Influences of Ni Content on the Microstructural and Catalytic Properties of Perovskite LaNixCr1−xO3 for Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
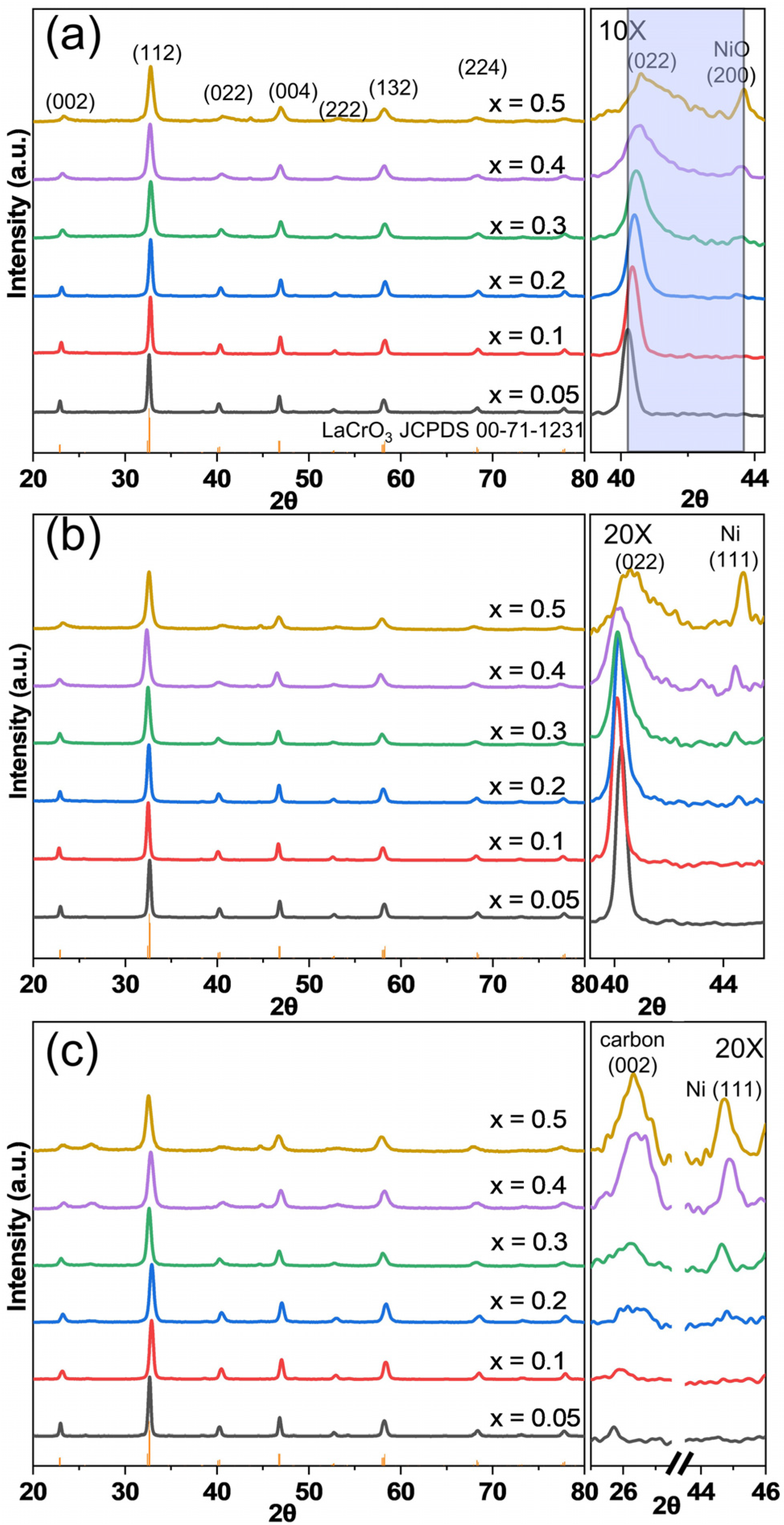
2.1. Crystalline Structure and Specific Surface Areas
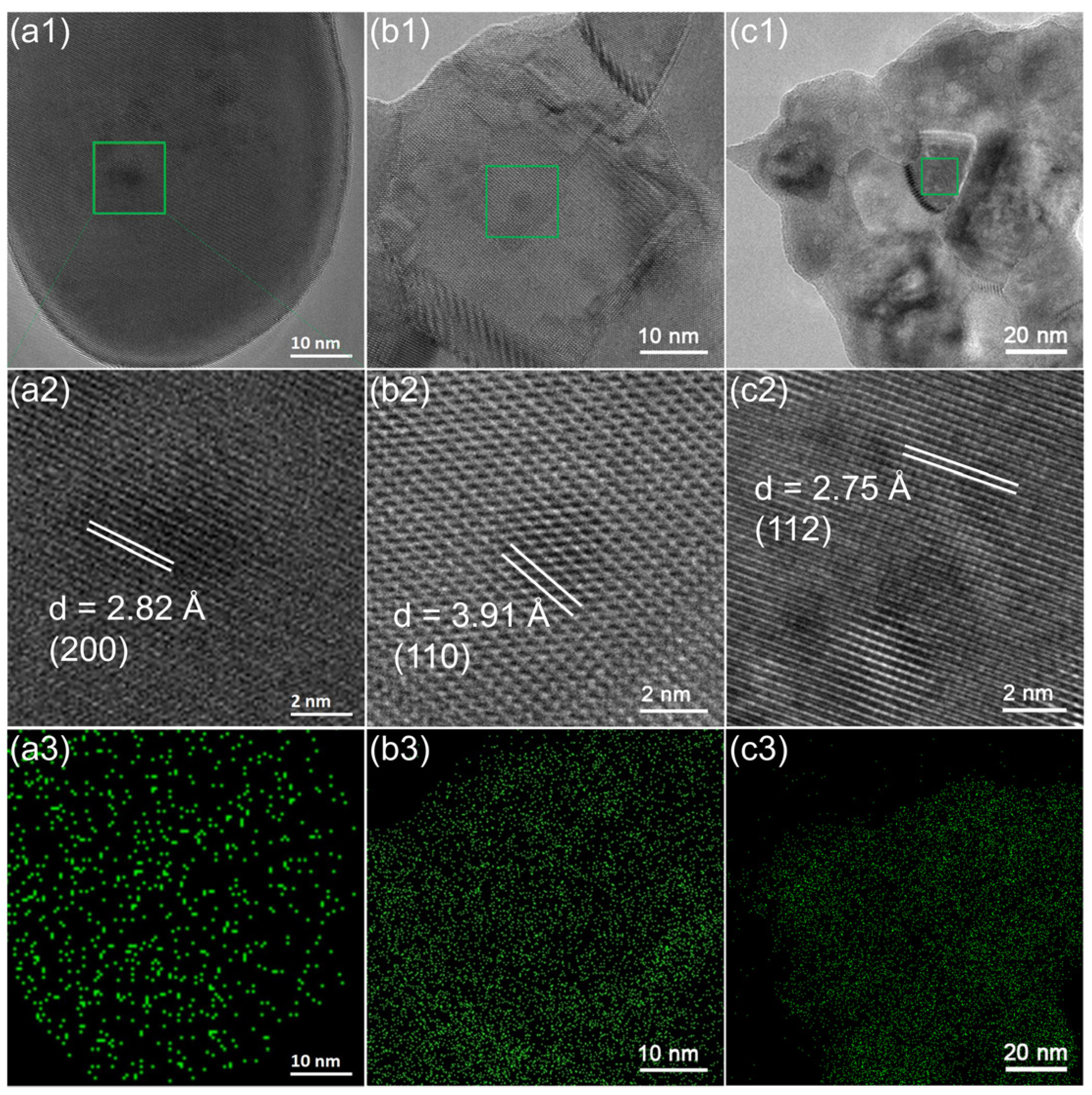
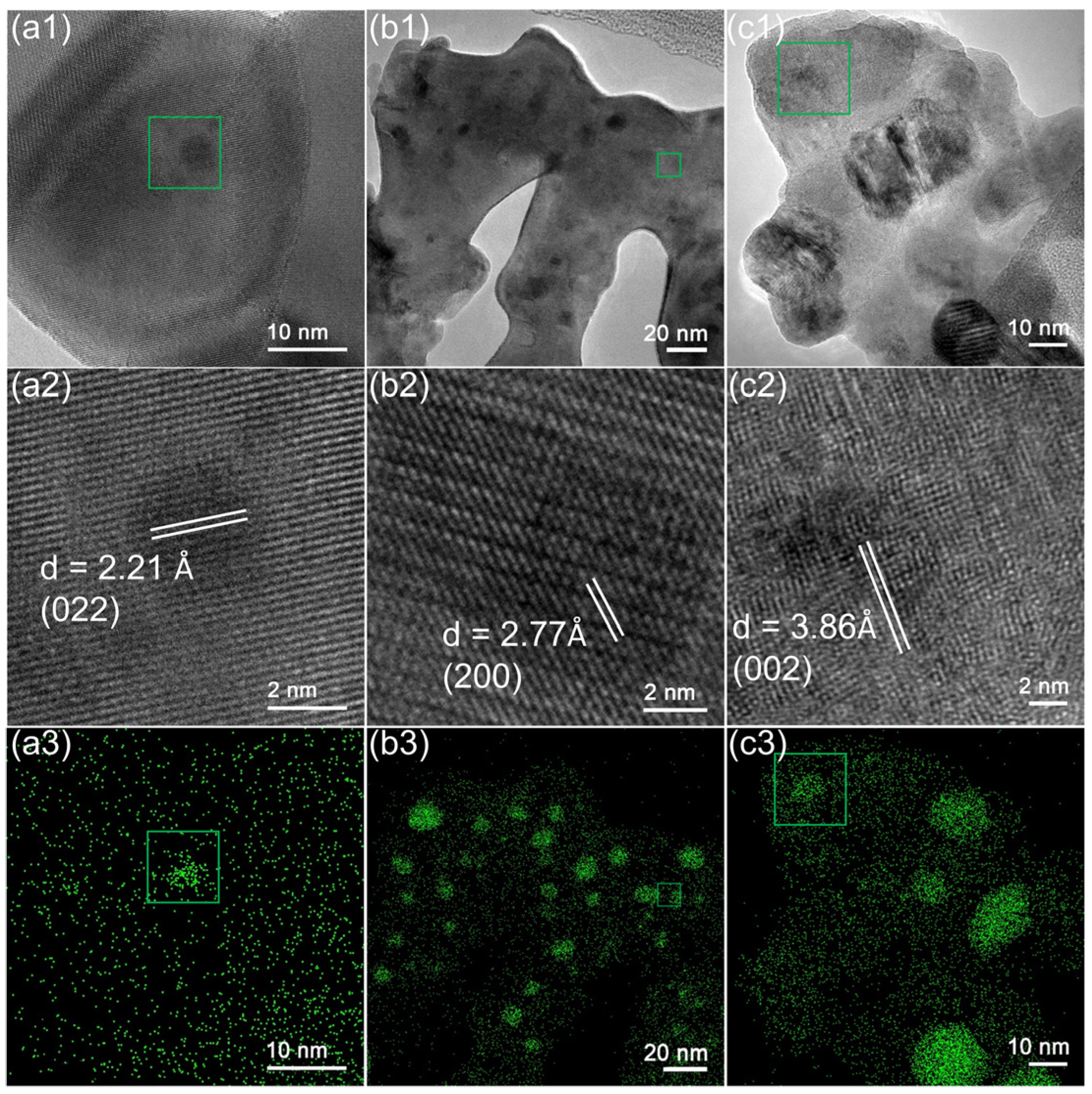
2.2. Microstructure
2.3. Electronic Structure
2.4. Reducibility
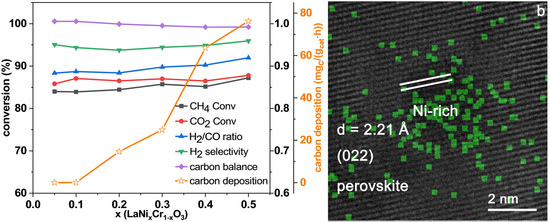
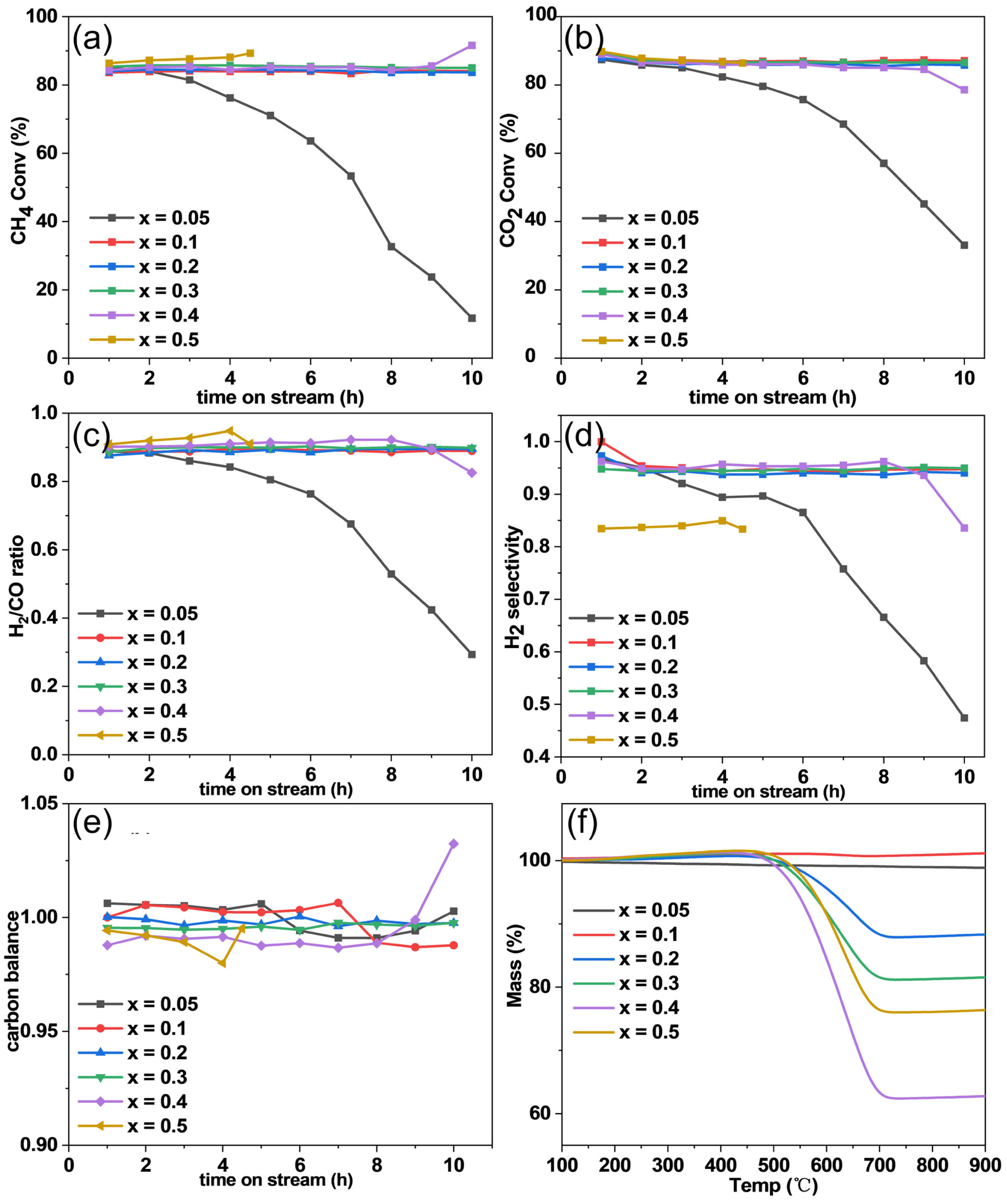
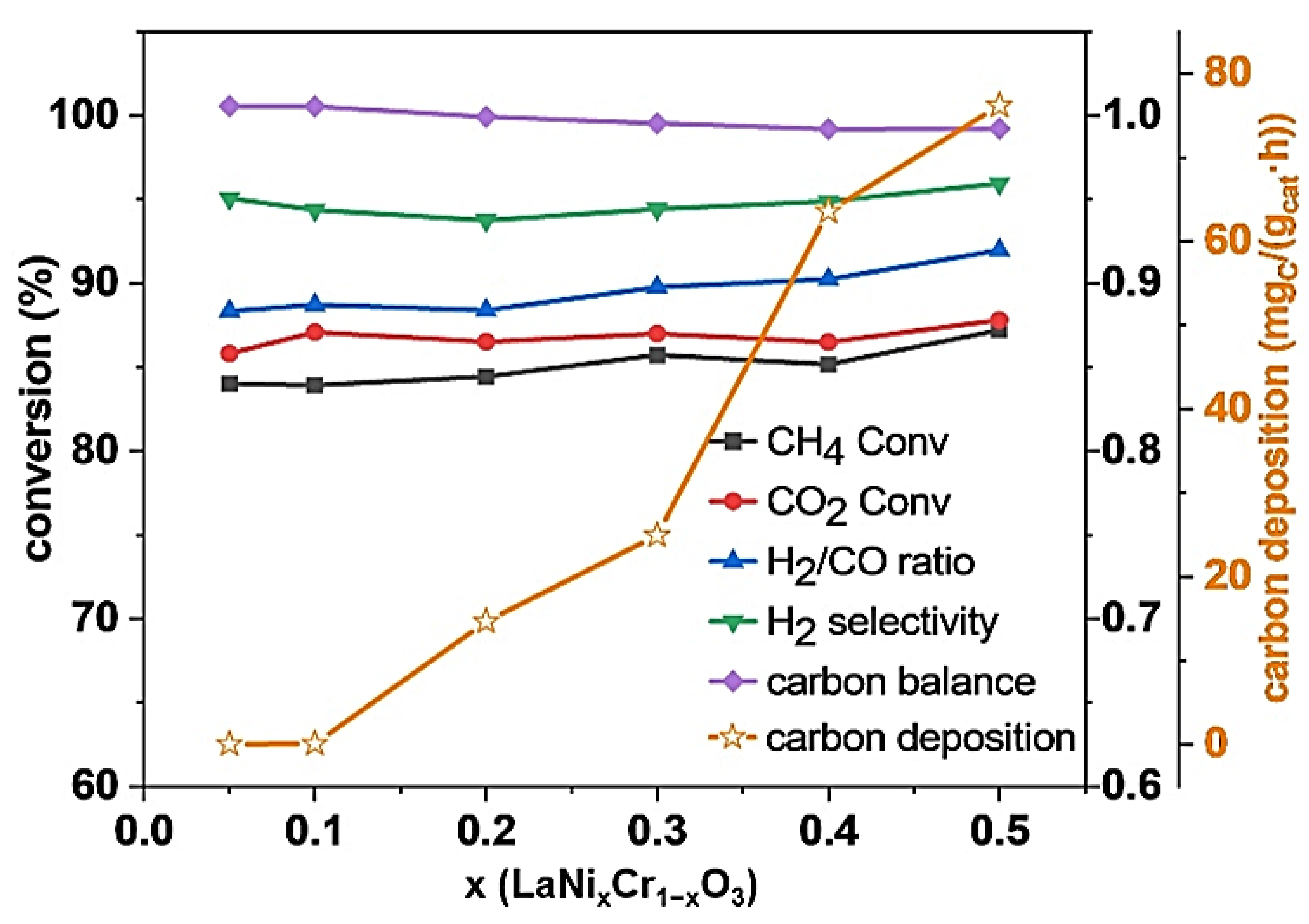
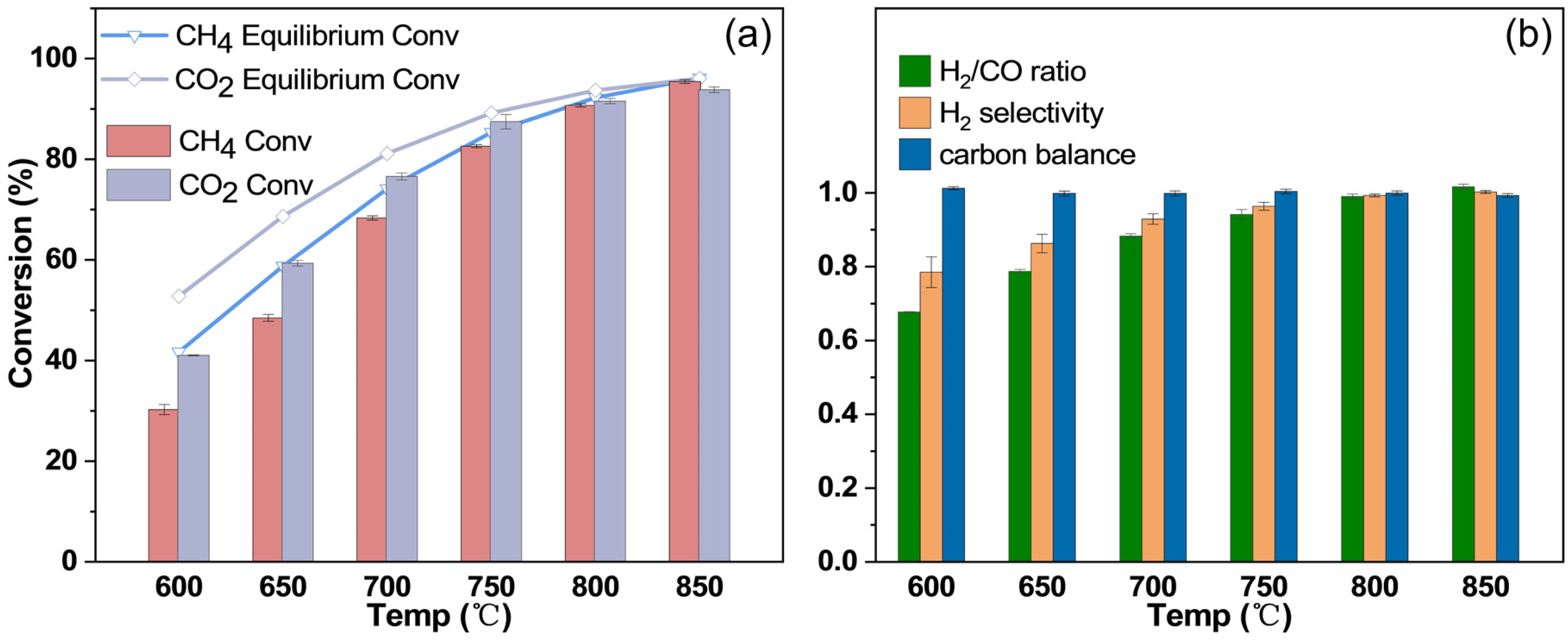
2.5. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O.; Batista, J.; Levec, J.; Pintar, A. Catalytic syngas production from greenhouse gasses: Performance comparison of Ru-Al2O3 and Rh-CeO2 catalysts. Chem. Eng. Process. Process Intensif. 2011, 50, 1054–1062. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Wang, Y.; Chu, W.; Liu, M. A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier. Catal. Today 2013, 212, 98–107. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ. 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; de Navarro, C.U.; Goldwasser, M.R. LaNi1−xMnxO3 perovskite-type oxides as catalysts precursors for dry reforming of methane. Appl. Catal. A Gen. 2018, 565, 26–33. [Google Scholar] [CrossRef]
- Sagar, T.V.; Sreelatha, N.; Hanmant, G.; Surendar, M.; Lingaiah, N.; Rama Rao, K.S.; Satyanarayana, C.V.V.; Reddy, I.A.K.; Sai Prasad, P.S. Influence of method of preparation on the activity of La-Ni-Ce mixed oxide catalysts for dry reforming of methane. RSC Adv. 2014, 4, 50226–50232. [Google Scholar] [CrossRef]
- Surendar, M.; Sagar, T.V.; Raveendra, G.; Ashwani Kumar, M.; Lingaiah, N.; Rama Rao, K.S.; Sai Prasad, P.S. Pt doped LaCoO3 perovskite: A precursor for a highly efficient catalyst for hydrogen production from glycerol. Int. J. Hydrogen Energy 2016, 41, 2285–2297. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Khatri, J.; Kumar, R.; Kumar Srivastava, V.; Osman, A.I.; AlGarni, T.S.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H.; Rooney, D.W. Role of Ca, Cr, Ga and Gd promotor over lanthana-zirconia–supported Ni catalyst towards H2-rich syngas production through dry reforming of methane. Energy Sci. Eng. 2022, 10, 866–880. [Google Scholar] [CrossRef]
- Lanre, M.S.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Al-Awadi, A.S.; Jumah, A.B.; Al-Mubaddel, F.S.; Al-Fatesh, A.S. Lanthanum-Cerium-Modified Nickel Catalysts for Dry Reforming of Methane. Catalysts 2022, 12, 715. [Google Scholar] [CrossRef]
- Lanre, M.S.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Alquraini, A.A.; AlReshaidan, S.B.; Al-Fatesh, A.S. Modification of CeNi0.9Zr0.1O3 Perovskite Catalyst by Partially Substituting Yttrium with Zirconia in Dry Reforming of Methane. Materials 2022, 15, 3564. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Seiyama, T. Total Oxidation of Hydrocarbons on Perovskite Oxides. Catal. Rev. 1992, 34, 281–300. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W., Jr.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef]
- Singh, U.G.; Li, J.; Bennett, J.W.; Rappe, A.M.; Seshadri, R.; Scott, S.L. A Pd-doped perovskite catalyst, BaCe1−xPdxO3−δ, for CO oxidation. J. Catal. 2007, 249, 349–358. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3 Perovskite Catalysts for Boosting Dry Reforming of CH4 Using CO2 for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Urasaki, K.; Sekine, Y.; Kawabe, S.; Kikuchi, E.; Matsukata, M. Catalytic activities and coking resistance of Ni/perovskites in steam reforming of methane. Appl. Catal. A Gen. 2005, 286, 23–29. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1−x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Song, X.; Dong, X.L.; Yin, S.L.; Wang, M.; Li, M.; Wang, H.Q. Effects of Fe partial substitution of La2NiO4/LaNiO3 catalyst precursors prepared by wet impregnation method for the dry reforming of methane. Appl. Catal. A-Gen. 2016, 526, 132–138. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, J.; Hou, H.; Xu, S.; Liu, G.; Hussain, S.; Qiao, G. Synthesis and microstructures of La1−xCaxCrO3 perovskite powders for optical properties. J. Mater. Sci. Mater. Electron. 2019, 30, 3472–3481. [Google Scholar] [CrossRef]
- Nakamura, T.; Petzow, G.; Gauckler, L.J. Stability of the perovskite phase LaBO3 (B = V, Cr, Mn, Fe, Co, Ni) in reducing atmosphere I. Experimental results. Mater. Res. Bull. 1979, 14, 649–659. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, J.; Tao, X.; Yu, H.; Li, M.; Zeng, J.; Wang, H. Highly active and thermostable submonolayer La(NiCo)OΔ catalyst stabilized by a perovskite LaCrO3 support. Commun. Chem. 2022, 5, 70. [Google Scholar] [CrossRef]
- Yang, J. Structural analysis of perovskite LaCr1−xNixO3 by Rietveld refinement of X-ray powder diffraction data. Acta Cryst. B 2008, 64, 281–286. [Google Scholar] [CrossRef]
- Baraneedharan, P.; Siva, C.; Saranya, A.; Jayavel, R.; Nehru, K.; Sivakumar, M. Dual emissive Sn(1−2x)CuxCoxO2 nanostructures—A correlation study of doping concentration on structural, optical and electrical properties. Superlattices Microstruct. 2014, 68, 66–75. [Google Scholar] [CrossRef]
- Koli, P.B.; Kapadnis, K.H.; Deshpande, U.G.; Tupe, U.J.; Shinde, S.G.; Ingale, R.S. Fabrication of thin film sensors by spin coating using sol-gel LaCrO3 Perovskite material modified with transition metals for sensing environmental pollutants, greenhouse gases and relative humidity. Environ. Chall. 2021, 3, 100043. [Google Scholar] [CrossRef]
- Valderrama, G.; Urbina de Navarro, C.; Goldwasser, M.R. CO2 reforming of CH4 over Co–La-based perovskite-type catalyst precursors. J. Power Sources 2013, 234, 31–37. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Direct observation of Ni3+ and Ni2+ in correlated LaNiO3−δ films. EPL 2011, 93, 57002. [Google Scholar] [CrossRef]
- Liu, X.; Su, W.; Lu, Z.; Liu, J.; Pei, L.; Liu, W.; He, L. Mixed valence state and electrical conductivity of La1−xSrxCrO3. J. Alloys Compd. 2000, 305, 21–23. [Google Scholar] [CrossRef]
- Álvarez-Galván, M.C.; de la Peña O’Shea, V.A.; Arzamendi, G.; Pawelec, B.; Gandía, L.M.; Fierro, J.L.G. Methyl ethyl ketone combustion over La-transition metal (Cr, Co, Ni, Mn) perovskites. Appl. Catal. B Environ. 2009, 92, 445–453. [Google Scholar] [CrossRef]
- Rida, K.; Benabbas, A.; Bouremmad, F.; Peña, M.A.; Sastre, E.; Martínez-Arias, A. Effect of calcination temperature on the structural characteristics and catalytic activity for propene combustion of sol-gel derived lanthanum chromite perovskite. Appl. Catal. A Gen. 2007, 327, 173–179. [Google Scholar] [CrossRef]
- Zhang, G.J.; Liu, R.; Yang, Y.; Jia, Y.Q. Metal-insulator transition and variations of crystal structures and valence states of Cr and Ni ions in LaCr1−xNixO3. Phys. Status Solidi A 1997, 160, 19–27. [Google Scholar] [CrossRef]
- Liang, H.; Hong, Y.; Zhu, C.; Li, S.; Chen, Y.; Liu, Z.; Ye, D. Influence of partial Mn-substitution on surface oxygen species of LaCoO3 catalysts. Catal. Today 2013, 201, 98–102. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Zhang, R.; Liu, T.; Xia, L.; Chen, Z.; Fang, X.; Xu, X.; Xu, J.; Wang, X. Ni/LaBO3 (B = Al, Cr, Fe) Catalysts for Steam Reforming of Methane (SRM): On the Interaction Between Ni and LaBO3 Perovskites with Differed Fine Structures. Catal. Surv. Asia 2021, 25, 424–436. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Q.; Cui, J. XPS study of surface absorbed oxygen of ABO3 mixed oxides. J. Rare Earths 2008, 26, 511–514. [Google Scholar] [CrossRef]
- Rida, K.; Peña, M.A.; Sastre, E.; Martinez-Arias, A. Effect of calcination temperature on structural properties and catalytic activity in oxidation reactions of LaNiO3 perovskite prepared by Pechini method. J. Rare Earths 2012, 30, 210–216. [Google Scholar] [CrossRef]
- Wei, T.; Jia, L.; Luo, J.-L.; Chi, B.; Pu, J.; Li, J. CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 2020, 506, 144699. [Google Scholar] [CrossRef]
- Guo, T.; Dong, X.; Shirolkar, M.M.; Song, X.; Wang, M.; Zhang, L.; Li, M.; Wang, H. Effects of Cobalt Addition on the Catalytic Activity of the Ni-YSZ Anode Functional Layer and the Electrochemical Performance of Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 16131–16139. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, M.; Haverkamp, R.G.; Mims, C.A.; Moudallal, H.; Jacobson, A.J. Synthesis and Characterization of LaCr1−xNixO3Perovskite Oxide Catalysts. J. Catal. 1997, 166, 315–323. [Google Scholar] [CrossRef]
- Zhou, R.; Mohamedali, M.; Ren, Y.; Lu, Q.; Mahinpey, N. Facile synthesis of multi-layered nanostructured Ni/CeO2 catalyst plus in-situ pre-treatment for efficient dry reforming of methane. Appl. Catal. B Environ. 2022, 316, 121696. [Google Scholar] [CrossRef]
- Odedairo, T.; Zhou, W.; Chen, J.; Zhu, Z. Flower-like perovskite LaCr0.9Ni0.1O3−δ-NiO nanostructures: A new candidate for CO2 reforming of methane. RSC Adv. 2014, 4, 21306–21312. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Preparing catalytic materials by the sol-gel method. Ind. Eng. Chem. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]



| x in LaNixCr1−xO3 | Average Crystallite Size/nm | ||
|---|---|---|---|
| Fresh | Reduced | Used | |
| 0.05 | 29.1 | 26.1 | 28.4 |
| 0.1 | 25.8 | 25.8 | 19.3 |
| 0.2 | 21.1 | 21.8 | 16.0 |
| 0.3 | 15.1 | 17.3 | 16.3 |
| 0.4 | 13.7 | 14.9 | 12.4 |
| 0.5 | 12.7 | 13.9 | 12.0 |
| x in LaNixCr1−xO3 | Specific Surface Area (m2 g−1) | Correlation Coefficient |
|---|---|---|
| 0.05 | 9.7 | 0.9999 |
| 0.1 | 9.4 | 0.9977 |
| 0.2 | 7.2 | 0.9984 |
| 0.3 | 9.4 | 0.9996 |
| 0.4 | 9.9 | 0.9997 |
| 0.5 | 9.5 | 0.9998 |
| x in LaNixCr1−xO3 | ΔE of Cr 3s for Fresh Samples (eV) | ΔE of Cr 3s for Used Samples (eV) |
|---|---|---|
| 0.1 | 4.2 | 3.8 |
| 0.3 | 4.5 | 4.0 |
| 0.5 | 5.0 | 4.6 |
| Catalyst | GHSV | Temperature (°C) | CH4 Conv (%) | CO2 Conv (%) | Ref. |
|---|---|---|---|---|---|
| LaNiO3 | 15 L g−1 h−1 | 750 | 99 | 95 | [11] |
| LaNi0.8Mn0.2O3 | 15 L g−1 h−1 | 750 | 97 | 95 | [11] |
| LaNi0.4Ce0.6O3 | 12 L g−1 h−1 | 800 | 93 | 93 | [12] |
| La0.6Ce0.4Ni0.5Fe0.5O3 | 12 L g−1 h−1 | 750 | 62 | 72 | [14] |
| La0.6Ce0.4Ni0.9Zr0.01Y0.09O3 | 42 L g−1 h−1 | 800 | 89 | 91 | [16] |
| CeNi0.9Zr0.01Y0.09O3 | 42 L g−1 h−1 | 800 | 90 | 91 | [17] |
| LaCr0.95Ir0.05O3−δ | 4000 h−1 | 750 | 81 | 82 | [23] |
| 10 wt% Pd–LaCr0.9Ni0.1O3−δ | 19.2 L g−1 h−1 | 750 | 63 | 96 | [47] |
| LaNi0.05Co0.05Cr0.9O3 | 12 L g−1 h−1 | 750 | 85 | 88 | [29] |
| LaNi0.1Cr0.9O3 | 12 L g−1 h−1 | 750 | 84 | 87 | this work |
| x in LaNixCr1−xO3 | La:Ni:Cr (Atomic Ratio) |
|---|---|
| 0.05 | 100:4.5:91.0 |
| 0.1 | 100:9.1:86.3 |
| 0.2 | 100:19.0:76.5 |
| 0.3 | 100:27.9:66.7 |
| 0.4 | 100:38.0:55.6 |
| 0.5 | 100:47.3:48.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Yu, H.; Tao, X.; Yu, F.; Li, M.; Wang, H. Influences of Ni Content on the Microstructural and Catalytic Properties of Perovskite LaNixCr1−xO3 for Dry Reforming of Methane. Catalysts 2022, 12, 1143. https://doi.org/10.3390/catal12101143
Zhao T, Yu H, Tao X, Yu F, Li M, Wang H. Influences of Ni Content on the Microstructural and Catalytic Properties of Perovskite LaNixCr1−xO3 for Dry Reforming of Methane. Catalysts. 2022; 12(10):1143. https://doi.org/10.3390/catal12101143
Chicago/Turabian StyleZhao, Tingting, Haoran Yu, Xuyingnan Tao, Feiyang Yu, Ming Li, and Haiqian Wang. 2022. "Influences of Ni Content on the Microstructural and Catalytic Properties of Perovskite LaNixCr1−xO3 for Dry Reforming of Methane" Catalysts 12, no. 10: 1143. https://doi.org/10.3390/catal12101143
APA StyleZhao, T., Yu, H., Tao, X., Yu, F., Li, M., & Wang, H. (2022). Influences of Ni Content on the Microstructural and Catalytic Properties of Perovskite LaNixCr1−xO3 for Dry Reforming of Methane. Catalysts, 12(10), 1143. https://doi.org/10.3390/catal12101143