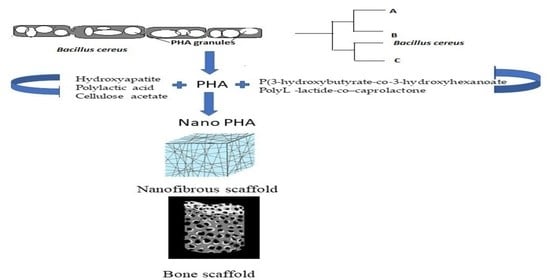
Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds
Abstract
:1. Introduction
2. PHA: A Biodegradable Microbial Biopolymer Synthesized as a Component of a Microbial Cell
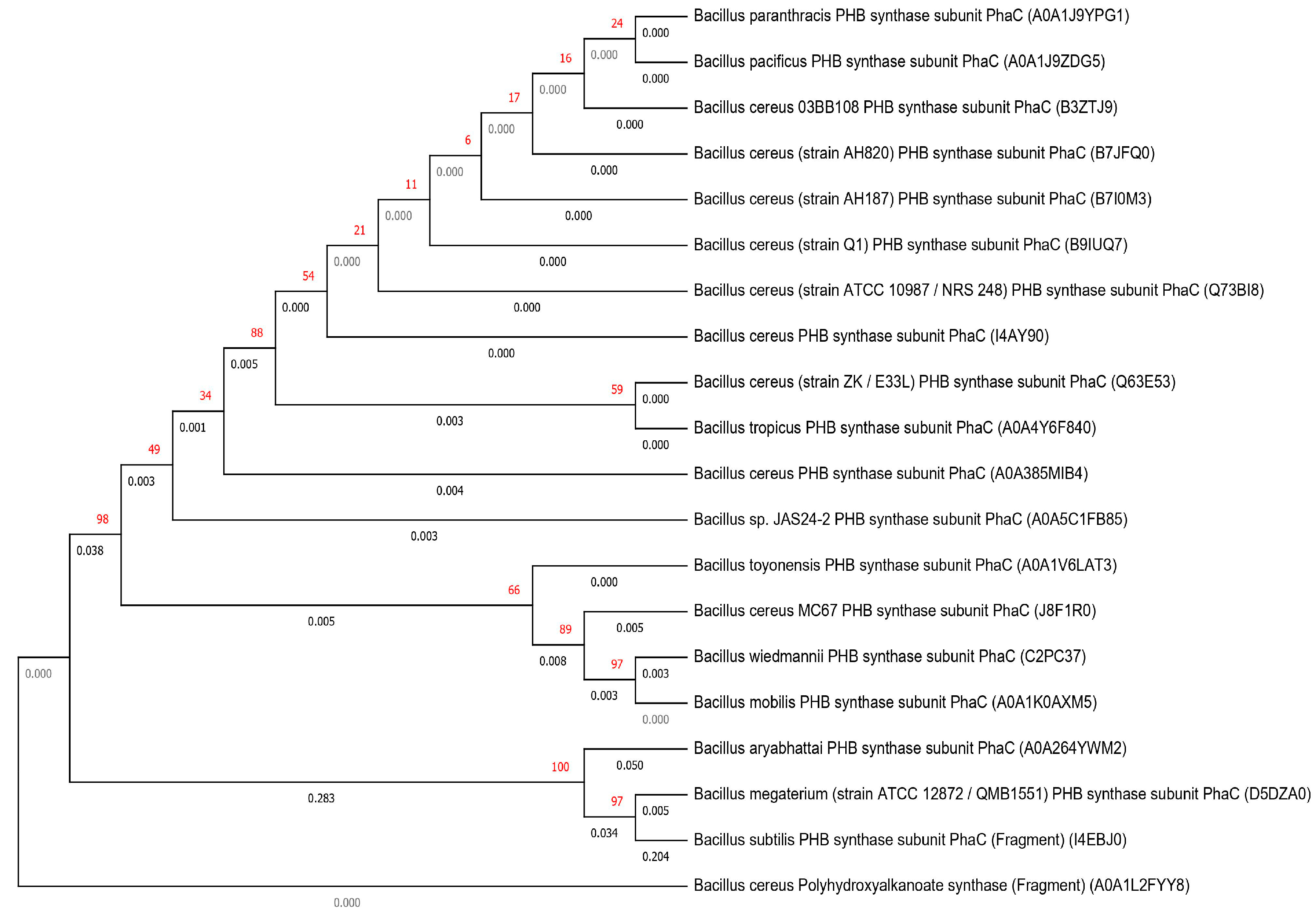
Bacillus as Novel PHA Producer
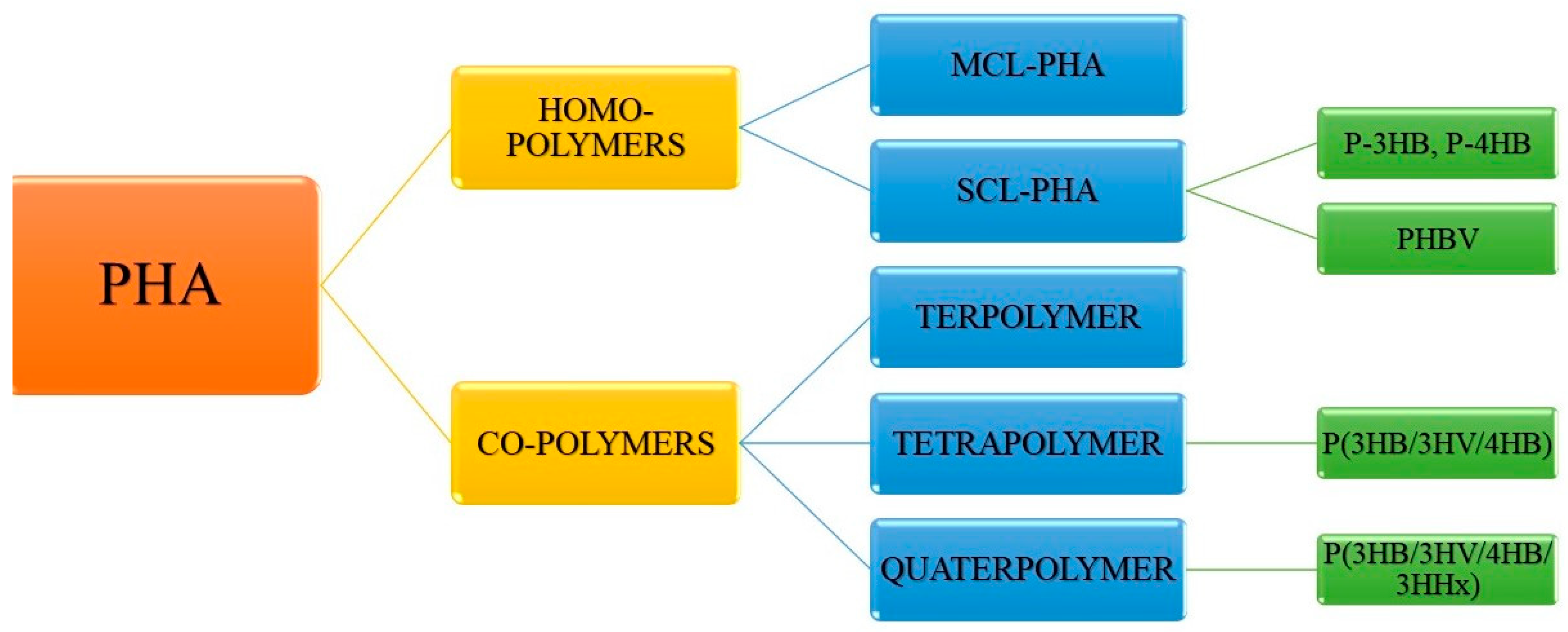
3. Structural Diversity of PHA
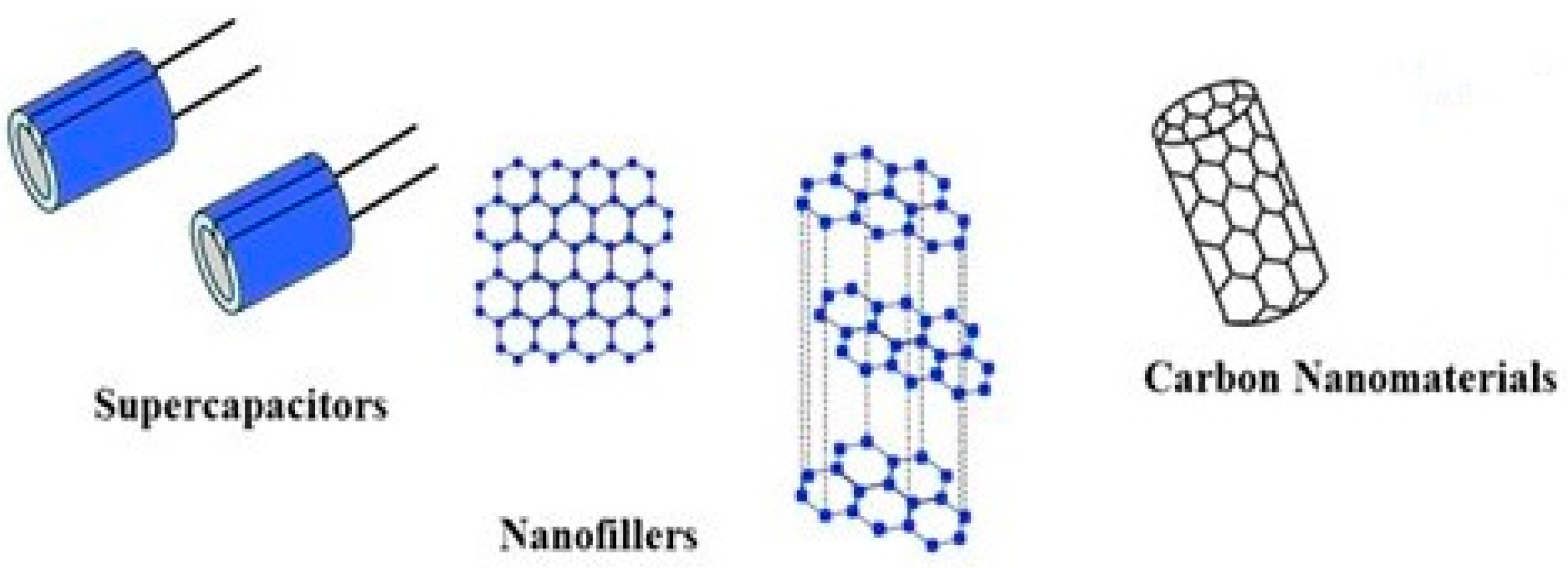
4. PHA as a Potential Nanocomposite
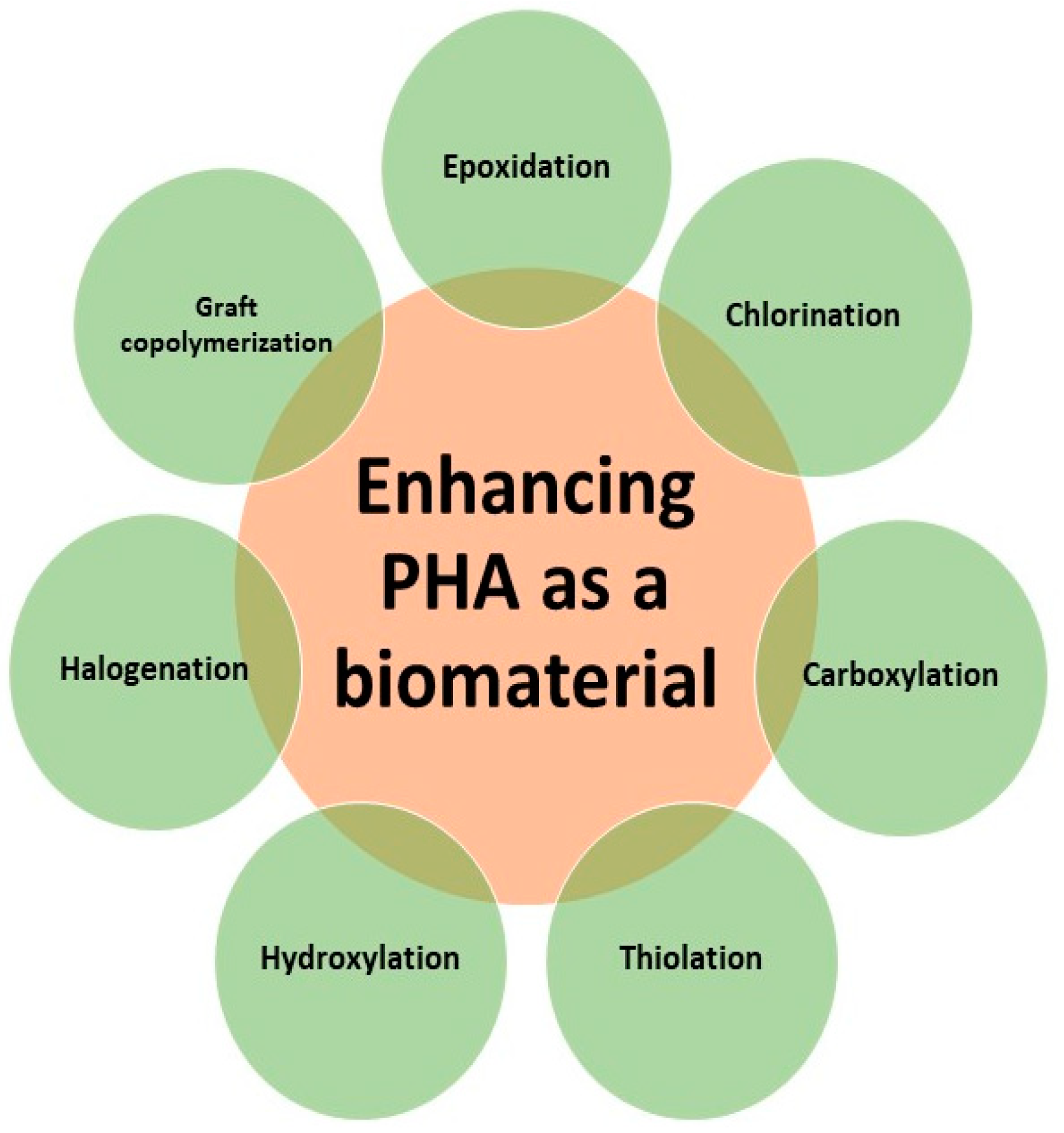
5. Chemical Modification for Enhancement of Functional Properties of PHA
6. Applications of PHA and Its Nanocomposites as a Potential Biomaterial for Medical Applications
6.1. Biosensors
6.2. Delivery of Plasmid DNA
6.3. Packaging of Essential Hormones and Microcapsules
6.4. Restoration of Insulin Production and Release
6.5. Microbeads for Targeted Drug Release
6.6. Bone Marrow Scaffolds
6.7. Scaffolds for Bone Tissue Engineering and Processing of Biomaterials
7. Strategies for Designing Bone Scaffolds for Bone Regeneration
7.1. Solvent Casting Particle Leaching Method
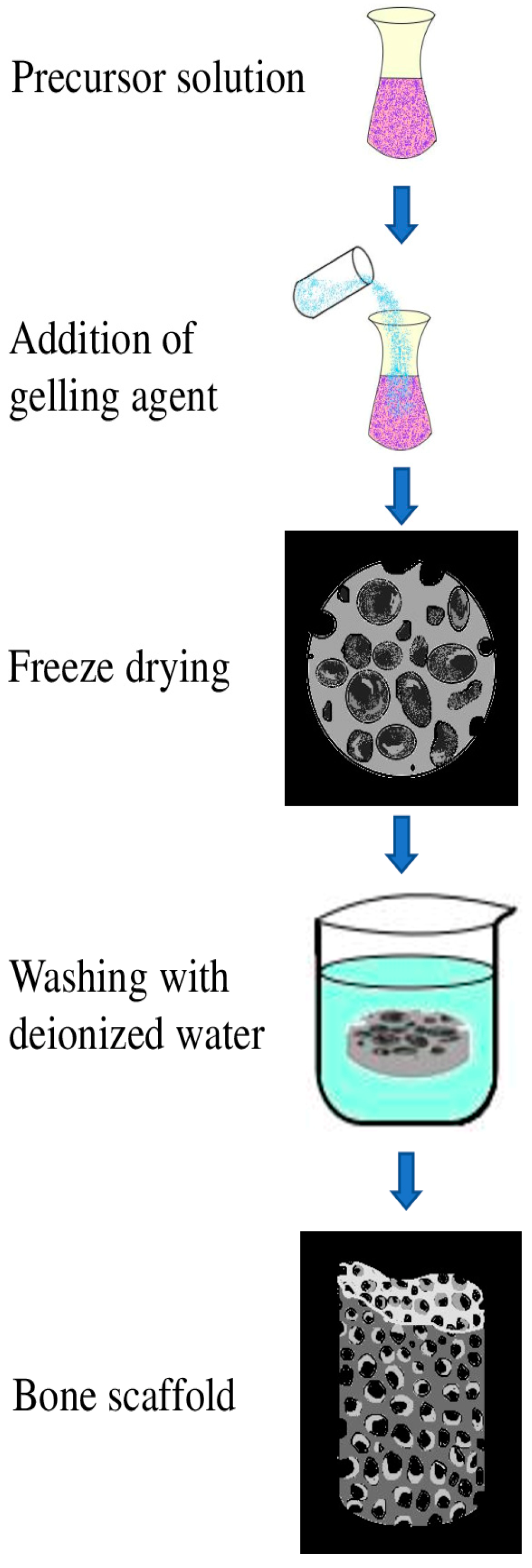
7.2. Sol–Gel Technique
7.3. Gas Foaming Method
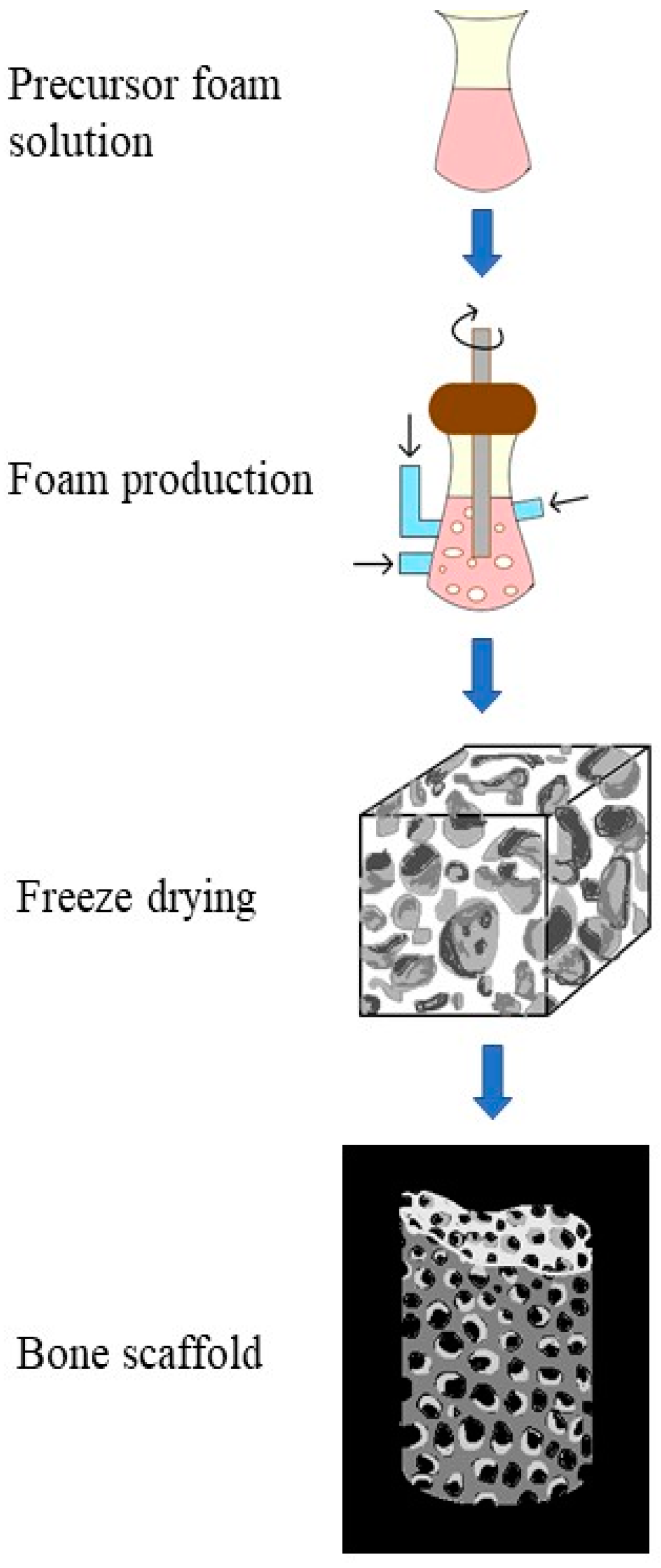
7.4. Lyophilization
7.5. Electrospinning
8. Future Scope and Overall Versatility of PHA as a Nanocomposite
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef] [PubMed]
- Gabr, G.A. Isolation and Identification of Bacterial Strains Able to Biopolymer Polyhydroxybutyrate (Phb) Production from Soil of Al-Kharj Probes, Saudi Arabia. J. Pharm. Res. Int. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Juengert, J.; Bresan, S.; Jendrossek, D. Determination of Polyhydroxybutyrate (PHB) Content in Ralstonia eutropha Using Gas Chromatography and Nile Red Staining. Bio Protoc. 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Pillai, A.B.; Kumar, A.J.; Thulasi, K.; Kumarapillai, H. Evaluation of short-chain-length polyhydroxyalkanoate accumulation in Bacillus aryabhattai. Braz. J. Microbiol. 2017, 48, 451–460. [Google Scholar] [CrossRef]
- Penkhrue, W.; Jendrossek, D.; Khanongnuch, C.; Pathom-Aree, W.; Aizawa, T.; Behrens, R.L.; Lumyong, S. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 2020, 15, e0230443. [Google Scholar] [CrossRef] [Green Version]
- Shen, R.; Ning, Z.-Y.; Lan, Y.-X.; Chen, J.-C.; Chen, G.-Q. Manipulation of polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis. Metab. Eng. 2019, 54, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Sanz, J.M. Polyhydroxyalkanoate-associated phasins as phylogenetically heterogeneous, multipurpose proteins. Microb. Biotechnol. 2017, 10, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.; Blanco, F.G.; Maestro, B.; Sanz, J.M.; Pérez-Gil, J.; Prieto, M.A. Dissecting the Polyhydroxyalkanoate-Binding Domain of the PhaF Phasin: Rational Design of a Minimized Affinity Tag. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Chi, L.; Fuchs, H.; Pötter, M.; Steinbüchel, A. Studies on the influence of phasins on accumulation and degradation of PHB and nanostructure of PHB granules in Ralstonia eutropha H16. Biomacromolecules 2007, 8, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus equi using different carbon sources. Arab. J. Sci. Eng. 2017, 42, 2371–2379. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Nunes, J.M.N.; Lordelo, L.N.; Druzian, J.I. Assessment of polyhydroxyalkanoate synthesis in submerged cultivation of Cupriavidus necator and Burkholderia cepacia strains using soybean as substrate. Braz. J. Chem. Eng. 2019, 36, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Bresan, S.; Jendrossek, D. New insights into PhaM-PhaC-mediated localization of polyhydroxybutyrate granules in Ralstonia eutropha H16. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Kihara, T.; Hiroe, A.; Ishii-Hyakutake, M.; Mizuno, K.; Tsuge, T. Bacillus cereus-type polyhydroxyalkanoate biosynthetic gene cluster contains R-specific enoyl-CoA hydratase gene. Biosci. Biotechnol. Biochem. 2017, 81, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Tobón, D.I.; Gul, M.; Elias, A.L.; Sauvageau, D. Polyhydroxybutyrate (PHB) biodegradation using bacterial strains with demonstrated and predicted PHB depolymerase activity. Appl. Microbiol. Biotechnol. 2018, 102, 8049–8067. [Google Scholar] [CrossRef] [PubMed]
- Pötter, M.; Madkour, M.H.; Mayer, F.; Steinbüchel, A. Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 2002, 148, 2413–2426. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Maity, S.; Dash, H.R.; Das, S.; Pattnaik, S.; Rath, C.C.; Samantaray, D. Bacillus and biopolymer: Prospects and challenges. Biochem. Biophys. Rep. 2017, 12, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, N.; Kumar, M.; Thakur, I.S.; Srivastava, S. Production, process optimization and molecular characterization of polyhydroxyalkanoate (PHA) by CO2 sequestering B. cereus SS105. Bioresour. Technol. 2018, 254, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Abdul-Salam, M.; Yasin, T.; Hameed, A. Effect of glucose and olive oil as potential carbon sources on production of PHAs copolymer and tercopolymer by Bacillus cereus FA11. 3 Biotechnology 2017, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.; Behera, H.T.; Dekebo, A.; Ray, L. Optimization of the culture conditions for production of Polyhydroxyakanoate and its characterization from a new Bacillus cereus sp. BNPI-92 strain, isolated from plastic waste dumping yard. Int. J. Biol. Macromol. 2020, 156, 1064–1080. [Google Scholar] [CrossRef]
- Mohandas, S.P.; Balan, L.; Jayanath, G.; Anoop, B.S.; Philip, R.; Cubelio, S.S.; Singh, I.B. Biosynthesis and characterization of polyhydroxyalkanoate from marine Bacillus cereus MCCB 281 utilizing glycerol as carbon source. Int. J. Biol. Macromol. 2018, 119, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Yashchuk, O.; Hermida, É.B. Influence of Culture Conditions on Poly-3-Hydroxybutyrate Production by a Newly Isolated Bacillus cereus Y23. Clean Soil Air Water 2020, 48, 1900415. [Google Scholar] [CrossRef]
- Suryawanshi, S.S.; Sarje, S.S.; Loni, P.C.; Bhujbal, S.; Kamble, P.P. Bioconversion of Sugarcane Molasses into Bioplastic (Polyhydroxybutyrate) using Bacillus cereus 2156 under Statistically Optimized Culture Conditions. Anal. Chem. Lett. 2020, 10, 80–92. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Wecker, P.; Moppert, X.; Simon-Colin, C.; Costa, B.; Berteaux-Lecellier, V. Discovery of a mcl-PHA with unexpected biotechnical properties: The marine environment of French Polynesia as a source for PHA-producing bacteria. AMB Express 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; He, F.; Liu, X.; Shi, J.; Liang, J.; Wang, S.; Yang, C.; Liu, R. Metabolic engineering of Pseudomonas mendocina NK-01 for enhanced production of medium-chain-length polyhydroxyalkanoates with enriched content of the dominant monomer. Int. J. Biol. Macromol. 2020, 154, 1596–1605. [Google Scholar] [CrossRef]
- Oliveira, G.H.; Zaiat, M.; Rodrigues, J.A.D.; Ramsay, J.A.; Ramsay, B.A. Towards the Production of mcl-PHA with Enriched Dominant Monomer Content: Process Development for the Sugarcane Biorefinery Context. J. Polym. Environ. 2020, 28, 844–853. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Wongsirichot, P.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Winterburn, J.; Retegi, A. Application of cider by-products for medium chain length polyhydroxyalkanoate production by Pseudomonas putida KT2440. Eur. Polym. J. 2018, 108, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 1454. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Keshavarz, T.; Roy, I. Novel Poly(3-hydroxyoctanoate)/Poly(3-hydroxybutyrate) blends for medical applications. React. Funct. Polym. 2013, 73, 1340–1348. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kageyama, Y. Increased Production and Molecular Weight of Artificial Polyhydroxyalkanoate Poly(2-hydroxybutyrate) Above the Glass Transition Temperature Threshold. Front. Bioeng. Biotechnol. 2019, 7, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi-Takeuchi, M.; Morisaki, K.; Toyooka, K.; Numata, K. Synthesis of High-Molecular Weight Polyhydroxyalkanoates by Marine Photosynthetic Purple Bacteria. PLoS ONE 2016, 11, e0160981. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.J.; Snell, K.D.; Hogan, S.A.; Stubbe, J.; Rha, C.; Sinskey, A.J. PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat. Biotechnol. 1997, 15, 63–67. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wen, Q.; Zhang, H. Biosynthesis of polyhydroxyalkanoate by Gamma proteobacterium WD-3 from volatile fatty acids. Chemosphere 2011, 82, 1209–1213. [Google Scholar] [CrossRef]
- Yuan, W.; Jia, Y.; Tian, J.; Snell, K.D.; Müh, U.; Sinskey, A.J.; Lambalot, R.H.; Walsh, C.T.; Stubbe, J. Class I and III polyhydroxyalkanoate synthases from Ralstonia eutropha and Allochromatium vinosum: Characterization and substrate specificity studies. Arch. Biochem. Biophys. 2001, 394, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Patel, S.K.S.; Kalia, V.C. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb. Cell Factories 2009, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valappil, S.P.; Rai, R.; Bucke, C.; Roy, I. Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J. Appl. Microbiol. 2008, 104, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, S.; Hyakutake, M.; Saito, Y.; Agus, J.; Mizuno, K.; Abe, H.; Tsuge, T. Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules 2011, 12, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Gapes, D.J.; Newman, R.H.; Dare, P.H. Physico-chemical properties of polyhydroxyalkanoate produced by mixed-culture nitrogen-fixing bacteria. Appl. Microbiol. Biotechnol. 2009, 82, 545–555. [Google Scholar] [CrossRef]
- Visakh, P.M. Chapter 1: Polyhydroxyalkanoates (PHAs), their Blends, Composites and Nanocomposites: State of the Art, New Challenges and Opportunities. Green Chem. Ser. 2014, 1–17. [Google Scholar] [CrossRef]
- Esposti, D.M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mat. Sci. Eng. C Mater. 2019, 100, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Zhang, Y.; Chen, G.-Q. Nanofibrous polyhydroxyalkanoate matrices as cell growth supporting materials. Biomaterials 2008, 29, 3720–3728. [Google Scholar] [CrossRef]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.A.; Fu, H.; Wu, D.; Yang, Z. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymeters 2018, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Bassas-Galià, M.; Gonzalez, A.; Micaux, F.; Gaillard, V.; Piantini, U.; Schintke, S.; Zinn, M.; Mathieu, M. Chemical Modification of Polyhydroxyalkanoates (PHAs) for the Preparation of Hybrid Biomaterials. Chim. Int. J. Chem. 2015, 69, 627–630. [Google Scholar] [CrossRef]
- Raza, Z.A.; Riaz, S.; Banat, I.M. Polyhydroxyalkanoates: Properties and chemical modification approaches for their functionalization. Biotechnol. Prog. 2018, 34, 29–41. [Google Scholar] [CrossRef]
- Kwiecień, M.; Adamus, G.; Kowalczuk, M. Selective reduction of PHA biopolyesters and their synthetic analogues to corresponding PHA oligodiols proved by structural studies. Biomacromolecules 2013, 14, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Samsuddin, F.M.; Benjamin, R.L.; Sriram, Y.; Shant, A.; Dennis, W.S. Green Polymer Chemistry: Biocatalysis and Materials II. Am. Chem. Soc. 2013, 1144, 291–301. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Mota, R.C.D.A.G.; da Silva, E.O.; de Menezes, L.R. Polymer nanocomposites used as scaffolds for bone tissue regeneration. MSA 2018, 9, 679–697. [Google Scholar]
- Ji, Y.; Li, X.-T.; Chen, G.-Q. Interactions between a poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolyester and human keratinocytes. Biomaterials 2008, 29, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Gamal, M.A.B.J. Water resistance, mechanical properties, and biodegradability of poly(3-hydroxybutyrate)/starch composites. Appl. Polym. Sci. 2010, 115, 2813–2819. [Google Scholar] [CrossRef]
- Ten, E.; Turtle, J.D.; Bahr, D.; Jiang, L.; Wolcott, M. Thermal and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites. Carbohydr. Polym. 2010, 51, 2652–2660. [Google Scholar] [CrossRef]
- Butt, F.I.; Muhammad, N.; Hamid, A.; Moniruzzaman, M.; Sharif, F. Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications—Review. Int. J. Biol. Macromol. 2018, 120, 1294–1305. [Google Scholar] [CrossRef]
- Saska, S.; Pires, L.C.; Cominotte, M.A.; de Mendes, L.S.; Oliveira, M.F.; Maia, I.A.; Da Silva, J.V.L.; Ribeiro, S.J.L.; Cirelli, J.A. Three-dimensional printing and in vitro evaluation of poly(3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering. Mater. Sci. Eng. C 2018, 89, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldi, P.; Steiner, P.; Raine, T.; Lin, K.; Kocabas, C.; Young, R.J.; Bissett, M.; Kinloch, I.A.; Papageorgiou, D.G. Multifunctional biocomposites based on polyhydroxyalkanoate and graphene/carbon nanofiber hybrids for electrical and thermal applications. ACS Appl. Polym. Mater. 2020, 2, 3525–3534. [Google Scholar] [CrossRef]
- Yao, H.; Wu, L.P.; Chen, G.Q. Synthesis and characterization of electroconductive PHA-graft-graphene nanocomposites. Biomacromolecules 2018, 20, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pickrell, G.; Sriranganathan, N.; Kumar, V.; Homa, D. Review and the state of the art: Sol-gel and melt quenched bioactive glasses for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1248–1275. [Google Scholar] [CrossRef]
- Phukon, P.; Radhapyari, K.; Konwar, B.K.; Khan, R. Natural polyhydroxyalkanoate–gold nanocomposite-based biosensor for detection of antimalarial drug artemisinin. Mater. Sci. Eng. C 2014, 37, 314–320. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Cristovan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Yao, J.-M.; Qin, Z.Y.; Liu, L.; Yang, X.G. Comparison of covalent and noncovalent interactions of carbon nanotubes on the crystallization behavior and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2013, 130, 4299–4307. [Google Scholar] [CrossRef]
- Sanpui, P.; Zheng, X.; Loeb, J.C.; Bisesi, J.H., Jr.; Khan, I.A.; Afrooz, A.R.M.N.; Liu, K.; Badireddy, A.R.; Wiesner, M.R.; Ferguson, P.L.; et al. Single-walled carbon nanotubes increase pandemic influenza A H1N1 virus infectivity of lung epithelial cells. Part. Fibre Toxicol. 2014, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sparks, J.; Scholz, C. Evaluation of a Cationic Poly(β-hydroxyalkanoate) as a Plasmid DNA Delivery System. Biomacromolecules 2009, 10, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, Z.; Chi, W.; Yang, X.; Wang, W.; Zhang, B. Mono-methoxy-poly(3-hydroxybutyrate-co-4-hydroxybutyrate)-graft-hyper-branched polyethylenimine copolymers for siRNA delivery. Biomaterials 2012, 33, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. BioMed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.H. Biphasic TiO2 microspheres/reduced graphene oxide for effective simultaneous photocatalytic reduction and oxidation processes. Appl. Catal. A Gen. 2017, 541, 25–34. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, Z.-R.; Gong, T.; Chen, G.-Q.; Sun, X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials 2012, 33, 1583–1588. [Google Scholar] [CrossRef]
- Papaneophytou, C.; Katsipis, G.; Halevas, E.; Pantazaki, A.A. Polyhydroxyalkanoates Applications in Drug Carriers. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 77–124. [Google Scholar]
- Govindasamy, S.; Syafiq, I.M.; Amirul, A.A.A.; Amin, R.M.; Bhubalan, K. Dataset on controlled production of polhydroxyalkanoate-based microbead using double emulsion solvent evaporation technique. Data Brief 2019, 23, 103675. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.; Wang, Y.; Chan, P.L.; Chan, S.W.; Tsang, Y.F.; Chua, H.; Yu, P.H.F. Production of polyhydroxyalkanoates (PHA) using sludge from different wastewater treatment processes and the potential for medical and pharmaceutical applications. Environ. Technol. 2017, 38, 1779–1791. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C 2017, 79, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; McAdams, D.A.; Grunlan, J.C. Nano/Micro-Manufacturing of Bioinspired Materials: A Review of Methods to Mimic Natural Structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar] [CrossRef]
- Puppi, D.; Morelli, A.; Chiellini, F. Additive Manufacturing of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/poly(ε-caprolactone) Blend Scaffolds for Tissue Engineering. Bioengineering 2017, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Galego, N.; Rozsa, C.; Sánchez, R.; Fung, J.; Vázquez, A.; Tomás, J.S. Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polym. Test. 2000, 19, 485–492. [Google Scholar] [CrossRef]
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A Micro-Ark for Cells: Highly Open Porous Polyhydroxyalkanoate Microspheres as Injectable Scaffolds for Tissue Regeneration. Sci. Adv. Mater. 2018, 30, 1802273. [Google Scholar] [CrossRef]
- Wang, M.; Duan, B. Nanocomposite scaffolds for bone tissue engineering: Design, fabrication, surface modification and sus-tained release of growth factor. Mater. Res. Soc. Symp. Proc. 2011, 1301, 99–110. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of art on solvent casting particulate leaching method for orthopedic scaffolds fab-rication. Mater. Today 2017, 4, 898–907. [Google Scholar]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Nassar, E.J.; Ciuffi, K.J.; Calefi, P.S.; Rocha, L.A.; De Faria, E.H.; Marcio, L.A.; Luz, P.P.; Bandeira, L.C.; Cestari, A.; Fernandes, C.N. Biomaterials and Sol–Gel Process: A Methodology for the Preparation of Functional Materials. In Biomaterials Science and Engineering; IntechOpen: London, UK, 2011. [Google Scholar]
- Ding, Y.; Li, W.; Correia, A.; Yang, Y.; Zheng, K.; Liu, D.; Schubert, D.W.; Boccaccini, A.R.; Santos, H.A.; Roether, J.A. Electrspun phb/pcl/sol-gel derived silica hybrid scaffolds with drug releasing function for bone tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 14540–14548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, A.; Oliviero, M.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef]
- Le Moigne, N.; Sauceau, M.; Benyakhlef, M.; Jemai, R.; Bénézet, J.C.; Rodier, E.; Fages, J. Foaming of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/organo-clays nanobiocomposites by a continuous supercritical CO2 assisted extrusion process. Eur. Polym. J. 2014, 61, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wray, L.S.; Burke, K.A.; Torregrosa, T.; Golinski, J.M.; Huang, W.; Kaplan, D.L. Lyophilized Silk Sponges: A Versatile Biomaterial Platform for Soft Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing in Situ-Stabilized Silver Nanoparticles. Nanomaterials 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoria, A.; Sinha-Ray, S. A review on biopolymer-based fibers via electrospinning and solution blowing and their applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Toloue, E.B.; Karbasi, S.; Salehi, H.; Rafienia, M. Evaluation of mechanical properties and cell viability of poly (3-hydroxybutyrate)-chitosan/Al2O3nanocomposite scaffold for cartilage tissue engineering. J. Med. Signals Sens. 2019, 9, 111–116. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. A Review: Electrospinning of Biopolymer Nanofibers and their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Datta, S.; Menon, G. Nanofibers from Polyhydroxyalkanoates and Their Applications in Tissue Engineering. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 409–420. [Google Scholar]
- Koller, M. Biodegradable and biocompatible polyhydroxyalkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Gahlawat, G.; Soni, S.K. Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour. Technol. 2017, 243, 492–501. [Google Scholar] [CrossRef]
- Israni, N.; Shivakumar, S. Polyhydroxyalkanoate (PHA) biosynthesis from directly valorized ragi husk and sesame oil cake by Bacillus megaterium strain Ti3: Statistical optimization and characterization. Int. J. Biol. Macromol. 2020, 148, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Odermatt, E.K.; Funk, L.; Bargon, R.; Martin, D.P.; Rizk, S.; Williams, S.F. MonoMax Suture: A New Long-Term Absorbable Monofilament Suture Made from Poly-4-Hydroxybutyrate. Int. J. Polym. Sci. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]




| Bacterial Species | PHA Yield before Optimization (w/v)% | PHA Yield after Optimization (w/v)% | Reference |
|---|---|---|---|
| Bacillus cereus SS105 | 53.36 % | 55.16 % | [18] |
| B. cereus FA11 | 22.66% | 62.03% | [19] |
| Bacillus cereus BNPI-92 | 40.38% | 60.67% | [20] |
| Bacillus cereus MCCB 281 | 58.00% | 62.23 % | [21] |
| B. cereus Y23 | 42.7% | 67.9% | [22] |
| Bacillus cereus 2156 | 59.30 % | 68.78 % | [23] |
| B. aryabhattai PHB10 | 32.64% | 75% | [5] |
| B. drentensis BP17 | 19.9% | 55.5% | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, M.; Rekhi, P.; Debnath, M.; Ramakrishna, S. Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules 2021, 26, 860. https://doi.org/10.3390/molecules26040860
Goswami M, Rekhi P, Debnath M, Ramakrishna S. Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules. 2021; 26(4):860. https://doi.org/10.3390/molecules26040860
Chicago/Turabian StyleGoswami, Moushmi, Pavni Rekhi, Mousumi Debnath, and Seeram Ramakrishna. 2021. "Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds" Molecules 26, no. 4: 860. https://doi.org/10.3390/molecules26040860
APA StyleGoswami, M., Rekhi, P., Debnath, M., & Ramakrishna, S. (2021). Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules, 26(4), 860. https://doi.org/10.3390/molecules26040860