 0.5 | Ysyngas = 140.53 mmol g−1plastic | 0.48 | |
| TDR = 1073 K | Ni–Co/Al2O3 | Ysyngas = 148.56 mmol g−1plastic | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawelczyk, E.; Wysocka, I.; Gębicki, J. Pyrolysis Combined with the Dry Reforming of Waste Plastics as a Potential Method for Resource Recovery—A Review of Process Parameters and Catalysts. Catalysts 2022, 12, 362. https://doi.org/10.3390/catal12040362
Pawelczyk E, Wysocka I, Gębicki J. Pyrolysis Combined with the Dry Reforming of Waste Plastics as a Potential Method for Resource Recovery—A Review of Process Parameters and Catalysts. Catalysts. 2022; 12(4):362. https://doi.org/10.3390/catal12040362
Chicago/Turabian StylePawelczyk, Ewelina, Izabela Wysocka, and Jacek Gębicki. 2022. "Pyrolysis Combined with the Dry Reforming of Waste Plastics as a Potential Method for Resource Recovery—A Review of Process Parameters and Catalysts" Catalysts 12, no. 4: 362. https://doi.org/10.3390/catal12040362






