Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane
Abstract
:1. Introduction
2. Recent Advances on Catalysts for Syngas Production
2.1. Noble Metal-Based Catalysts
| Catalyst | Preparation Method | Reaction Conditions | H2/CO Ratio | Coke Deposition | Ref. | ||
|---|---|---|---|---|---|---|---|
| Noble metal-based catalysts | |||||||
| 0.1 wt% Rh- and 10 wt% Ni-based catalysts supported on γ-Al2O3 | Incipient wetness impregnation | 700–800 °C, CH4:CO2 = 1:1; GHSV = 12–108 h−1 | 92 | 98 | >0.97 | nr | [23] |
| 2 wt% Ru-, Pt- and Pd-substituted LaAlO3 | Combustion synthesis | 400–800 °C, CH4:CO2:N2 = 0.1:0.1:0.8, GHSV = 48,000 h−1 | >60 | >80 | 0.6–0.85 | nr | [22] |
| 0.16 wt% Pt-CeZrO2 supported on carbon fibers | Co-precipitation | 800 °C, CH4:CO2:Ar = 0.04:0.1:0.86, GHSV = 5000 h−1 | 40 | 52 | 0.30 | nr | [30] |
| Core-shell 0.6 wt%Ru@SiO2 | Reverse-phase microemulsion | 700 °C, CH4:CO2:N2 = 1:1:1, WHSV = 34.8 L h−1 g−1 | 68 | 70 | 0.90 | nr | [31] |
| Nickel-based catalysts | |||||||
| 10 wt%Ni/MgAl2O4 | Microwave-assisted combustion | 800 °C, CH4:CO2:N2 = 0.1:0.1:0.8, WHSV = 72 L h−1 g−1 | >75 | >80 | >0.90 | 2–6 wt% | [32] |
| 1–5 wt% K-promoted 10 wt%Ni/Al2O4 | Co-precipitation | 650 °C, CH4:CO2:N2 = 0.2:0.2:0.6, WHSV = 30 L h−1 g−1 | 35–55 | 42–58 | 0.50–0.86 | No coke for 5 wt% K promotion | [33] |
| 1–8 wt% Mn-promoted 3–8 wt% Ni/Al2O3 | Wet impregnation | 750 °C, CH4:CO2:Ar = 1:1:1, WHSV = 36 L h−1 g−1 | 70–80 | 80–90 | 0.70–0.76 | nr | [34] |
| Mg(Ni)Al2O4 spinel | Co-precipitation | 850 °C, CH4:CO2:N2 = 30:80:90, WHSV = 120 L h−1 g−1 | 90 | 98 | 1.4 | nr | [35] |
| 10 wt% Ni/Al2O3 | Microwave-assisted combustion | 700 °C, CH4:CO2:N2 = 0.1:0.1:0.8, WHSV = 72 L h−1 g−1 | 72–87 | 72–87 | 1.09 | 13.7 wt% | [36] |
| 1–2 wt% Fe promoted 5–15 wt% Ni/MgAl2O4 catalyst | Incipient wetness impregnation | 650–850 °C, CH4:CO2 = 1:1, WHSV = 30,000 L g−1 h−1 | 57–100 | 62–95 | 0.82–1.01 | 2.1–34.2 wt% | [37] |
| 10 wt% Mo promoted 10 wt% Ni/Al2O3 catalyst | Wet impregnation | 550–850 °C, CH4:CO2 = 1:1, GHSV = 20,000 h−1 | 6–91 | 8–93 | 0.40–0.93 | nr | [38] |
| 5 wt% La, Ce and Zr promoted 25 wt% Ni/Al2O3 | Ultrasound assisted co-precipitation | 550–700 °C, CH4:CO2 = 1:1, WHSV = 12,000 mL h−1 g−1 | 0–70 | 40–80 | 0.6–1.1 | nr | [39] |
| 4.5 wt% Ni yolk-shell catalyst on hollow silica spheres (HSS) | Micro-emulsion impregnation | 800 °C, CH4:CO2 = 1:1, WHSV = 36,000 mL h−1 g−1 | 94 | 98 | 0.95 | No coke | [40] |
| 6.5 wt% Ni core-shell catalyst on 3 wt% ZrO2/SiO2 | Micro-emulsion impregnation | 550–800 °C, CH4:CO2 = 1:1, Flow rate = 15 mL min−1 | 85–93 | 90–95 | 0.9–1.0 | No coke | [41] |
| DMS-supported 7 wt% Ni nanoparticles | Ethylene glycol impregnation | 700 °C, CH4:CO2 = 1:1, WHSV = 18 L h−1 g−1 | 76 | 83 | 0.9 | nr | [42] |
| Sandwiched SiO2@8.9wt%Ni@ZrO2 catalyst | Organic wet synthesis | 500–900 °C, CH4:CO2 = 1:1, WHSV = 180 L h−1 g−1 | 7–98 | 7–95 | 0.5–1.0 | 0–19 mg gcat−1 h−1 | [43] |
| 5 wt% Ni supported on CeO2-SiO2 | Incipient wetness impregnation | 600–800 °C, CH4:CO2 = 1:1, WHSV = 48 L h−1 g−1 | 66–97 | 51–92 | 0.77–0.94 | nr | [44] |
| 1–5 mol% Ce and Ca promoted Ni/MSC catalyst | Co-impregnation | 650–900 °C, CH4:CO2 = 1:1, Flow rate = 120 mL min−1 | 45–97 | 54–97 | 0.8–1.0 | nr | [45] |
| 3 wt% Sc, Y, Ce and Pr promoted 15wt% NiMgAl catalysts | Co-precipitation | 750 °C, CH4:CO2 = 1:1, Flow rate = 45 mL min-1, WHSV = 15 L g−1 h−1 | 86–88 | 95–96 | 0.97–0.98 | nr | [46] |
| Cobalt-based catalysts | |||||||
| 10–25 mol% Co/MgO catalyst | Sol-gel method | 750 °C, CH4:CO2 = 1:1, WHSV = 24 L h−1 g−1 | 85–90 | 80–85 | 0.93–0.95 | 0–0.22 mg gcat−1 h−1 | [47] |
| 0.1–2 wt% Ce-promoted 10 wt%Co/Al2O3 | Incipient wetness impregnation | 650–750 °C, CH4:CO2:N2 = 0.2:0.2:0.6, WHSV = 36 L h−1 g−1 | 75–82 | 63–72 | 0.86–0.91 | 7.5–12.8 wt% | [48] |
| 3 wt%, Y, La, Ce or Sm-promoted 10 wt%Co/MA | Incipient wetness impregnation | 750 °C, CH4:CO2:N2 = 1:1:3.1, WHSV = 36 L h−1 g−1 | 71–84 | 73–90 | 0.86–0.96 | 7–28 wt% | [49] |
| 3 wt% La-promoted 10 wt% Co/Al2O3 | Hydrothermal synthesis | 750 °C, CH4:CO2 = 1:1, WHSV = 36 L h−1 g−1 | 76–96 | 81–93 | 0.89–0.99 | 26–38 wt% | [50] |
| 0.2–0.5 wt% Rd-promoted12 wt% Co/SBA-15 | Two-solvent impregnation | 550 °C, CH4:CO2 = 1:1, WHSV = 67 L h−1 g−1 | 15–49 | 18–43 | 0.5–1.1 | nr | [51] |
| 0.5–2 wt% Sc-promoted 5 wt% Co/TiO2 | Incipient wetness impregnation | 700 °C, CH4:CO2:N2 = 17:17:2, WHSV = 3.6 L h−1 g−1 | 82–84 | 82–85 | 0.94–0.96 | 6.8–32.3 wt% | [52] |
| Other transition metal-based catalysts | |||||||
| LaNi0.34Co0.33Mn0.33O3 perovskite catalyst | Microwave-assisted Pechini method | 800 °C, CH4:CO2 = 1:1.05, WHSV = 12 L h−1 g−1 | 94 | 93 | 1.1–1.2 | nr | [53] |
| LaNiO3 perovskite | One-step sol-gel method with chitosan | 600–800 °C, CH4:CO2 = 1:1, WHSV = 6–24 L h−1 g−1 | 48–95 | 45–95 | 0.65–1.25 | 16–34 wt% | [54] |
| SmCoO3 perovskite | Sol-gel citrate method | 700–800 °C, CH4:CO2 = 1:1, WHSV = 30 L h−1 g−1 | 88–93 | 89–93 | 0.8–1.1 | nr | [55] |
| Bi-and trimetallic catalysts | |||||||
| 3–15 wt% Ce promoted 1 wt%Pt-1 wt%Pd-1 wt%Ni/MgO trimetallic | Co-precipitation | 700–900 °C, CH4:CO2 = 1:1 and 2:1, Flow rate = 30 mL min−1 | 60–83 | 39–98 | 0.4–1.1 | 2.4 wt% | [56] |
| 0.1–0.5 wt% Rh-4.5–4.9 wt% Co/Al2O3 | Wet co-impregnation | 600–700 °C, CH4:CO2 = 1:1, GHSV = 1000–2000 h−1 | 87–94 | 86–91 | 0.99–1.0 | No coke | [57] |
| 10.5 wt%Ni-4.5 wt%Co supported on Al2O3-MgO catalysts | Sol-gel method | 800 °C, CH4:CO2 = 1:1, Flow rate = 40 mL min−1 | 79 | 84 | 0.85–0.90 | nr | [58] |
| Co2Ni2Mg2Al2 mixed oxide catalyst | Hydrotalcite route synthesis | 500–800 °C, CH4:CO2 = 1:1, GHSV = 32,000 h−1 | 97 | 91–93 | 0.8 | nr | [59] |
| 12.5 wt% Ni and 12.5 wt%Ni-2 wt%Co supported on CeO2-ZnAl2O4 | Co-precipitation | 700 °C, CH4:CO2 = 1:1, WHSV = 180 L h−1 g−1 | 76 | 88 | 0.99 | 38 wt% | [60] |
| 7 wt% Ni and 7 wt%Ni-1 wt%Ru supported on SiO2, ZrO2, Al2O3 and MgAl2O4 | Wet impregnation and precipitation | 800 °C, CH4:CO2 = 1:1, Flow rate = 400 mL min−1 | 39–100 | 48–85 | 0.5–1.0 | 0.1–0.42 wt% | [61] |
2.2. Transition Metals-Based Catalysts
2.2.1. Nickel Catalysts
2.2.2. Cobalt Catalysts
2.2.3. Other Transition Metals
2.2.4. Bi- and Trimetallic Catalysts
2.2.5. Role of Catalyst Support
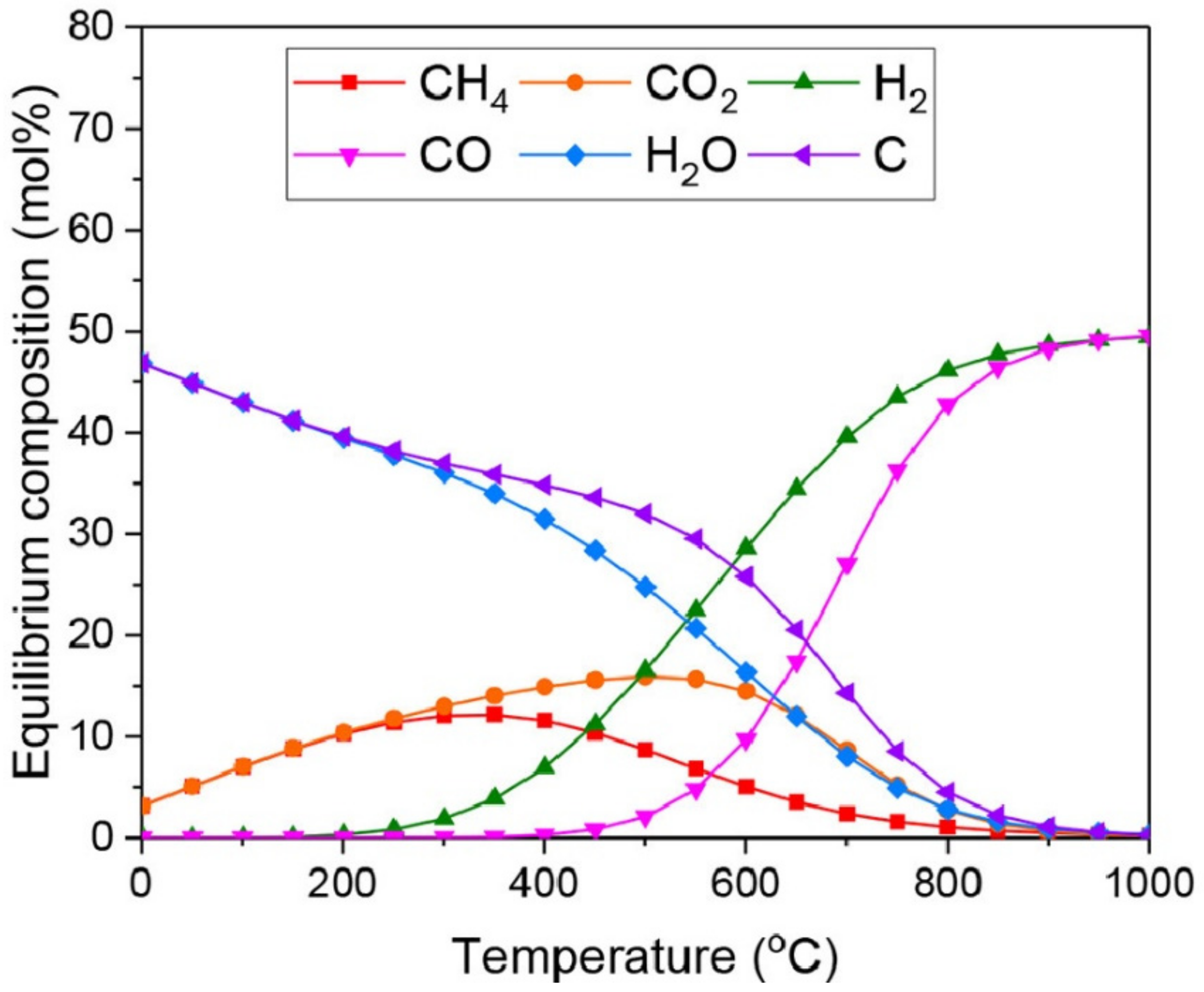
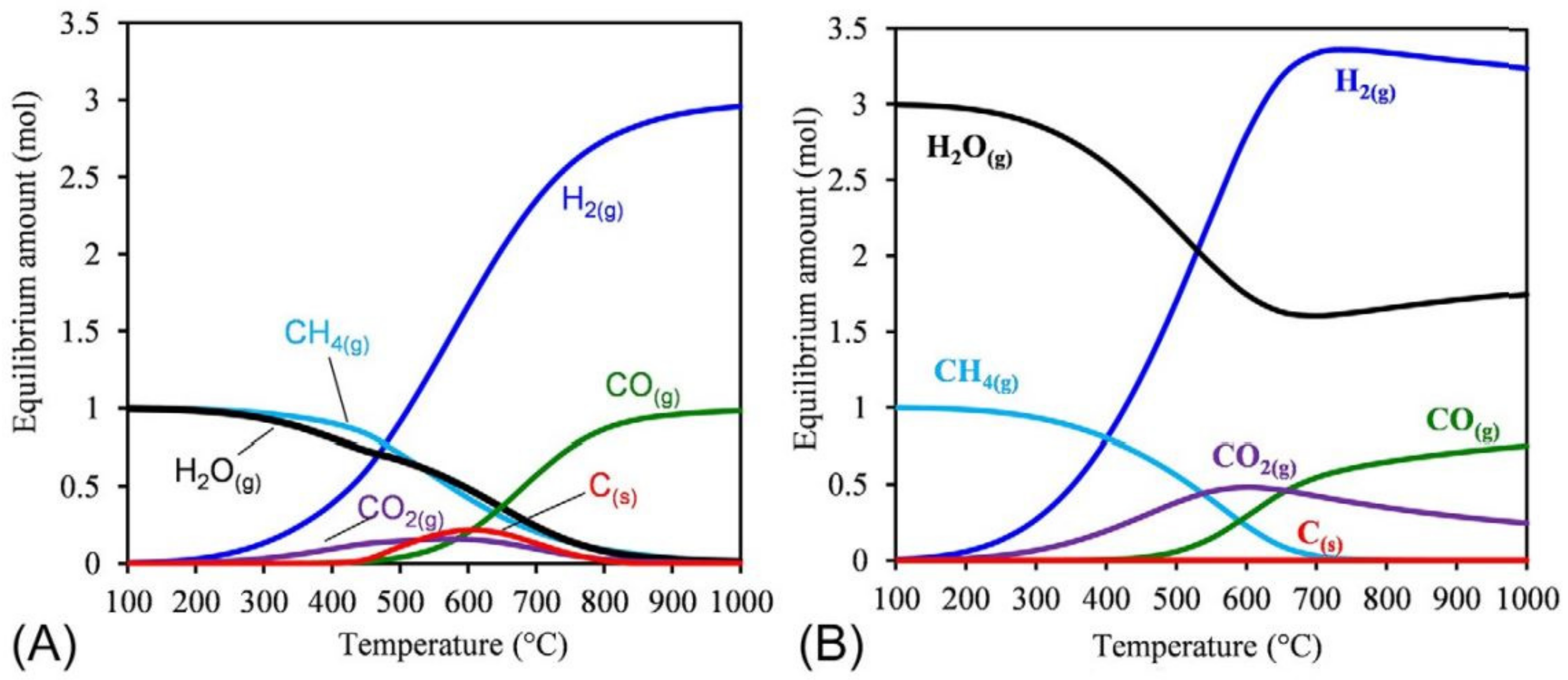
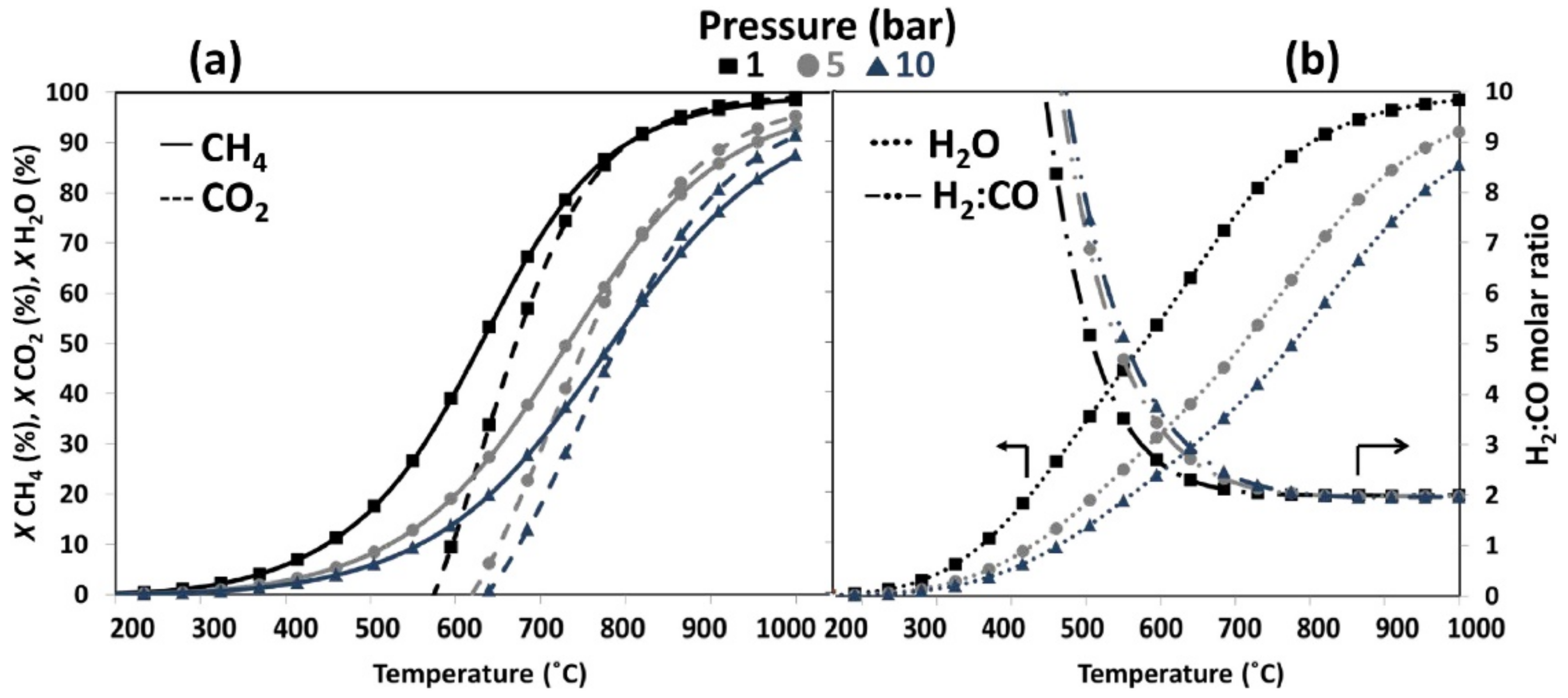
3. Alternative Processes for Increased H2/CO Ratio
3.1. Steam Reforming of Methane (SRM)
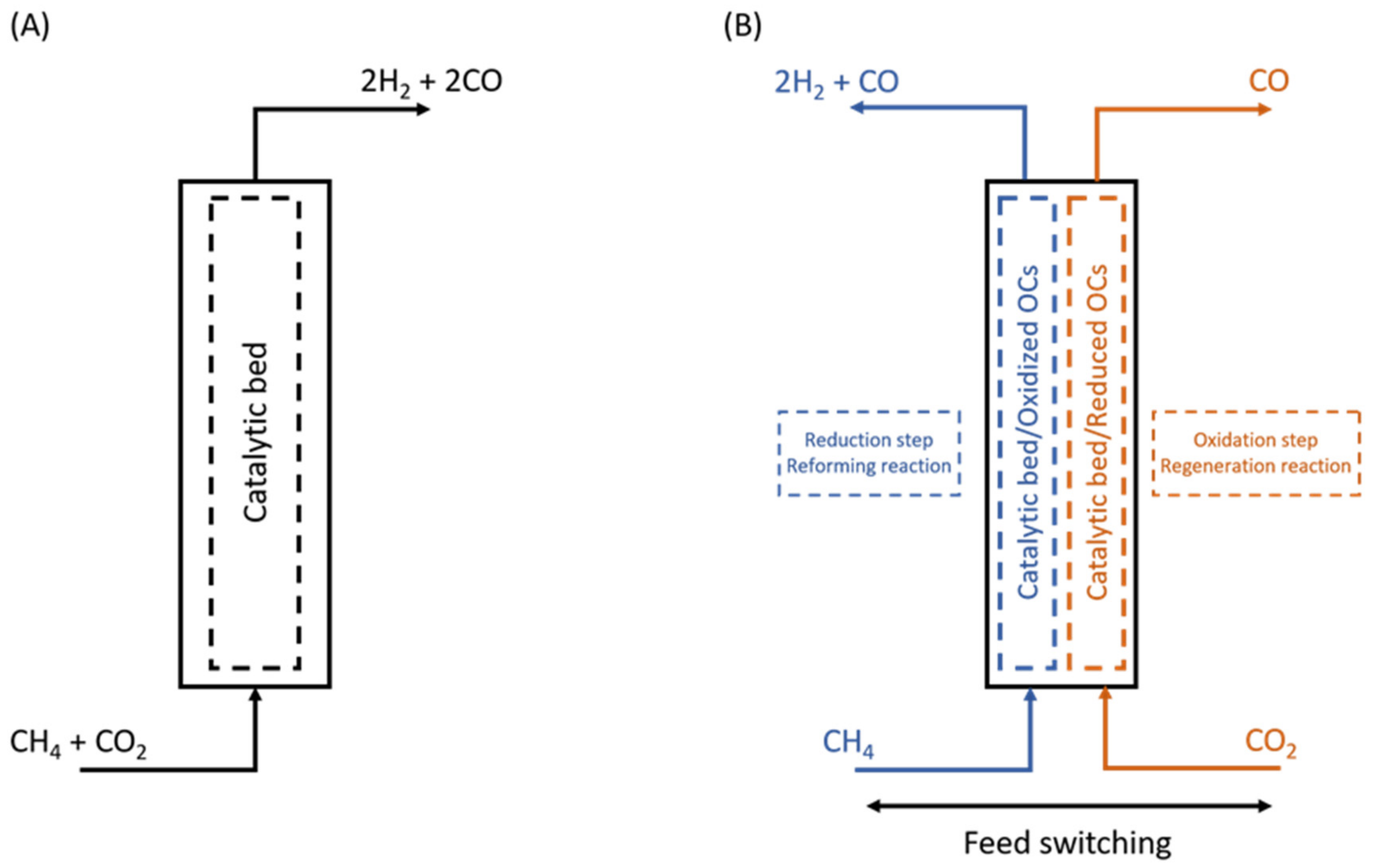
3.2. Chemical Looping Dry Reforming of Methane (CL-DRM)
3.3. Combined Steam and Dry Reforming of Methane (CSDRM)
3.4. Partial Oxidation of Methane (POM)
3.5. Methane Tri-Reforming (TRM)
4. Large-Scale Applications of DRM
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. The Future of Hydrogen; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 9 February 2022).
- Rego de Vasconcelos, B.; Lavoie, J.-M. Recent Advances in Power-to-X Technology for the Production of Fuels and Chemicals. Front. Chem. 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Hydrogen Production by Electrical Energy. In Sustainable Hydrogen Production; Elsevier: Cambridge, MA, USA, 2016; pp. 99–161. ISBN 9780128015636. [Google Scholar]
- le Saché, E.; Reina, T.R. Analysis of Dry Reforming as direct route for gas phase CO2 conversion. The past, the present and future of catalytic DRM technologies. Prog. Energy Combust. Sci. 2022, 89, 100970. [Google Scholar] [CrossRef]
- Chein, R.Y.; Chen, Y.C.; Yu, C.T.; Chung, J.N. Thermodynamic analysis of dry reforming of CH4 with CO2 at high pressures. J. Nat. Gas Sci. Eng. 2015, 26, 617–629. [Google Scholar] [CrossRef]
- Nishimura, A.; Takada, T.; Ohata, S.; Kolhe, M.L. Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure. Fuels 2021, 2, 194–209. [Google Scholar] [CrossRef]
- Thanh Son, P.; Abdoul Razac, S.; Rêgo de Vasconcelos, B.; Nzihou, A.; Sharrock, P.; Grouset, D.; Doan, P.M. Hydroxyapatite supported bimetallic cobalt and nickel catalysts for syngas production from dry reforming of methane. Appl. Catal. B Environ. 2017, 224, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Rego de Vasconcelos, B.; Pham Minh, D.; Martins, E.; Germeau, A.; Sharrock, P.; Nzihou, A. Highly-efficient hydroxyapatite-supported nickel catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2019, 45, 18502–18518. [Google Scholar] [CrossRef] [Green Version]
- Rego de Vasconcelos, B.; Pham Minh, D.; Lyczko, N.; Phan, T.S.; Sharrock, P.; Nzihou, A. Upgrading greenhouse gases (methane and carbon dioxide) into syngas using nickel-based catalysts. Fuel 2018, 226, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, A.S.; Al-Swai, B.M.; Ruslan, F.H.; Mohd Zabidi, N.A.; Saidur, R.; ad Syed Muhammad, S.A.F.; Abdullah, B. Catalytic conversion of greenhouse gases (CO2 and CH4) to syngas over Ni-based catalyst: Effects of Ce-La promoters. Arab. J. Chem. 2020, 13, 5740–5749. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry reforming of methane has no future for hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrogen Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.A.A.; Setiabudi, H.D.; Teh, L.P.; Annuar, N.H.R.; Jalil, A.A. A review of heterogeneous catalysts for syngas production via dry reforming. J. Taiwan Inst. Chem. Eng. 2019, 101, 139–158. [Google Scholar] [CrossRef]
- Bullock, R.M. Reaction: Earth-Abundant Metal Catalysts for Energy Conversions. Chem 2017, 2, 444–446. [Google Scholar] [CrossRef] [Green Version]
- Renda, S.; Ricca, A.; Palma, V. Study of the effect of noble metal promotion in Ni-based catalyst for the Sabatier reaction. Int. J. Hydrogen Energy 2020, 46, 12117–12127. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Yang, J.; Zhang, J.; Zhang, L.; Li, H.; Zhao, Y.; Li, H.X.; Wu, K.; Xu, G.Q.; et al. Enhanced catalytic performance of Ir catalysts supported on ceria-based solid solutions for methane dry reforming reaction. Catal. Today 2017, 281, 295–303. [Google Scholar] [CrossRef]
- Andraos, S.; Abbas-Ghaleb, R.; Chlala, D.; Vita, A.; Italiano, C.; Laganà, M.; Pino, L.; Nakhl, M.; Specchia, S. Production of hydrogen by methane dry reforming over ruthenium-nickel based catalysts deposited on Al2O3, MgAl2O4, and YSZ. Int. J. Hydrogen Energy 2019, 44, 25706–25716. [Google Scholar] [CrossRef]
- Chen, J.; Yao, C.; Zhao, Y.; Jia, P. Synthesis gas production from dry reforming of methane over Ce0.75Zr0.25O2-supported Ru catalysts. Int. J. Hydrogen Energy 2010, 35, 1630–1642. [Google Scholar] [CrossRef]
- Anil, C.; Modak, J.M.; Madras, G. Syngas production via CO2 reforming of methane over noble metal (Ru, Pt, and Pd) doped LaAlO3 perovskite catalyst. Mol. Catal. 2020, 484, 110805. [Google Scholar] [CrossRef]
- De Araujo Moreira, T.G.; de Carvalho Filho, J.F.S.; Carvalho, Y.; de Almeida, J.M.A.R.; Nothaft Romano, P.; Falabella Sousa-Aguiar, E. Highly stable low noble metal content rhodium-based catalyst for the dry reforming of methane. Fuel 2020, 287, 119536. [Google Scholar] [CrossRef]
- Ballarini, A.D.; Virgens, C.F.; Rangel, M.C.; de Miguel, S.R.; Grau, J.M. Characterization and behaviour of Pt catalysts supported on basic materials in dry reforming of methane. Braz. J. Chem. Eng. 2019, 36, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Arkatova, L.A.; Kasatsky, N.G.; Maximov, Y.M.; Pakhnutov, O.V.; Shmakov, A.N. Intermetallides as the catalysts for carbon dioxide reforming of methane. Catal. Today 2018, 299, 303–316. [Google Scholar] [CrossRef]
- Kobayashi, T.; Furuya, T.; Fujitsuka, H.; Tago, T. Synthesis of Birdcage-type zeolite encapsulating ultrafine Pt nanoparticles and its application in dry reforming of methane. Chem. Eng. J. 2019, 377, 120203. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; Abdullah, A.M. Engineering of Pt-based nanostructures for efficient dry (CO2) reforming: Strategy and mechanism for rich-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5901–5928. [Google Scholar] [CrossRef]
- Carvalho, D.C.; De Souza, H.S.A.; Filho, J.M.; Oliveira, A.C.; Campos, A.; Milet, É.R.C.; De Sousa, F.F.; Padron-Hernandez, E. A study on the modification of mesoporous mixed oxides supports for dry reforming of methane by Pt or Ru. Appl. Catal. A Gen. 2014, 473, 132–145. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, B.; Kattel, S.; Lee, J.H.; Yao, S.; Wu, Q.; Rui, N.; Gomez, E.; Liu, Z.; Xu, W.; et al. Dry reforming of methane over CeO2-supported Pt-Co catalysts with enhanced activity. Appl. Catal. B Environ. 2018, 236, 280–293. [Google Scholar] [CrossRef]
- Jagódka, P.; Matus, K.; Sobota, M.; Łamacz, A. Dry reforming of methane over carbon fibre-supported cezro2, ni-cezro2, pt-cezro2 and pt-ni-cezro2 catalysts. Catalysts 2021, 11, 563. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhao, J.; Bai, Y.; Du, H.; Wang, Q.; Jiang, B.; Li, H. CO2 conversion via dry reforming of methane on a core-shell Ru@SiO2 catalyst. J. CO2 Util. 2022, 57, 101893. [Google Scholar] [CrossRef]
- de Souza, M.G.; Melo, D.M.A.; Medeiros, R.L.B.A.; Maziviero, F.V.; Macedo, H.P.; Oliveira, Â.A.S.; Braga, R.M. NiO–MgAl2O4 systems for dry reforming of methane: Effect of the combustion synthesis route in the catalysts properties. Mater. Chem. Phys. 2022, 278, 125599. [Google Scholar] [CrossRef]
- Azancot, L.; Blay, V.; Blay-Roger, R.; Bobadilla, L.F.; Penkova, A.; Centeno, M.A.; Odriozola, J.A. Evidence of new Ni-O-K catalytic sites with superior stability for methane dry reforming. Appl. Catal. B Environ. 2022, 307, 121148. [Google Scholar] [CrossRef]
- Akansu, H.; Arbag, H.; Tasdemir, H.M.; Yasyerli, S.; Yasyerli, N.; Dogu, G. Nickel-based alumina supported catalysts for dry reforming of biogas in the absence and the presence of H2S: Effect of manganese incorporation. Catal. Today 2022. [Google Scholar] [CrossRef]
- Abbas, M.; Sikander, U.; Mehran, M.T.; Kim, S.H. Exceptional stability of hydrotalcite derived spinel Mg(Ni)Al2O4 catalyst for dry reforming of methane. Catal. Today 2021, 1–12. [Google Scholar] [CrossRef]
- Maziviero, F.V.; Medeiros, R.L.B.A.; Melo, D.M.A.; Macedo, H.P.; Oliveira, Â.A.S.; Araújo, T.R. Synthesis of alumina by microwave-assisted combustion method using low fuel content and its use as catalytic support for dry reforming of methane. Mater. Chem. Phys. 2021, 264, 124408. [Google Scholar] [CrossRef]
- Medeiros, R.L.B.A.; Macedo, H.P.; Melo, V.R.M.; Oliveira, Â.A.S.; Barros, J.M.F.; Melo, M.A.F.; Melo, D.M.A. Ni supported on Fe-doped MgAl2O4 for dry reforming of methane: Use of factorial design to optimize H2 yield. Int. J. Hydrogen Energy 2016, 41, 14047–14057. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Da Costa, P. Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 23500–23507. [Google Scholar] [CrossRef]
- Rahbar Shamskar, F.; Meshkani, F.; Rezaei, M. Preparation and characterization of ultrasound-assisted co-precipitated nanocrystalline La-, Ce-, Zr -promoted Ni-Al2O3 catalysts for dry reforming reaction. J. CO2 Util. 2017, 22, 124–134. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Ruan, Y.; Zhao, Y.; Wang, S.; Ma, X. Facile one-pot synthesis of Ni@HSS as a novel yolk-shell structure catalyst for dry reforming of methane. J. CO2 Util. 2018, 24, 190–199. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, X.; Han, X.; You, X.; Lin, S.; Chen, H.; Liu, W.; Wang, X.; Zhang, N.; Wang, Z.; et al. Catalysts in Coronas: A Surface Spatial Confinement Strategy for High-Performance Catalysts in Methane Dry Reforming. ACS Catal. 2019, 9, 9072–9080. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, R.; Hao, X.; Bao, Z.; Wu, T.; Wang, B.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B Environ. 2019, 254, 612–623. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Liu, J.; Xu, Y.; Lv, Y. Production of syngas via CO2 methane reforming process: Effect of cerium and calcium promoters on the performance of Ni-MSC catalysts. Int. J. Hydrogen Energy 2020, 45, 640–649. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Promotional effects of rare earth elements (Sc, Y, Ce, and Pr) on NiMgAl catalysts for dry reforming of methane. RSC Adv. 2016, 6, 112215–112225. [Google Scholar] [CrossRef]
- Sukri, M.F.F.; Khavarian, M.; Mohamed, A.R. Effect of cobalt loading on suppression of carbon formation in carbon dioxide reforming of methane over Co/MgO catalyst. Res. Chem. Intermed. 2018, 44, 2585–2605. [Google Scholar] [CrossRef]
- Cao, A.N.T.; Pham, C.Q.; Pham, L.K.H.; Le Tri Nguyen, D.; Phuong, P.T.T.; Tran, T.T.V.; Nguyen, V.P.; Nguyen, T.B.; Van Le, Q.; Nguyen, N.A.; et al. Boosted methane dry reforming for hydrogen generation on cobalt catalyst with small cerium dosage. Int. J. Hydrogen Energy 2021, 1–13. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Duy Nguyen, T.; Phuong, P.T.T.; Duc Truong, Q.; Abdul Jalil, A.; Ainirazali, N.; Vo, D.V.N. Insight into the influence of rare-earth promoter (CeO2, La2O3, Y2O3, and Sm2O3) addition toward methane dry reforming over Co/mesoporous alumina catalysts. Chem. Eng. Sci. 2020, 228, 115967. [Google Scholar] [CrossRef]
- Tran, N.T.; Van Le, Q.; Van Cuong, N.; Nguyen, T.D.; Huy Phuc, N.H.; Phuong, P.T.T.; Monir, M.U.; Aziz, A.A.; Truong, Q.D.; Abidin, S.Z.; et al. La-doped cobalt supported on mesoporous alumina catalysts for improved methane dry reforming and coke mitigation. J. Energy Inst. 2020, 93, 1571–1580. [Google Scholar] [CrossRef]
- El Hassan, N.; Kaydouh, M.N.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. The effect of Sc promoter on the performance of Co/TiO2-P25 catalyst in dry reforming of methane. Bull. Korean Chem. Soc. 2015, 36, 2081–2088. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Oliveira, Â.A.S.; Medeiros, R.L.B.A.; Figueredo, G.P.; Macedo, H.P.; Braga, R.M.; Maziviero, F.V.; Melo, M.A.F.; Melo, D.M.A.; Vieira, M.M. One-step synthesis of LaNiO3 with chitosan for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 9696–9704. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Setiabudi, H.D.; Rasid, R.A.; Cheng, C.K. Syngas production via methane dry reforming: A novel application of SmCoO3 perovskite catalyst. J. Nat. Gas Sci. Eng. 2017, 37, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Al-Doghachi, F.A.J.; Rashid, U.; Taufiq-Yap, Y.H. Investigation of Ce(III) promoter effects on the tri-metallic Pt, Pd, Ni/MgO catalyst in dry-reforming of methane. RSC Adv. 2016, 6, 10372–10384. [Google Scholar] [CrossRef]
- Itkulova, S.S.; Valishevskiy, K.A.; Boleubayev, Y.A. Bimetallic Co-Rh Systems as a Prospective Base for Design of CH4 Reforming Catalysts to Produce Syngas with a Controllable Composition. Catalysts 2022, 12, 105. [Google Scholar] [CrossRef]
- Abd Ghani, N.A.; Azapour, A.; Syed Muhammad, S.A.F.; Mohamed Ramli, N.; Vo, D.-V.N.; Abdullah, B. Dry reforming of methane for syngas production over Ni–Co-supported Al2O3–MgO catalysts. Appl. Petrochem. Res. 2018, 8, 263–270. [Google Scholar] [CrossRef]
- Tanios, C.; Gennequin, C.; Labaki, M.; Tidahy, H.L.; Aboukaïs, A.; Abi-Aad, E. Evaluation of a catalyst durability in absence and presence of toluene impurity: Case of the material Co2Ni2Mg2Al2 mixed oxide prepared by hydrotalcite route in methane dry reforming to produce energy. Materials 2019, 12, 1362. [Google Scholar] [CrossRef] [Green Version]
- Movasati, A.; Alavi, S.M.; Mazloom, G. Dry reforming of methane over CeO2-ZnAl2O4 supported Ni and Ni-Co nano-catalysts. Fuel 2019, 236, 1254–1262. [Google Scholar] [CrossRef]
- Wysocka, I.; Hupka, J.; Rogala, A. Catalytic Activity of Nickel and Ruthenium–Nickel Catalysts Supported on SiO2, ZrO2, Al2O3, and MgAl2O4 in a Dry Reforming Process. Catalysts 2019, 9, 540. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dhir, A.; Mohapatra, S.K.; Mahla, S.K. Dry reforming of methane using various catalysts in the process: Review. Biomass Convers. Biorefinery 2020, 10, 567–587. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z. Recent advances during CH4 dry reforming for syngas production: A mini review. Int. J. Hydrogen Energy 2020, 46, 5852–5874. [Google Scholar] [CrossRef]
- Karam, L.; Armandi, M.; Casale, S.; El Khoury, V.; Bonelli, B.; Massiani, P.; El Hassan, N. Comprehensive study on the effect of magnesium loading over nickel-ordered mesoporous alumina for dry reforming of methane. Energy Convers. Manag. 2020, 225, 113470. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, Q.; Wang, Y.; Hu, C.; Da Costa, P. One-Step Synthesis of Highly Active and Stable Ni-ZrOxfor Dry Reforming of Methane. Ind. Eng. Chem. Res. 2020, 59, 11441–11452. [Google Scholar] [CrossRef]
- Chein, R.Y.; Fung, W.Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- De la Cruz-Flores, V.G.; Martinez-Hernandez, A.; Gracia-Pinilla, M.A. Deactivation of Ni-SiO2 catalysts that are synthetized via a modified direct synthesis method during the dry reforming of methane. Appl. Catal. A Gen. 2020, 594, 117455. [Google Scholar] [CrossRef]
- Mahfouz, R.; Estephane, J.; Gennequin, C.; Tidahy, L.; Aouad, S.; Abi-Aad, E. CO2 reforming of methane over Ni and/or Ru catalysts supported on mesoporous KIT-6: Effect of promotion with Ce. J. Environ. Chem. Eng. 2021, 9, 104662. [Google Scholar] [CrossRef]
- Mourhly, A.; Kacimi, M.; Halim, M.; Arsalane, S. New low cost mesoporous silica (MSN) as a promising support of Ni-catalysts for high-hydrogen generation via dry reforming of methane (DRM). Int. J. Hydrogen Energy 2020, 45, 11449–11459. [Google Scholar] [CrossRef]
- Medeiros, R.L.B.A.; Figueredo, G.P.; Macedo, H.P.; Oliveira, Â.A.S.; Rabelo-Neto, R.C.; Melo, D.M.A.; Braga, R.M.; Melo, M.A.F. One-pot microwave-assisted combustion synthesis of Ni-Al2O3 nanocatalysts for hydrogen production via dry reforming of methane. Fuel 2021, 287, 119511. [Google Scholar] [CrossRef]
- Miri, S.S.; Meshkani, F.; Rastegarpanah, A.; Rezaei, M. Influence of Fe, La, Zr, Ce, and Ca on the catalytic performance and coke formation in dry reforming of methane over Ni/MgO.Al2O3 catalyst. Chem. Eng. Sci. 2022, 250, 116956. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, X.; Zeng, L.; Gong, J. Recent Advances on the Design of Group VIII Base-Metal Catalysts with Encapsulated Structures. ACS Catal. 2015, 5, 4959–4977. [Google Scholar] [CrossRef]
- San José-Alonso, D.; Illán-Gómez, M.J.; Román-Martínez, M.C. Low metal content Co and Ni alumina supported catalysts for the CO2 reforming of methane. Int. J. Hydrogen Energy 2013, 38, 2230–2239. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.J.; Román-Martínez, M.C. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Chen, S.; Zaffran, J.; Yang, B. Dry reforming of methane over the cobalt catalyst: Theoretical insights into the reaction kinetics and mechanism for catalyst deactivation. Appl. Catal. B Environ. 2020, 270, 118859. [Google Scholar] [CrossRef]
- Al Abdulghani, A.J.; Park, J.H.; Kozlov, S.M.; Kang, D.C.; AlSabban, B.; Pedireddy, S.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.L.; Basset, J.M.; et al. Methane dry reforming on supported cobalt nanoparticles promoted by boron. J. Catal. 2020, 392, 126–134. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.T.; Le Minh Pham, T.; Nguyen, T.D.; Van Cuong, N.; Siang, T.J.; Phuong, P.T.T.; Jalil, A.A.; Truong, Q.D.; Abidin, S.Z.; Hagos, F.Y.; et al. Improvements in hydrogen production from methane dry reforming on filament-shaped mesoporous alumina-supported cobalt nanocatalyst. Int. J. Hydrogen Energy 2021, 46, 24781–24790. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef]
- Li, R.; Shahbazi, A.; Wang, L.; Zhang, B.; Chung, C.C.; Dayton, D.; Yan, Q. Nanostructured molybdenum carbide on biochar for CO2 reforming of CH4. Fuel 2018, 225, 403–410. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Xiao, X.; Ye, Z.-M.; Zhao, S.; Shen, R.; He, C.-T.; Zhang, J.-P.; Li, Y.; Chen, X.-M. Cage-Confinement Pyrolysis Route to Ultrasmall Tungsten Carbide Nanoparticles for Efficient Electrocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 5285–5288. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Zhang, G.; Liu, J.; Lv, Y.; Zhang, Y. Highly stable activity of cobalt based catalysts with tungsten carbide-activated carbon support for dry reforming of methane: Role of tungsten carbide. Fuel 2022, 311, 122512. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Li, G.; Liu, J.; Wang, Y.; Xu, Y.; Lyu, Y. Understanding structure-activity relationships of the highly active and stable La promoted Co/WC-AC catalyst for methane dry reforming. Int. J. Hydrogen Energy 2022, 47, 7823–7835. [Google Scholar] [CrossRef]
- Dehimi, L.; Gaillard, M.; Virginie, M.; Erto, A.; Benguerba, Y. Investigation of dry reforming of methane over Mo-based catalysts. Int. J. Hydrogen Energy 2020, 45, 24657–24669. [Google Scholar] [CrossRef]
- Vroulias, D.; Gkoulemani, N.; Papadopoulou, C.; Matralis, H. W–modified Ni/Al2O3 catalysts for the dry reforming of methane: Effect of W loading. Catal. Today 2020, 355, 704–715. [Google Scholar] [CrossRef]
- Sasson Bitters, J.; He, T.; Nestler, E.; Senanayake, S.D.; Chen, J.G.; Zhang, C. Utilizing bimetallic catalysts to mitigate coke formation in dry reforming of methane. J. Energy Chem. 2022, 68, 124–142. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Contemporary trends in composite Ni-based catalysts for CO2 reforming of methane. Chem. Eng. Sci. 2021, 229, 116072. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Pérez, J.M.M.; Lucio-Ortiz, C.J.; Rosa, J.R.; Maldonado, C.S.; De Haro Del Río, D.A.; Sandoval-Rangel, L.; Garza-Navarro, M.A.; Martínez-Vargas, D.X.; Morales-Leal, F.J. Dry Reforming of Methane for Hydrogen Production Using Bimetallic Catalysts of Pt-Fe Supported on γ-Alumina. ChemistrySelect 2021, 6, 12685–12695. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abu-Dahrieh, J.K.; Atia, H.; Armbruster, U.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Effect of pre-treatment and calcination temperature on Al2O3-ZrO2 supported Ni-Co catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2019, 44, 21546–21558. [Google Scholar] [CrossRef] [Green Version]
- Turap, Y.; Wang, I.; Fu, T.; Wu, Y.; Wang, Y.; Wang, W. Co–Ni alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 6538–6548. [Google Scholar] [CrossRef]
- Jin, F.; Fu, Y.; Kong, W.; Wang, J.; Cai, F.; Zhang, J.; Xu, J. Dry reforming of methane over trimetallic NiFeCu alloy catalysts. Chem. Phys. Lett. 2020, 750, 137491. [Google Scholar] [CrossRef]
- Ibrahim, A.; Fakeeha, A.; Abasaeed, A.; Al-Fatesh, A. Dry Reforming of Methane Using Ni Catalyst Supported on ZrO2: The Effect of Different Sources of Zirconia. Catalysts 2021, 11, 827. [Google Scholar] [CrossRef]
- Bagabas, A.; Al-Fatesh, A.S.; Kasim, S.O.; Arasheed, R.; Ibrahim, A.A.; Ashamari, R.; Anojaidi, K.; Fakeeha, A.H.; Abu-Dahrieh, J.K.; Abasaeed, A.E. Optimizing MgO Content for Boosting γ-Al2O3-Supported Ni Catalyst in Dry Reforming of Methane. Catalysts 2021, 11, 1233. [Google Scholar] [CrossRef]
- Gao, X.; Ge, Z.; Zhu, G.; Wang, Z.; Ashok, J.; Kawi, S. Anti-Coking and Anti-Sintering Ni/Al2O3 Catalysts in the Dry Reforming of Methane: Recent Progress and Prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- Song, D.H.; Jung, U.H.; Kim, Y.E.; Im, H.B.; Lee, T.H.; Lee, K.B.; Koo, K.Y. Influence of Supports on the Catalytic Activity and Coke Resistance of Ni Catalyst in Dry Reforming of Methane. Catalysts 2022, 12, 216. [Google Scholar] [CrossRef]
- Özdemir, H.; Faruk Öksüzömer, M.A.; Ali Gürkaynak, M. Preparation and characterization of Ni based catalysts for the catalytic partial oxidation of methane: Effect of support basicity on H2/CO ratio and carbon deposition. Int. J. Hydrogen Energy 2010, 35, 12147–12160. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Chaudhary, M.L.; Fakeeha, A.H.; Ibrahim, A.A.; Al-Mubaddel, F.; Kasim, S.O.; Albaqmaa, Y.A.; Bagabas, A.A.; Patel, R.; Kumar, R. Role of mixed oxides in hydrogen production through the dry reforming of methane over nickel catalysts supported on modified γ-Al2O3. Processes 2021, 9, 157. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt% Ni/Ce1−xMxO2−δ (M=Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Gao, D.Z.; Strand, J.; Munde, M.S.; Shluger, A.L. Mechanisms of oxygen vacancy aggregation in SiO2 and HfO2. Front. Phys. 2019, 7, 43. [Google Scholar] [CrossRef]
- Faria, E.C.; Neto, R.C.R.; Colman, R.C.; Noronha, F.B. Hydrogen production through CO2 reforming of methane over Ni/CeZrO2/Al2O3 catalysts. Catal. Today 2014, 228, 138–144. [Google Scholar] [CrossRef]
- Arias-Duque, C.; Bladt, E.; Muñoz, M.A.; Hernández-Garrido, J.C.; Cauqui, M.A.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Bals, S.; Calvino, J.J.; Pérez-Omil, J.A.; et al. Improving the Redox Response Stability of Ceria-Zirconia Nanocatalysts under Harsh Temperature Conditions. Chem. Mater. 2017, 29, 9340–9350. [Google Scholar] [CrossRef]
- Rapier, R. Life Cycle Emissions of Hydrogen. Available online: https://4thgeneration.energy/life-cycles-emissions-of-hydrogen/ (accessed on 6 December 2021).
- Tristantini, D.; Lögdberg, S.; Gevert, B.; Borg, Ø.; Holmen, A. The effect of synthesis gas composition on the Fischer–Tropsch synthesis over Co/γ-Al2O3 and Co–Re/γ-Al2O3 catalysts. Fuel Process. Technol. 2007, 88, 643–649. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Akiki, E.; Akiki, D.; Italiano, C.; Vita, A.; Abbas-Ghaleb, R.; Chlala, D.; Drago Ferrante, G.; Laganà, M.; Pino, L.; Specchia, S. Production of hydrogen by methane dry reforming: A study on the effect of cerium and lanthanum on Ni/MgAl2O4 catalyst performance. Int. J. Hydrogen Energy 2020, 45, 21392–21408. [Google Scholar] [CrossRef]
- Wurzel, T.; Malcus, S.; Mleczko, L. Reaction engineering investigations of CO2 reforming in a fluidized-bed reactor. Chem. Eng. Sci. 2000, 55, 3955–3966. [Google Scholar] [CrossRef]
- Mantripragada, H.C.; Veser, G. Hydrogen production via chemical looping dry reforming of methane: Process modeling and systems analysis. AIChE J. 2022, e17612. [Google Scholar] [CrossRef]
- Pajak, M.; Mozdzierz, M.; Chalusiak, M.; Kimijima, S.; Szmyd, J.S.; Brus, G. A numerical analysis of heat and mass transfer processes in a macro-patterned methane/steam reforming reactor. Int. J. Hydrogen Energy 2018, 43, 20474–20487. [Google Scholar] [CrossRef]
- Navas-Anguita, Z.; García-Gusano, D.; Dufour, J.; Iribarren, D. Revisiting the role of steam methane reforming with CO2 capture and storage for long-term hydrogen production. Sci. Total Environ. 2021, 771, 145432. [Google Scholar] [CrossRef] [PubMed]
- Minh, D.P.; Siang, T.J.; Vo, D.V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri-Reforming of Methane; Elsevier Ltd.: Cambridge, MA, USA, 2018; ISBN 9780128111970. [Google Scholar]
- Carapellucci, R.; Giordano, L. Steam, dry and autothermal methane reforming for hydrogen production: A thermodynamic equilibrium analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Noh, Y.S.; Lee, K.Y.; Moon, D.J. Hydrogen production by steam reforming of methane over nickel based structured catalysts supported on calcium aluminate modified SiC. Int. J. Hydrogen Energy 2019, 44, 21010–21019. [Google Scholar] [CrossRef]
- Cho, E.; Yu, Y.J.; Kim, Y.; Phan, T.N.; Park, D.; Ko, C.H. Egg-shell-type Ni supported on MgAl2O4 pellets as catalyst for steam methane reforming: Enhanced coke-resistance and pellet stability. Catal. Today 2020, 352, 157–165. [Google Scholar] [CrossRef]
- Kim, C.H.; Han, J.Y.; Lim, H.; Lee, K.Y.; Ryi, S.K. Methane steam reforming using a membrane reactor equipped with a Pd-based composite membrane for effective hydrogen production. Int. J. Hydrogen Energy 2018, 43, 5863–5872. [Google Scholar] [CrossRef]
- Wang, M.; Tan, X.; Motuzas, J.; Li, J.; Liu, S. Hydrogen production by methane steam reforming using metallic nickel hollow fiber membranes. J. Membr. Sci. 2021, 620, 118909. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, R.L.B.A.; Melo, V.R.M.; Melo, D.M.A.; Macedo, H.P.; Moure, G.T.; Adánez-Rubio, I.; Melo, M.A.F.; Adánez, J. Double perovskite (La2−xCa-Bax)NiO4 oxygen carriers for chemical looping reforming applications. Int. J. Hydrogen Energy 2019, 45, 1681–1696. [Google Scholar] [CrossRef]
- Moghtaderi, B. Review of the recent chemical looping process developments for novel energy and fuel applications. Energy Fuels 2012, 26, 15–40. [Google Scholar] [CrossRef]
- Barros do Nascimento, R.A.; Pimenta de Macedo, H.; Melo, D.M.A.; Santiago, R.C.; Rodrigues de Araújo, T.; Medeiros, R.L.B.A.; Adánez, J. Structure and Reactivity of Brazilian Iron Ores as Low-Cost Oxygen Carriers for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2022, 2469–2482. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Chen, S.; Li, M.; Zhang, L.; Xiang, W. Chemical looping dry reforming of methane with hydrogen generation on Fe2O3/Al2O3 oxygen carrier. Chem. Eng. J. 2019, 368, 812–823. [Google Scholar] [CrossRef]
- Chein, R.Y.; Hsu, W.H. Thermodynamic analysis of syngas production via chemical looping dry reforming of methane. Energy 2019, 180, 535–547. [Google Scholar] [CrossRef]
- Löfberg, A.; Kane, T.; Guerrero-Caballero, J.; Jalowiecki-Duhamel, L. Chemical looping dry reforming of methane: Toward shale-gas and biogas valorization. Chem. Eng. Process. Process Intensif. 2017, 122, 523–529. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Gu, Z.; Zhu, X.; Qing, S.; Li, K. Chemical-Looping Conversion of Methane: A Review. Energy Technol. 2020, 8, 1900925. [Google Scholar] [CrossRef]
- Guerrero-Caballero, J.; Kane, T.; Haidar, N.; Jalowiecki-Duhamel, L.; Löfberg, A. Ni, Co, Fe supported on Ceria and Zr doped Ceria as oxygen carriers for chemical looping dry reforming of methane. Catal. Today 2019, 333, 251–258. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.S.; Lee, M.; Lee, J.W. Ni-Fe-Al mixed oxide for combined dry reforming and decomposition of methane with CO2 utilization. Catal. Today 2021, 368, 86–95. [Google Scholar] [CrossRef]
- Sastre, D.; Galván, C.Á.; Pizarro, P.; Coronado, J.M. Enhanced performance of CH4 dry reforming over La0.9Sr0.1FeO3/YSZ under chemical looping conditions. Fuel 2022, 309, 122122. [Google Scholar] [CrossRef]
- Soria, M.A.; Mateos-Pedrero, C.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Thermodynamic and experimental study of combined dry and steam reforming of methane on Ru/ZrO2-La2O3 catalyst at low temperature. Int. J. Hydrogen Energy 2011, 36, 15212–15220. [Google Scholar] [CrossRef]
- Park, N.; Park, M.J.; Baek, S.C.; Ha, K.S.; Lee, Y.J.; Kwak, G.; Park, H.G.; Jun, K.W. Modeling and optimization of the mixed reforming of methane: Maximizing CO2 utilization for non-equilibrated reaction. Fuel 2014, 115, 357–365. [Google Scholar] [CrossRef]
- Jokar, S.M.; Parvasi, P.; Basile, A. The evaluation of methane mixed reforming reaction in an industrial membrane reformer for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 15321–15329. [Google Scholar] [CrossRef]
- Jabbour, K. Tuning combined steam and dry reforming of methane for “metgas” production: A thermodynamic approach and state-of-the-art catalysts. J. Energy Chem. 2020, 48, 54–91. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Lazar, M.D. Hydrogen and/or syngas production by combined steam and dry reforming of methane on nickel catalysts. Int. J. Hydrogen Energy 2020, 45, 26254–26264. [Google Scholar] [CrossRef]
- Batebi, D.; Abedini, R.; Mosayebi, A. Combined steam and CO2 reforming of methane (CSCRM) over Ni–Pd/Al2O3 catalyst for syngas formation. Int. J. Hydrogen Energy 2020, 45, 14293–14310. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Cuenya, B.R.; Fierro, J.L.G. Partial oxidation of methane to syngas over nickel-based catalysts: Influence of support type, addition of rhodium, and preparation method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseno, K.T.C.; Schmal, M.; Brackmann, R.; Alves, R.M.B.; Giudici, R. Partial oxidation of methane on neodymium and lanthanium chromate based perovskites for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 8166–8177. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Ding, C.; Wang, J.; Guo, S.; Ma, Z.; Li, Y.; Ma, L.; Zhang, K. Abundant hydrogen production over well dispersed nickel nanoparticles confined in mesoporous metal oxides in partial oxidation of methane. Int. J. Hydrogen Energy 2019, 44, 30171–30184. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Gossage, J.L.; Lou, H.H.; Benson, T.J. Thermodynamic analyses of tri-reforming reactions to produce syngas. Energy Fuels 2014, 28, 2717–2726. [Google Scholar] [CrossRef]
- Pham Minh, D.; Pham Xuan, H.; Siang, T.J.; N.Vo, D.-V. A review on the impact of operating conditions, catalyst deactivation and regeneration in tri-reforming of methane. Appl. Catal. A Gen. 2021, 621, 118202. [Google Scholar] [CrossRef]
- MacIel, L.J.L.; De Souza, A.E.M.; Cavalcanti-Filho, V.O.; Knoechelmann, A.; De Abreu, C.A.M. Kinetic evaluation of the tri-reforming process of methane for syngas production. React. Kinet. Mech. Catal. 2010, 101, 407–416. [Google Scholar] [CrossRef]
- Majewski, A.J.; Wood, J. Tri-reforming of methane over Ni@SiO2 catalyst. Int. J. Hydrogen Energy 2014, 39, 12578–12585. [Google Scholar] [CrossRef] [Green Version]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Hydrogen production by methane tri-reforming process over Ni-ceria catalysts: Effect of La-doping. Appl. Catal. B Environ. 2011, 104, 64–73. [Google Scholar] [CrossRef]
- García-Vargas, J.M.; Valverde, J.L.; Dorado, F.; Sánchez, P. Influence of the support on the catalytic behaviour of Ni catalysts for the dry reforming reaction and the tri-reforming process. J. Mol. Catal. A Chem. 2014, 395, 108–116. [Google Scholar] [CrossRef]
- Chutichairattanaphum, N.; Narataruksa, P.; Pana-Suppamassadu, K.; Tungkamani, S.; Prapainainar, C.; Chotiwan, S.; Wattanathana, W. Effects of Raschig Ring Packing Patterns on Pressure Drop, Heat Transfer, Methane Conversion, and Coke Deposition on a Semi-pilot-scale Packed Bed Reformer. Chem. Biochem. Eng. Q. 2019, 33, 191–211. [Google Scholar] [CrossRef]
- Kahle, L.C.S.; Roussière, T.; Maier, L.; Herrera Delgado, K.; Wasserschaff, G.; Schunk, S.A.; Deutschmann, O. Methane dry reforming at high temperature and elevated pressure: Impact of gas-phase reactions. Ind. Eng. Chem. Res. 2013, 52, 11920–11930. [Google Scholar] [CrossRef]
- Wang, H.; Duan, X.; Liu, X.; Ye, G.; Gu, X.; Zhu, K.; Zhou, X.; Yuan, W. Influence of tubular reactor structure and operating conditions on dry reforming of methane. Chem. Eng. Res. Des. 2018, 139, 39–51. [Google Scholar] [CrossRef]
- Aouad, S.; Labaki, M.; Ojala, S.; Seelam, P.; Turpeinen, E.; Gennequin, C.; Estephane, J.; Aad, E.A. A Review on the Dry Reforming Processes for Hydrogen Production: Catalytic Materials and Technologies. In Catalytic Materials for Hydrogen Production and Electro-Oxidation Reactions; Bentham Books: Sharjah, United Arab Emirates, 2018; pp. 60–128. [Google Scholar]
- Caloric GmbH Carbon Monoxide Generation Plants and Production Technologies. Available online: https://www.caloric.com/en/product/carbon-monoxide-generation-plants/ (accessed on 9 February 2022).
- Teuner, S.C.; Neumann, P.; Von Linde, F. The Calcor standard and Calcor economy processes. Oil Gas Eur. Mag. 2001, 27, 44–46. [Google Scholar]
- Van Diepen, A.E.; Kapteijn, F.; Makkee, M.; Moulijn, J.A. Contribution of Catalysis Towards the Reduction of Atmospheric Air Pollution: CO2, CFCs, N2O, Ozone. In Environmental Catalysis; Janssen, F.J.J., van Santen, R.A., Eds.; Imperial College Press: London, UK, 1999; pp. 219–256. [Google Scholar]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Midrex Technologies Inc. MIDREX Plants. Available online: https://www.midrex.com/about-midrex/midrex-plants-map/ (accessed on 10 February 2022).
- Sarkar, S.; Bhattacharya, R.; Roy, G.G.; Sen, P.K. Modeling MIDREX Based Process Configurations for Energy and Emission Analysis. Steel Res. Int. 2018, 89, 1700248. [Google Scholar] [CrossRef]
- Farhadi, F.; Motemed Hashemi, M.Y.; Bahrami Babaheidari, M. Modelling and simulation of syngas unit in large scale direct reduction plant. Ironmak. Steelmak. 2003, 30, 18–24. [Google Scholar] [CrossRef]
- Linde Engineering. Smaller Carbon Footprint. Higher Process Efficiency. Available online: https://www.engineering.linde.com/dryref (accessed on 9 February 2022).
- Bartesch, T.; Wawrzinek, K.; Behrens, A.; Peschel, A. DRYREF & SYNSPIRE Innovation for HyCO Applications. In Proceedings of the Global Syngas Technologies Conference, Virtual Conference, 20–21, 27–28 October 2020. [Google Scholar]
- York, A.P.E.; Xiao, T.; Green, M.L.H.; Claridge, J.B. Methane Oxyforming for Synthesis Gas Production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Shah, Y.T.; Gardner, T.H. Dry Reforming of Hydrocarbon Feedstocks. Catal. Rev. 2014, 56, 476–536. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Rezaei, E.; Dzuryk, S. Techno-economic comparison of reverse water gas shift reaction to steam and dry methane reforming reactions for syngas production. Chem. Eng. Res. Des. 2019, 144, 354–369. [Google Scholar] [CrossRef]
- Osaki, T.; Horiuchi, T.; Suzuki, K.; Mori, T. Suppression of carbon deposition in CO2-reforming of methane on metal sulfide catalysts. Catal. Lett. 1995, 35, 39–43. [Google Scholar] [CrossRef]
- Midrex Technologies Inc. About Midrex. Available online: https://www.midrex.com/about-midrex/ (accessed on 10 February 2022).
- Linde Engineering. Technologies that Do More with Less. Available online: https://www.linde-engineering.com/en/about-linde-engineering/success-stories/technologies-more-with-less.html (accessed on 9 February 2022).
- Kelling, R.; Eigenberger, G.; Nieken, U. Ceramic counterflow reactor for autothermal dry reforming at high temperatures. Catal. Today 2016, 273, 196–204. [Google Scholar] [CrossRef]
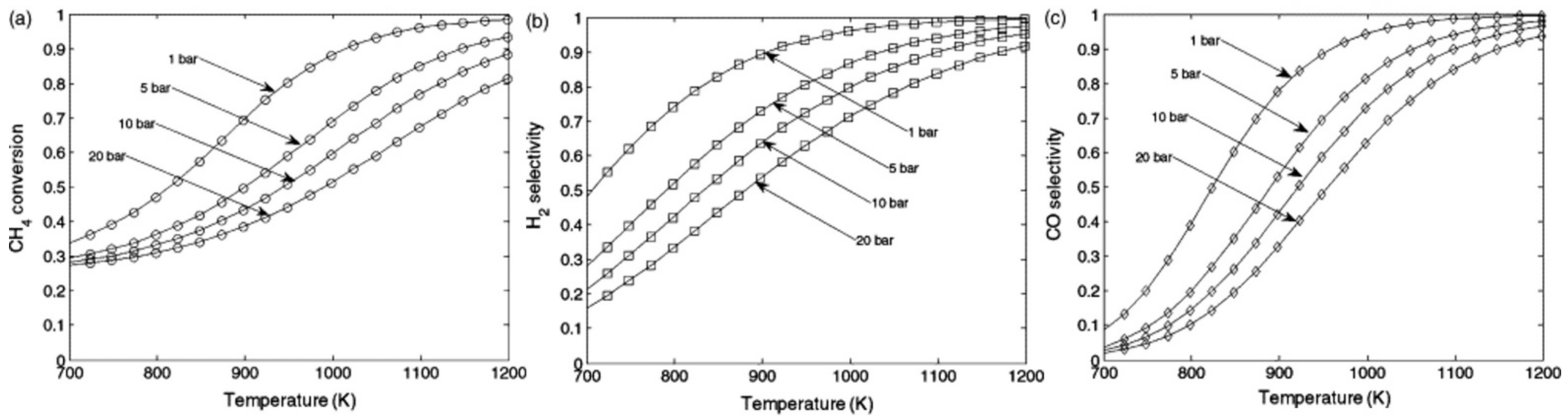
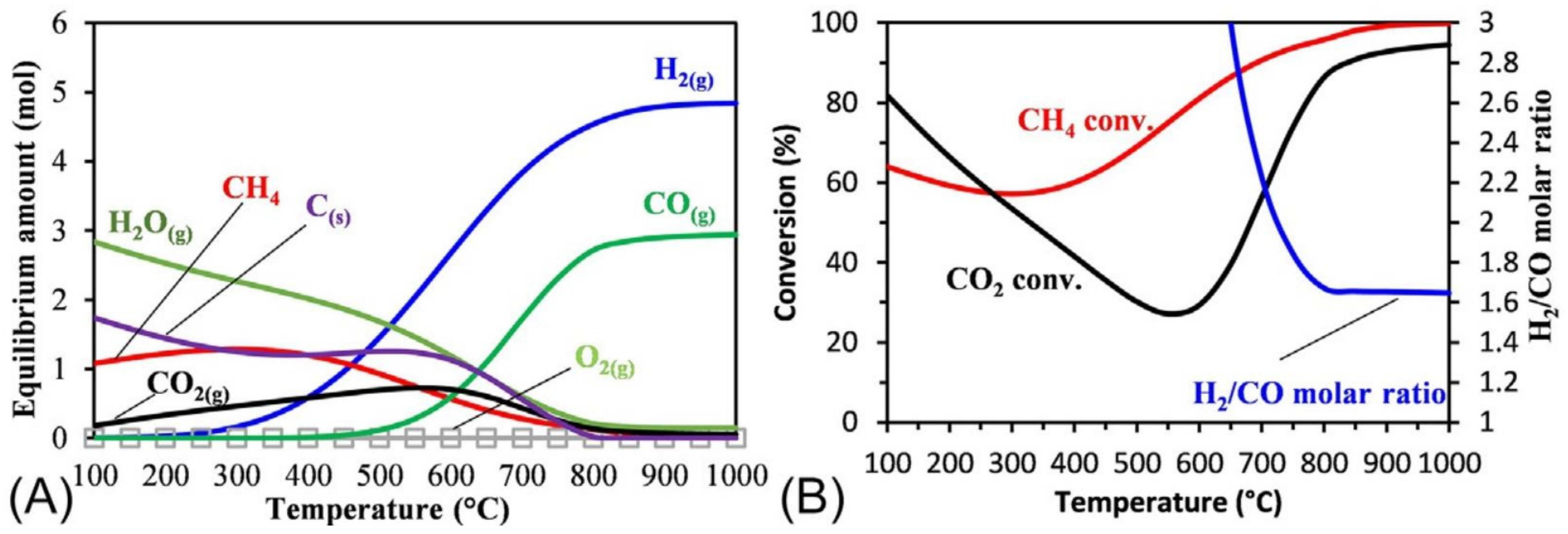

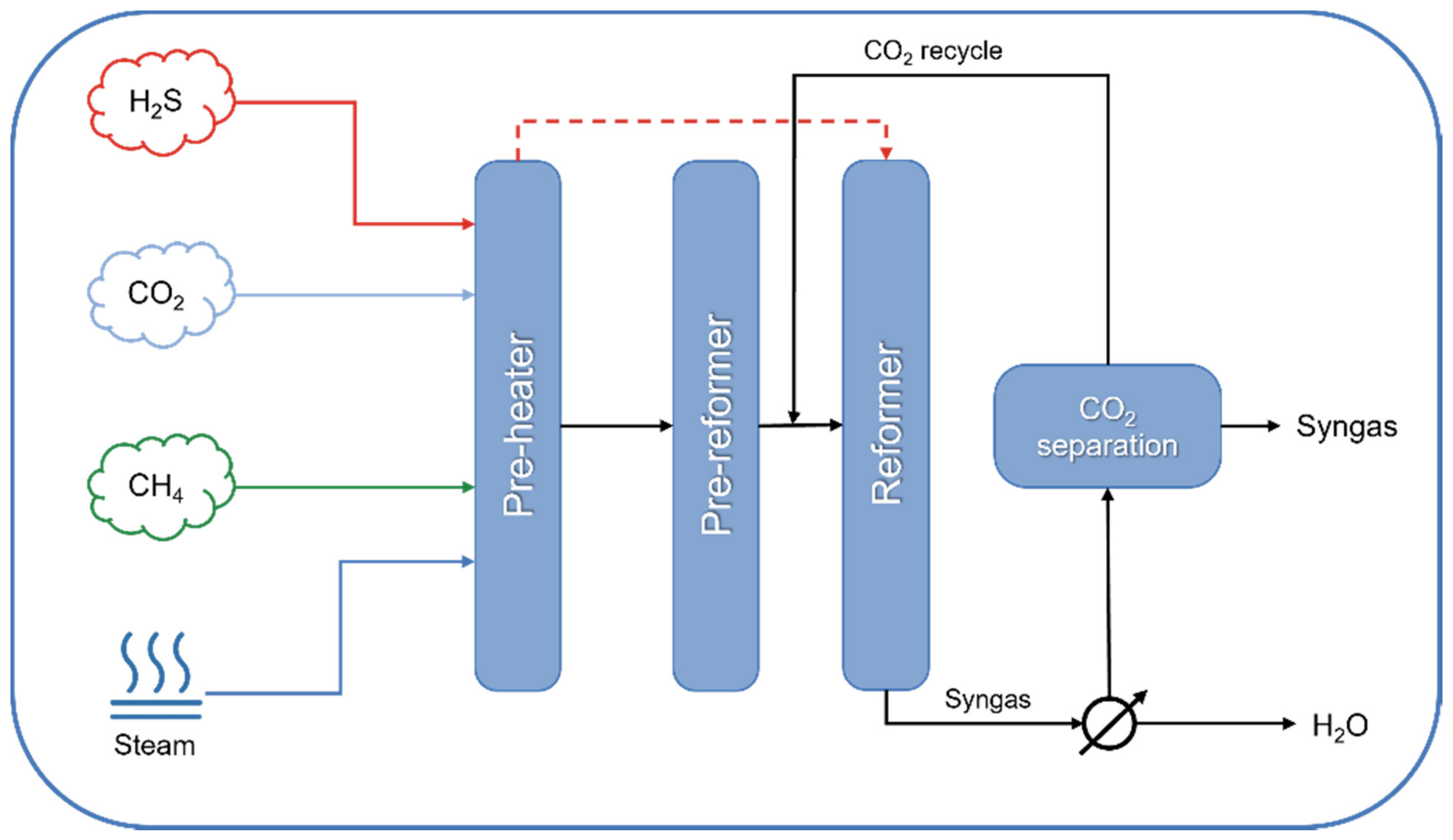
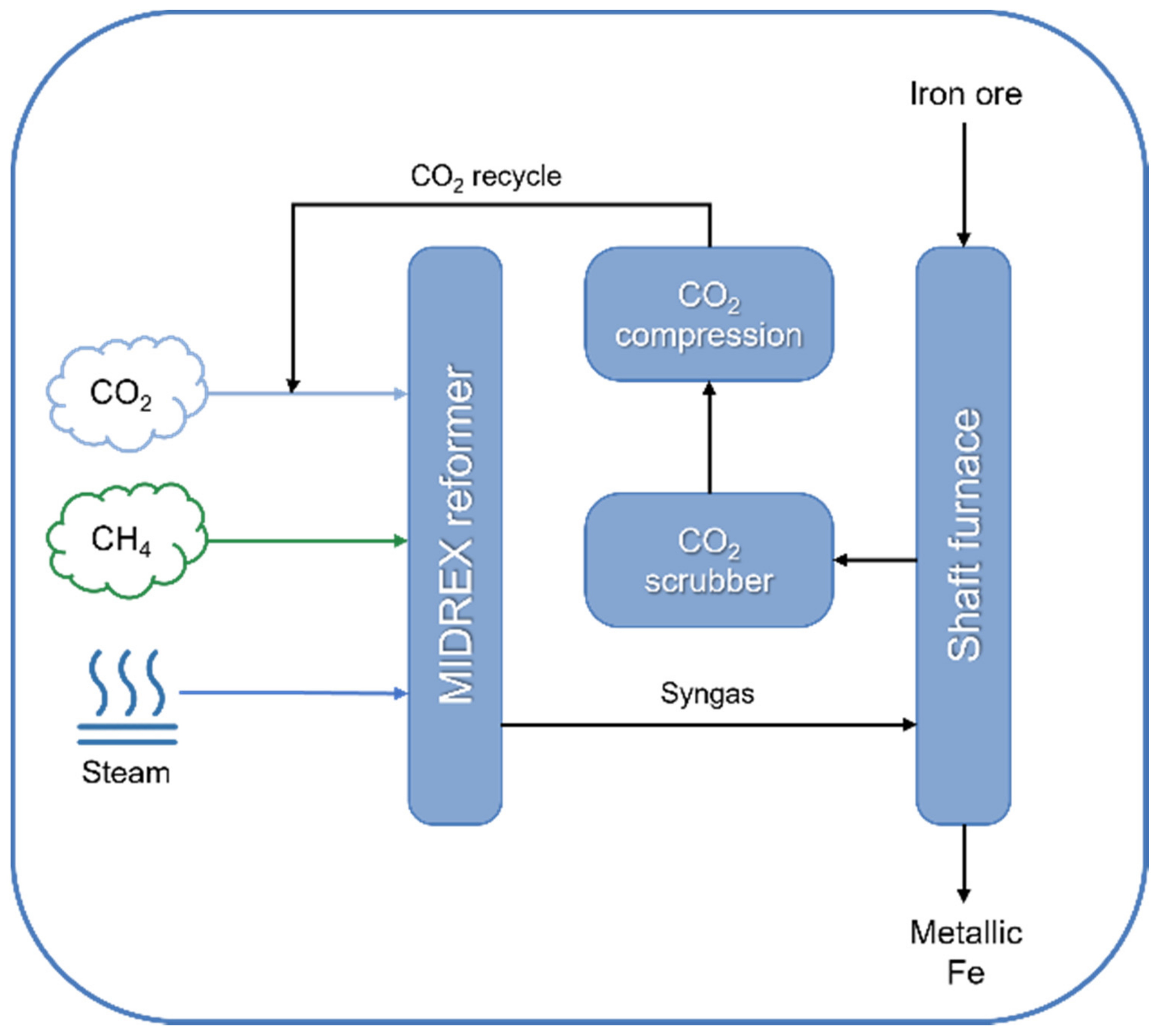
| Technology | Catalyst | Feed Composition | Operation Conditions | H2/CO Ratio | TRL | Process Application | Ref. |
|---|---|---|---|---|---|---|---|
| DRM | 10 wt% Ni/Al2O3-MgO | CO2:CH4:N2 = 2:1:0.5 | 600 °C, 1 bar | >1.0 | Semi-pilot scale | Syngas production | [150] |
| DRM | Pt-based | CH4:CO2:Ar = 1:1:0.05 | 850–950 °C, 20 bar | 0.7–0.8 | Pilot-scale | Syngas production | [151] |
| CALCOR™ (Caloric GmbH) | Ni-based | CH4, LPG, CO2. Feed composition not reported. | Low pressure, high temperature | 0.42–1.0 | Commercial | CO production | [154,155] |
| SPARG™ (Haldor-Topsoe) | Partially sulfur-poisoned Ni | CH4, H2O, CO2, H2S (pre-reforming, for catalyst passivation). CO2/CH4 = 0.07–2.5; H2O/CH4: 0.1–0.26 | 875–945 °C | 0.55–3.2 | Commercial | Syngas production | [156,157,158] |
| MIDREX™ (Midrex Technologies) | Ni-based, supported on α-Al2O3 and/or MgO | CH4:CO2:H2O = 1:1:1 | 850–1000 °C, 2–3 bar | 1.7–2.5 | Commercial | Metallic Fe production | [159,160,161] |
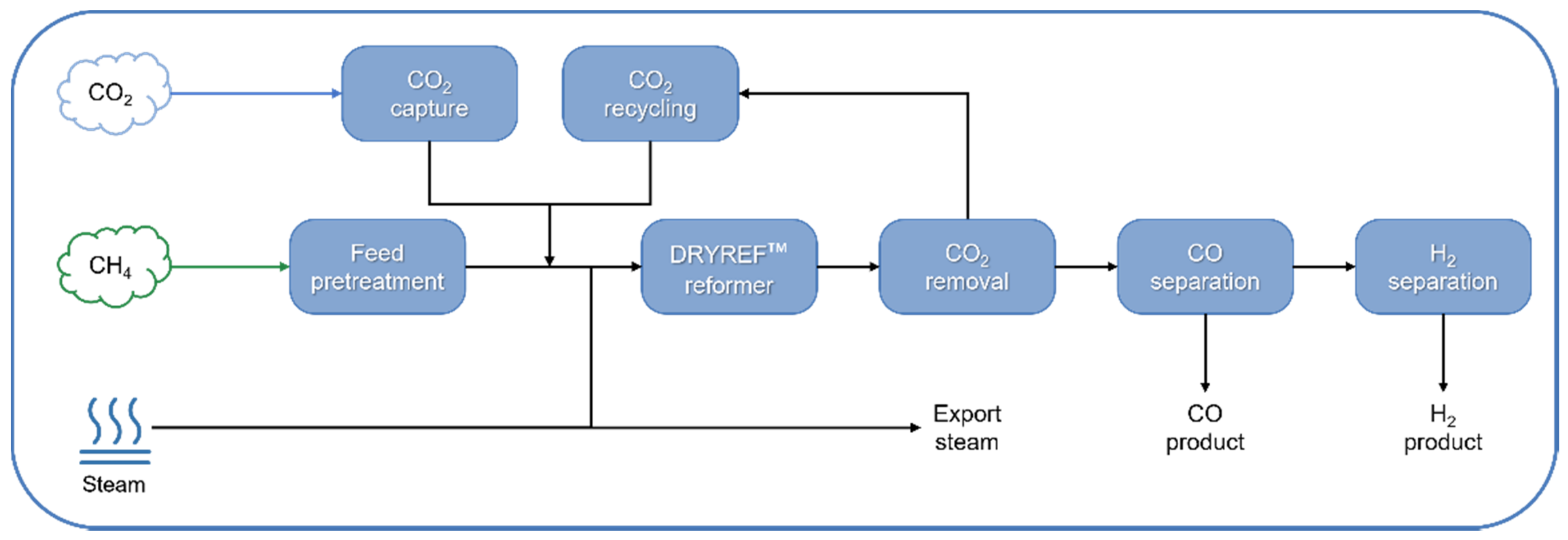
| DRYREF™ (Linde and BASF) | Ni-based oxide-supported tablets (4-hole quadrilobes) | CH4, H2O, CO2 (S/C < 1.8) | 800–950 °C, 20–40 bar | 1.0–3.0 | Commercial demonstration (since 2019) | CO and H2 production | [162,163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Medeiros, F.G.M.; Lopes, F.W.B.; Rego de Vasconcelos, B. Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane. Catalysts 2022, 12, 363. https://doi.org/10.3390/catal12040363
de Medeiros FGM, Lopes FWB, Rego de Vasconcelos B. Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane. Catalysts. 2022; 12(4):363. https://doi.org/10.3390/catal12040363
Chicago/Turabian Stylede Medeiros, Fábio Gonçalves Macêdo, Francisco Wendell Bezerra Lopes, and Bruna Rego de Vasconcelos. 2022. "Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane" Catalysts 12, no. 4: 363. https://doi.org/10.3390/catal12040363
APA Stylede Medeiros, F. G. M., Lopes, F. W. B., & Rego de Vasconcelos, B. (2022). Prospects and Technical Challenges in Hydrogen Production through Dry Reforming of Methane. Catalysts, 12(4), 363. https://doi.org/10.3390/catal12040363







