Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer
Abstract
1. Introduction
2. Results
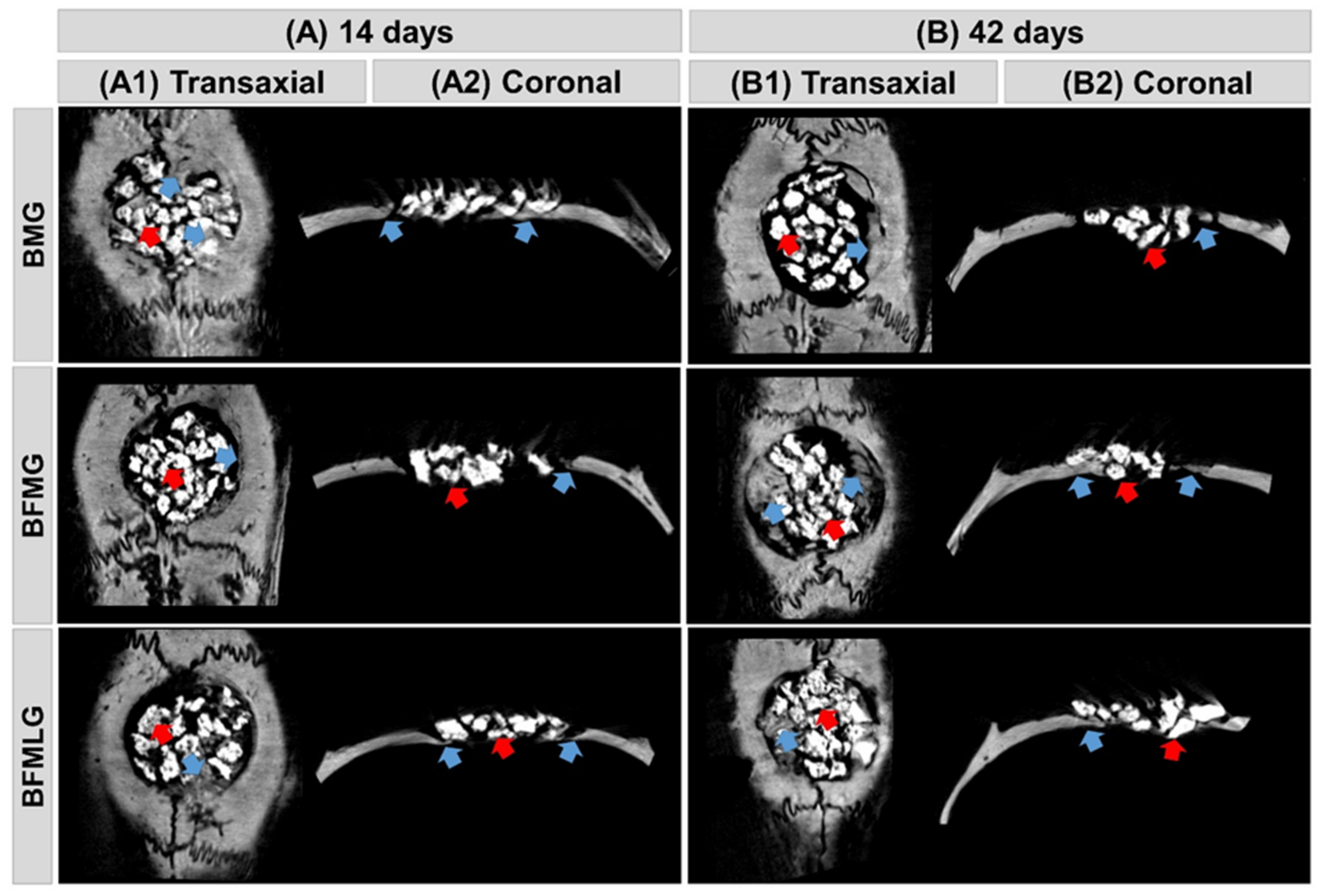
2.1. Microtomographic Analysis
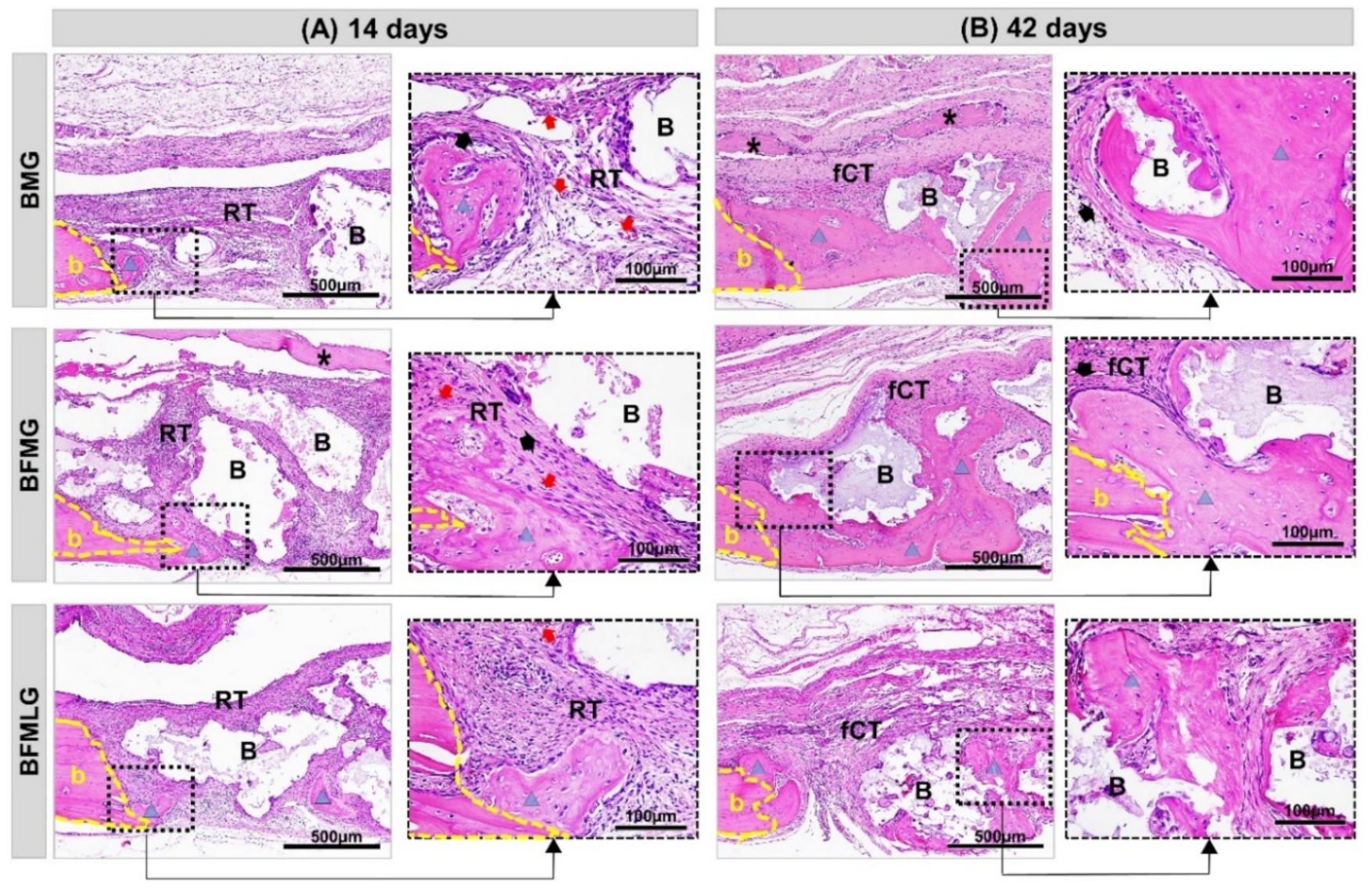
2.2. Histomorphological Analysis
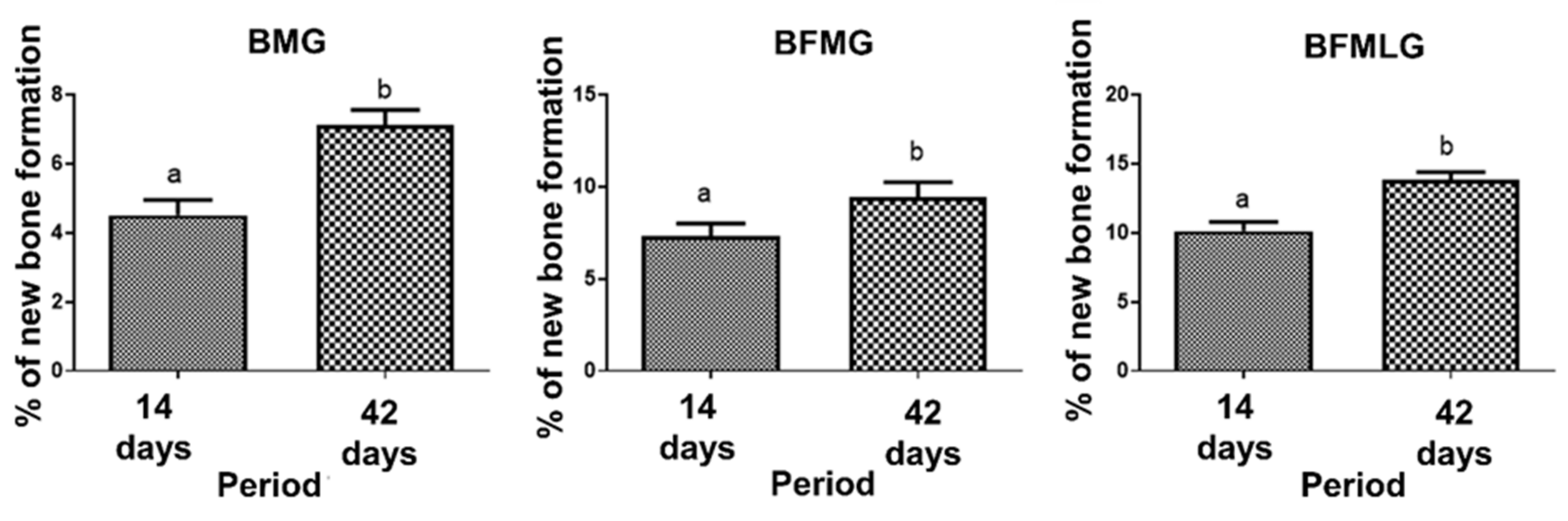
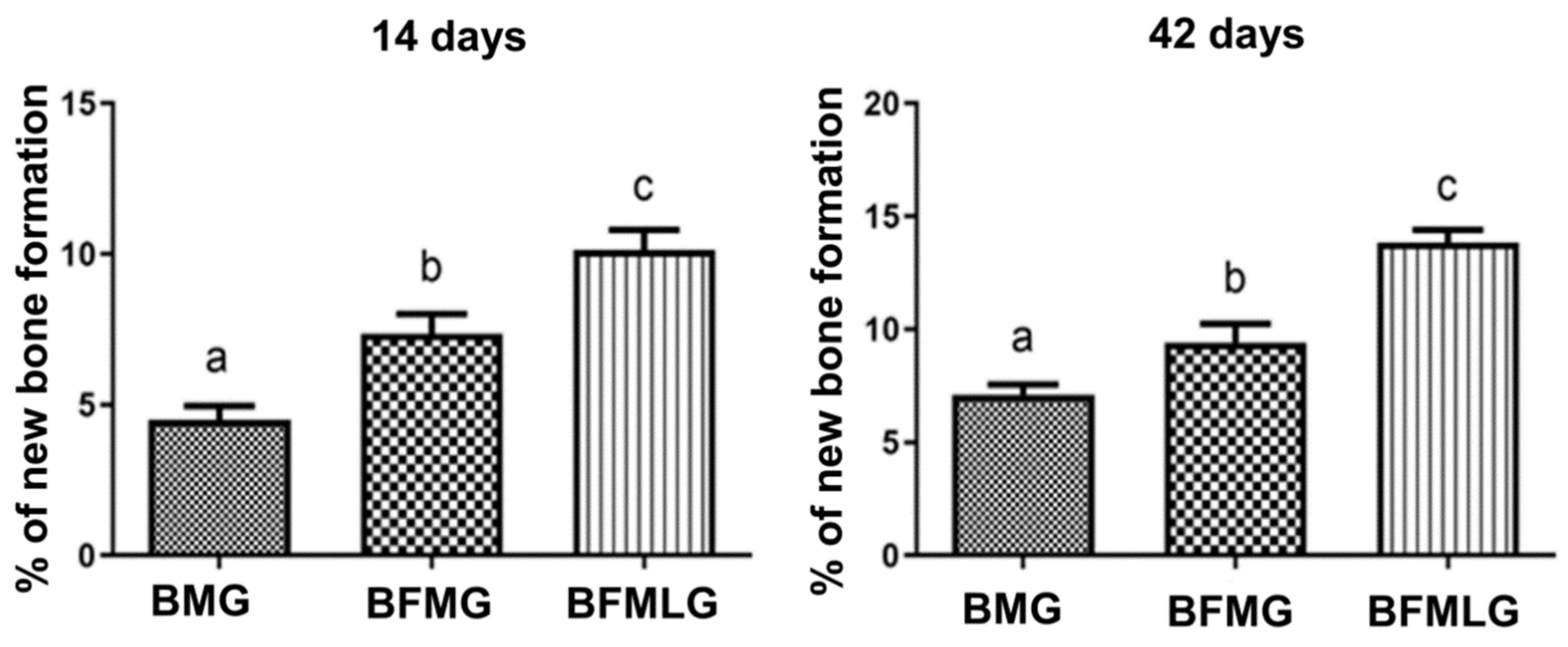
2.3. Histomorphometric Analysis
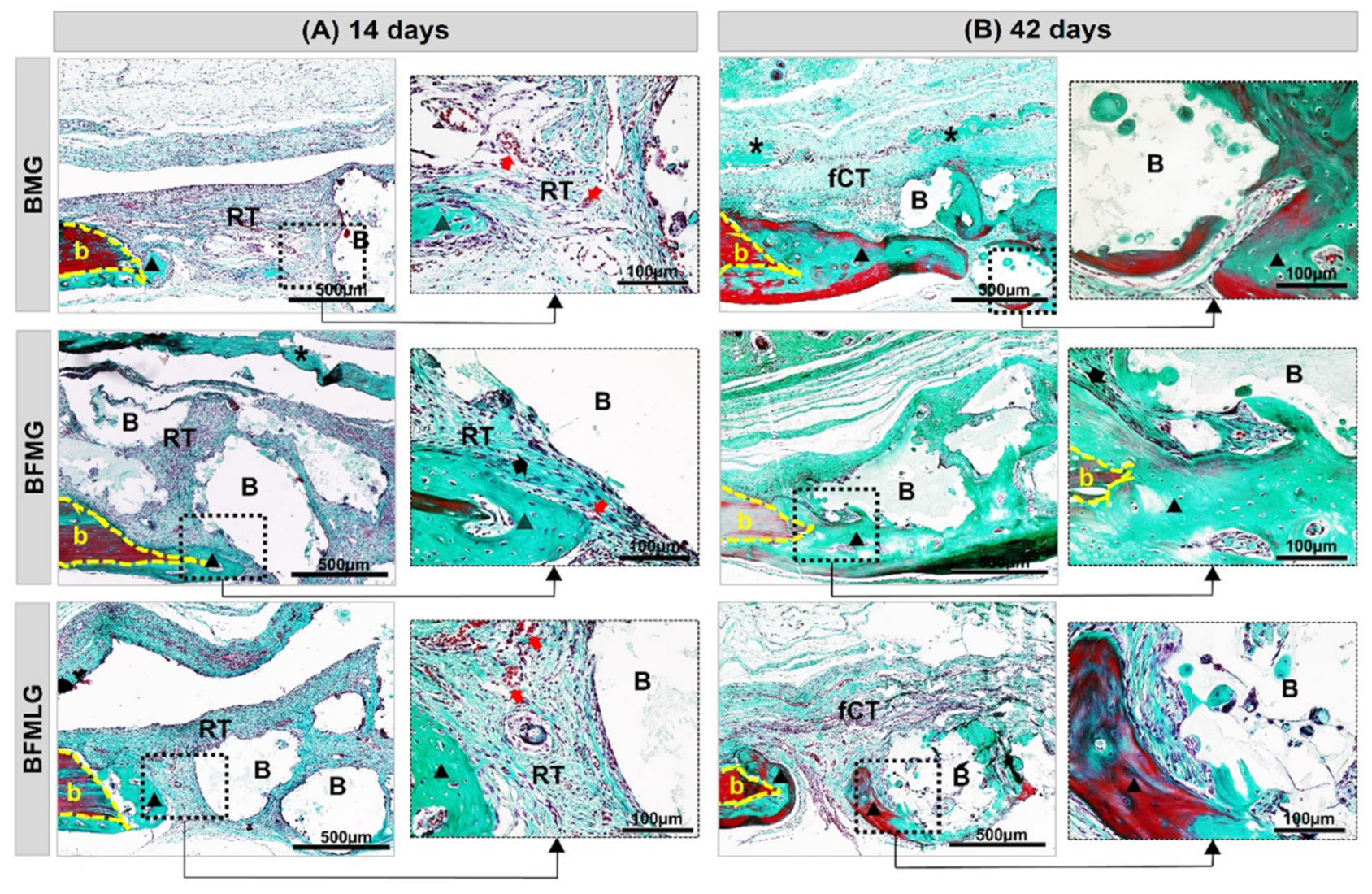
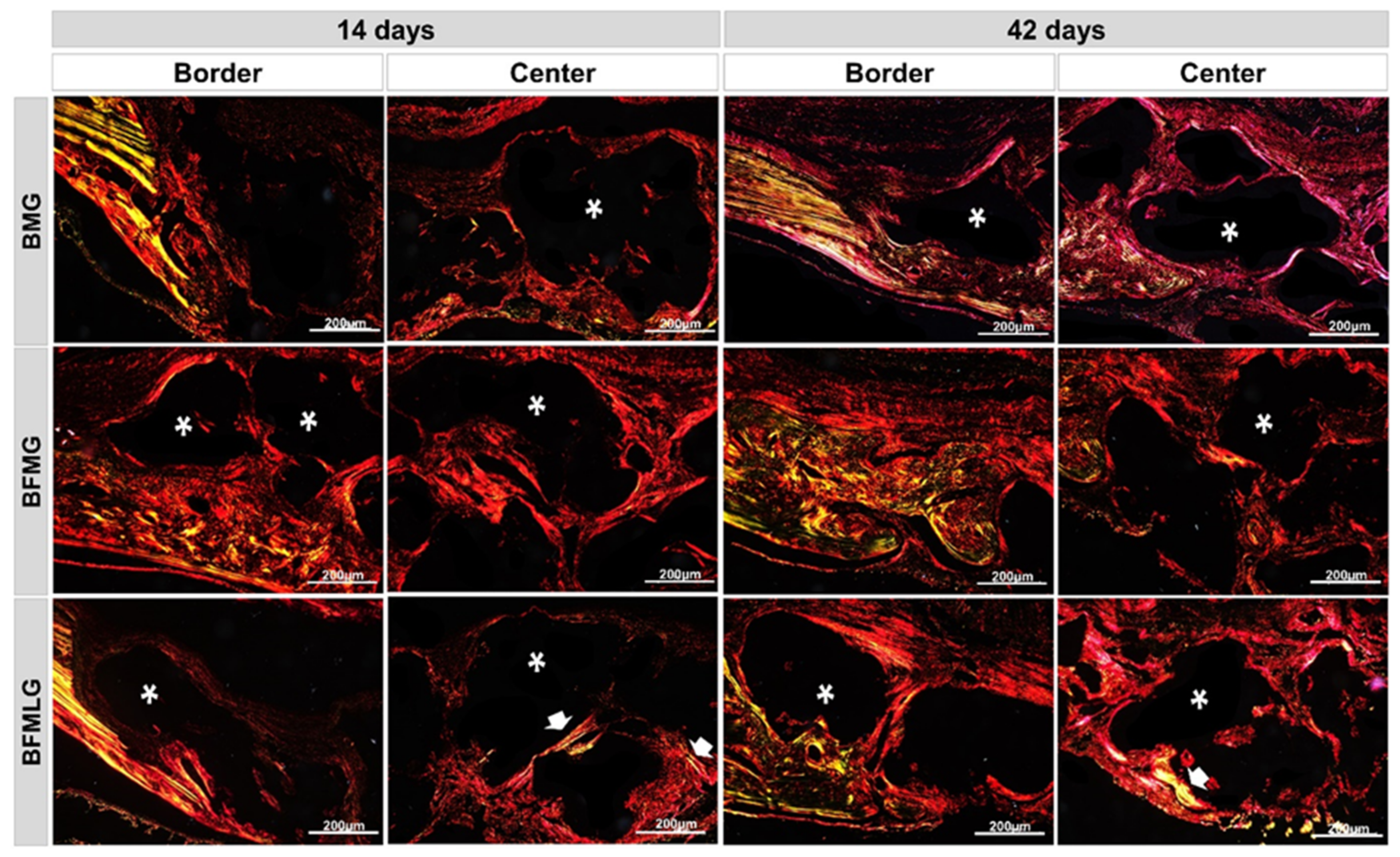
2.4. Analysis of Birefringence of Collagen Fibers
3. Discussion
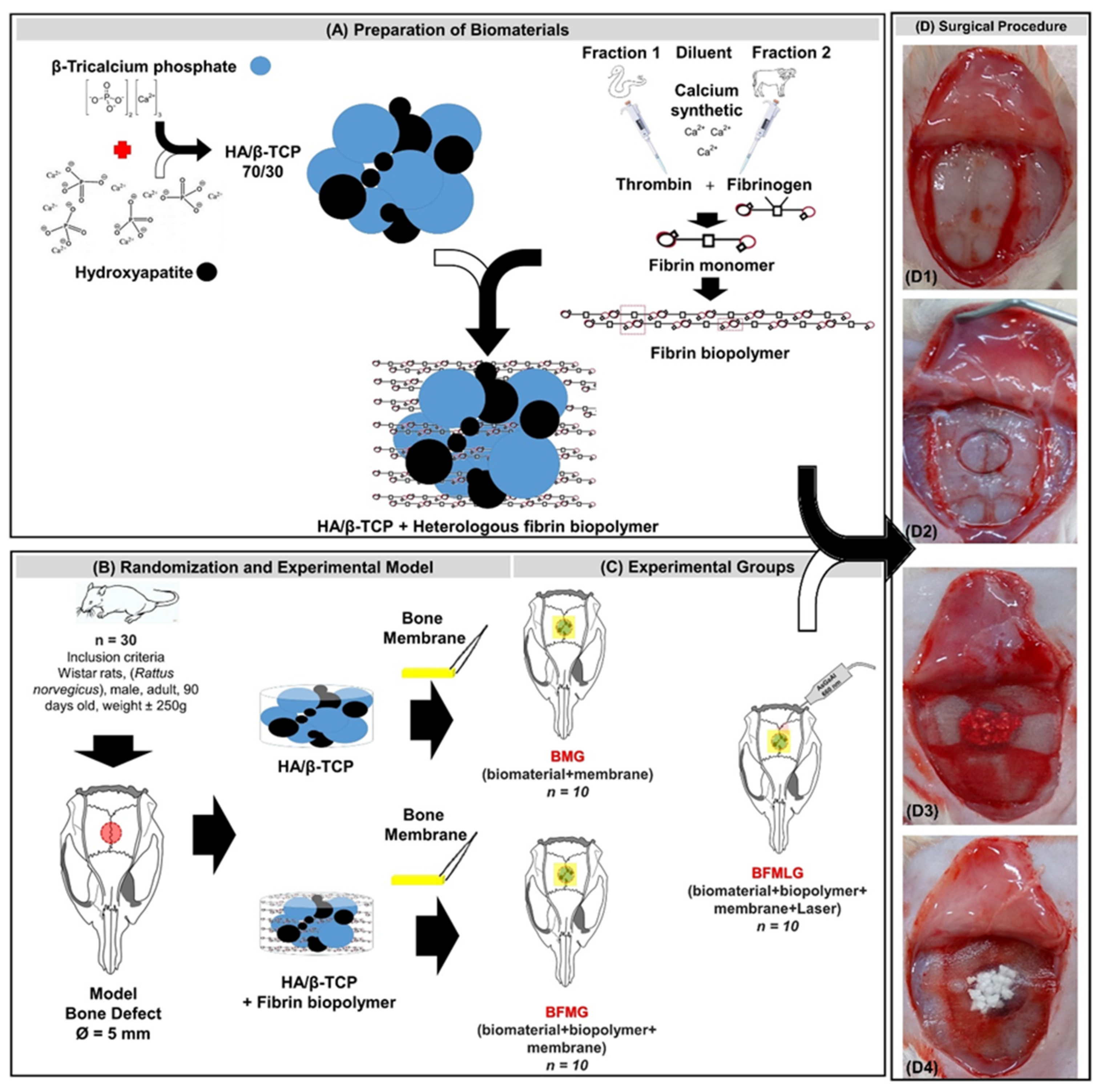
4. Materials and Methods
4.1. Alloplastic Biomaterial
4.2. Fibrin Biopolymer
- -
- Fraction 1: thrombin-like or gyroxin purified from the poison of Crotalus durissus terrificus, 0.4 mL.
- -
- Diluent: calcium chloride, 0.6 mL.
- -
- Fraction 2: fibrinogen, cryoprecipitate derived from the blood of buffalo (Bubalus bubalis), 1 mL.
Preparation of the Biopolymer
4.3. Guided Bone Regeneration (GBR)
4.4. Experimental Design
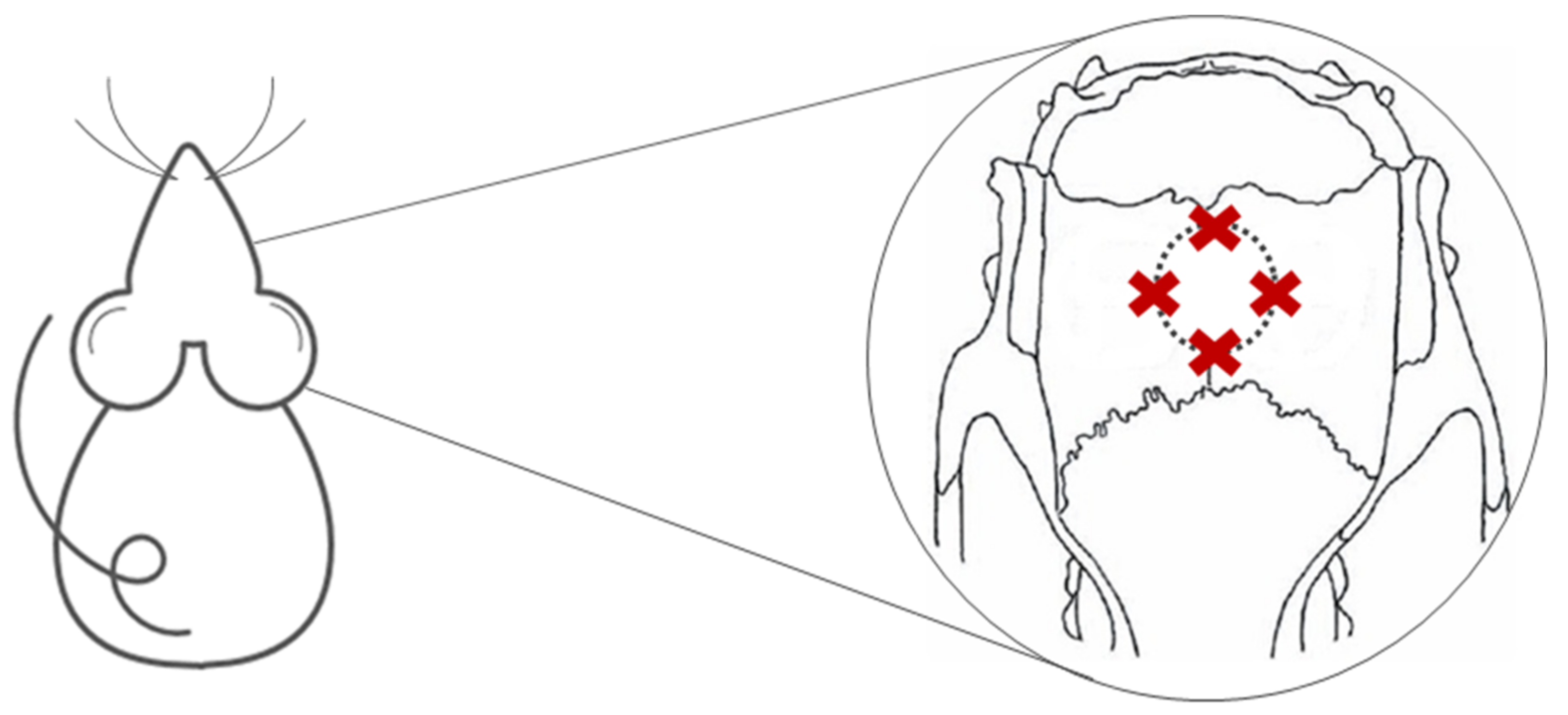
4.5. Surgical Procedure
4.6. Photobiomodulation Therapy
4.7. Sampling and Histological Procedures
4.8. Micro-CT
4.9. Histotechnical Processing
4.10. Histomorphological and Histomorphometric Analyses
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; I Mataliotakis, G.; Calori, G.M.; Giannoudis, P. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Rogers, G.F.; Greene, A.K. Autogenous bone graft: Basic science and clinical implications. J. Craniofac. Surg. 2012, 23, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Layrolle, P.; Daculsi, G.; Redl, H.; Pandit, A.; Czernuszka, J. A review of bioceramics and fibrin sealant. Eur. Cell Mater. 2004, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Le Nihouannen, D.; Goyenvalle, E.; Aguado, E.; Pilet, P.; Bilban, M.; Daculsi, G.; Layrolle, P. Hybrid composites of calcium phosphate granules, fibrin glue, and bone marrow for skeletal repair. J. Biomed. Mater. Res. Part A 2007, 81, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Wiltfang, J.; Pistner, H.; Yildirim, M.; Ploder, B.; Chapman, M.; Schiestl, N.; Hantak, E. Bone formation with a biphasic calcium phosphate combined with fibrin sealant in maxillary sinus floor elevation for delayed dental implant. Clin. Oral Implant. Res. 2012, 23, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, V.P.O.; Landim-Alvarenga, F.C.; Oliveira, A.L.R.; Simões, G.F.; Lima-Neto, J.F.; Barraviera, B.; Ferreira, R.S. A new fibrin sealant as a three-dimensional scaffold candidate for mesenchymal stem cells. Stem Cell Res. Ther. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Orsi, P.R.; Landim-Alvarenga, F.D.C.; Justulin, L.A.; Kaneno, R.; Assis, M.G.; Dos Santos, D.C.; Creste, C.F.Z.; Oba, E.; Maia, L.; Barraviera, B.; et al. A unique heterologous fibrin sealant (HFS) as a candidate biological scaffold for mesenchymal stem cells in osteoporotic rats. Stem Cell Res. Ther. 2017, 8, 205. [Google Scholar] [CrossRef]
- Cassaro, C.V., Jr.; De Lima, P.R.; Golim, M.D.A.; Biscola, N.P.; De Castro, M.V.; De Oliveira, A.L.R.; Doiche, D.P.; Pereira, E.J., Jr.; Barraviera, B.; Justulin, J.L.; et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Aciole, J.M.D.S.; Aciole, G.T.D.S.; Soares, L.G.P.; Barbosa, A.F.S.; Dos Santos, J.N.; Pinheiro, A.L.B.; Longo, L. Bone Repair on Fractures Treated with Osteosynthesis, ir Laser, Bone Graft and Guided Bone Regeneration: Histomorfometric Study. In Advances in Laserology-Selected Papers of Laser Florence 2010: The 50th Birthday of Laser Medicine World; AIP: Melville, NY, USA, 2011; Volume 1364, pp. 60–65. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Khadra, M.; Kasem, N.; Haanaes, H.R.; E Ellingsen, J.; Lyngstadaas, S.P. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Andreo, J.C.; Junior, R.S.F.; Barraviera, B.; Rodrigues, A.D.C.; Macedo, M.D.C.; Junior, G.M.R.; Shinohara, A.L.; German, I.J.S.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef]
- Rosso, M.P.D.O.; Buchaim, R.L.; Pomini, K.T.; Della Coletta, B.B.; Reis, C.H.B.; Pilon, J.P.G.; Júnior, G.D.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) Applied in Bone Reconstructive Surgery Using Bovine Bone Grafts: A Systematic Review. Materials 2019, 12, 4051. [Google Scholar] [CrossRef]
- De Freitas, N.R.; Guerrini, L.B.; Esper, L.A.; Sbrana, M.C.; Dalben, G.D.S.; Soares, S.; De Almeida, A.L.P.F. Evaluation of photobiomodulation therapy associated with guided bone regeneration in critical size defects. In vivo study. J. Appl. Oral Sci. 2018, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.S.B.; Perez, M.G.B.; Rosso, M.P.D.O.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef]
- Rosso, M.P.D.O.; Buchaim, D.V.; Kawano, N.; Furlanette, G.; Pomini, K.T.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Gerbi, M. Photo-engineering of bone repair processes. Photomed. Laser Surg. 2006, 24, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.F.; Faleiros, P.L.; Carmona, L.R.; Garcia, V.G.; Theodoro, L.H.; De Araujo, N.J.; Nagata, M.J.H.; De Almeida, J.M. Effects of low-level laser therapy on bone healing of critical-size defects treated with bovine bone graft. J. Photochem. Photobiol. B Biol. 2016, 163, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.B.D.O.; Buchaim, D.V.; Bueno, C.R.D.S.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; Rodrigues, A.D.C.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2016, 162, 663–668. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.D.O.; Della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Junior, G.M.R.; Shindo, J.V.T.C.; et al. Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Dalvi, S.; Amaroli, A.; De Angelis, N.; Benedicenti, S. Effects of photobiomodulation on bone defects grafted with bone substitutes: A systematic review of in vivo animal studies. J. Biophotonics 2021, 14. [Google Scholar] [CrossRef]
- Forte, C.P.F.; Matos, A.P.; Mendes, F.H.; Dias, C.C.; Ferreira, A.E.C.; Bezerra, T.P.; Sousa, F.B.; Silva, P.G.D.B. Photobiomodulation Therapy Reduces the Inflammatory Process without Inhibiting Bone Deposition in Rats in an Extraction Model. Photobiomodulation Photomed. Laser Surg. 2020, 38, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimões, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral Implant. Res. 2013, 25, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2011, 40, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.S.; Dos Santos, J.N.; Monteiro, J.S.; Amorim, P.G.; Pinheiro, A.L. Does the Use of Laser Photobiomodulation, Bone Morphogenetic Proteins, and Guided Bone Regeneration Improve the Outcome of Autologous Bone Grafts? An in Vivo Study in a Rodent Model. Photomed. Laser Surg. 2008, 26, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of light parameters and photobiomodulation efficacy: Dive into complexity. J. Biomed. Opt. 2018, 23, 120901. [Google Scholar] [CrossRef] [PubMed]
- Basford, J.R. Low intensity laser therapy: Still not an established clinical tool. Lasers Surg. Med. 1995, 16, 331–342. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef]
- Fávaro–Pípi, E.; Ribeiro, D.A.; Ribeiro, J.U.; Bossini, P.; Oliveira, P.; Parizotto, N.A.; Tim, C.R.; De Araújo, H.S.S.; Renno, A.C.M. Low-Level Laser Therapy Induces Differential Expression of Osteogenic Genes During Bone Repair in Rats. Photomed. Laser Surg. 2011, 29, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.K.; Song, L.; Yu, P.; Mehrara, B.J.; Maeda, C.Y.; Gold, L.I.; Longaker, M.T. Osteogenesis in Cranial Defects: Reassessment of the Concept of Critical Size and the Expression of TGF-β Isoforms. Plast. Reconstr. Surg. 2000, 106, 360–371. [Google Scholar] [CrossRef]
- Wang, J.; Xue, D.; Yuan, W.; Wang, W.; Shen, D.; Tong, X.; Shi, D.; Liu, L.; Zheng, Q.; Gao, C.; et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur. Cells Mater. 2010, 20, 109–120. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and Modulating Inflammation in Strategies for Bone Regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.B.; Pastoreli, M.T.; Damasceno, L.H.F.; Defino, H.L.A. Estudo experimental da influência das dimensões dos grânulos de hidroxiapatita na integração óssea. Acta Ortop. Bras. 2003, 11, 240–250. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Tamai, N.; Murase, T.; Myoui, A. Interconnected porous hydroxyapatite ceramics for bone tissue engineering. J. R. Soc. Interface 2008, 6, S341–S348. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.S.; Cestari, T.M.; Paulin, J.B.; Martins, R.; Rocha, C.A.; Arantes, R.V.N.; Costa, B.C.; Dos Santos, C.M.; Assis, G.F.; Taga, R. Osteoinductive porous biphasic calcium phosphate ceramic as an alternative to autogenous bone grafting in the treatment of mandibular bone critical-size defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Sung, H.-M.; You, H.-K.; Lee, J. Effects of fibrinogen concentration on fibrin glue and bone powder scaffolds in bone regeneration. J. Biosci. Bioeng. 2014, 118, 469–475. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Della Coletta, B.B.; Pomini, K.T.; Rosso, M.P.D.O.; Campos, L.M.G.; Jr, R.S.F.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Creste, C.F.Z.; Orsi, P.R.; Landim-Alvarenga, F.D.C.; Justulin, L.A.; Golim, M.D.A.; Barraviera, B.; Ferreira, J.R.S. Highly Effective Fibrin Biopolymer Scaffold for Stem Cells Upgrading Bone Regeneration. Materials 2020, 13, 2747. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.G.; Leite, A.P.S.; Sartori, A.A.; Tibúrcio, F.C.; Barraviera, B.; Junior, R.S.F.; Filadelpho, A.L.; De Carvalho, S.C.; Matheus, S.M.M. Heterologous fibrin biopolymer associated to a single suture stitch enables the return of neuromuscular junction to its mature pattern after peripheral nerve injury. Injury 2020. [Google Scholar] [CrossRef]
- De Oliveira, C.T.B.; Leonel, B.C.; De Oliveira, A.C.; Paiva, M.D.B.; Ramos, J.; Barraviera, B.; Ferreira, R.S.; Shimano, A.C. Effects of fibrin sealant and bone fragments on defect regeneration performed on rat tibiae: An experimental study. J. Mech. Behav. Biomed. Mater. 2020, 104, 103662. [Google Scholar] [CrossRef]
- Rosso, M.P.D.O.; Oyadomari, A.T.; Pomini, K.T.; Botteon, B.; Coletta, D.; Cosin, T.; Seabra, R.; Ferreira, J.; Barraviera, B.; Cassaro, C.V.; et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules 2020, 10, 1–17. [Google Scholar] [CrossRef]
- State, P.; Regina, S.; Sartori, C.; State, P.; Silvares, M.R.; Satate, P. A new fibrin sealant derived from snake venom candidate to treat chronic venous ulcers. J. Am. Acad. Dermatol. 2015, 72, AB271. [Google Scholar]
- Abbade, L.P.F.; Ferreira, J.R.S.; Santos, L.; Barraviera, B. Chronic venous ulcers: a review on treatment with fibrin sealant and prognostic advances using proteomic strategies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190101. [Google Scholar] [CrossRef] [PubMed]
- Kempe, P.R.G.; Chiarotto, G.B.; Barraviera, B.J.R.S.F., Jr.; De Oliveira, A.L.R. Neuroprotection and immunomodulation by dimethyl fumarate and a heterologous fibrin biopolymer after ventral root avulsion and reimplantation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, 1–18. [Google Scholar] [CrossRef]
- Mozafari, R.; Kyrylenko, S.; Castro, M.V.; Ferreirajr, R.S.; Barraviera, B.; Oliveira, A.L.R. Combination of heterologous fibrin sealant and bioengineered human embryonic stem cells to improve regeneration following autogenous sciatic nerve grafting repair. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.L.D.L.; Granjeiro, J.M.; Cestari, T.M.; Taga, R. Healing of critical-size cranial defects in guinea pigs using a bovine bone-derived resorbable membrane. Int. J. Oral Maxillofac. Implant. 2008, 23, 427–436. [Google Scholar]
- Bernabe, P.F.E.; Melo, L.G.N.; Cintra, L.T.A.; Filho, J.E.G.; Jr, E.D.; Nagata, M.J.H. Bone healing in critical-size defects treated with either bone graft, membrane, or a combination of both materials: A histological and histometric study in rat tibiae. Clin. Oral Implant. Res. 2011, 23, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, S.; Wong, L.O.; Ma, L.; Hao, J.; Kasugai, S.; Lang, N.P.; Mattheos, N. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin. Oral Implant. Res. 2015, 27, e206–e214. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Júnior, G.M.R.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; De Souza, R.G.; Pereira, M.; Júnior, I.A.F.; Bueno, C.R.D.S.; Gonçalves, J.B.D.O.; et al. Stimulation of morphofunctional repair of the facial nerve with photobiomodulation, using the end-to-side technique or a new heterologous fibrin sealant. J. Photochem. Photobiol. B: Biol. 2017, 175, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.R.; Menezes, F.A.; Dos Santos, G.R.; Pinto, C.A.L.; Barraviera, B.; Martins, V.D.C.A.; Plepis, A.M.D.G.; Junior, R.S.F. Hydroxyapatite and a New Fibrin Sealant Derived from Snake Venom as Scaffold to Treatment of Cranial Defects in Rats. Mater. Res. 2015, 18, 196–203. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Karystinou, A.; Guillot, P.V.; Fisk, N.M.; Jones, E.A.; McGonagle, D.; Khan, I.M.; Archer, C.W.; Mitsiadis, T.A.; et al. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheum. 2008, 58, 240–250. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V.; Chung, T.-Y.; Baxter, G.D. Laser Photobiomodulation of Proliferation of Cells in Culture: A Review of Human and Animal Studies. Photomed. Laser Surg. 2010, 28, S3–S40. [Google Scholar] [CrossRef]
- Mokoena, D.R.; Houreld, N.N.; Kumar, S.S.D.; Abrahamse, M.H.A.H. Photobiomodulation at 660 nm Stimulates Fibroblast Differentiation. Lasers Surg. Med. 2019, 52, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Tim, C.R.; Bossini, P.S.; Kido, H.W.; Malavazi, I.; Kress, M.R.V.Z.; Carazzolle, M.F.; Rennó, A.C.; Parizotto, N.A. Low-level laser therapy induces an upregulation of collagen gene expression during the initial process of bone healing: a microarray analysis. J. Biomed. Opt. 2016, 21, 88001. [Google Scholar] [CrossRef]
- Bossini, P.S.; Renno, A.C.M.; Ribeiro, D.A.; Fangel, R.; Ribeiro, A.C.; Lahoz, M.D.A.; Parizotto, N.A. Low level laser therapy (830 nm) improves bone repair in osteoporotic rats: Similar outcomes at two different dosages. Exp. Gerontol. 2012, 47, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Garavello-Freitas, I.; Baranauskas, V.; Joazeiro, P.; Padovani, C.R.; Pai-Silva, M.D.; Da Cruz-Höfling, M.A. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J. Photochem. Photobiol. B Biol. 2003, 70, 81–89. [Google Scholar] [CrossRef]
- Mitchell, J.; Heteren, A.H. Van A literature review of the spatial organization of lamellar bone. C. R. Palevol. 2016, 15, 23–31. [Google Scholar] [CrossRef]
- Pomini, K.T.; Andreo, J.C.; De Rodrigues, A.C.; De Gonçalves, J.B.O.; Daré, L.R.; German, I.J.S.; Rosa, G.M.; Buchaim, R.L. Effect of low-intensity pulsed ultrasound on bone regeneration biochemical and radiologic analyses. J. Ultrasound Med. 2014, 33, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Deniz, E.; Arslan, A.H.; Diker, N.; Olgac, V.; Kilic, E. Evaluation of light-emitting diode photobiomodulation on bone healing of rat calvarial defects. Biotechnol. Biotechnol. Equip. 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Santos, J.D.A.F.; Campelo, M.B.D.; De Oliveira, R.A.; Nicolau, R.A.; Rezende, V.E.A.; Arisawa, E. Ângela L. Effects of Low-Power Light Therapy on the Tissue Repair Process of Chronic Wounds in Diabetic Feet. Photomed. Laser Surg. 2018, 36, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Arashiro, F.N.; Jardim, E.C.G.; da Silva, J.C.L. Enucleation of Odontogenic Cyst with Bone Graft. Int. J. Odontostomatol. 2019, 13, 433–436. [Google Scholar] [CrossRef]
- Encarnação, I.C.; Ferreira Xavier, C.C.; Bobinski, F.; Dos Santos, A.R.S.; Corrêa, M.; De Freitas, S.F.T.; Aragonez, A.; Goldfeder, E.M.; Cordeiro, M.M.R. Analysis of bone repair and inflammatory process caused by simvastatin combined with PLGA+HA+βTCP scaffold. Implant. Dent. 2016, 25, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Vieira, L.; Barraviera, B.; Barraviera, S. Treatment of venous ulcers with fibrin sealant derived from snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 226–229. [Google Scholar] [CrossRef]
- Paini, S.; Bighetti, A.C.C.; Cestari, T.M.; Arantes, R.V.N.; Santos, P.S.; Mena-Laura, E.E.; Garlet, G.; Taga, R.; Assis, G.F. Concentration-dependent effects of latex F1 -protein fraction incorporated into deproteinized bovine bone and biphasic calcium phosphate on the repair of critical-size bone defects. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2020, 108, 3270–3285. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.B.; Aciole, G.T.S.; Aciole, J.M.S.; Silveira, L.; Dos Santos, J.N.; Pinheiro, A.L.B. Assessment of bone healing on tibial fractures treated with wire osteosynthesis associated or not with infrared laser light and biphasic ceramic bone graft (HATCP) and guided bone regeneration (GBR): Raman spectroscopy study. SPIE BiOS 2011, 7887, 78870. [Google Scholar] [CrossRef]
- Costa, N.M.; Yassuda, D.H.; Sader, M.S.; Fernandes, G.V.; Soares, G.D.A.; Granjeiro, J.M. Osteogenic effect of tricalcium phosphate substituted by magnesium associated with Genderm® membrane in rat calvarial defect model. Mater. Sci. Eng. C 2016, 61, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Rodrigues, A.D.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; Bueno, C.R.D.S.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef] [PubMed]
| 14 Days | 42 Days | p Value | |
|---|---|---|---|
| BMG | 4.51 ± 0.44aA | 7.11 ± 0.44bA | p < 0.0001 |
| BFMG | 7.35 ± 0.66aB | 9.41 ± 0,84bB | p = 0.0026 |
| BFMLG | 10.12 ± 0.67aC | 13.85 ± 0.54bC | p < 0.0001 |
| Parameter | Unit/Description |
|---|---|
| Type of laser | GaAlAs (gallium-aluminum-arsenide) |
| Output power | 30 mW |
| Wavelength | 830 nm |
| Power density | 258.6 mW/cm2 |
| Energy density | 6.2 J/cm2 |
| Beam area | 0.116 cm2 |
| Total power | 2.9 J |
| Beam type | Positioned perpendicular to the skull |
| Emission mode | Continuous |
| Form of application | Four points around the surgical area |
| Irradiation duration | 24 s per point |
| Total time of each application | 96 s |
| Treatment time | Immediately after surgery and three times a week until euthanasia. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.d.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.d.S.B.M.; Roque, D.D.; Rosso, M.P.d.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. https://doi.org/10.3390/molecules26040847
Della Coletta BB, Jacob TB, Moreira LAdC, Pomini KT, Buchaim DV, Eleutério RG, Pereira EdSBM, Roque DD, Rosso MPdO, Shindo JVTC, et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules. 2021; 26(4):847. https://doi.org/10.3390/molecules26040847
Chicago/Turabian StyleDella Coletta, Bruna Botteon, Thiago Borges Jacob, Luana Aparecida de Carvalho Moreira, Karina Torres Pomini, Daniela Vieira Buchaim, Rachel Gomes Eleutério, Eliana de Souza Bastos Mazuqueli Pereira, Domingos Donizeti Roque, Marcelie Priscila de Oliveira Rosso, João Vitor Tadashi Cosin Shindo, and et al. 2021. "Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer" Molecules 26, no. 4: 847. https://doi.org/10.3390/molecules26040847
APA StyleDella Coletta, B. B., Jacob, T. B., Moreira, L. A. d. C., Pomini, K. T., Buchaim, D. V., Eleutério, R. G., Pereira, E. d. S. B. M., Roque, D. D., Rosso, M. P. d. O., Shindo, J. V. T. C., Duarte, M. A. H., Alcalde, M. P., Júnior, R. S. F., Barraviera, B., Dias, J. A., Andreo, J. C., & Buchaim, R. L. (2021). Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules, 26(4), 847. https://doi.org/10.3390/molecules26040847








