Sustainable Catalytic Synthesis of 2,5-Diformylfuran from Various Carbohydrates
Abstract
:1. Introduction
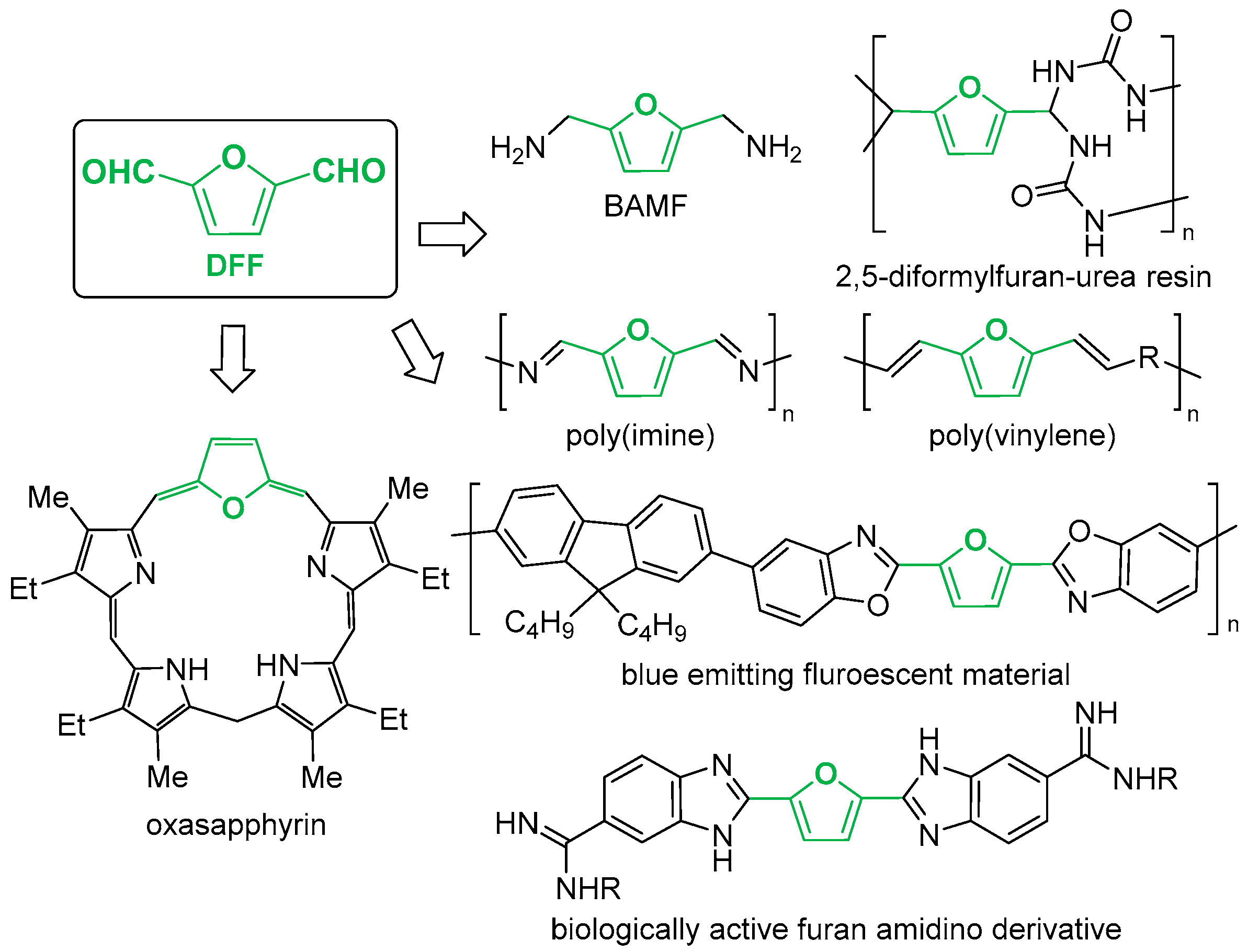
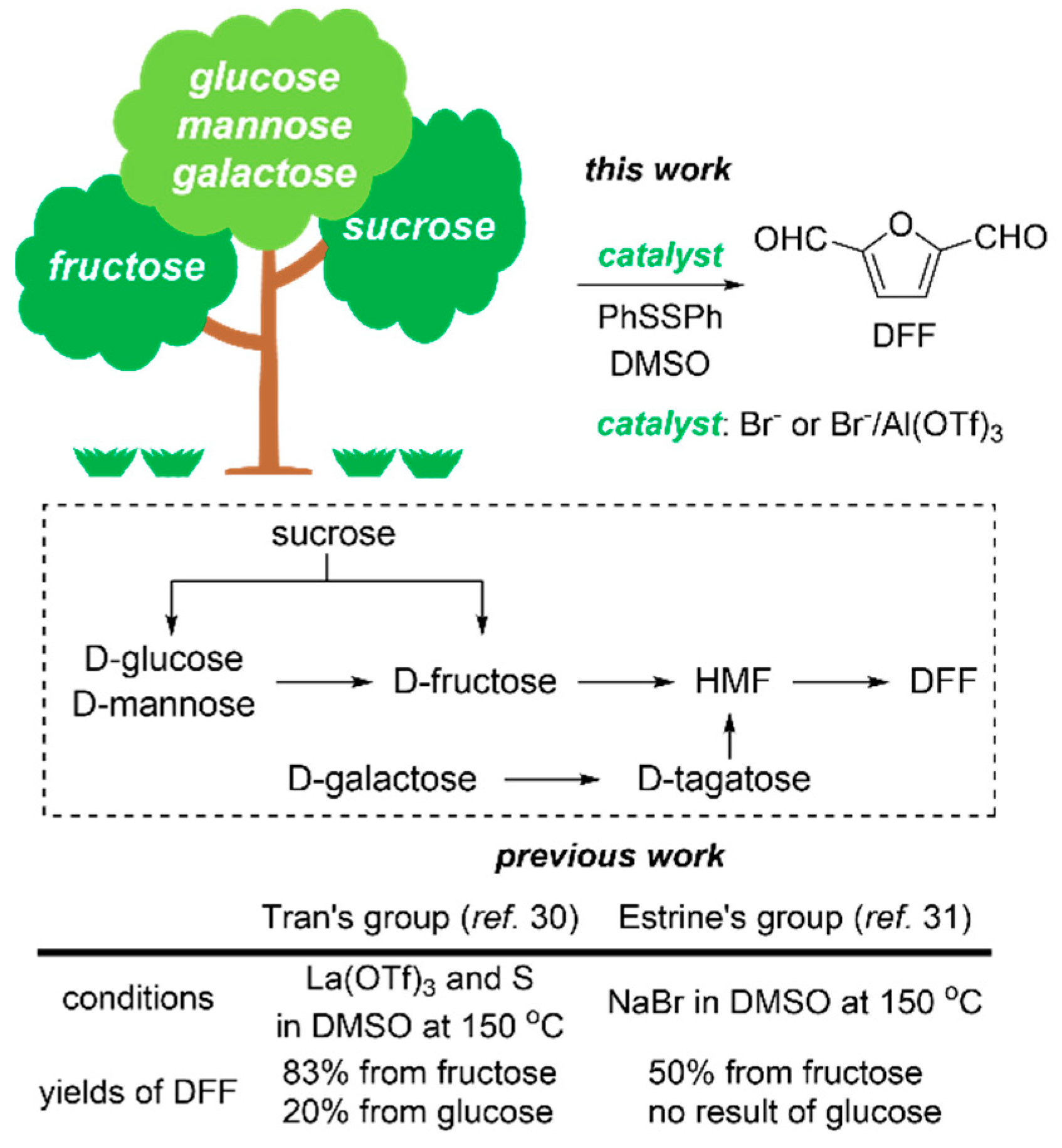
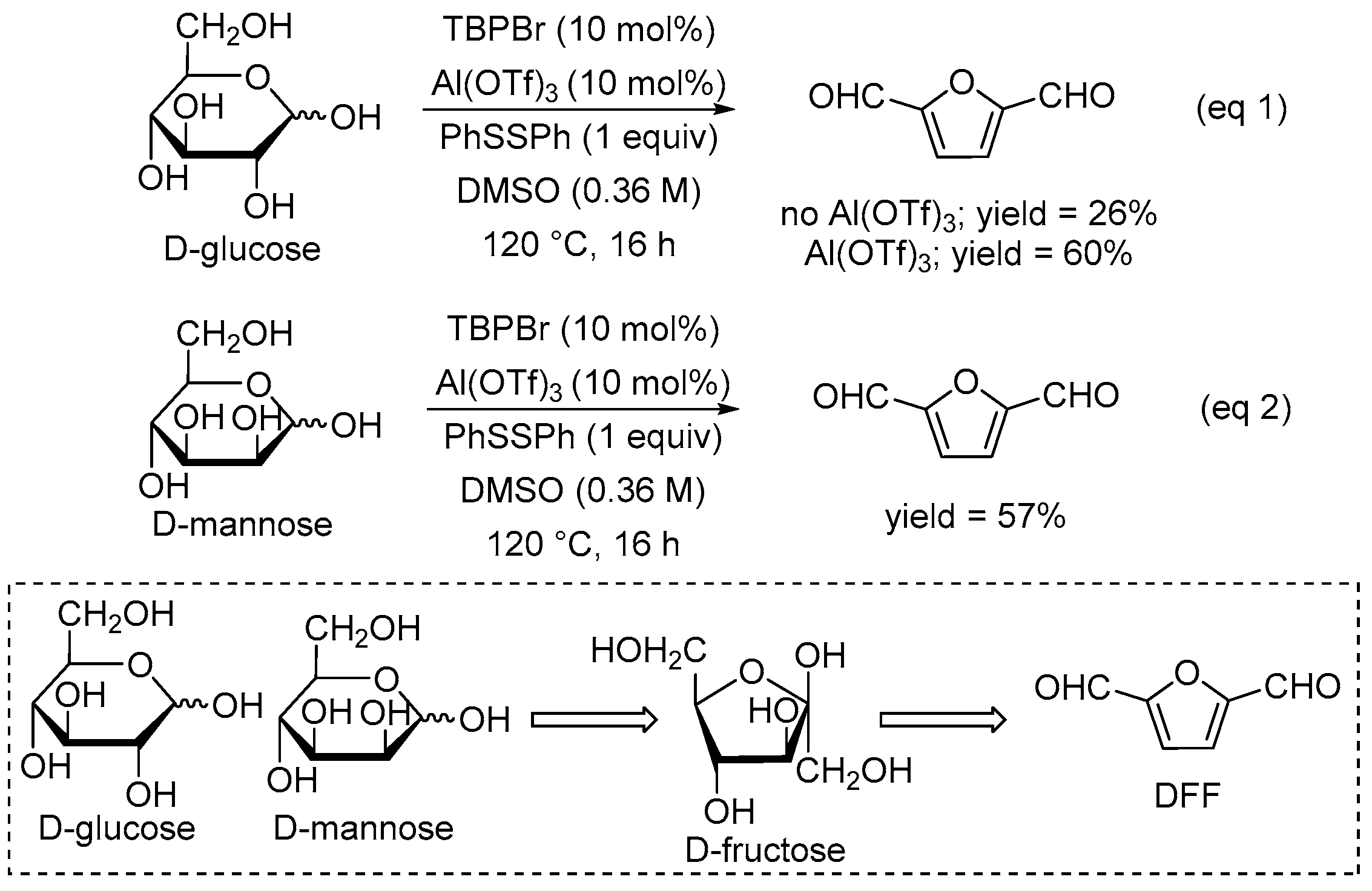
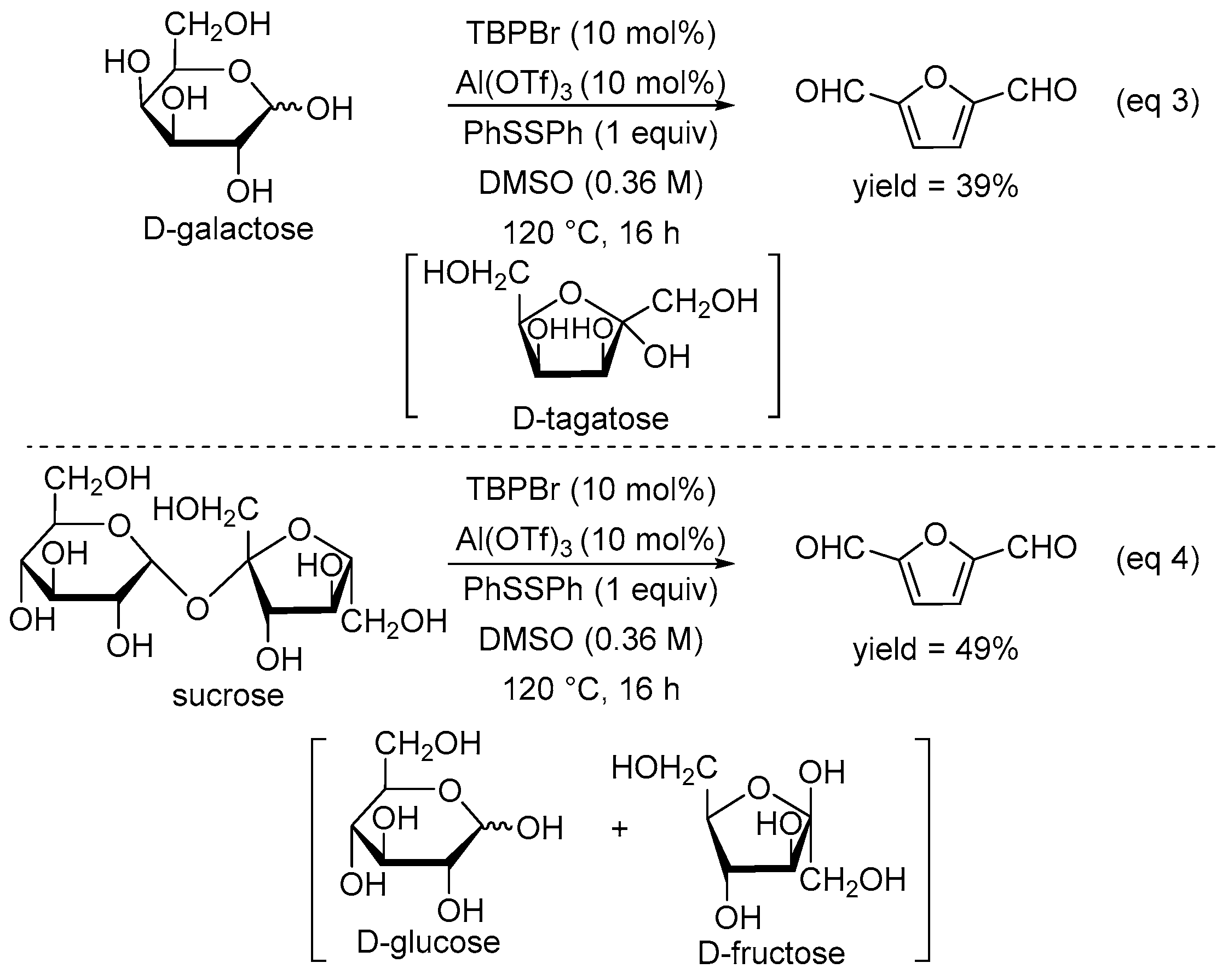
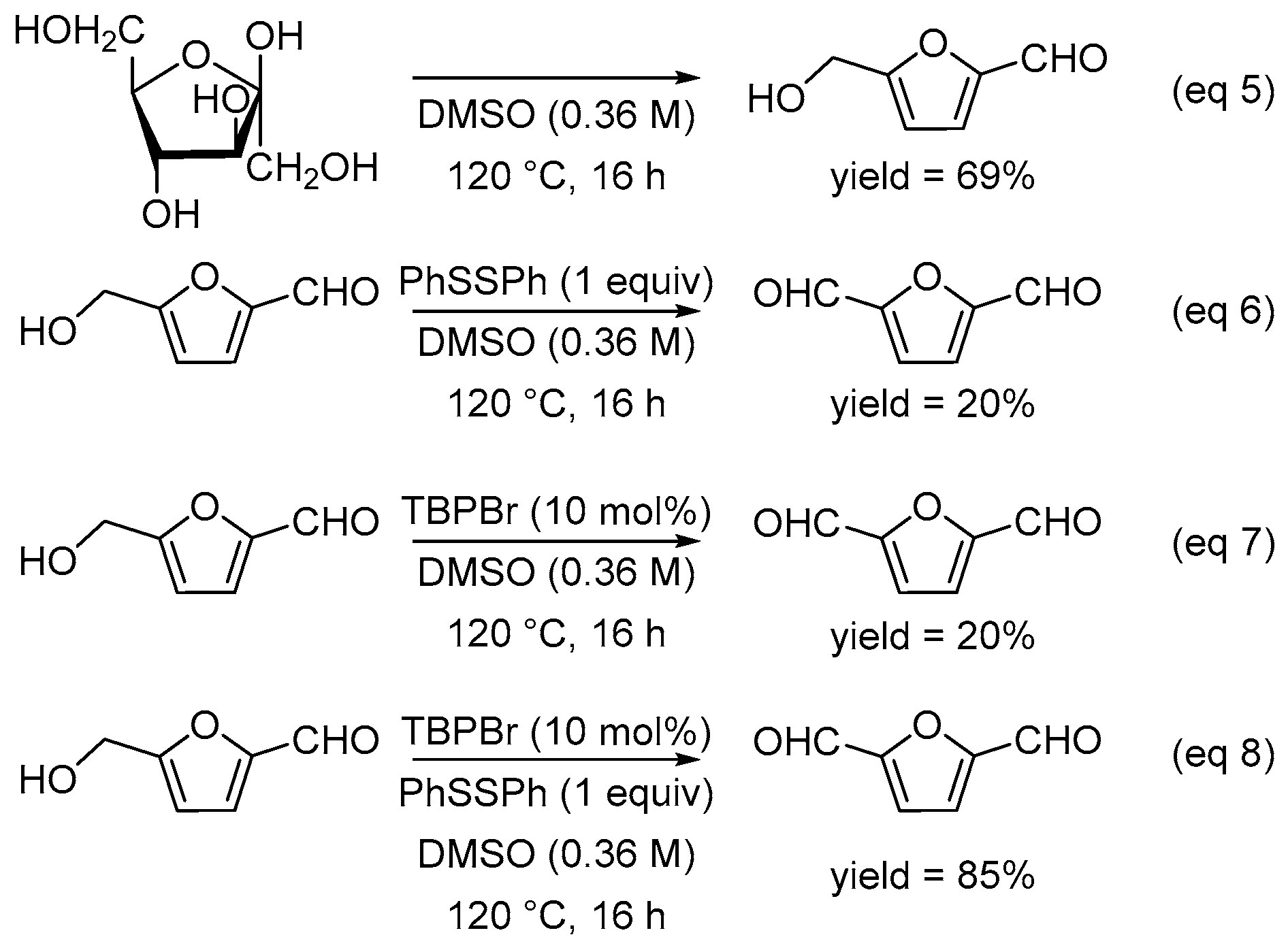
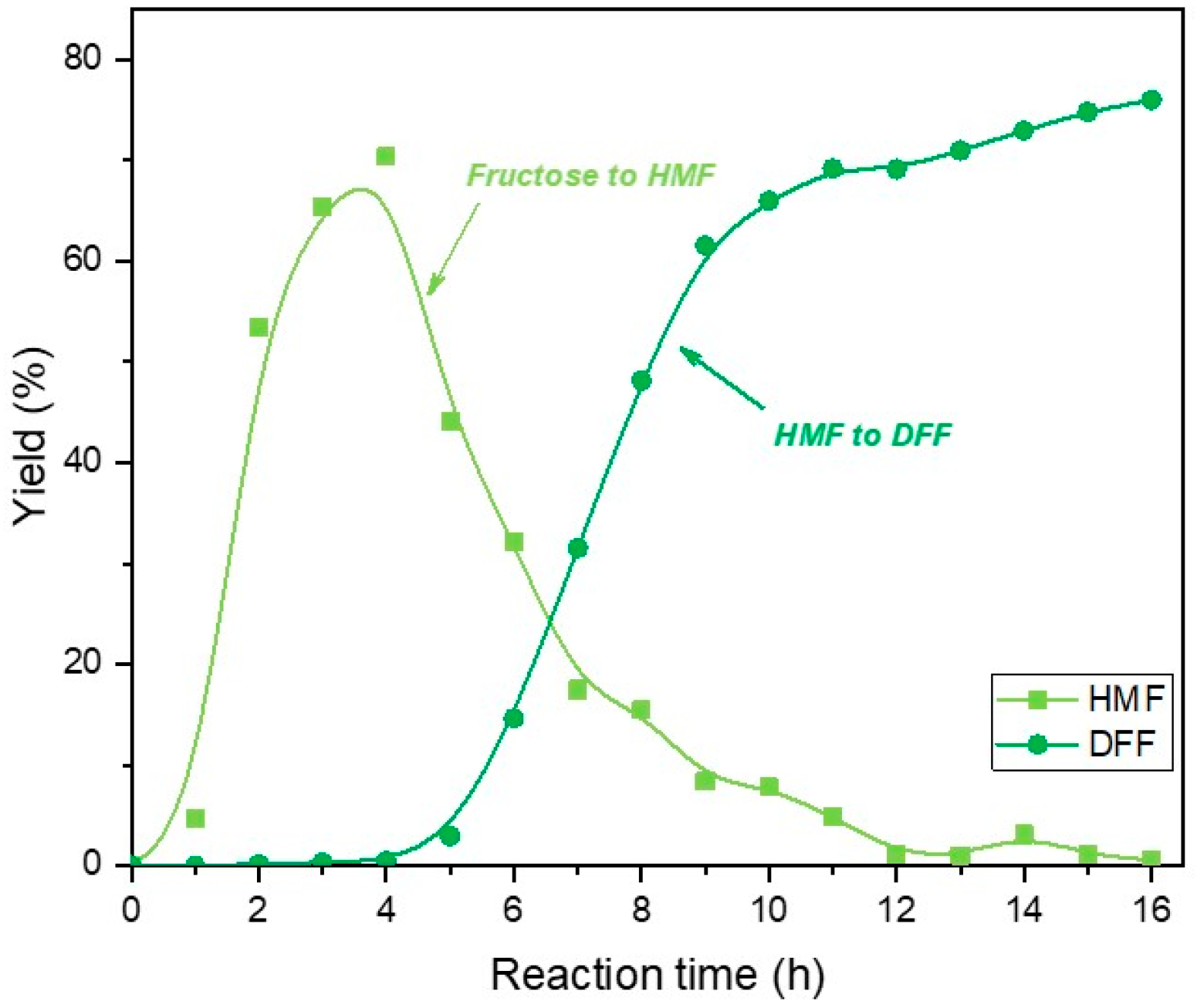
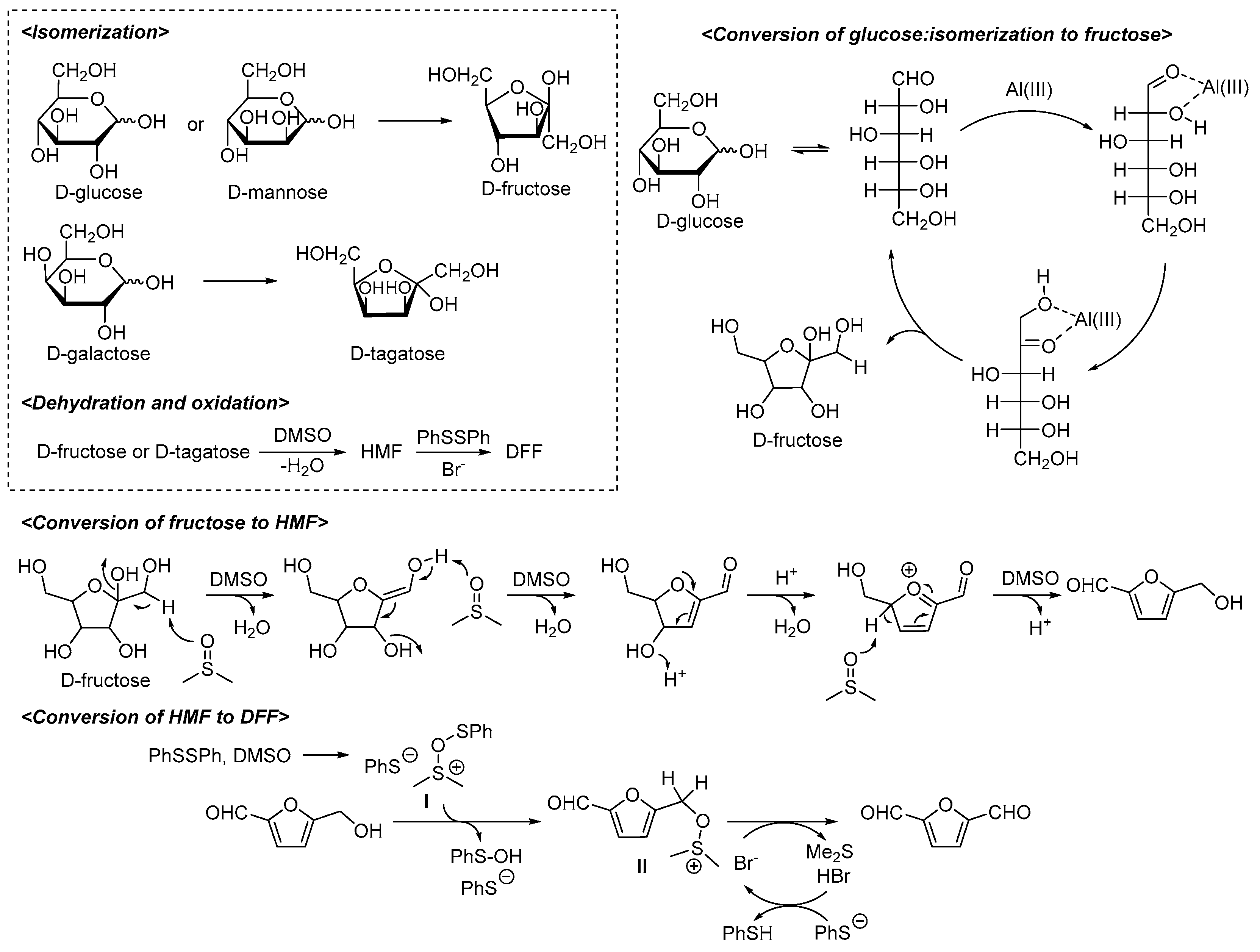
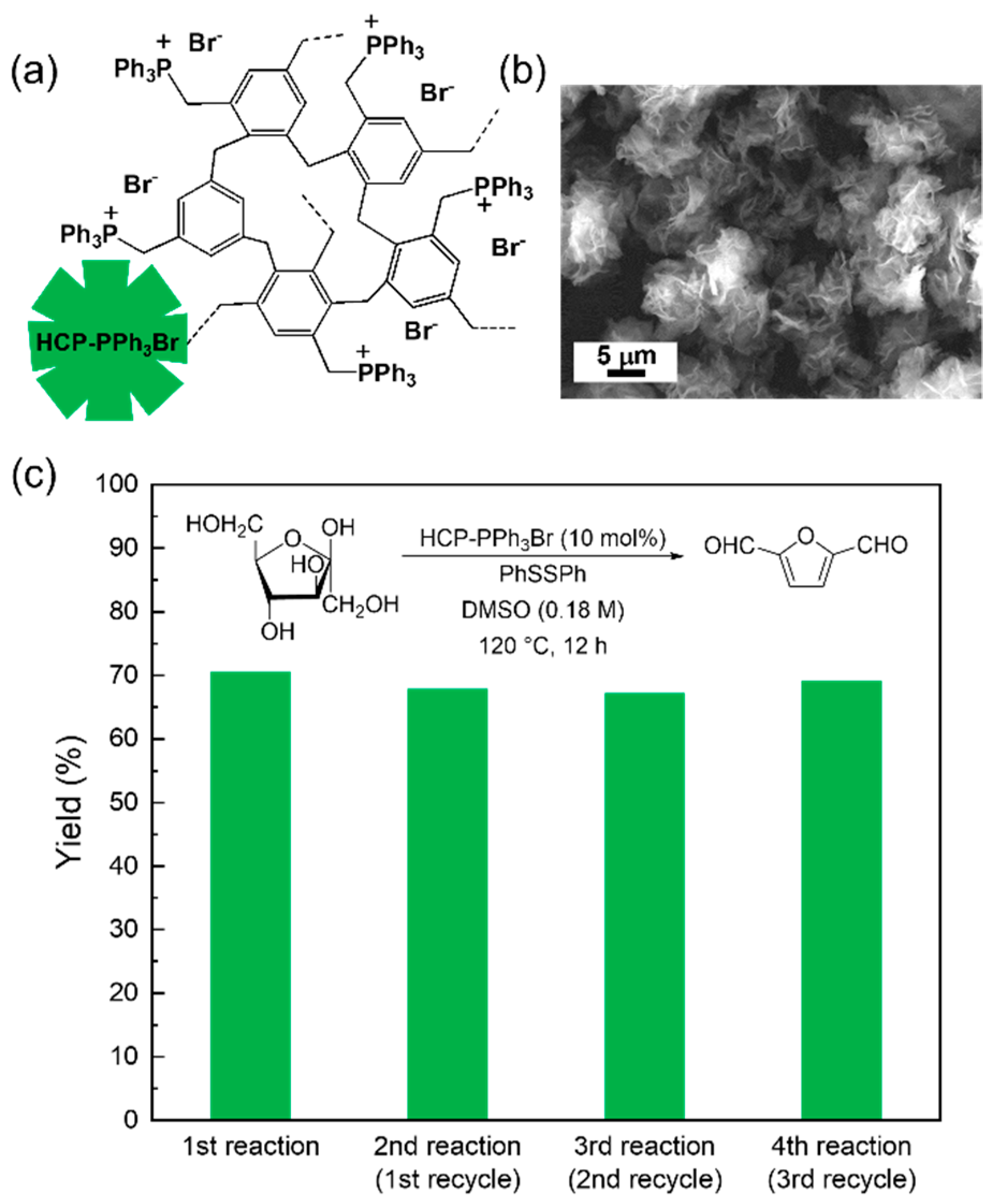
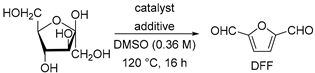
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Zhang, Z. One-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural. Catal. Sci. Technol. 2016, 6, 3694–3712. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Huber, G.W. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2009, 2, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Upare, P.P.; Hwang, Y.K.; Lee, J.-M.; Hwang, D.W.; Chang, J.-S. Chemical Conversions of Biomass-Derived Platform Chemicals over Copper-Silica Nanocomposite Catalysts. ChemSusChem 2015, 8, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. One-Pot Conversion of Carbohydrates into Furan Derivatives via Furfural and 5-Hydroxylmethylfurfural as Intermediates. ChemSusChem 2016, 9, 2015–2036. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Benahmed-Gasmi, A.S.; Frere, P.; Jubault, M.; Gorgues, A.; Cousseau, J.; Garrigues, B. 2,5-bis(1,4-dithiafulven-6-yl) substituted furans, thiophenes and N-methyl pyrroles as precursors for organic metals. Synth. Met. 1993, 56, 1751–1755. [Google Scholar] [CrossRef]
- Hopkins, K.T.; Wilson, D.; Bender, B.C.; McCurdy, D.R.; Hall, J.E.; Tidwell, R.R.; Kumar, A.; Bajic, M.; Boykin, D.W. Extended Aromatic Furan Amidino Derivatives as Anti-Pneumocystis carinii Agents. J. Med. Chem. 1998, 41, 3872–3878. [Google Scholar] [CrossRef]
- Richter, D.T.; Lash, T.D. Oxidation with dilute aqueous ferric chloride solutions greatly improves yields in the ‘4+1’ synthesis of sapphyrins. Tetrahedron Lett. 1999, 40, 6735–6738. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Green, D.; Williams, L.D. Renewable resources based polymers: Synthesis and characterization of 2,5-diformylfuran–urea resin. Eur. Polym. J. 2009, 45, 595–598. [Google Scholar] [CrossRef]
- Gandini, A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: A review of recent progress. Polym. Chem. 2010, 1, 245–251. [Google Scholar] [CrossRef]
- Ma, J.; Du, Z.; Xu, J.; Chu, Q.; Pang, Y. Efficient Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran, and Synthesis of a Fluorescent Material. ChemSusChem 2011, 4, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, M.; Du, Z.; Chen, C.; Gao, J.; Xu, J. Synthesis and properties of furan-based imine-linked porous organic frameworks. Polym. Chem. 2012, 3, 2346–2349. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative monomers based on ligno-cellulose and their use for polymer production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Haas, T.; Tacke, T.; Pfeffer, J.C.; Klasovsky, F.; Rimbach, M.; Volland, M.; Ortelt, M. Process for Producing 2,5-Diformylfuran and Derivatives Thereof. World Patent WO2012004069A1, 12 January 2012. [Google Scholar]
- Xu, J.; Ma, J.; Wang, D.; Du, Z.; Gao, J.; Wang, F. Biomass-Based phenolic Resin and Preparation Method Thereof. Patent CN 102827336 A, 19 December 2012. [Google Scholar]
- Michoud, C.; Doisneau, D. Aqueous Adhesive Composition with a Base of Biosourced Aldehyde and Polyphenol. World Patent WO 2015007641A1, 22 January 2015. [Google Scholar]
- Liu, D.; Chen, E.Y.-X. Organocatalysis in biorefining for biomass conversion and upgrading. Green Chem. 2014, 16, 964–981. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef]
- Partenheimer, W.; Grushin, V. Synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by catalytic air-oxidation of 5-hydroxymethylfurfrual; Unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal/bromide catalysts. Adv. Synth. Catal. 2001, 343, 102–111. [Google Scholar] [CrossRef]
- Carlini, C.; Patrono, P.; Galletti, A.M.R.; Sbrana, G.; Zima, V. Selective oxidation of 5-hydroxymethyl-2-furaldehyde to furan-2,5-dicarboxaldehyde by catalytic systems based on vanadyl phosphate. Appl. Catal. A Gen. 2005, 289, 197–204. [Google Scholar] [CrossRef]
- Navarro, O.C.; Canós, A.C.; Chornet, S.I. Chemicals from Biomass: Aerobic Oxidation of 5-Hydroxymethyl-2-Furaldehyde into Diformylfurane Catalyzed by Immobilized Vanadyl-Pyridine Complexes on Polymeric and Organofunctionalized Mesoporous Supports. Top. Catal. 2009, 52, 304–314. [Google Scholar] [CrossRef]
- Nie, J.; Xie, J.; Liu, H. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on supported Ru catalysts. J. Catal. 2013, 301, 83–91. [Google Scholar] [CrossRef]
- Artz, J.; Mallmann, S.; Palkovits, R. Selective aerobic oxidation of HMF to 2,5-diformylfuran on covalent triazine frame-works-supported Ru catalysts. ChemSusChem 2015, 8, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.A.; Young, R.J.; Grushin, V.V. One-Pot, Two-Step, Practical Catalytic Synthesis of 2,5-Diformylfuran from Fructose. Org. Lett. 2003, 5, 2003–2005. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Tang, J.; Liu, M.; Cheng, R.; Hu, C. One-pot, One-step Synthesis of 2,5-Diformylfuran from Carbohydrates over Mo-Containing Keggin Heteropolyacids. ChemSusChem 2014, 7, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Ghezali, W.; Vigier, K.D.O.; Kessas, R.; Jérôme, F. A choline chloride/DMSO solvent for the direct synthesis of diformylfuran from carbohydrates in the presence of heteropolyacids. Green Chem. 2015, 17, 4459–4464. [Google Scholar] [CrossRef]
- Nguyen, Q.N.B.; Le, H.A.N.; Ly, P.D.; Phan, H.B.; Tran, P.H. One-step synthesis of 2,5-diformylfuran from monosaccharides by using lanthanum(iii) triflate, sulfur, and DMSO. Chem. Commun. 2020, 56, 13005–13008. [Google Scholar] [CrossRef] [PubMed]
- Laugel, C.; Estrine, B.; Le Bras, J.; Hoffmann, N.; Marinkovic, S.; Muzart, J. NaBr/DMSO-Induced Synthesis of 2,5-Diformylfuran from Fructose or 5-(Hydroxymethyl)furfural. ChemCatChem 2014, 6, 1195–1198. [Google Scholar] [CrossRef]
- Lv, G.; Wang, H.; Yang, Y.; Deng, T.; Chen, C.; Zhu, Y.; Hou, X. Direct synthesis of 2,5-diformylfuran from fructose with graphene oxide as a bifunctional and metal-free catalyst. Green Chem. 2016, 18, 2302–2307. [Google Scholar] [CrossRef]
- Takagaki, A.; Takahashi, M.; Nishimura, S.; Ebitani, K. One-Pot Synthesis of 2,5-Diformylfuran from Carbohydrate Derivatives by Sulfonated Resin and Hydrotalcite-Supported Ruthenium Catalysts. ACS Catal. 2011, 1, 1562–1565. [Google Scholar] [CrossRef]
- Rathod, P.V.; Nale, S.D.; Jadhav, V.H. Metal free acid bae catalyst in the selective synthesis of 2,5-diformylfuran from hy-droxymethylfurfural, fructose, and glucose. ACS Sustain. Chem. Eng. 2017, 5, 701–707. [Google Scholar] [CrossRef]
- Binder, J.B.; Cefali, A.V.; Blank, J.J.; Raines, R.T. Mechanistic insights on the conversion of sugars into 5-hydroxymethylfurfural. Energy Environ. Sci. 2010, 3, 765–771. [Google Scholar] [CrossRef]
- Su, K.; Liu, X.; Ding, M.; Yuan, Q.; Li, Z.; Cheng, B. Effective conversion sucrose into 5-hydroxymethylfurfural by tyrosine in [Emim]Br. J. Mol. Catal. A Chem. 2013, 379, 350–354. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Lobo, R.F.; Vlachos, D.G.; Sandler, S.I. Comparison of Homogeneous and Heterogeneous Catalysts for Glucose-to-Fructose Isomerization in Aqueous Media. ChemSusChem 2013, 6, 2369–2376. [Google Scholar] [CrossRef]
- Tang, J.; Guo, X.; Zhu, L.; Hu, C. Mechanistic Study of Glucose-to-Fructose Isomerization in Water Catalyzed by [Al(OH)2(aq)]+. ACS Catal. 2015, 5, 5097–5103. [Google Scholar] [CrossRef]
- Li, Y.-N.; Wang, J.-Q.; He, L.-N.; Yang, Z.-Z.; Liu, A.-H.; Yu, B.; Luan, C.-R. Experimental and theoretical studies on imidazolium ionic liquid-promoted conversion of fructose to 5-hydroxymethylfurfural. Green Chem. 2012, 14, 2752–2758. [Google Scholar] [CrossRef]
- Zhao, J.; Si, Z.; Shan, H.; Cai, D.; Li, S.; Li, G.; Lin, H.; Baeyens, J.; Wang, G.; Zhao, H.; et al. Highly Efficient Production of 5-Hydroxymethylfurfural from Fructose via a Bromine-Functionalized Porous Catalyst under Mild Conditions. Ind. Eng. Chem. Res. 2020, 59, 14569–14577. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Nguyen, L.A.; Retailleau, P. Strategy for Contiguous Tetramination of Cyclohexanones with o-Phenylenediamines with Elemental Sulfur and DMSO. Org. Lett. 2019, 21, 6570–6574. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Hou, J.-y.; Retailleau, P. Sulfur-promoted synthesis of 2-aroylquinazolin-4(3H)-ones by oxidative condensation of anthranilamide and acetophenones. Adv. Synth. Catal. 2019, 361, 3337–3341. [Google Scholar] [CrossRef]
- Nguyen, L.A.; Dang, T.D.; Ngo, Q.A.; Nguyen, T.B. Sulfur-Promoted Synthesis of Benzoxazoles from 2-Aminophenols and Aldehydes. Eur. J. Org. Chem. 2020, 2020, 3818–3821. [Google Scholar] [CrossRef]
- Gilbert, H.F. Effects of solvation on the nucleophilic reaction of stable carbanions with diaryl disulfides. J. Am. Chem. Soc. 1980, 102, 7059–7065. [Google Scholar] [CrossRef]
- Gu, Y.; Son, S.U.; Li, T.; Tan, B. Low-Cost Hypercrosslinked Polymers by Direct Knitting Strategy for Catalytic Applications. Adv. Funct. Mater. 2020, 31, 2008265. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.; Suárez, R.V.; López-Manchado, M.A.; Fernández, J.F. Relavant features of bentonite modification with a phosphonium salt. J. Nanosci. Nanotechnol. 2006, 6, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, X.; Liang, Y.-F.; Tang, C.; Li, X.; Jiao, N. From simple organobromides or olefins to highly value added bromohydrins: A versatile performace of dimethyl sulfoxide. Green Chem. 2015, 17, 2727–2731. [Google Scholar] [CrossRef]
- Chakraborty, D.; Nandi, S.; Sinnwell, M.A.; Liu, J.; Kushwaha, R.; Thallapally, P.K.; Vaidhyanathan, R. Hyper-Cross-linked Porous Organic Frameworks with Ultramicropores for Selective Xenon Capture. ACS Appl. Mater. Interfaces 2019, 11, 13279–13284. [Google Scholar] [CrossRef] [PubMed]
 | ||||
| Entry | Catalyst (Mol%) | Additive (Equiv) | Conversion of Fructose | Yield a |
| 1 | NaBr (5) | PhSSPh (1) | >99% | 54% |
| 2 | TBABr (5) | PhSSPh (1) | >99% | 70% |
| 3 | TBPBr (5) | PhSSPh (1) | >99% | 65% |
| 4 | TBPBr (10) | PhSSPh (1) | >99% | 92% |
| 5 | TPBPBr (10) | PhSSPh (1) | >99% | 84% |
| 6 | BTPPBr (10) | PhSSPh (1) | >99% | 80% |
| 7 | BTPPCl (10) | PhSSPh (1) | >99% | 1% b,c |
| 8 | TBPBr (10) | - | >99% | 20% b,d |
| 9 | - | PhSSPh (1) | >99% | 2% b,e |
| 10 | TBPBr (10) | PhSPh (1) | >99% | 31% |
| 11 | TBPBr (10) | BnSSBn (1) | >99% | 62% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.A.; Sung, K.; Jang, J.Y.; Bang, S.; Son, S.U.; Jang, H.-Y. Sustainable Catalytic Synthesis of 2,5-Diformylfuran from Various Carbohydrates. Catalysts 2022, 12, 360. https://doi.org/10.3390/catal12040360
Kim SA, Sung K, Jang JY, Bang S, Son SU, Jang H-Y. Sustainable Catalytic Synthesis of 2,5-Diformylfuran from Various Carbohydrates. Catalysts. 2022; 12(4):360. https://doi.org/10.3390/catal12040360
Chicago/Turabian StyleKim, Si Ae, Kihyuk Sung, June Young Jang, Sohee Bang, Seung Uk Son, and Hye-Young Jang. 2022. "Sustainable Catalytic Synthesis of 2,5-Diformylfuran from Various Carbohydrates" Catalysts 12, no. 4: 360. https://doi.org/10.3390/catal12040360
APA StyleKim, S. A., Sung, K., Jang, J. Y., Bang, S., Son, S. U., & Jang, H.-Y. (2022). Sustainable Catalytic Synthesis of 2,5-Diformylfuran from Various Carbohydrates. Catalysts, 12(4), 360. https://doi.org/10.3390/catal12040360