Copper (II) Heterocyclic Thiosemicarbazone Complexes as Single-Source Precursors for the Preparation of Cu9S5 Nanoparticles: Application in Photocatalytic Degradation of Methylene Blue
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of the Ligands and Complexes
2.1.1. Infrared Study
2.1.2. Thermal Decomposition Studies of Precursors
2.2. Characterisation of Copper Sulphide Nanoparticles
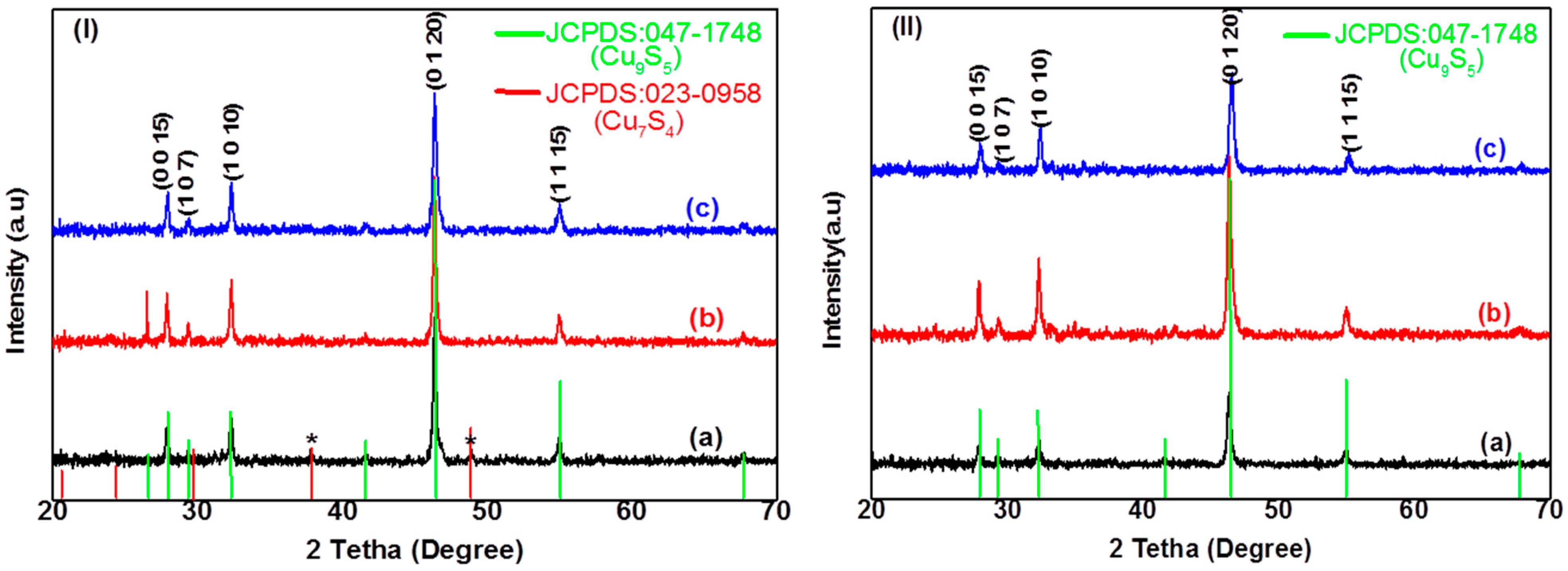
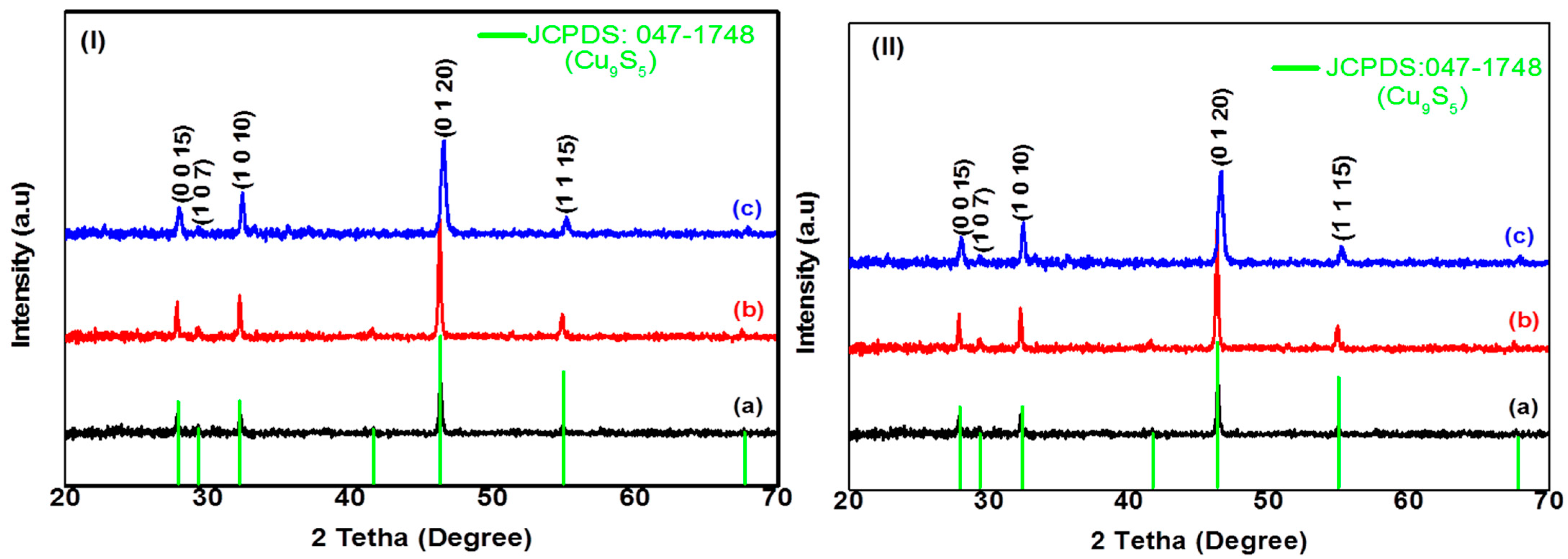
2.2.1. Structural Characterization of CuxSy Nanoparticles
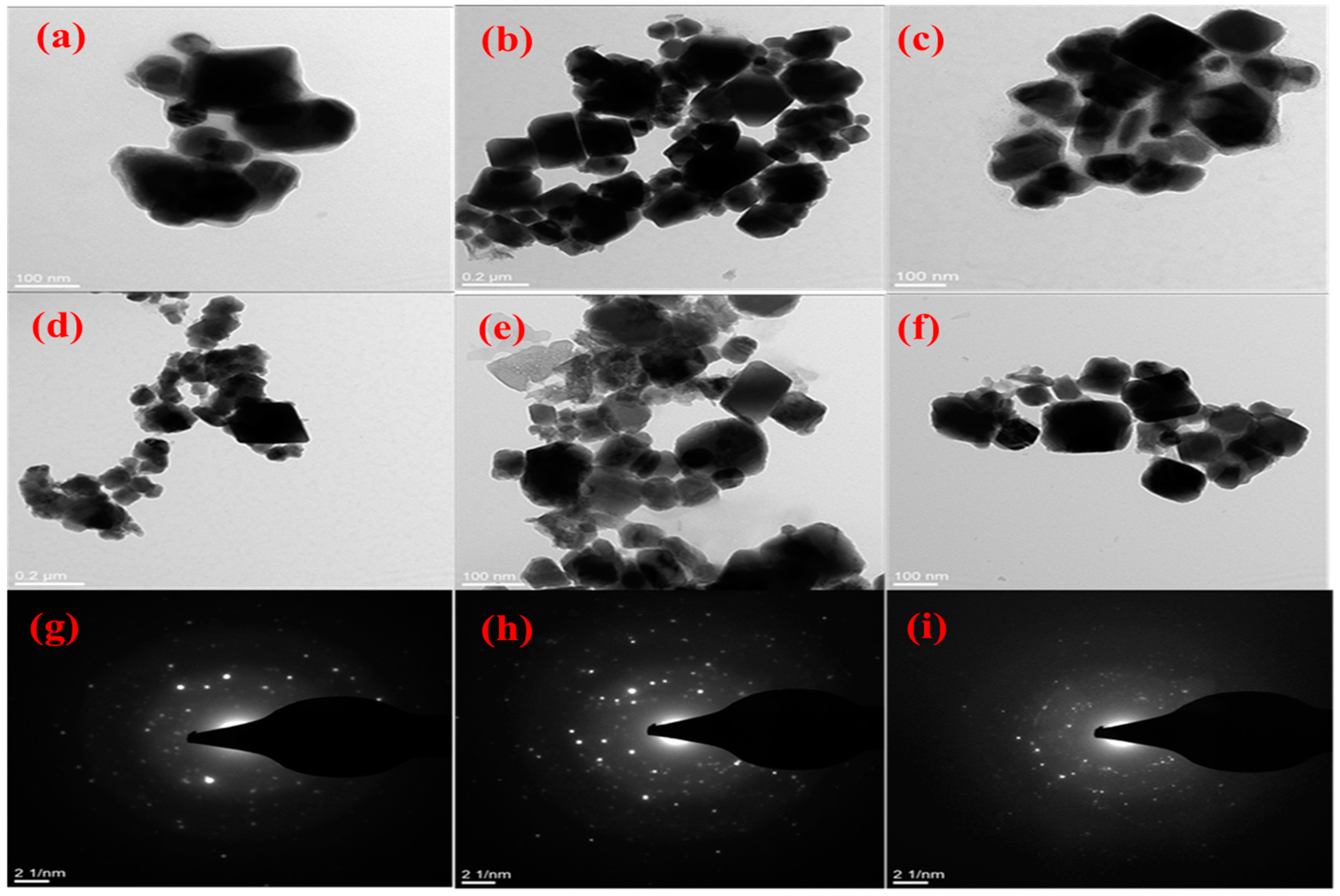
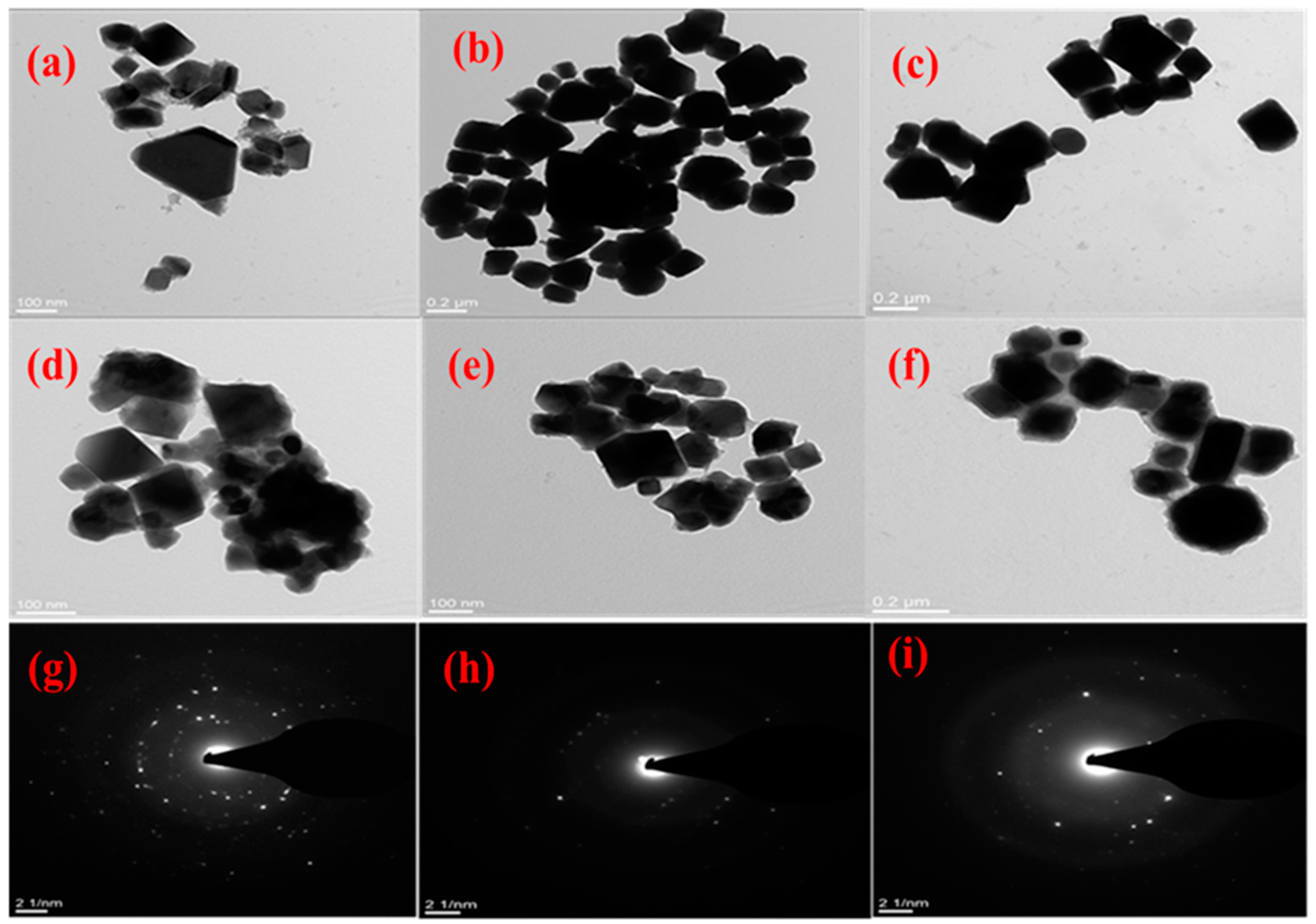
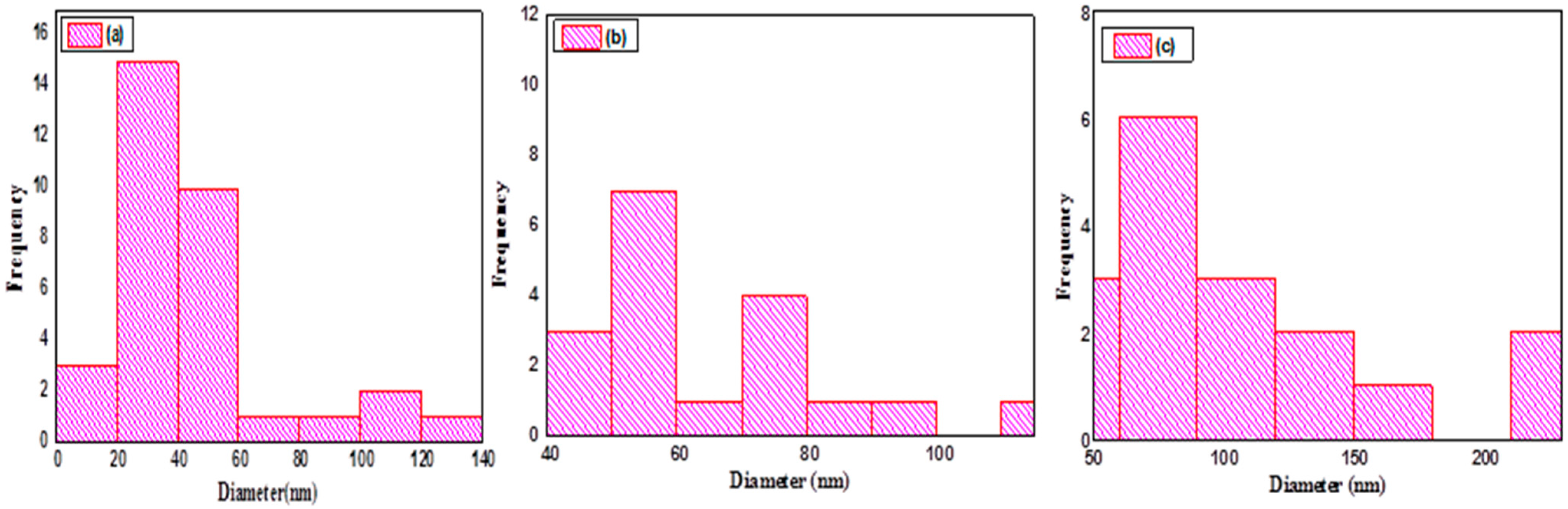
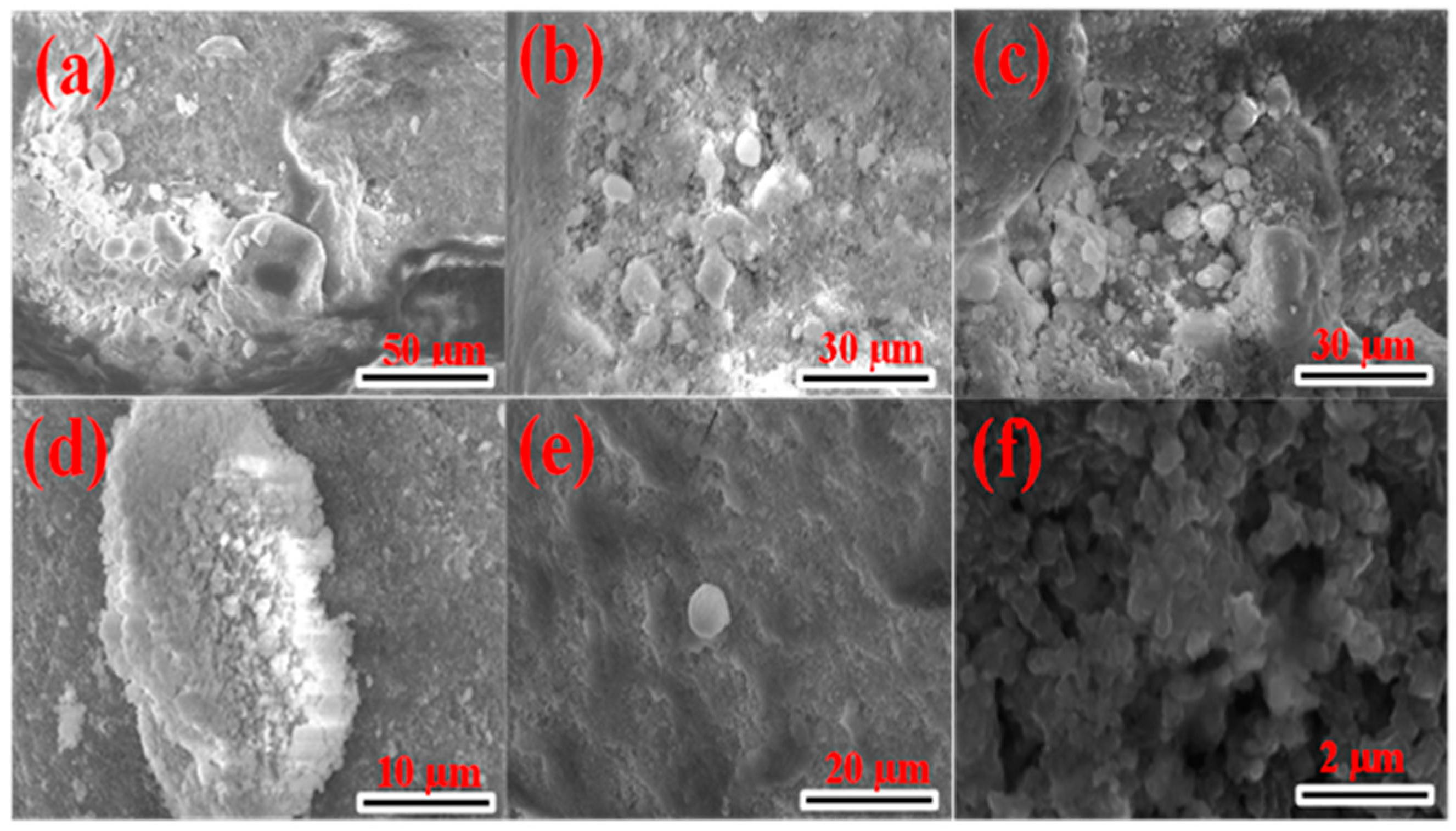
2.2.2. Morphological Characterisation of CuxSy Nanoparticles
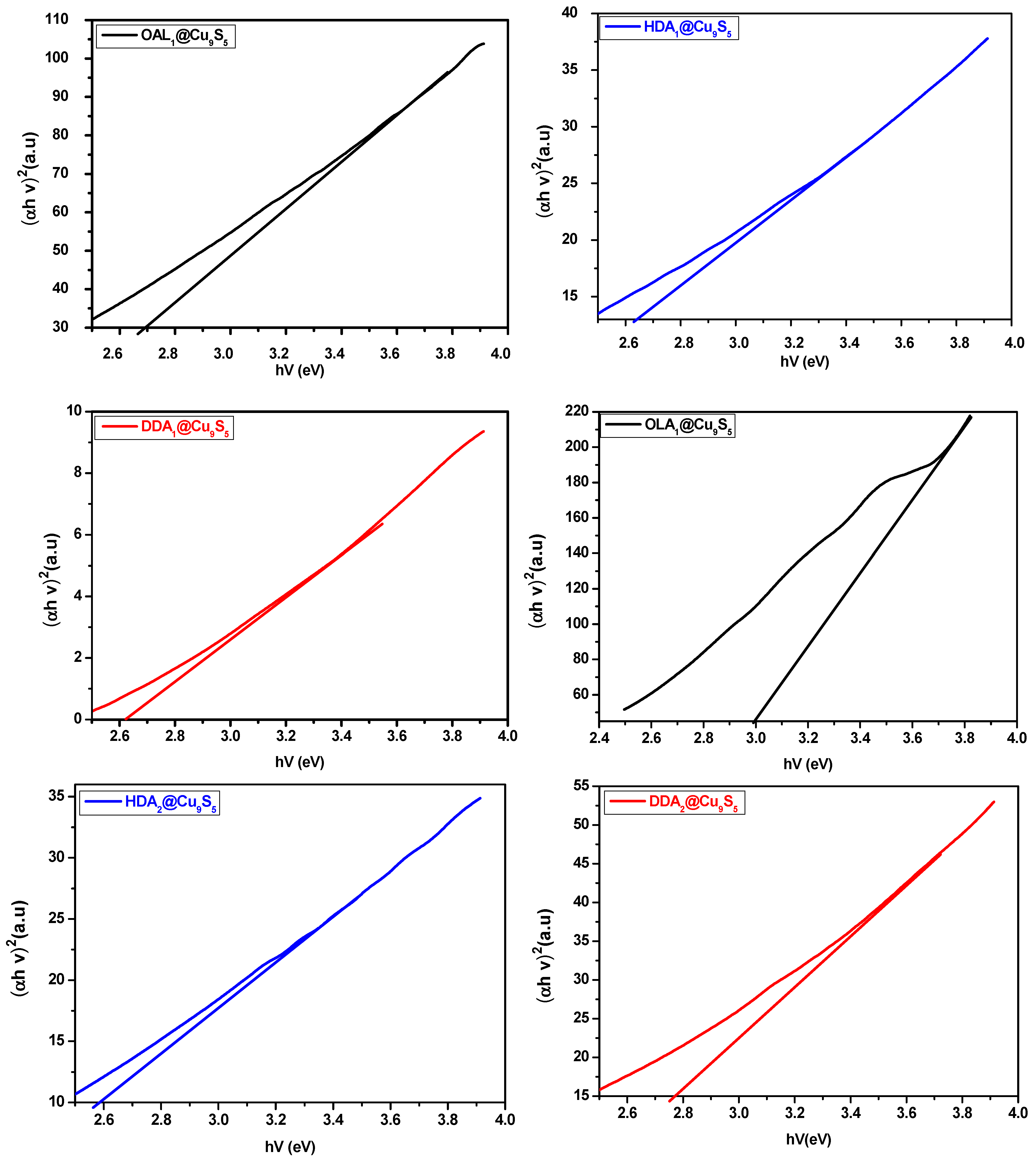
2.2.3. Optical Properties of CuxSy Nanoparticles
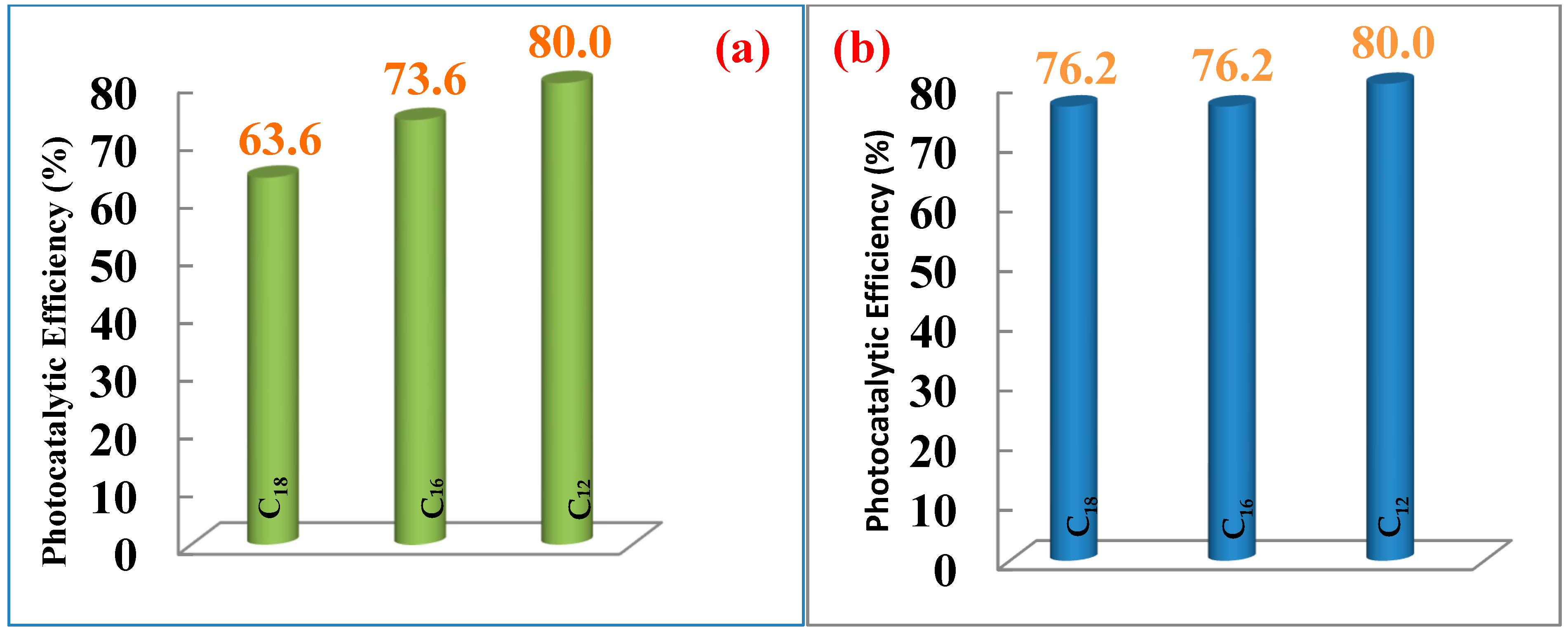
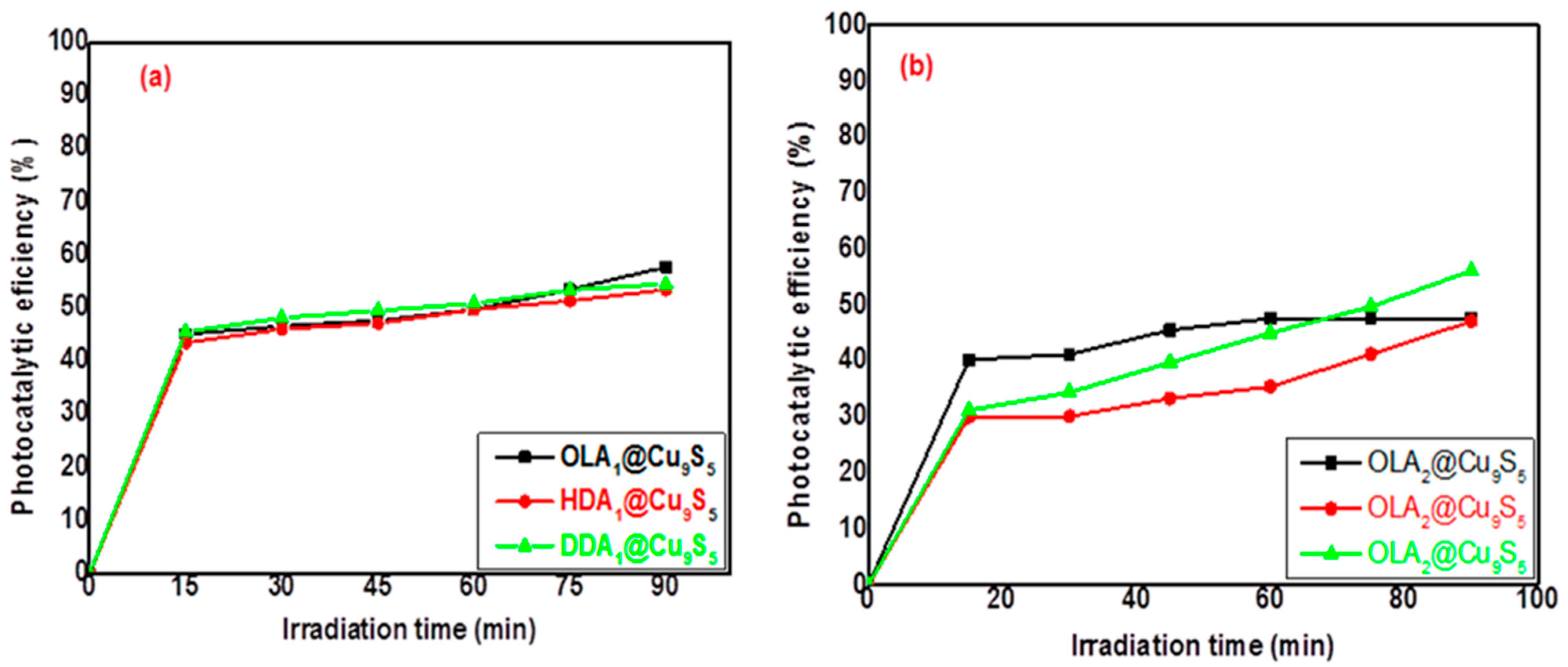
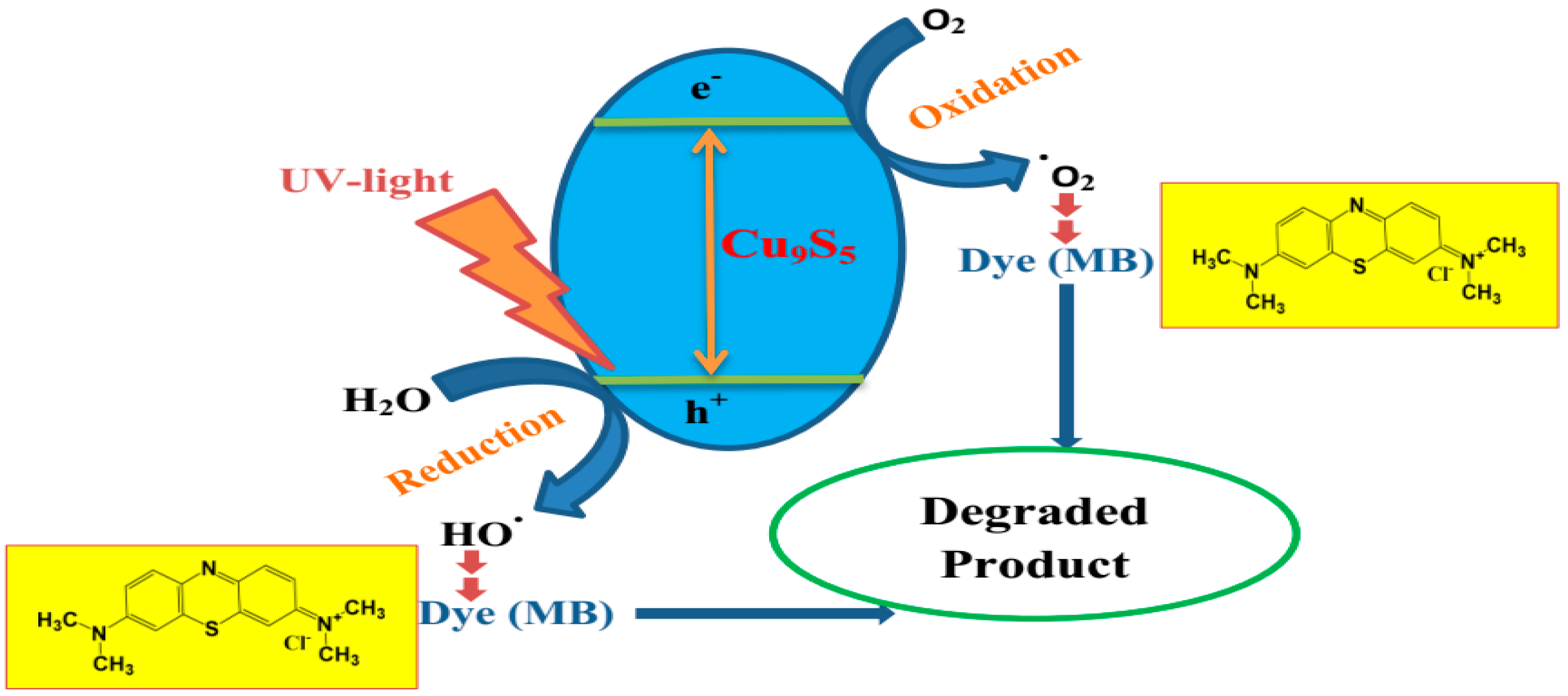
2.3. Photocatalytic Activity of CuxSy Nanoparticles on Methylene-Blue Dye
3. Materials and Methods Experimental
3.1. Chemicals
3.2. Instrumentation
3.3. Preparation of Thiosemicarbazone Ligands
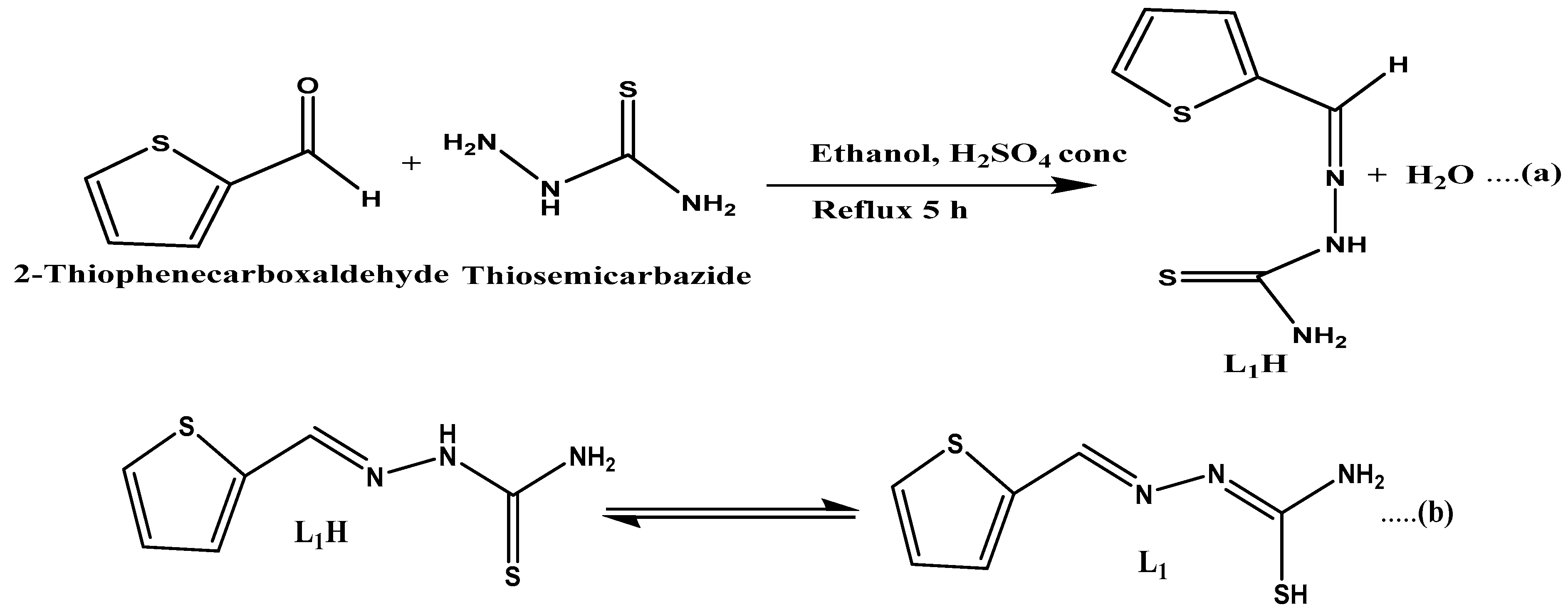
3.3.1. Synthesis of 2-Thiophenecarboxaldehyde Thiosemicarbazone (L1H) Ligand
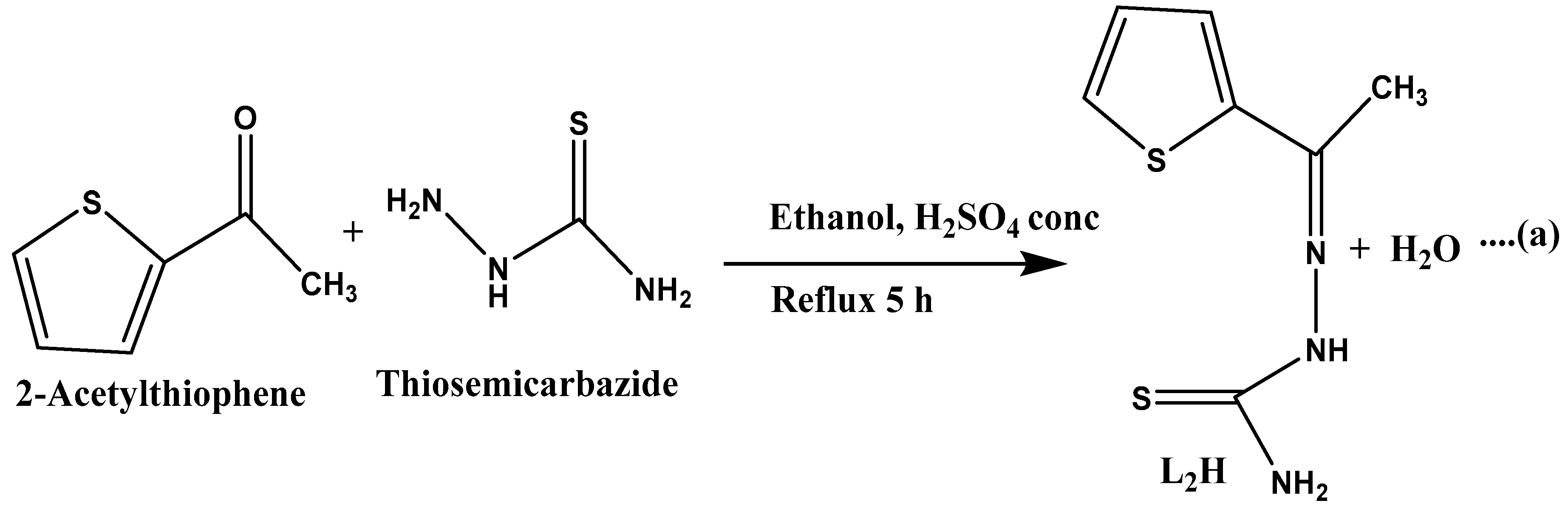
3.3.2. Synthesis of 2-Acetylthiophene Thiosemicarbazone (L2H) Ligand
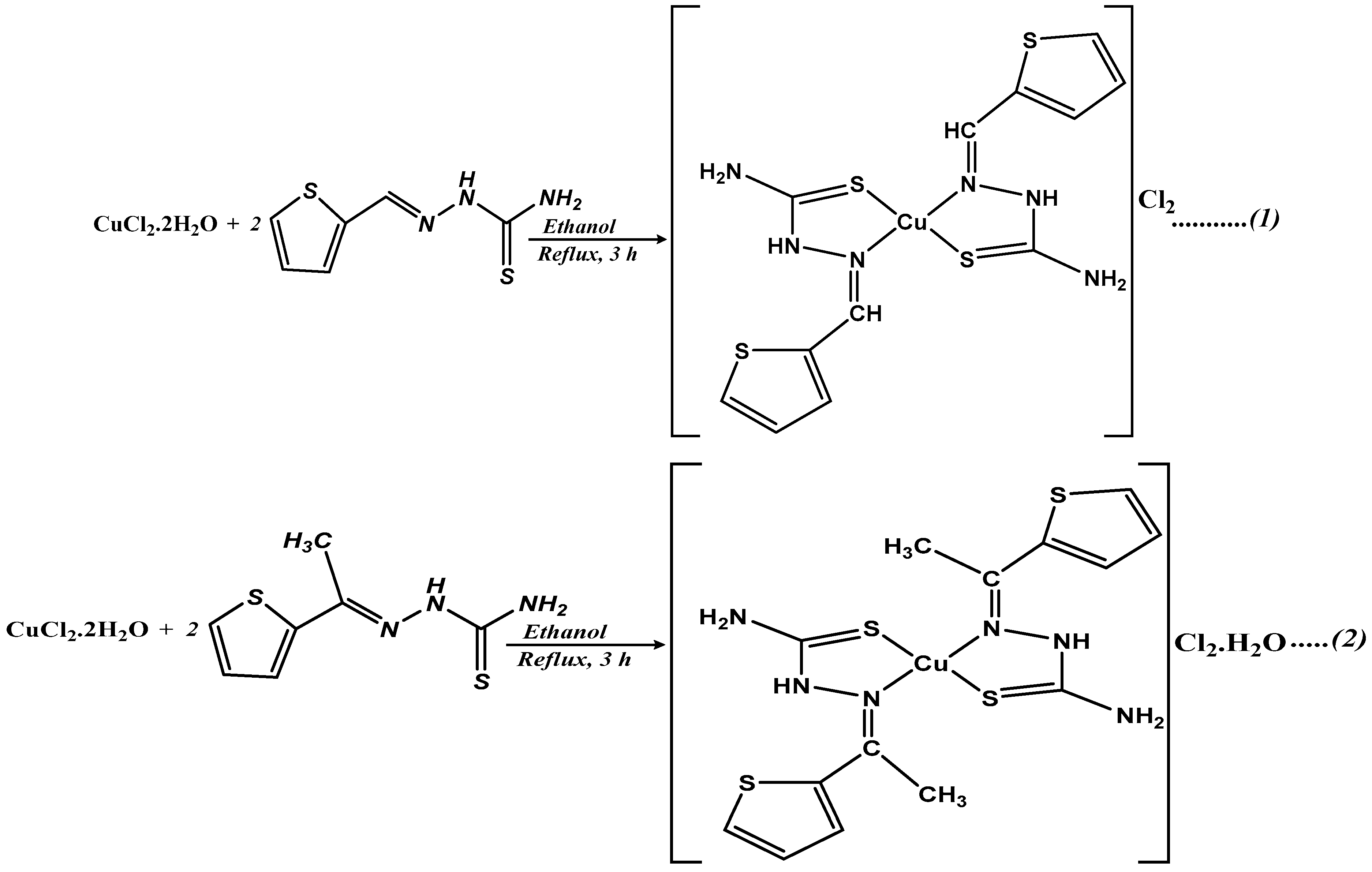
3.4. Preparation of Cu(II) Complexes (Complex 1 and 2)
3.5. Preparation of Copper Sulphide Nanoparticles
3.6. Photocatalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef] [PubMed]
- Jiade, P.A.A.; Nqombolo, A. Synthesis and structural studies of nickel sulphide and palladium sulphide nanocrystals. Chalcogenide Lett. 2016, 13, 427–434. [Google Scholar]
- Chakraborty, P.; Adhikary, J.; Chatterjee, S.; Biswas, B.; Chattopadhyay, T. Facile syntheses of copper sulfide nanoparticles: Antibacterial and antifungal activity study. Rasayan J. Chem. 2016, 9, 77–83. [Google Scholar]
- Olatunde, O.C.; Onwudiwe, D.C. Temperature Controlled Evolution of Pure Phase Cu9S5 Nanoparticles by Solvothermal Process. Front. Mater. 2021, 8, 211. [Google Scholar] [CrossRef]
- Lei, H.; Qin, P.; Ke, W.; Guo, Y.; Dai, X.; Chen, Z. Performance enhancement of polymer solar cells with high work function CuS modifed ITO as anodes. Org. Electron. 2015, 22, 173–179. [Google Scholar] [CrossRef]
- Srinivas, B.; Kumar, B.G.; Muralidharan, K. Stabilizer free copper sulphide nanostructures for rapid photocatalytic decomposition of rhodamine B. J. Mol. Catal. A Chem. 2015, 410, 8–18. [Google Scholar] [CrossRef]
- Zhang, K.; Khan, M.W.; Zuo, X.; Yang, Q.; Tang, H.; Wu, M. Controllable Synthesis and Photoelectric Properties of Interconnected and Self-Assembled Nanocomposite of Porous Hollow Cu7S4/CuS and Nitrogen-Doped Graphene Oxide. Electrochim. Acta 2019, 307, 64–75. [Google Scholar] [CrossRef]
- Khan, M.D.; Akhtar, M.; Malik, M.A.; Revaprasadu, N.; O’Brien, P. New examples of phase control in the preparation of copper sulfde nanoparticles and deposition of thin flms by AACVD from bis (piperidinedithiocarbamato) copper(II) complex. ChemistrySelect 2018, 3, 2943–2950. [Google Scholar] [CrossRef]
- Ketchemen, K.I.Y.; Khan, M.D.; Mlowe, S.; Nyamen, L.D.; Ndifon, P.T.; O’Brien, P.; Revaprasadu, N. Tailoring Shape and Crystallographic Phase of Copper Sulfide Nanostructures Using Novel Thiourea Complexes as Single Source Precursors. J. Inorg. Organomet. Polym. Mater. 2019, 29, 917–927. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G. Synthesis of copper sulfde nanowire arrays for high-performance supercapacitors. Electrochim. Acta 2014, 139, 401–407. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wang, J.F.; Tao, X.X. Synthesis of CuS and Cu1.1Fe1.1S2 crystals and their electrochemical properties. Mater. Charact. 2011, 62, 354–359. [Google Scholar] [CrossRef]
- Ayodhya, D.; Venkatesham, M.; Kumari, A.S.; Reddy, G.B.; Ramakrishna, D.; Veerabhadram, G. Photocatalytic degradation of dye pollutants under solar, visible and UV lights using green synthesised CuS nanoparticles. J. Exp. Nanosci. 2016, 11, 418–432. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Babu, S.M. Synthesis and characterization of hexagonal faceted copper sulfide (Cu1.8S) nanodisks. Mater. Sci. Semicond. Process. 2015, 40, 203–208. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Botha, N.L. Synthesis and structural studies of copper sulfide nanocrystals. Results Phys. 2016, 6, 581–589. [Google Scholar] [CrossRef]
- Ravele, M.P.; Oyewo, O.A.; Onwudiwe, D.C. Controlled Synthesis of CuS and Cu9S5 and Their Application in the Photocatalytic Mineralization of Tetracycline. Catalysts 2021, 11, 899. [Google Scholar] [CrossRef]
- Liao, X.-H.; Chen, N.-Y.; Xu, S.; Yang, S.-B.; Zhu, J.-J. A microwave assisted heating method for the preparation of copper sulfide nanorods. J. Cryst. Growth 2003, 252, 593–598. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Chen, X.; Jia, H.; Zheng, Z. In situ fabricate Cu2S thin film with hierarchical petal-like nanostructures. Mater. Res. Bull 2013, 48, 2940–2943. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, S.; Si, D.; Geng, B. Controlled synthesis of copper sulfide 3D nanoarchitectures through a facile hydrothermal route. J. Alloys Compd. 2010, 492, 44–49. [Google Scholar] [CrossRef]
- Botha, N.L.; Ajibade, P.A. Effect of temperature on crystallite sizes of copper sulfide nanocrystals prepared from copper (II) dithiocarbamate single source precursor. Mater. Sci. Semicond. Processing 2016, 43, 149–154. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Peng, L.; Xu, B.; Du, Y.; Deng, M.; Xu, H.; Wang, Q. Generalized synthesis of metal sulfide nanocrystals from single-source precursors: Size, shape and chemical composition control and their properties. CrystEngComm 2011, 13, 4572–4579. [Google Scholar] [CrossRef]
- Jen-La Plante, I.; Zeid, T.W.; Yang, P.; Mokari, T. Synthesis of metal sulfide nanomaterials via thermal decomposition of single-source precursors. J. Mater. Chem. 2010, 20, 6612–6617. [Google Scholar] [CrossRef]
- Paca, A.M.; Ajibade, P.A. Synthesis and structural studies of iron sulphide nanocomposites from iron(III) dithiocarbamate single source precursors. Mater. Chem. Phys. 2017, 202, 143–150. [Google Scholar] [CrossRef]
- Ma, Y.; Wan, H.; Ye, Y.; Chen, L.; Li, H.; Zhou, H.; Chen, J. In-situ synthesis of size tunable silver sulfide nanoparticles to improve tribological properties of the polytetrafluoroethylene-based nanocomposite lubricating coatings. Tribol. Int. 2020, 148, 106324. [Google Scholar] [CrossRef]
- Pawar, A.S.; Masikane, S.C.; Mlowe, S.; Garje, S.S.; Akerman, M.P.; Revaprasadu, N. Zinc thiosemicarbazone complexes: Single source precursors for alkylamine capped ZnS nanoparticles. Inorg. Chim. Acta 2017, 463, 7–13. [Google Scholar] [CrossRef]
- Khan, M.D.; Malik, M.A.; Akhtar, J.; Mlowe, S.; Revaprasadu, N. Phase pure deposition of flower-like thin flms by aerosol assisted chemical vapor deposition and solvent mediated structural transformation in copper sulfde nanostructures. Thin Solid Films 2017, 638, 338–344. [Google Scholar] [CrossRef]
- Du, X.-S.; Yu, Z.-Z.; Dasari, A.; Ma, J.; Meng, Y.-Z.; Mai, Y.-W. Facile synthesis and assembly of Cu2S nanodisks to corncoblike nanostructures. Chem. Mater. 2006, 18, 5156–5158. [Google Scholar] [CrossRef]
- Abdelhady, A.L.; Ramasamy, K.; Malik, M.A.; O’Brien, P.; Haighb, S.J.; Raftery, J. New routes to copper sulfide nanostructures and thin films. J. Mater. Chem. 2011, 21, 17888–17895. [Google Scholar] [CrossRef]
- Krishna, P.M.; Reddy, N.B.G.; Kottam, N.; Yallur, B.C.; Katreddi, H.R. Design and Synthesis of Metal Complexes of (2E)-2-[(2E)-3-Phenylprop-2-en-1-ylidene]hydrazine carbothioamide and Their Photocatalytic Degradation of Methylene Blue. Sci. World J. 2013, 2013, 828313. [Google Scholar] [CrossRef]
- Jain, M.; Babar, D.G.; Garje, S.S. Ligand-based stoichiometric tuning in copper sulfde nanostructures and their catalytic ability. Appl. Nanosci. 2019, 9, 353–367. [Google Scholar] [CrossRef]
- Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassa, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Saied, E.M. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef]
- Wilson, J.T.; Jiang, X.; McGill, B.C.; Lisic, E.C.; Deweese, J.E. Examination of the Impact of Copper(II) α-(N)-Heterocyclic Thiosemicarbazone Complexes on DNA Topoisomerase Iiα. Chem. Res. Toxicol. 2016, 29, 649–658. [Google Scholar] [CrossRef]
- Conner, D.; Medawala, W.; Stephens, M.T.; Morris, W.H.; Deweese, J.E.; Kent, P.L.; Rice, J.J.; Jiang, X.; Lisic, E.C. Cu(II) Benzoylpyridine Thiosemicarbazone Complexes: Inhibition of Human Topoisomerase IIα and Activity against Breast Cancer Cells. Open J. Inorg. Chem. 2016, 6, 146. [Google Scholar] [CrossRef]
- Lisic, E.C.; Rand, V.G.; Ngo, L.; Kent, P.; Rice, J.; Gerlach, D.; Papish, E.T.; Jiang, X. Cu(II) Propionyl-Thiazole Thiosemicarbazone Complexes: Crystal Structure, Inhibition of Human Topoisomerase IIα, and Activity against Breast Cancer Cells. Open J. Med. Chem. 2018, 8, 30–46. [Google Scholar] [CrossRef][Green Version]
- Sarker, D.; Hossen, M.F.; Zahan1, M.K.; Haque, M.M.; Zamir, R.; Asraf, M.A. Synthesis, Characterization, Thermal Analysis and Antibacterial Activity of Cu (II) and Ni(II) Complexes with Thiosemicarbazone Derived from Thiophene-2-aldehyde. J. Mater. Sci. Res. Rev. 2020, 5, 15–25. [Google Scholar]
- Mohan, C.; Kumar, V.; Kumari, S. synthesis, characterization, and antibacterial activity of schiff bases derived from thiosemicarbazide, 2-acetylthiophene and thiophene-2-aldehyde. Int. Res. J. Pharm. 2018, 9, 153–158. [Google Scholar] [CrossRef]
- Tyagi, M.; Chandra, S.; Tyagi, P. Mn(II) and Cu(II) complexes of a bidentate Schiff’s base ligand: Spectral, thermal, molecular modelling and mycological studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 117, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Goel, S.; Dwivedi, S.D. spectroscopic and biological studies on newly synthesized copper(II) and nickel(II) complexes with p-dimethylaminobanzaldehyde semicarbazone and p-dimethylaminobanzaldehyde thiosemicarbazone. Int. J. Appl. biol. Pharm. 2012, 3, 149–156. [Google Scholar]
- El-Saied, F.; El-Aarag, B.; Salem, T.; Said, G.; Khalifa, S.A.M.; El-Seedi, H.R. Synthesis, Characterization, and In Vivo Anti-Cancer Activity of New Metal Complexes Derived from Isatin-N(4) antipyrinethiosemicarbazone Ligand Against Ehrlich Ascites Carcinoma Cells. Molecules 2019, 24, 3313. [Google Scholar] [CrossRef]
- Pahontu, E.; Fala, V.; Gulea, A.; Poirier, D.; Tapcov, V.; Rosu, T. Synthesis and Characterization of Some New Cu(II), Ni(II) and Zn(II) Complexes with Salicylidene Thiosemicarbazones: Antibacterial, Antifungal and in Vitro Antileukemia Activity. Molecules 2013, 18, 8812–8836. [Google Scholar] [CrossRef]
- Jing, M.; Li, F.; Chen, M.; Zhang, J.; Long, F.; Jing, L.; Lv, X.; Ji, X.; Wu, T. Facile synthetic strategy to uniform Cu9S5 embedded into carbon: A novel anode for sodium-ion batteries. J. Alloys Compd. 2018, 762, 473–479. [Google Scholar] [CrossRef]
- Mbewana-Ntshanka, N.G.; Moloto, M.J.; Mubiayi, P.K. Antimicrobial Activity of the Synthesized of Copper Chalcogenide Nanoparticles. J. Nanotechnol. 2021, 2021, 6675145. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Hrubaru, M.; Ebenso, E.E. Synthesis, Structural and Optical Properties of TOPO and HDA Capped Cadmium Sulphide Nanocrystals, and the Effect of Capping Ligand Concentration. J. Nanomater. 2015, 9, 143632. [Google Scholar] [CrossRef]
- Akhtar, M.; Alghamdi, Y.; Akhtar, J.; Aslam, Z.; Revaprasadu, N.; Malik, M.A. Phase controlled synthesis of copper sulfide nanoparticles by colloidal and non-colloidal methods. Mater. Chem. Phys. 2016, 180, 404–412. [Google Scholar] [CrossRef]
- Ketchemen, K.I.Y.; Mlowe, S.; Nyamen, L.D.; Ndifon, P.T.; Revaprasadu, N. Comparative study on the effect of precursors on the morphology and electronic properties of CdS nanoparticles. Turk. J. Chem. 2021, 45, 400–409. [Google Scholar] [CrossRef]
- Botha, N.L.; Ajibade, P.A. Optical and structural characterization of copper sulphide nanoparticles from copper(II) piperidine dithiocarbamate. Opt. Quantum Electron. 2020, 52, 337. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Room-Temperature Synthesis of ZnS Nanoparticles Using Zinc Xanthates as Molecular Precursors. Materials 2020, 13, 171. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Sikakane, B.M.; Botha, N.L.; Oluwalana, A.E.; Omondi, B. Synthesis and crystal structures of bis(dibenzyl dithiocarbamato)Cu(II) and Ag(I) complexes: Precursors for Cu1.8S and Ag2S nano-photocatalysts. J. Mol. Struct. 2020, 1221, 128791. [Google Scholar] [CrossRef]
- Pal, M.; Mathews, N.R.; Sanchez-Mora, E.; Pal, U.; Paraguay-Delgado, F.; Mathew, X. Synthesis of CuS nanoparticles by a wet chemical route and their photocatalytic activity. J. Nanoparticle Res. 2015, 17, 301. [Google Scholar] [CrossRef]
- Yepseu, A.P.; Isac, L.; Nyamen, L.D.; Cleymand, F.; Duta, A.; Ndifon, P.T. Optical and Photocatalytic Properties of CuxS/ZnO Composite Thin Films Deposited by Robotic Spray Pyrolysis Deposition. J. Nanomater 2021, 2021, 9975600. [Google Scholar] [CrossRef]

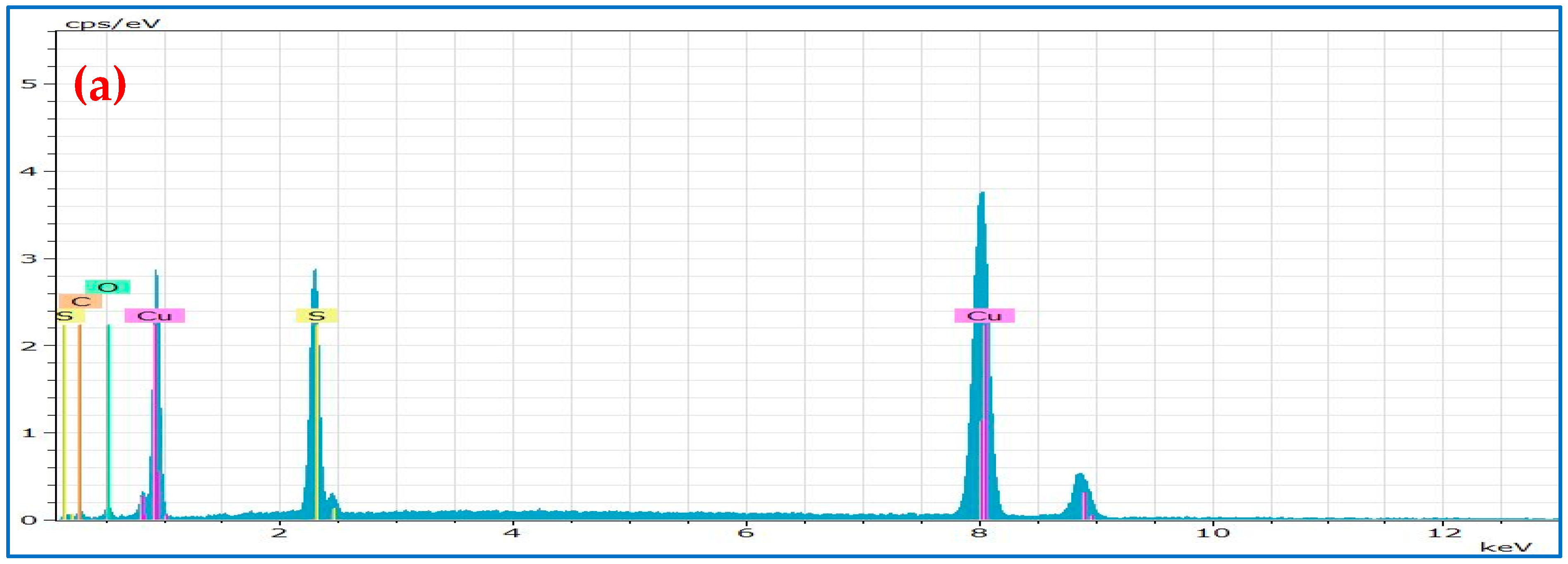
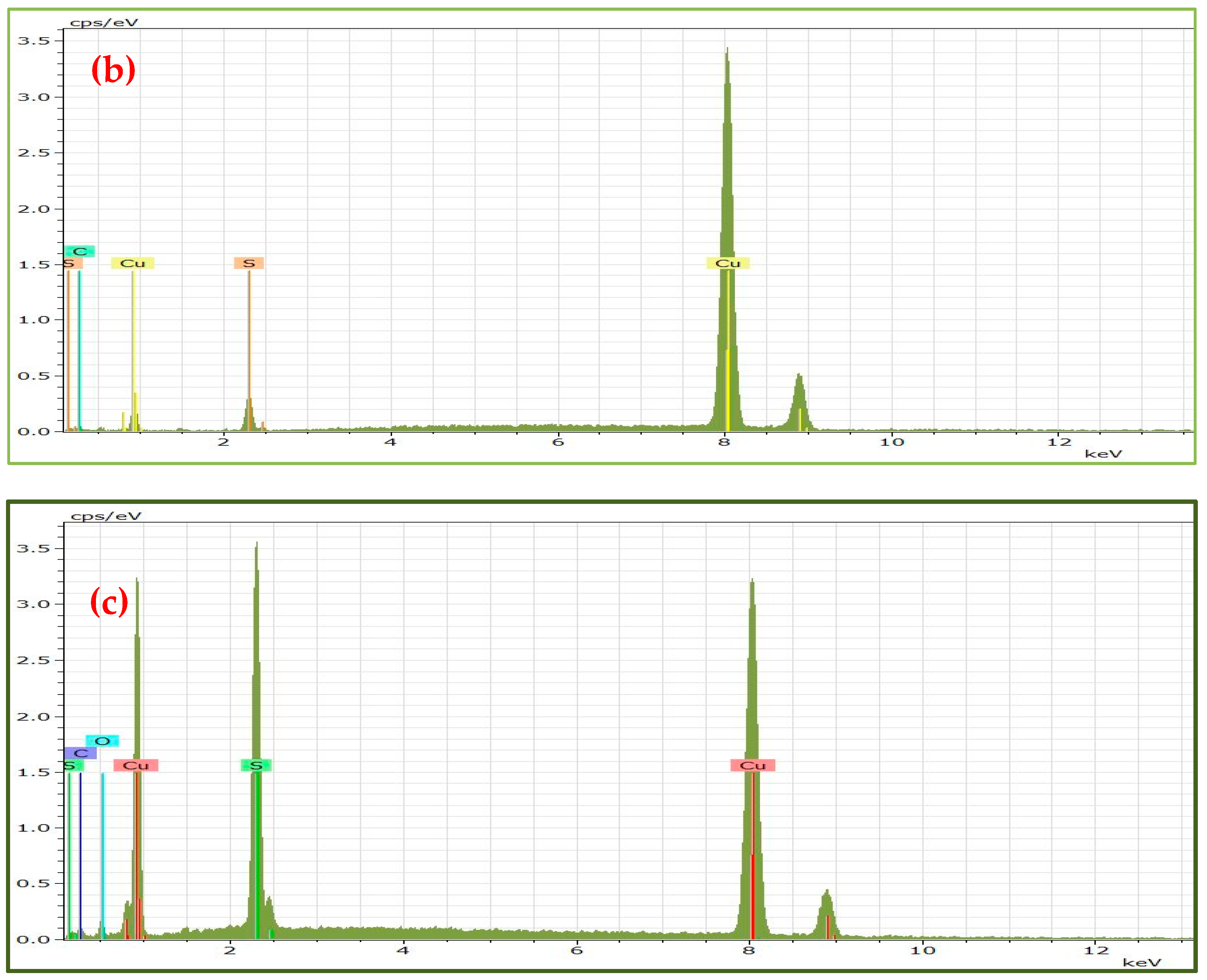



| OLA1@Cu9S5 | HDA1@Cu9S5 | DDA1@Cu9S5 | OLA2@Cu9S5 | HDA2@Cu9S5 | DDA2@Cu9S5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Weight % | Element | Weight % | Element | Weight % | Element | Weight % | Element | Weight % | Element | Weight % |
| Cu | 49.50 | Cu | 61.06 | Cu | 46.47 | Cu | 27.77 | Cu | 31.67 | Cu | 31.07 |
| S | 25.44 | S | 27 | S | 25.33 | S | 13.90 | S | 18.50 | S | 17.66 |
| O | 4.92 | O | / | O | 6.61 | O | 7.90 | O | 19.52 | O | 19.22 |
| C | 20.14 | C | 11.94 | C | 21.5 | C | 50.43 | C | 30.31 | C | 32.05 |
| Cu/S | 1.94:1 | Cu/S | 2.26:1 | Cu/S | 1.83:1 | Cu/S | 1.99:1 | Cu/S | 1.71:1 | Cu/S | 1.76:1 |
| OLA1@Cu9S5 | HDA1@Cu9S5 | DDA1@Cu9S5 | OLA2@Cu9S5 | HDA2@Cu9S5 | DDA2@Cu9S5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Weight % | Element | Weight % | Element | Weight % | Element | Weight % | Element | Weight % | Element | Weight % |
| Cu | 59.86 | Cu | 60.53 | Cu | 61.56 | Cu | 58.81 | Cu | 59.57 | Cu | 56.54 |
| S | 31.94 | S | 33.27 | S | 32.78 | S | 3197 | S | 33.32 | S | 29.56 |
| O | 8.20 | O | 6.20 | O | 5.66 | O | 9.22 | O | 7.11 | O | 13.90 |
| C | 59.86 | C | / | C | / | C | / | C | / | C | / |
| Cu/S | 1.87:1 | Cu/S | 1.81:1 | Cu/S | 1.87:1 | Cu/S | 1.84:1 | Cu/S | 1.80:1 | Cu/S | 1.91:1 |
| t (min) | 15 | 30 | 45 | 60 | 75 | 90 | |
|---|---|---|---|---|---|---|---|
| OLA1@Cu9S5 | η (%) | 33.3 | 46.0 | 49.7 | 57.1 | 62.4 | 63.6 |
| HDA1@Cu9S5 | η (%) | 48.7 | 58.2 | 60.3 | 70.4 | 70.4 | 73.6 |
| DDA1@Cu9S5 | η (%) | 54.6 | 60.0 | 64.6 | 69.3 | 74.1 | 80.0 |
| OLA2@Cu9S5 | η (%) | 58.7 | 60.0 | 66.7 | 68.8 | 73.0 | 76.2 |
| HDA2@Cu9S5 | η (%) | 60.0 | 63.5 | 67.7 | 71.0 | 73.5 | 76.2 |
| DDA2@Cu9S5 | η (%) | 62.4 | 65.1 | 70.4 | 74.6 | 75.7 | 80.0 |
| t (min) | 15 | 30 | 45 | 60 | 75 | 90 | |
|---|---|---|---|---|---|---|---|
| OLA1@Cu9S5 | η (%) | 45.0 | 46.6 | 47.6 | 49.7 | 53.4 | 57.7 |
| HDA1@Cu9S5 | η (%) | 43.4 | 46.0 | 47.1 | 49.7 | 51.3 | 53.4 |
| DDA1@Cu9S5 | η (%) | 45.5 | 48.1 | 49.5 | 50.8 | 53.4 | 54.5 |
| OLA2@Cu9S5 | η (%) | 40.2 | 41.2 | 45.5 | 47.6 | 47.6 | 47.6 |
| HDA2@Cu9S5 | η (%) | 30.0 | 30.1 | 33.3 | 35.4 | 41.2 | 47.1 |
| DDA2@Cu9S5 | η (%) | 31.2 | 34.4 | 39.7 | 45.0 | 49.7 | 56.1 |
| Complexes | Capping Agent | Reaction Temp (°C) | Energy Bandgap (eV) | Phase (Formula) | Morphology | Average Size (nm) | Degradation Rates (%) after 90 min |
|---|---|---|---|---|---|---|---|
| 1 | OLA | 190 | 2.70 | Mixture of Cu9S5 & Cu7S5 | Agglomerated Irregular | 49–80 | 63.6 |
| HDA | 190 | 2.64 | Cu9S5 | Rectangular and cubic | 46–134 | 73.6 | |
| DDA | 190 | 2.57 | Cu9S5 | Rectangular and irregular | 53–154 | 80.0 | |
| OLA | 230 | 2.60 | Cu9S5 | Rectangular and truncated | 40–120 | 57.7 | |
| HDA | 230 | 2.76 | Cu9S5 | Irregular cubic and rectangular | 101–200 | 53.4 | |
| DDA | 230 | 2.64 | Cu9S5 | Irregular cubic | 61–119 | 54.5 | |
| 2 | OLA | 190 | 3.00 | Cu9S5 | Agglomerated | 32–75 | 76.2 |
| HDA | 190 | 2.58 | Cu9S5 | Semi-spherical and rectangular | 43–125 | 76.2 | |
| DDA | 190 | 2.55 | Cu9S5 | Irregular cubic | 49–196 | 80.0 | |
| OLA | 230 | 2.83 | Cu9S5 | Agglomerated | 34–128 | 47.6 | |
| HDA | 230 | 2.76 | Cu9S5 | Irregular cubic | 45–115 | 47.1 | |
| DDA | 230 | 2.73 | Cu9S5 | Rectangular and cubic | 66–225 | 56.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepseu, A.P.; Girardet, T.; Nyamen, L.D.; Fleutot, S.; Ketchemen, K.I.Y.; Cleymand, F.; Ndifon, P.T. Copper (II) Heterocyclic Thiosemicarbazone Complexes as Single-Source Precursors for the Preparation of Cu9S5 Nanoparticles: Application in Photocatalytic Degradation of Methylene Blue. Catalysts 2022, 12, 61. https://doi.org/10.3390/catal12010061
Yepseu AP, Girardet T, Nyamen LD, Fleutot S, Ketchemen KIY, Cleymand F, Ndifon PT. Copper (II) Heterocyclic Thiosemicarbazone Complexes as Single-Source Precursors for the Preparation of Cu9S5 Nanoparticles: Application in Photocatalytic Degradation of Methylene Blue. Catalysts. 2022; 12(1):61. https://doi.org/10.3390/catal12010061
Chicago/Turabian StyleYepseu, Adrien P., Thomas Girardet, Linda D. Nyamen, Solenne Fleutot, Kevin I. Y. Ketchemen, Franck Cleymand, and Peter T. Ndifon. 2022. "Copper (II) Heterocyclic Thiosemicarbazone Complexes as Single-Source Precursors for the Preparation of Cu9S5 Nanoparticles: Application in Photocatalytic Degradation of Methylene Blue" Catalysts 12, no. 1: 61. https://doi.org/10.3390/catal12010061
APA StyleYepseu, A. P., Girardet, T., Nyamen, L. D., Fleutot, S., Ketchemen, K. I. Y., Cleymand, F., & Ndifon, P. T. (2022). Copper (II) Heterocyclic Thiosemicarbazone Complexes as Single-Source Precursors for the Preparation of Cu9S5 Nanoparticles: Application in Photocatalytic Degradation of Methylene Blue. Catalysts, 12(1), 61. https://doi.org/10.3390/catal12010061







