Abstract
Traditionally, biochar is generally proposed to substitute Canadian Sphagnum peat moss. Sugarcane bagasse, filter press mud and sugarcane bagasse ash were mixed in different ratios and proposed as an alternative to substitute commercial Sphagnum peat moss (SPM) for the soilless cultivation of tomato (S. lycopersicum Linnaeus). The choice of the agroindustrial waste ratio was performed from physical-chemical sample characterization. During vegetable production, an adequate ratio was evaluated based on plant height, substrate pH, number of leaves, yield, and fruit size. The addition of essential macro- and micro-nutrients was performed manually. The results show that as-received materials contain different minerals with a structure that corresponds to the quartz, cristobalite, and feldspar phases. A morphology composed of lamellate structures was observed for sugarcane bagasse, whereas the filter press mud and the sugarcane bagasse ash presented compact agglomerates with a surface area of 1.60, 3.78, and 1.07 m2 g−1, respectively. The properties of each component promote the water adsorption, retention and releasing capacity. Important differences were observed as the quantity of filter press mud increased, but also it is required an amount of sugarcane bagasse above of 18 wt% to reach a superior performance. This work demonstrated that, in adequate quantities, agroindustrial wastes can be used as a soilless substrate for growing tomatoes in greenhouse, reducing their disposal problems.
1. Introduction
Worldwide exists a growing crisis in three main categories: energy, water, and food. Alarmingly, the number of hungry people globally has risen for three consecutive years leading to 2017 up to 821 million people [1]. One factor that has contributed to the constant food crisis is the climate change [2]. In the last 10 years, climate change has been the most discussed environmental problem in the world, and alternatives to mitigate it have been requested. Given the currently projections of global climate change, where it is predicted that temperature will increase between 2 °C and 4 °C for 2080 [2,3], the impact in the available natural resources and energy supply will also exacerbate the shortage problems [4]. Two of the main alternatives to diminish climate change are based on the gradual elimination of the energy consumption from fossil fuels and the development of a sustainable agriculture [5]. Particularly, in Mexico, there are ~52.4 million poor people (43.6% of total population) and another ~62 million people (50.6%) have income below the welfare limit [6]. For this reason, alternatives to improve the food production at low cost with sustainable materials and techniques are highly demanded. In fact, it is expected that by 2050, the demand for agricultural products will increase by 100% compared to 2005 [7,8].
On the other hand, tomato (S. lycopersicum Linnaeus) is one of the most important and popular fruit vegetable in the world. It is a self-pollinated annual crop and belongs to the family Solanaceae. Actually, the tomato production is the pivotal point on horticultural industry and is growing worldwide either for fresh market and processing [9,10]. There are three main farming systems that are commonly used for tomato production, the first, known as traditional agriculture, is based on the empirical knowledge of low-income farmers [11,12]; the second, current agriculture that promotes the use of nitrogen with agrochemicals; and, the third, greenhouse tomato production. A wide variety of methods to evaluate vegetable production from greenhouse systems and their environmental impact have been extensively studied [9,13,14]. Greenhouses have emerged as an alternative to improve yield of crops, especially in soilless process, enhancing the operational efficiency during irrigation by recirculation of nutrient solutions (zero runoff sub-irrigation) [15]. There is a wide variety of materials that can be used as substrate during tomato or vegetables growth such as: rock wool [16], perlite [17], gravel [18], synthetic foam [19], SPM [20], and different kinds of residues [10]. Residues of leaf and corn stalks [21], wheat straw [22], barley and beans [23], cotton shell [24], and others, have been reported with adequate yields [25]. Among these materials, SPM stands out for displaying excellent production [26]. This substrate is produced by the accumulation of organic material forming several thick layers throughout the years from 0.9–7.3 cm year−1. The substrate decomposition degree modifies some physical properties such as: apparent density (0.1–0.5 g cm−3), porosity (80–94%), water absorption capacity (1–287 g/100 g dry matter (DM)), easy water extraction (24–33.5%), reserve water (4.7–6.5%), and cationic exchange capacity (110–250 meq/100 g DM), providing a medium to grow a wide variety of vegetables. Unfortunately, there is an overexploitation of this material, affecting the biodiversity and the hydric cycle of different ecosystems [27]. Additionally, the bogs are considered as worldwide carbon accumulators; estimating that contain twice the carbon stored in all the forests of the world, a compare quantity with all the carbon existing in the atmosphere [28]. Similarly, it is considered as the only habitat of evolved animal and vegetable species through thousands of years adapted to this particular environment [29]. Thus, it is evident that the uncontrolled exploitation of peats presented in the last years, is a generalized concern around the world, since its extraction liberates massive quantities of carbon in the planet, generating highly contaminated greenhouse gas production [27,30]. Additional factors to be considered are the fuel cost for extraction, processing and transportation, which elevate the final cost of horticultural plant production. Different studies have proposed substitution of SPM using different kind of compost; such as: vermicomposting, biochar, and green manure [10,31,32,33,34]. However, alternatives for peat substitution in soil free-substrates including biomass waste products are still under studies and represent a challenge [35,36,37,38]. Among agroindustrial wastes, the different by-products obtained during sugar manufacture can be interesting alternative biomasses to be used as substrate in horticulture applications [12,30]. Specifically, sugarcane bagasse (SCB), filter press mud (FPM), and sugarcane bagasse ash (SCBA) are the most unappreciated biomasses.
SCB is a fibrous material residue resulting from the cleaning, preparation and extraction of sugarcane juice and contains about 50% cellulose, 25% lignin, and 25% hemicellulose [39]. SCB is actually used during the production of paper pulp and ethanol, also significant amounts still burned in sugarcane mills to produce energy and electricity [40,41]. It is estimated a worldwide SCB production of 280 million tons per year [42]. Initial physical properties of SCB fibers such as total porous space (93.7%), total water availability (37.3%), apparent density (0.09 g cm−3) and high capacity of water retention (699 mL L−1) make it a good candidate to form a compost for soil-free substrates [43]. Additionally, during sugarcane juice clarification and filtrations, solid fibrous residues are also generated (FPM). In Mexico, this by-product is commonly discarded by the sugar mill without giving a productive use due to the lack of knowledge of its value as a fertilizer [44]. FPM is characterized in a dry weight basis by 9–14% wax, oil, resin, 10–18% protein, 11–17% cellulose, 15–27% hemicellulose and 9–14% lignin [45,46,47]. Additionally, it is known that contains phosphorous, calcium, nitrogen, zinc, boron and organic material, with an average pH of 8.2 [48]. Then, its incorporation in soils can enhance filtration and the solubility of calcium carbonate. Another waste of the sugar industry, is the SCBA, which is the last by-product derived of the bagasse combustion over 700 °C, a process that allows the breakup of the bond of the biomass product generating nutrients with vegetable assimilation [49,50]. The SCBA composition is mostly SiO2 (58–76%) according to the sugarcane species, it also contains in a lower degree Al2O3 (5.8–11.8%), Fe2O3 (4.5–6.4%), CaO (3.3–9%), MgO (2.2–4.3%), K2O (2–7.3%), and Na2O (1.1–1.3%) [51]. It is usually used as reinforcement of concrete and red ceramics production [52,53,54].
On the basis of this background, the goal of this study was to evaluate a new sugarcane byproducts-based substrate (FPM, SCB, and SCBA) as alternative for substitution of commercial SPM using the greenhouse soilless harvest technique in tomato plant “Alvaro” species (S. lycopersicum Linnaeus). Initial physico-chemical characterizations were performed to evaluate the amount of protein, crude fiber, ash, DM, ether extract, humidity, as well as porosity and morphology to propose the most prominent substrate compositions. The effect of the different substrate compositions on the height of the plant, number of leaves, fruit yield, and fruit size was analyzed. As control for crop performance, it was carried out a set of experiments with SPM under similar conditions.
2. Materials and Methods
2.1. Raw Materials
The agroindustrial waste matter, SCB, FPM, and SCBA, typically generated as by-product in sugar mills, was used in this study. These raw materials were obtained as a donation by ZUCARMEX SA de CV locate in “El Higo”, Veracruz, México (21°77′37″ N, 98°45′54″ W). The average humidity of this area is 88% with an average temperature of 30 °C. All the agroindustrial wastes were received in 2019 (June) and the experiments were started in 2019 (September). Commercial SPM was purchased from a distribution center. This product is manufactured for Premier Horticulture Inc. under the name of Sphagnum peat moss (premier). The total pore space of the commercial SPM was about 70% with a coarse particle size.
2.2. Characterization
The as-received agroindustrial wastes were characterized by proximate analyses using the methods of the Association of Official Analytical Chemists [55], specifically ash (942.05) from Equation (1):
Ash (%) = (Weight of ash)/(Weight of sample) × 100
Crude fiber (962.09) using Equation (2):
Crude fiber (%) = (((Dried crude fiber + moisture content) − Moisture content))/(Moisture content) × 100
Ether extract (945.16) and dried matter basis computed from the following formula:
where, W_1 is the weight of the original sample with a filter bag before drying at the pre-extraction stage, W_2 is the sample weight with filter bag after drying at the post-extraction stage. Finally, it was determined crude protein (CP, Kjeldahl, N × 6.25, 990.03) using the Equation (4):
Crude oil(%) = ((W_1 − W_2))/((W_1 − W_o)) × 100
Crude protein (%) = ((A − B × N × 1.4007 × 6.25)/(Weight of sample)) × 100
In this formula, A is the volume of 0.1 N HCl used in the sample titration, B refers to the volume of 0.1N HCl used in the blank titration and N is the normality of HCl. It is important to mention that all characterization presented in this work was carried out in triplicate.
The moisture tests were performed with 30 g of agroindustrial wastes, which were dried at 100 °C and thereafter, the moisture content was computed from the following equation:
Moisture (%) = ((initial weight–final weight)/(initial weight)) × 100
A structural characterization of the as-received raw materials was performed to evaluate possible impurities. X-ray diffraction (XRD) patterns were recorded on a Bruker D8-Advance diffractometer (Brucker, Billerica, MA, USA) operating at 35 kV and 15mA (CuKα = 1.54056 Å). The spectra were acquired from 10 to 90° using a step size of 0.05° s−1.
Fourier transform infrared spectroscopy (FT-IR) was carried out to analyze chemical groups contained in the agroindustrial wastes [56]. The IR spectra were performed in a Spectrum One Perkin-Elmer spectrometer (Perkin Elmer, Waltham, MA, USA) equipped with a diffuse reflectance accessory. The SCB, FPM and SCBA spectra were acquired in the wavenumber interval from 4000 to 600 cm−1 under room conditions with 10 scans and a resolution of 4 cm−1. Morphological aspects and semi-quantitative composition of the materials were determined by scanning electron microscopy (SEM)/energy dispersive X-ray spectroscopy (EDS) using a JSM-7800F field emission electron microscope (JEOL Ltd., Akishima, Tokyo, Japan), the observations were performed at 15 kV.
Specific surface area of the as-receive materials and substrates that present an adequate fruit yield were studied by Brunauer–Emmett–Teller (BET) method. A micrometrics instruments corporation, Tristar II 3020 equipment (Norcross, GA, USA) was used. The evaluated temperatures were in the range of 30–300 °C using a rate of 5 °C min−1 and 120 min of acquisition time. The pore size distribution of the samples was also determined by Barret–Joyner–Halenda (BJH) method.
2.3. Substrate Preparation
Experiments were carried out in a greenhouse located at the Research Center in Applied Science and Advance Technology in Altamira Tamaulipas, Mexico (22°13′39″ N, 97°53′43″ W). The predominant climate is damp warm, with summer rain regime. The annual average temperature in this region is 26.5 °C, an average humidity of 79.8%, a cumulative annual total precipitation 868.69 mm and average wind speed of 10.8 km h−1. Tomato (S. lycopersicum Linnaeus) “Álvaro” seedlings were donated from National Research Institute for Forestry, Agriculture and Livestock (INIFAP) located at (22°33′58″ N, 98°09′51″ W) Cuauhtémoc, Tamaulipas Mexico. Five seeds were sown in 5 g of SPM using unicel plug trays. After 40 days seedlings were sent to greenhouse and transplanted using a mixture volume of 5 L (substrate) using plastic pots (180 mm × 160 mm × 250 mm). The Figure 1a,b shows photographs of the as-received seedlings, their growth and transplantation in the plastic pots.

Figure 1.
Photographs summarizing (a) the seedlings growth and (b) transplantation using plastic pots.
The experiments were completely randomized block design with five replicates. The evaluation was performed during a total period of 130 days. During the experiments, the average temperature was 27.5 °C, with a minimum and maximum temperatures of 17 °C and 33 °C and a humidity within the green house of 80% in average.
2.4. Nutritive Solution Management
The plan density in the greenhouse was about 2.4 crops m−2; the culture gutters contains about 17 plastic pots with a separation between them of 200 mm. The greenhouse had the following dimensions 6 m in length, 4 m in width and 5.0 m in height.
Water irrigation system consisted of a semicircular system of polyvinyl chloride pipe of 12.7 mm in diameter and was operated three times a week after 5:00 PM with a flow rate of 1.0 L h−1. The drip water system was collocated at 5 cm of each plastic pot and operated until added ~1.2 L in each sample.
On the other hand for the fertigation process, 14 mL of the nutritive solution was manually added in each plastic pot. The macro- and micro-nutrients were added every day between 9:00 am and 11:00 am. The nutritive solution was prepared by adding in 2 L of water the following proportions: KNO3 (969 mg), Ca(NO3)2•4H2O (1758.2 mg), KH2PO4 (8 mg), K2SO4 (318 mg), MgSO4•7H2O (667 mg), H3PO4 (204 µL), and 50 mg of micronourishment. The micronutrients were added to the solution with the following composition (weight percent, wt%): nitrogen (3.0 wt%), sulfur (13.0 wt%), boron (0.10 wt%), iron (12.5 wt%), manganese (1.0 wt%), and zinc (7.50 wt%). The nutritive solution drained directly in the soil in order to evaluate the characteristics of each substrate during the plant growth. The initial electrical conductivity (EC) of nutritive solution was about 2.5 dS m−1 with pH 6.3. The pH in each evaluated substrate compositions was daily monitored using an agricultural meter TPM QL-KC300B model (Shenzhen Wellbom Technology Co. Ltd., Shenzhen, Guandong, China).
2.5. Plant Height, Number of Leaves, Substrate pH, and Fruit Yield Evaluation
In order to allow adaptation to seedlings (stress period), the plant height and leaf quantity were measured, after 20 days of growing. Thereafter, data were collected once a week until the end of experiment. Substrate pH was measured from the first day of transplant throughout the experiment.
The ripening period was considered when the fruit become red. Then, the harvest stage, for all tomato plants was performed from 10 February to 10 April 2019. It is important to highlight that fruits were harvested individually by hand. At the end of the cropping season, the fresh yield of each harvest was summed and reported as total fresh fruit weight. Similarly was performed for dehydrate weight, but in this case the samples were heat treat at 50 °C for 36 h. Before these analyses, fruits were washed under running water and dried with absorbent paper. Then, the crop yield (%) was expressed for each substrate as a ratio of the total number of fruits per plant and the average fruit weight. Also, the yield was considered in terms of the fresh fruit weight, dehydrated fruit weight, polar and equatorial diameters. The procedure was repeated by triplicate.
2.6. Statistical Analyses
Statistical analyses were performed using IBM statistics SPSS v.21 (IBM, Armonk, NY, USA). Tomato data were analyzed according to the randomized complete block design in order to compare the control SPM with the proposed substrates and determine their influence with the growth parameters. The analysis of variance (ANOVA) was performed to find the effect of different substrate ratios on substrate pH, plant height, number of leaves and fruit yield. Potential interactions were considered when a parameter varies depending on the substrate composition using a p ≤ 0.05. Effects of each variable were analyzed separately for each factor. Post-hoc analysis of interactions was also performed separately using a Tukey’s honestly significant difference (HSD) test, p ≤ 0.05.
3. Results
3.1. Characterization of Substrate Components
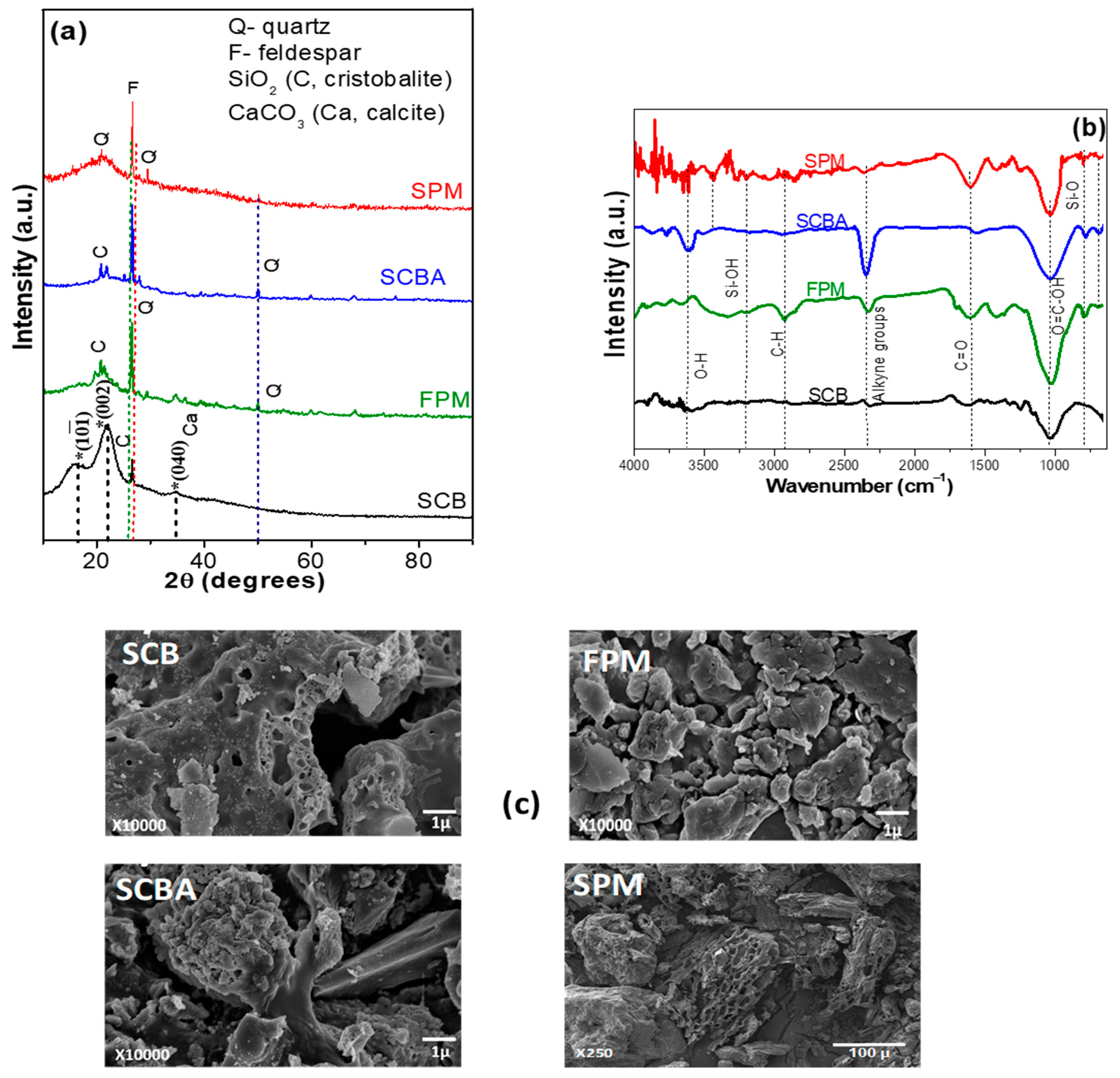
Proximate analysis is a commonly used method to characterized biomass. The properties of agro-industrial wastes were analyzed through AOAC analytical methods [55] and the results are shown in Table 1. It is well known that these parameters have a high impact on the seedling growth, especially in the ash (minerals), and crude fiber. This table shows that moisture percentage was significantly higher in SCB (8.34 ± 0.15%) and FPM (6.38 ± 0.64%) compared to commercial SPM (3.85 ± 0.33%) and SCBA (3.68 ± 0.36%). Apart of SCBA; the material with the highest ash content was the FPM (39.88 ± 2.23%), followed by SPM (5.96 ± 0.34%) and finally, SCB with 3.98 ± 0.22%. The substrate rich in crude protein related with the total nitrogen was FPM (6.94 ± 0.40%) but it was very close to the commercial SPM (6.8 ± 0.9%), whereas that SCB and SCBA display about 1.3 ± 0.19 and 1.16 ± 0.09%, respectively. FPM shows equal apparent density (0.34 ± 0.02 g cm−3) than the commercial SPM followed by SCBA and SCB. Recently, the soilless culture is considered as the major technological component of the greenhouses thus, efficient monitoring in the physical and chemical characteristics can influence the pathogens control without application of soil fumigation [57,58]. The information of the proximate analysis confirmed that an adequate relation between the agroindustrial wastes proposed as substrates can create a system (matrix) that propitiate both water and air retaining that favoring the plant growth and fruit yield. To identify the contained mineral in the as-received industrial wastes, the initial soil particles were analyzed by XRD patterns (Figure 2a). For comparison purposes, XRD of SPM was also presented. SCB showed typical peaks at 16.6°, 22.2°, and 34.7° that corresponded to the crystalline cellulose type I [59,60]. In addition to this phase, there was a peak at 26.8° that was associated with cristobalite (SiO2) structure. Some other impurities were overlapped with some peaks of cellulose type I and corresponded to calcite phase (CaCO3) PDF 00-056-1717, 00-056-1718, and 00-056-1719 charts. The XRD patterns of FPM showed a great quantity of peaks that were correlated with the presence of feldspar group including wairakite (CaAl2(SiO3)4•2H2O), mordenite ((Ca,Na2,K2)4(Al8Si40O96)28H2O), ferroaxinite (FeCa2Al2(BO3OH)(SiO3)4), latiumite (Ca, K2)8 (Al, Mg, Fe)(Si,Al)10O25(SO4), and some quantities of cristobalite and calcite structures, calcium [61,62]. In the case of SCBA, XRD presented some peaks that matched to the quartz, cristobalite, and feldspar phases. It is well known that SiO2 is the main component of SCBA (58.6–76.4%) and in this case, it was presented as cristobalite and quartz phases [53,63]. On the other hand, commercial SPM showed similar XRD signals than that observed for FPM and SCB, which corresponded to the main components of organic matter: cellulose, hemicellulose, and lignin [64].

Table 1.
Proximate analysis and physico-chemical properties of the raw materials used as growing substrates in this study.
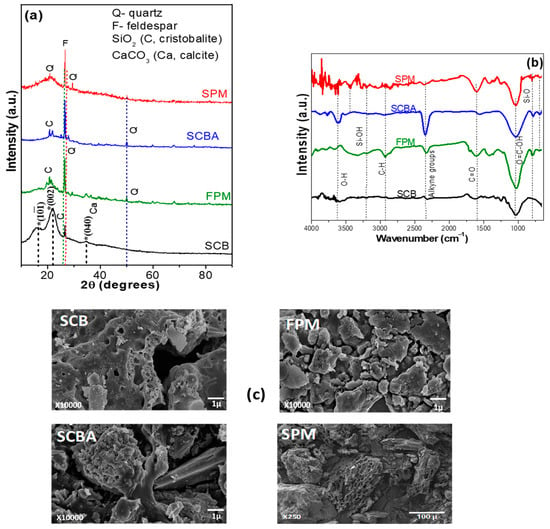
Figure 2.
(a) X-ray diffraction patterns, (b) Fourier-transform infrared analysis, and (c) scanning electron microscopy images of SCB, FPM, SCBA, and commercial SPM before of the experimental design.
FTIR analysis is used as important indicator of the chemical composition of the raw materials (Figure 2b). SCB showed typical fingerprint of cellulose, hemicellulose, and lignin compounds. The widely band centered at ~3620 cm−1 corresponded to O-H stretching vibrations and at 2917 cm−1 appeared the C-H stretching vibration. It was also observed that the signal at ~2330 represents alkyne groups, which can be contained in the lignin. The band at 1730 cm−1 corresponded to the C=O stretching vibration band of polysaccharides, hemicellulose or ester linkages in lignin, hemicellulose, pectin [65]. In the 1100–1650 cm−1 range the signals related to the aromatic ring contained in lignin and the absorbed water were observed, as well as C-O out of plane stretching, due to the aryl group in lignin.
The signal at ~1030 cm−1 corresponded to an overlapping of O=C-OH stretching bonds and β-glycosidic linkages of glucose ring cellulose [41,66,67,68]. FPM showed the hydroxyl linkage at 3620 cm−1, with a wide band between 3500–3000 cm−1 due to the presence of hydrogen bonded OH groups and Si-OH group [69]. The other bands were similar to SCB; additionally, the signal at ~788 cm−1 was attributed to the presence of Si-O bonds (quartz) [69,70]. As expected, the bands between 3500–3000 cm−1 corresponding to the presence of hydrogen bonded OH groups and the band at 1730 cm−1 due to C=O stretching vibration, almost disappeared. The signal of asymmetric stretching vibration Si-O bonds was shifted from 788 and 780 cm−1 whereas the other one at 689 cm−1 belonged to in-plane bending Si(Al)-O-Si vibrations of the aluminosilicate network. The organic matter that remained in the SCBA was also seen in the region of 1100–2500 cm−1. Additionally, the hydroxyl linkage at 3620 cm−1 remained in the spectrum [71]. In the case of commercial SPM, small signals were observed between 3500–3000 cm−1, which corresponded to the hydroxyl groups (O-H). The signal at 2917 cm−1 was the result of asymmetric and symmetric stretching vibration of C-H bonds within aliphatic alkyl groups. Absorption resulting from stretching vibration of C=O bands appeared at 1602 cm−1, whereas the stretching vibrations of C-OH bonds of cellulose were observed at 1039 cm−1.
The morphology of the agroindustrial wastes displayed important differences depending on the step of the sugar mill process (Figure 2c). SCB showed lamellar-type structures similar to commercial SPM, whereas a compact agglomerates and irregular shaped particles structures were observed for FPM and SCBA materials [72]. The SEM/EDS analysis showed that SCB was mainly composed of carbon, oxygen, and silicon (Table 2), whereas that FPM and SCBA samples displayed an improved of silicon amount and oxygen, which is characteristic of these materials [73,74].

Table 2.
SEM/EDS analysis of as-received raw materials and comparison with SPM.
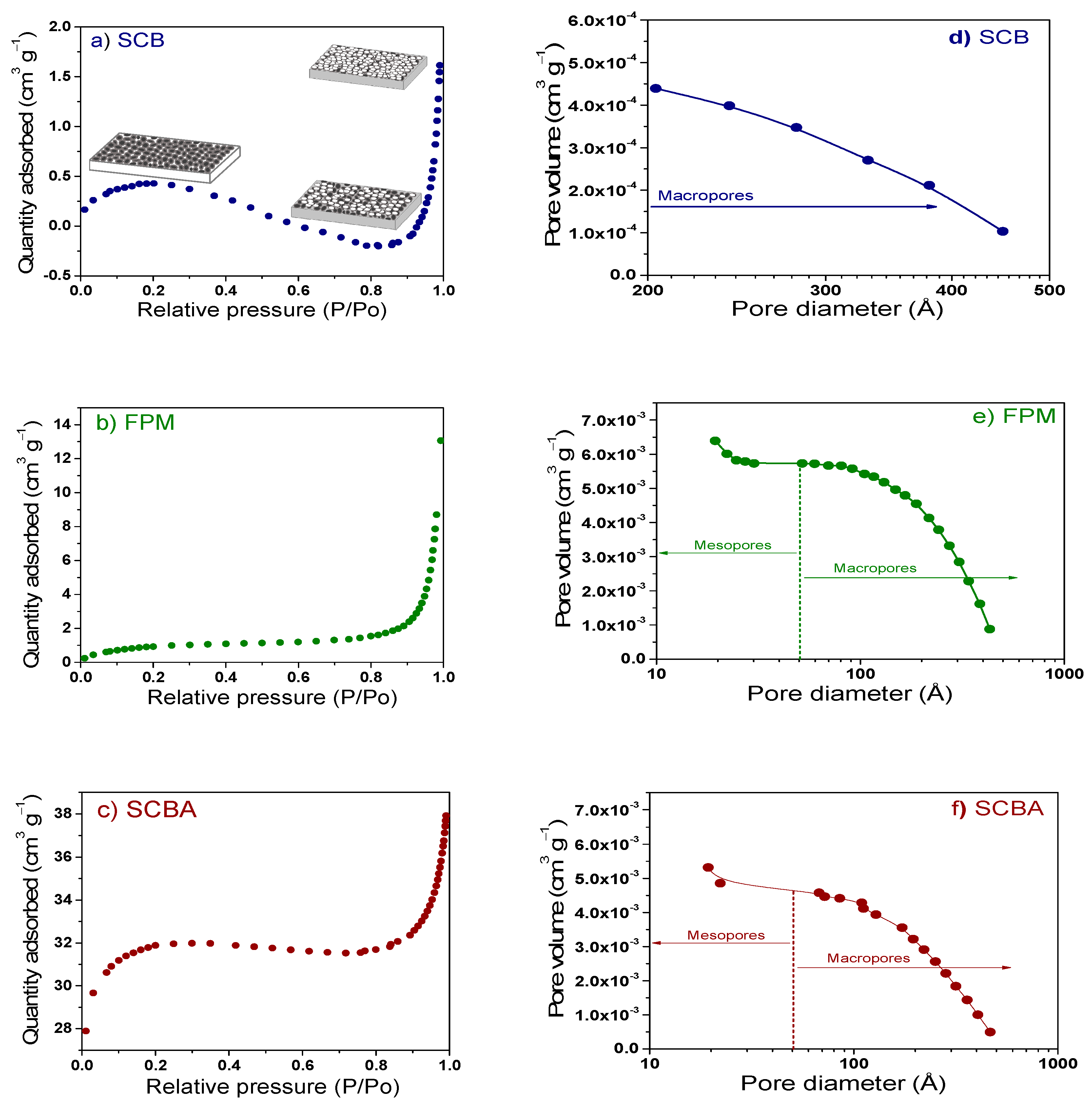
The surface area and pore size distribution of the proposed substrates were determined by BET analysis (Figure 3a–c). In general, the BET isotherms showed as initial step where monolayer was formed. This stage was followed by the pore filling and formation of multilayers (gas molecules clump together). The shape of BET isotherms, where the absorption increased and then decreased again, suggested that SCB and SCBA had a large number of pores that remained without gas filled (combination of isotherms type I and II). The pore size distribution, determined by BJH method, indicated that SCB presented a distribution of macropores (203–450 Å), which is desirable in materials used as substrates helping to the aeration and roots oxygenation process (Figure 3d). On the other hand, SCBA (466–20 Å) and FPM (430–19 Å) presented a combination of meso- and macro-pores where the significant adsorbed amount was given at intermediate-high relative pressures (Figure 3e,f). Specifically, the FPM showed an isotherm type III with a poor quantity of absorption at low and intermediate P/Po and thereafter increased to form multilayers; i.e., it showed a stronger absorbate-absorbate interactions than absorbate-adsorbent interactions [75]. The surface area of the substrates was about 1.60, 3.78 and 1.07 m2 g−1, for SCB, FPM and SCBA, respectively; which were in the range of the commercial SPM (130 m2 g−1). The surface area can promote the water adsorption, retention, and releasing capacity as well as improve the cation exchange capacity [76,77].
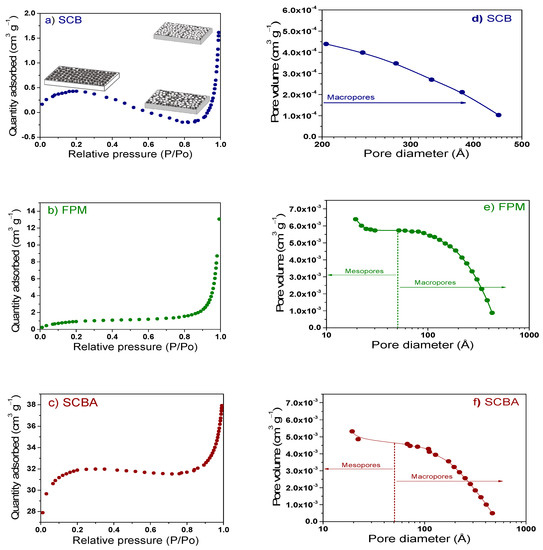
Figure 3.
Nitrogen adsorption isotherms for raw materials obtained from sugar cane processing; (a) SCB, (b) FPM, and (c) SCBA. In this figure, it is also presented the pore distribution determined by BJH method for (d) SCB, (e) FPM, and (f) SCBA.
3.2. Characterization of Soilless Substrates and Phenological Development of Tomato in Different Substrates
Based on the proximate analysis and physico-chemical properties of each agroindustrial waste, the main composition of the analyzed substrates was selected with high amounts of FPM (60, 70 or 80 wt%) and the balance varying the amount of SCB and SCBA (Table 3). In this table, the experiments were labeled from T1 to T15 depending on the amount of FPM. Additionally, control samples of commercial SPM were included in the experimental design and labeled as C1.

Table 3.
Compositions of the proposed substrates and effect of the substrate composition on the plant height, number of leaves and substrate pH, after 20, 74, and 130 days after transplanting (DAT). Different letters after means indicate significant differences at p ≤ 0.05 (Tukey’s HSD test).
The parameters plant height, substrate pH, number of leaves, and fruit yield were measured every week from 20 days after transplanting (DAT) until the end of experiments (130 days), but only representative days were discussed. The quantitative values of these parameters were subjected to one-way ANOVA to explain the changes as function of the substrate composition. The comparison of all treatments was also verified with Tukey’s honest significant difference (p ≤ 0.05). The analysis was carried out to corroborate any statistical effect of the plant growth depending on the composition of the substrate. Table 3 shows the treatments that presented statistically significant differences during the evaluation of parameters considering a p ≤ 0.05 as well as the interaction between substrates that did not present statistical differences.
In general, all the samples at 20 DAT presented values between 20.80 ± 3.15 and 29.17 ± 3.09. The high values for the plant height was observed for mixtures T15, T14, T9, T8, and T10 with average values of 29.17 ± 3.09 cm, 28.46 ± 2.55 cm, 28.45 ± 0.35, 28.1 ± 0.43 cm, and 26.23 ± 2.39, respectively. On the contrary, treatments T11 with 21.4 ± 3.83, T1 with 21.50 ± 2.55 cm, and T13 with 20.80 ± 3.15 cm displayed the low height values. The best values were higher than the average values in height for plants grown in commercial SPM (26.85 ± 4.03 cm). After Tukey’s test, it was found that overall samples did not present significant differences in the plant height with respect to the ratio of each component (p = 0.206). At this stage, a high amount of FPM positively favored the plant height, but in the best case it only increased ~7% compared to the control sample, indicating that they were statistically equivalent. This fact highlights that tomato plants required an adaptation time in each proposed substrate composition.
At 74 DAT, the progress of the plant was better in the T13 (62.00 ± 0.00 cm) ≅ T12 (61.96 ± 1.98 cm) followed by the treatment with T15 (57.80 ± 8.42.) ≅ T6, (56.43 ± 6.71). The poorest performance was obtained with the substrate labeled as T3, 25.7 ± 0.00 cm, and T2, 31.30 ± 0.00 cm. Tukey’s test indicated that can exist significant differences in the plant height with respect to the ratio of each component (p = 0.002), which modified the evolution of the plant growth. At this time, the general trend again suggested that the vegetation growth was favored by a high content of FPM and SCB. However, after the adaptation process, the samples that presented close performance, with statistically equivalent differences in comparison with commercial control, were the samples T13 (1.05%), T12 (1.03%), T15 (0.96%). The more important differences were observed in T13 ≅ T12−T3 (~50%) and T13 ≅ T12−T2 (~40%).
Subsequently, at 130 DAT when it was performed the harvest fruit, the treatments that showed the tallest plant were T15, 99.80 ± 7.92 cm which was followed by T13, 90.2 ± 0.00 cm, T12, 84.60 ± 4.10 cm, T14, 77.25 ± 7.99 cm, T6, 79.00 ± 2.26 ≅ T11, 76.50 ± 7.88 cm, and T1, 66.10 ± 8.20. In the best cases, the observed plant height values were comparable or higher than the observed height for commercial SPM. The substrate with a composition of T15 presented a vegetation growth of about 4% higher than the control (95.45 ± 11.24 cm); although, other treatments that were also statistically equivalent were T13, T12, T14. A significant increase can be observed between the samples T15−T2 (~47%) with a p = 0.001. In general, the best performance in this variable was observed with a high FPM content.
On the other hand, it has been established that plants with a high number of leaves usually present a high yield, thus, leaves quantity was also measured during the experiments [78]. At 20 DAT, the plants with a high number of leaves were in the following order T6 (31.50 ± 0.71) > T8 (25.50 ± 4.92) > T15 ≅ C1 (24.5 ± 0.70) > T11 (24.0 ± 0.00) ≅ T9 (22.0 ± 2.83) > T14 (21.67 ± 1.52) > T12 ≅ T1 ≅ T4 (21.00 ± 2.0) whereas samples with a low number of leaves were T3 (16.67 ± 5.51) < T5 ≅ T7 (16.00 ± 0.0) < T13 (15.50 ± 4.94). In this step, the substrates T6, T8, T15, T11, and T9 showed a higher or similar yield compared to the control, while, T14, T12, T1 and T4 showed a slightly low yield (8% less). The evaluation of this parameter confirmed that the plants are under adaptation without a clear trend but with important variations. For example, T6 presented a significant increase of ~22% compared with the control (p = 0.001) and with T13 (~51%, p = 0.001); whereas the statistically equivalent samples were T8, T15, T11, and T9. Then, a more accurate performance of the tomato plants affected by the substrate composition was observed at 50 days.
The trend in the number of leaves was slightly different at 74 DAT; the substrates that presented the highest number of leaves were T6 (48.00 ± 2.82), T11 (44.67 ± 2.08) and T12 (42.50 ± 2.12); which were inferior to the leaf number of C1 (56.00 ± 8.48). Tukey’s HSD indicated that samples T6–C1 can be statistically comparable, but presented a significantly superior performance (between 16–62%) in comparison with their counterparts (p = 0.012).
The significant differences prevailed at 130 DAT, samples T15 (289.67 ± 14.29), T12 (277.00 ± 9.16), T6 (244.00 ± 12.72), T11 (230.00 ± 1.41) showed a high number of leaves but the values were lower than those observed for commercial SPM (335.00 ± 7.07); which again represents a difference between 16–70% (p = 0.003). The samples that presented a low number of leaves were the mixtures T1 (95.5 ± 2.82) and T2 (104 ± 0.00), which had a high SCB composition (28–36%). Then, the quantity of SCB is negatively affecting the plant performance. Thus, the factor that most affected the number of leaves in tomato plant was the content of FPM.
Another important parameter that can be affected by the substrate composition was the evolution of substrate pH. The initial pH of the agroindustrial wastes, proposed as substrates, varies from 5.8–7.7; whereas, SPM presented a pH of 4.7. The initial substrate pH varied with the composition between 3.0 ± 0.0 and 6.67 ± 0.29, which was reached after adding 14 mL of a nutritive solution with a pH of 6.3. Significant differences were observed in the substrate pH with respect to the substrate composition, at 20 DAT (p = 0.003), 74 DAT (p = 0.001) and 130 DAT (p = 0.004). Samples that showed highest growth were obtained with an average substrate pH range from 3.0 ± 0.0 to 5.83 ± 0.29. In this case, the source of FPM acidified the media and reduced the overall pH. In the case of commercial SPM (C1), the substrate pH was about 5.83 ± 0.29 at 20 DAT and 5.33 ± 0.29 at 130 DAT. Statistically equivalences were observed in samples T6, T7, T11, and T12, whereas significant differences were observed between T15, T9 and T10. It is known that substrate pH plays an important role in the plant growth, due to a large number of processes such as nutrients availability, availability of toxic ion species and uptake rate [79]. However, in this study, acidification in the substrate pH did not affect the height and yield of the plant; probably, because the substrate pH values were still adequate to supply the necessary quantity of nutrients (Fe, Mg, K, N, and P) [80,81].
3.3. Harvest Stage
The fruiting stage started at 74 DAT and the harvest stage began after 130 DAT (for 60 days). During this stage, all the samples presented fruits, but only the harvested tomatoes were correlated with the yield of the number of leaves. The yield of the plant was estimated from the total yield excluding the fruits with a weight less than 50 g, for these reason only the trials that showed trustworthy results were discussed in this section. Table 4 shows the effect of the substrate composition on fruit yield using a p ≤ 0.05. The evolution in the plant tomato growth as well as the typical fresh fruit, dry fruit, polar, and equatorial diameter during the evaluation period are also shown in Figure 4. It is important to highlight that the total yield was only correlated with the substrate compositions, but other factors such as nutritional disorder and nutrients adsorption mechanism are under study.

Table 4.
Effect of the substrate composition on fruit quality. Different letters after means indicate significant differences at p ≤ 0.05 (Tukey´s HSD test).

Figure 4.
Evolution of the tomato plant growth emphasizing the specimens during the measurement of fresh weight, dry weight, polar diameter, and equatorial diameter after the harvesting stage (130 DAT).
The average fresh weights in the plants that displayed verifiable fruits depending on the substrate composition were: 55.00 ± 3.46 g for T6, 47.20 ± 3.53 g for T11 and with similar fresh weight the samples T15 (43.00 ± 0.0 g) ≅ T1 (42.5 ± 2.12 g) ≅ T12 (42.50 ± 3.53 g). The average tomato fruit weight was higher than commercial SPM, which displayed an average weight of 41.00 ± 1.15 g. Samples T6 and T1 show a significant increase (34% and 15%, respectively) in the fresh fruit weight compared to control samples. In this cases, the experiments presented significant differences with a p = 0.019. On the other hand, the samples T1, T15, and T12 were statistically equivalent to commercial SPM.
After made a drying at 50 °C, for 36 h, the results showed similar trend in comparison with the fresh fruit weight; i.e., the average dehydrated fruit weight was 9.63 ± 0.57 g for T6, 6.48 ± 0.50 g for T11, 3.99 ± 0.20 g obtained for the sample T12 ≅ T15 with 3.71 ± 0.26 g. Substrate labeled as T1, presented the lowest dehydrated fruit weight with an average value of 3.00 ± 0.14 g, whereas the dehydrated fruit weight for SPM was 3.33 ± 0.25 g. The significant differences were observed between the samples T6–C1 and T11–C1. These samples were up to 2.89 and 0.94 times higher than control with a statistical significance of p = 0.001. Similarly, samples statistically comparable were T1, T12, T15, and C1.
On the other hand, the highest polar diameters were found in the following order: T6 (65.17 ± 3.21 mm) >T11 (64.28 ± 3.21 mm) > T15 (54.17 ± 0.00 mm) >T1 (51.49 ± 0.71 mm) > T12 (46.17 ± 3.21 mm) > C1 (45.24 ± 3.51 mm); whereas in the equatorial diameter the highest values were observed in samples T6 (53.91 ± 4.24 mm), T11 (46.63 ± 2.08 mm), and T15 (43.98 ± 4.24 mm) and the commercial SPM presented an average of 37.29 ± 2.09 mm. In both parameters, notable significant differences prevailed between samples T6–C1 (p = 0.003) and T11–C1 (p = 0.030). It is clear that the yield and quality of the fruit were directly related to the substrate composition. In terms of these parameters, the results indicated that substrates with a composition of T6 were the most consistent for tomato growth in soilless culture. This substrate showed a fresh fruit weight of 55.00 ± 3.46, a polar and equatorial diameter of 65.17 ± 3.21 mm and 53.91 ± 4.24 respectively; whereas the dehydrated fruit weight was about 10.11 g. The characteristics of this mixture caused a positive relationship between the nutrients absorption and growth plant, which in turn improve the yield and quality of fruit. The results are also in good agreement with the crop yield (%) commercial SPM. Additionally, the performance of the samples T11, T15, or even T12 suggested that they could also be used for the tomato growth.
3.4. BET Surface Area Analysis of Samples
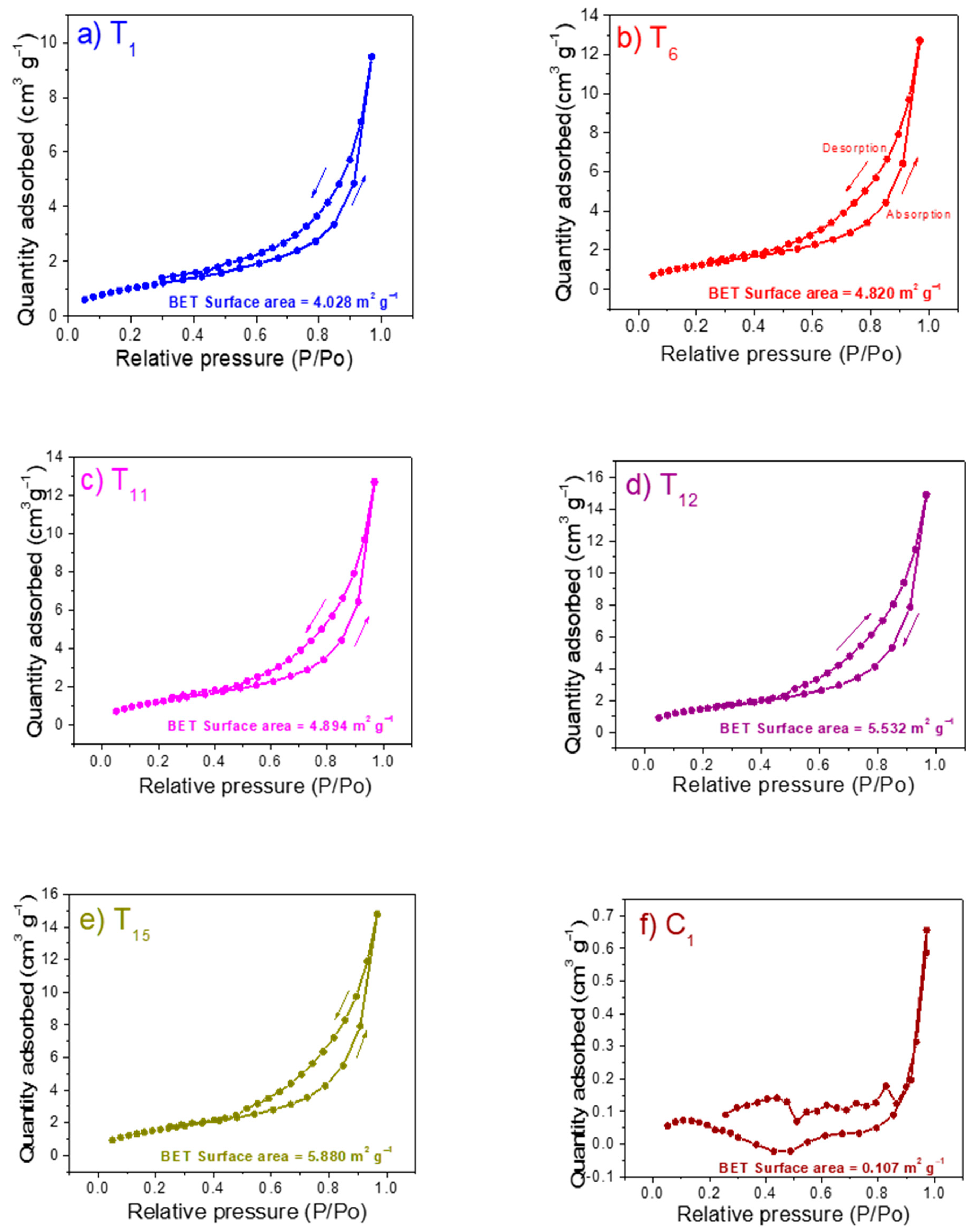
The evaluation of physical adsorptions of the substrates that presented the best performance in the plant height, number of leaves, and fruit yield was also performed by BET surface area analysis. Nitrogen absorption-desorption isotherm of the soilless substrates after 130 days of evaluation was presented. The Figure 5a–f shows the isotherms of samples T1, T6, T11, T12, T15, and C1. It is important to highlight that all the absorption-desorption isotherms presented similar shape with a type IV and/or V isotherms indicating, as expected, a macro-meso-porous nature of the materials proposed as substrate. All the evaluated samples showed isotherms that displayed the hysteresis phenomenon, which is associated with capillary condensation in the mesoporous structures in combination with the limiting uptake over a range of high P/Po. The initial part in the isotherms showed a gradual increase at low relative range because the mesoporous filling effect; followed by a behavior where multilayer sorption and capillary condensation occurred (at high pressure). In the backward loop, started the multilayer desorption, followed by a hysteresis process (differences between samples were due to thermodynamic and/or network effects) and finally, the remaining adsorbed nitrogen, in our case, indicated the quantity of mesoporous. Then, the isotherms showed the existence of both mesoporous and macroporous in the substrates [82]. The adsorption capacity decreased in the following order with a P/Po value of 0.9695: T12 (14.92 cm3 g−1), T15 (14.81 cm3 g−1), T11 (12.73 cm3 g−1), T6 (12.71 cm3 g−1), T1 (9.48 cm3 g−1), and C1 (0.65 cm3 g−1). On the other hand, at low relative pressures (below 0.29) nitrogen adsorption content in the sample T12 (1.69 cm3 g−1) was around 18 times than the commercial SPM (lowest nitrogen content). In general, the analyzed substrates were richer in mesoporous instead of macroporous, which increased in the following order T12 (2.07 cm3 g−1), T15 (1.86 cm3 g−1), T6 (1.55 cm3 g−1), T11 (1.46 cm3 g−1), T1 (1.39 cm3 g−1), and C1 (0.09 cm3 g−1). Therefore, the results indicated that substrates with an adequate ratio of their components (FPM, SCB and SCBA) modified the amount of macro and mesopores in the sample. Both mesopores and macropores are essential for the availability of water to the plant root system.
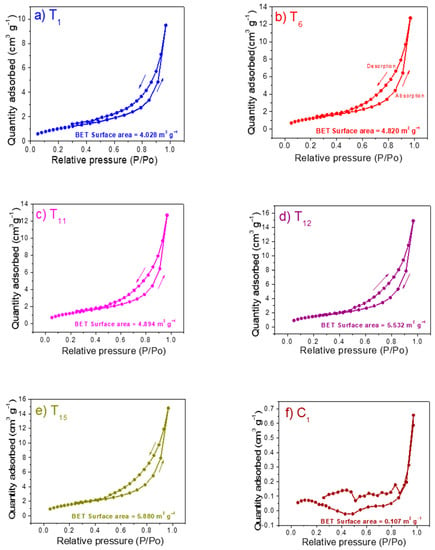
Figure 5.
N2 adsorption-desorption isotherms and pores size distributions of as-prepared samples (a) T1, (b) T6, (c) T11, (d) T12, (e) T15, and control (f) C1.
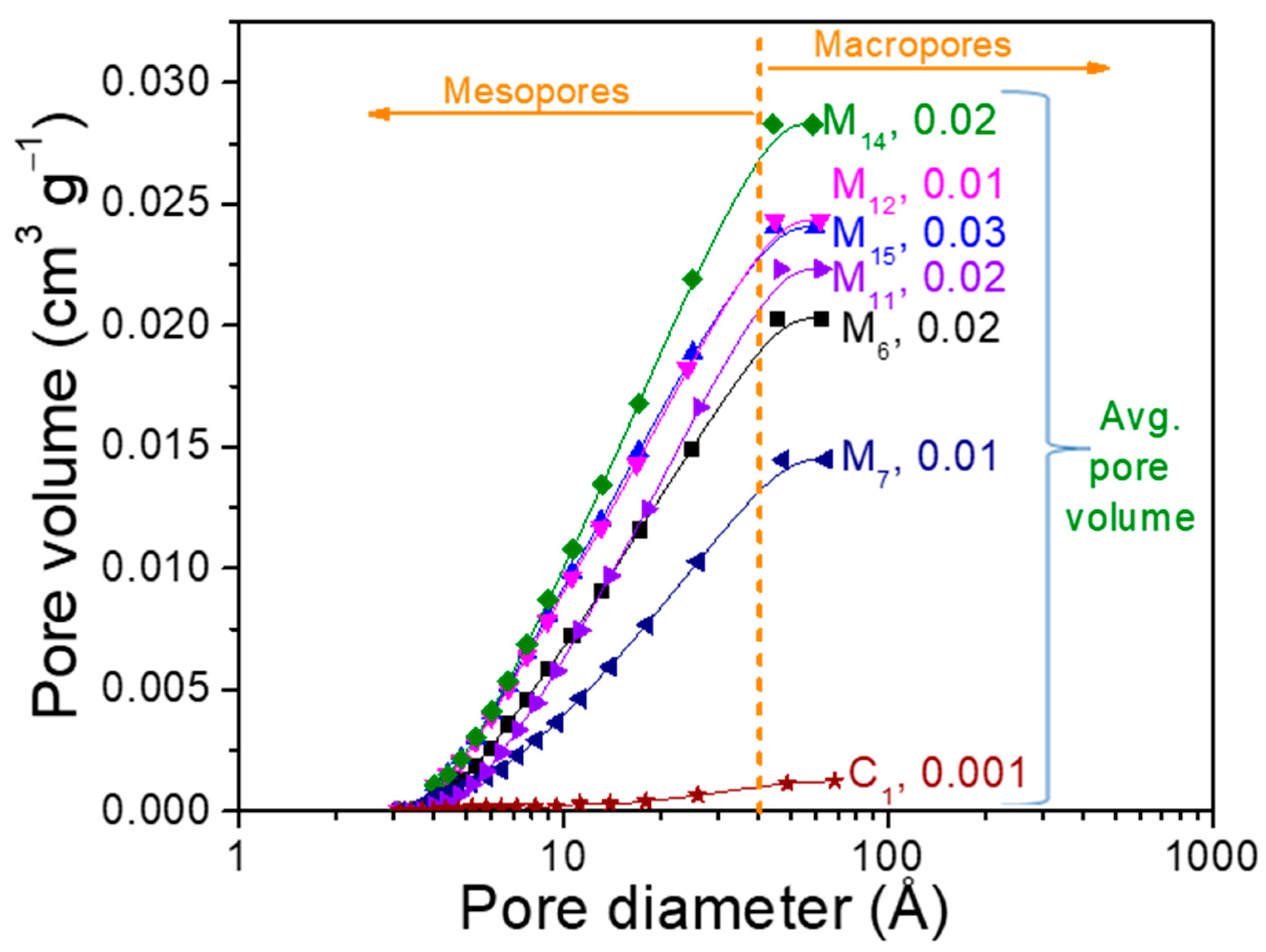
The surface area is congruent with the nitrogen adsorption-desorption isotherms and average pore volume observed in Figure 6. Porosity was also a key parameter to evaluate the potential of water retention and avoid the blocking effects through the substrates. All the samples at the end of assays, contain about 85% of mesopores and 15% of macropores, therefore, most pore diameters were under 50 Å. An interesting feature is that the pore volume increased similarly to the pore diameter with the PFM content, with even better performance than commercial SPM. Then, the surface characteristics suggest that these agroindustrial wastes can be effectively applied to substitute the SPM for the soilless culture used to grow tomato (S. lycopersicum Linnaeus).
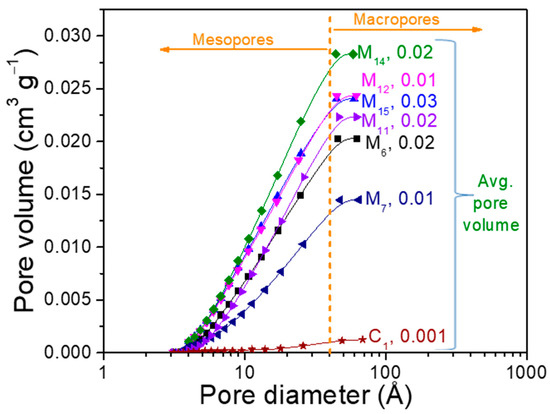
Figure 6.
Comparison of the accumulative pore volume versus pore size for the samples evaluate as substrates.
4. Discussion
It is well known that unbalanced nutrients reduce the soilless culture [83], thus, differences in the vegetation performance are related to the physical and chemical characteristics, however, the understanding of both features are still under debate. The substrate characterization and statistical analysis confirmed that the adequate combination of FPM/SCB/SCBA industrial wastes could be an alternative for substitution of SPM using the soilless harvest technique in tomato plant. During the vegetation, the tomato plant grew in different substrates developed differently. All the assessed groups were favored with the amount of FPM, although they presented significant differences with the SCB content, which is justified by the characteristics of these fibers.
Specifically, the treatments with adequate combination of FPM/SCB, T6 and T11, displayed the overall highest performance. This behavior could be associated with the microbial activity, which in turn can enhance the substrate mineralization increasing the overall nutrients [84,85,86]. Thus, the interaction between the substrates modulated the plant height, number of leaves fruit production and substrate pH. Then, an important aspect was to maintain a high quantity of SCB in the mixture. In accordance with compost systems, the adherence of essential nutrients to SCB can reduce nutrient leaching favoring the plant performance [87]. The as-received SCB was obtained from two sources of sugar mill processes during the harvest and juice extraction, therefore, other compounds such as clays or other materials that remain after the process can be presented in the fibers, favoring additional nutriments. The SCB was used as received after sun dried for 15 days; then in the first step (20 DAT) the composition of SCB (cellulose, hemicellulose, lignin) helped vegetative growth; however, it was observed that after 50 DAT the substrates with higher SCB content presented positive effects in the vegetation growth. Thus, SCB in the substrate could be beneficial for the plant height, the plant leaves and finally in the fruit yield [88]. Additionally, in adequate quantities SCB increased soil nutrients such as N, P, Ca, and Mg which were essential for plant growth [89]. According to with previous researches, small amounts in the substrates of SCBA also positively affect the parameters evaluated [90], although there was not a consistent trend between their SCBA amount and the plant growth as is the case of the T15 [91]. The main difference was obtained with plant growth, due to the increase of the FPM amount, up to reach a mean value of 99.85 ± 7.92 cm. The second most affected variable was the number of leaves with a mean average of 274.00 ± 0.00 in comparison with fruit yield 55.00 ± 3.46 g. Recent studies have indicated that FPM, as bio-fertilizer, reduces the height and weight in tomato plants [92], whereas others claim that improves the chemical and physical properties such as bulk density, soil pH, electrical conductivity and soluble cations which promote the growth of tomato plant [93,94]. The results obtained in this study were consistent with the last studies where the FPM contains enough amounts of macro- and micronutrients helping to water retention during the tomato plant growth. The addition of SCB and SCBA in adequate quantities can favor the performance of tomato plants. SCB is commonly added to the soilless substrates as biochar and its effects can vary depending on the types and amounts of biochar; however, in this work was added directly as fiber to favor the plant growth of tomato production, which in general favored the growth [95].
In these experiments, the addition of the micro and macronutrients was constant; then, the evolution in each mixture was related to the nutrients adsorption depending on substrate composition. It was also clear that substrate pH was affected by the substrate composition and moved to acidic values; however, according to the vegetation performance it never reached detrimental values to the plants growth. Fruit quality, fresh and DM were also quite affected by the FPM amount. It has been recognized that polar and equatorial diameters grew with the typical three stages: cellular division, cellular elongation, and maximum growth in the harvest step [96], but in this case, although it was followed the same growth mechanism, it was clearly influenced by the substrate composition. From the above information, it can be seen that the key factor that favored the tomato growth in greenhouse was the amount of FPM.
FPM has been previously used as organic fertilizer and co-substrate in soils to improve the feasibility of anaerobic digestion for biogas production [97,98], bioethanol production [99], stabilization of composting [100], as well as in the application of biofertilizer/compost for the vegetables and greens in soil [101]. The versatility of FPM is due to the fact that acted as a source of organic matter and other nutrients such as N, P, K, Ca, Mg, Fe, Zn, Cu, and Mn. For this reason, it can be used to improve physical, chemical and biological properties of the soil [94]. In this study, the best performance to produce tomato in a greenhouse culture was obtained with the mixtures T6 and T11 which had a high amount of FPM and SCB; however, the agronomical attributes were mainly affected by the amount of FPM; i.e., the nutrient that FPM can provide. The main value of this work is that it demonstrates that the substitution of commercial SPM with agroindustrial wastes is viable for growing tomatoes in greenhouses reducing their environmental impact and, at the same time, obtaining a value-added product.
5. Conclusions
In this work, it was evaluated three different agroindustrial wastes FPM, SCB, and SCBA in order to decide the adequate ratio to be proposed as substrate in soilless production of S. lycopersicum Linnaeus. From the above results the following conclusions can be withdrawn:
Proximate analyses indicated that among the as-received agroindustrial wastes, the FPM presented higher quantities of total nitrogen comparable with commercial SPM, which contained enough nutrients that matched with the feldspar group. The morphological aspects and the qualitative chemical composition of the raw materials supported that a substrate with the evaluated compounds (SCB, FPM, and SCBA) must be mainly composed of FPM, which in turn ensure the capacities of absorption, retaining, and cation exchange capacity during the supply of nutritive solution. The present study confirmed that the vegetative growth of tomato plant in soilless culture could be adjusted by modifying the substrate composition. The trend in the differences between the substrate in the plant height, number of leaves, substrate pH and fruit yield were not easily seen during the ANOVA analysis; however, Tukey´s test showed that the samples with best performance were those that contained a high amount of SCB, T6, and T11. The proposed substrates in adequate composition can be suitable to the SPM substitution in a soilless tomato production, at the same time that it is obtained a product with a value-added. The results showed that these agroindustrial wastes help to reduce the investments of soilless production. Finally, the specific role and interaction for each substrate in a nutrient recovery system is under study in different growth seasons.
Author Contributions
All authors whose names appear on the submission approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. V.N.O.-G.: Conceptualization, methodology, formal analysis. J.A.L.-C.: Methodology, formal analysis, resources, writing—review and editing. M.A.D.-C.: Writing—original draft, methodology, formal analysis, resources, writing—review and editing. J.P.-P.: Supervision, funding acquisition. A.M.T.-H.: Writing—Original draft, methodology, formal analysis, resources, writing—review and editing, supervision, funding acquisition. A.E.R.-S.: Writing—review and editing. Á.I.L.-A.: Data curation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Instituto Politécnico Nacional and Consejo Nacional de ciencia y tecnología de México.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Vanessa N. Orta Guzmán and Ángeles I. Licona-Aguilar are grateful for their postgraduate fellowship to CONACYT, COFAA and Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional. The authors are also grateful for the financial support provided by Instituto Politécnico Nacional through the SIP2020-1278, SIP2020-1279, SIP2020-1280 projects; CONACyT CB2015-252181 project; as well as SNI-CONACyT. In memory of Jorge A. Lois Correa, who was the main promoter of this research topic and dedicated his career to engineering and sustainable development.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Farfan, J.; Lohrmann, A.; Breyer, C. Integration of greenhouse agriculture to the energy infrastructure as an alimentary solution. Renew. Sustain. Energy Rev. 2019, 110, 368–377. [Google Scholar] [CrossRef]
- Orduño Torres, M.A.; Kallas, Z.; Ornelas Herrera, S.I. Farmers’ environmental perceptions and preferences regarding climate change adaptation and mitigation actions; towards a sustainable agricultural system in México. Land Use Policy 2020, 99, 105031. [Google Scholar] [CrossRef]
- López Feldman, A.J.; Hernández Cortés, D. Cambio climático y agricultura: Una revisión de la literatura con énfasis en América Latina. Trimest. Econímico 2016, 83, 459–496. [Google Scholar] [CrossRef]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. PNAS 2014, 111, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, D.; Cavicchioli, D.; Donzelli, F.; Ferrazzi, G.; Frisio, D.G.; Pretolani, R.; Ricci, E.C.; Ventura, V. Recent contributions of agricultural economics research in the field of sustainable development. Agriculture 2018, 8, 200. [Google Scholar] [CrossRef]
- Cárdenas-Elizalde, M.R.; Cortés-Cáceres, F.A.; Escobar-Latapí, A.; Nahmad-Sittón, S.; Scott-Andretta, J.; Teruel-Belismelis, G.A. Informe de Evaluación de la Política de Desarrollo Social 2018; CONEVAL: Ciudad de México, Mexico, 2018; pp. 1–60. Available online: https://www.coneval.org.mx/Evaluacion/IEPSM/IEPSM/Documents/RESUMEN_EJECUTIVO_IEPDS2018.pdf (accessed on 18 January 2021).
- Mubiru, D.N.; Namakula, J.; Lwasa, J.; Otim, G.A.; Kashagama, J.; Nakafeero, M.; Nanyeenya, W.; Coyne, M.S. Conservation farming and changing climate: More beneficial than conventional methods for degraded ugandan soils. Sustainability 2017, 9, 1084. [Google Scholar] [CrossRef]
- Torres Pineda, I.; Lee, Y.D.; Kim, Y.S.; Lee, S.M.; Park, K.S. Review of inventory data in life cycle assessment applied in production of fresh tomato in greenhouse. J. Clean. Prod. 2020, 282, 124395. [Google Scholar] [CrossRef]
- Maham, S.G.; Rahimi, A.; Subramanian, S.; Smith, D.L. The environmental impacts of organic greenhouse tomato production based on the nitrogen-fixing plant (Azolla). J. Clean. Prod. 2020, 245, 118679. [Google Scholar] [CrossRef]
- Margenot, A.J.; Griffin, D.E.; Alves, B.S.Q.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind. Crop. Prod. 2018, 112, 160–169. [Google Scholar] [CrossRef]
- Saavedra, T.M.; Figueroa, G.A.; Cauih, J.G.D. Origin and evolution of tomato production Lycopersicon esculentum in México. Cienc. Rural 2017, 47, 3. [Google Scholar] [CrossRef]
- Soto Mora, C. La agricultura comercial de los distritos de riego en México y su impacto en el desarrollo agrícola. Investig. Geográficas 2003, 50, 173–195. [Google Scholar]
- Schröder, J.J. An evaluation of whole-farm nitrogen balances and related indices for efficient nitrogen use. Eur. J Agron. 2003, 20, 33–44. [Google Scholar] [CrossRef]
- Chauhan, M.K.; Varun; Chaudhary, S.; Kumar, S.; Samar. Life cycle assessment of sugar industry: A review. Renew. Sustain. Energy Rev. 2011, 15, 3445–3453. [Google Scholar] [CrossRef]
- Santamaria, P.; Campanile, G.; Parente, A.; Elia, A. Subirrigation vs. drip-irrigation: Effects on yield and quality of soilless grown cherry tomato. J. Hortic. Sci. Biotech. 2003, 78, 290–296. [Google Scholar] [CrossRef]
- Horinouchi, H.; Muslim, A.; Suzuki, T.; Hyakumachi, M. Fusarium equiseti GF191 as an effective biocontrol agent against Fusarium crown and root rot of tomato in rock wool systems. J. Crop Prot. 2007, 26, 1514–1523. [Google Scholar] [CrossRef]
- Clematis, F.; Minuto, A.; Gullino, M.L.; Garibaldi, A. Suppressiveness to fusarium oxysporum f. sp. radicis lycopersici in re-used perlite and perlite–peat substrates in soilless tomatoes. Biol. Control 2009, 48, 108–114. [Google Scholar] [CrossRef]
- Ronga, D.; Zaccardelli, M.; Lovelli, S.; Perrone, D.; Francia, E.; Milc, J.; Ulrici, A.; Pecchioni, N. Biomass production and dry matter partitioning of processing tomato under organic vs conventional cropping systems in a Mediterranean environment. Sci. Hortic. 2017, 224, 163–170. [Google Scholar] [CrossRef]
- Tringovska, I.; Yankova, V.; Markova, D.; Mihov, M. Effect of companion plants on tomato greenhouse production. Sci. Hortic. 2015, 186, 31–37. [Google Scholar] [CrossRef]
- Carlile, W.R.; Cattivello, C.; Zaccheo, P. Organic growing media: Constituents and properties. Vadose Zone J. 2015, 14. [Google Scholar] [CrossRef]
- Guo, R.; Qin, W.; Jiang, C.; Kang, L.; Nendel, C.; Chen, Q. Sweet corn significantly increases nitrogen retention and reduces nitrogen leaching as summer catch crop in protected vegetable production systems. Soil Tillage Res. 2018, 180, 148–153. [Google Scholar] [CrossRef]
- Ma, X.; Hu, J.; Wang, X.; Choi, S.; Zhang, T.-A.; Tsang, Y.F.; Gao, M.-T. An integrated strategy for the utilization of rice straw: Production of plant growth promoter followed by ethanol fermentation. Process Saf. Environ. 2019, 129, 1–7. [Google Scholar] [CrossRef]
- Ronga, D.; Gallingani, T.; Zaccardelli, M.; Perrone, D.; Francia, E.; Milc, J.; Pecchioni, N. Carbon footprint and energetic analysis of tomato production in the organic vs the conventional cropping systems in Southern Italy. J. Clean. Prod. 2019, 220, 836–845. [Google Scholar] [CrossRef]
- Wakelyn, P.J.; Chaudhry, M.R. 11—Organic cotton: Production practices and post-harvest considerations. In Sustainable Textiles; Blackburn, R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 231–301. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrates for production of vegetable transplants II.: The effect of wood fiber substrates and their volume weights on the growth of tomato transplants. Sci. Hortic. 2004, 100, 333–340. [Google Scholar] [CrossRef]
- Lévesque, V.; Jeanne, T.; Dorais, M.; Ziadi, N.; Hogue, R.; Antoun, H. Biochars improve tomato and sweet pepper performance and shift bacterial composition in a peat-based growing medium. Appl. Soil Ecol. 2020, 153, 103579. [Google Scholar] [CrossRef]
- Könönen, M.; Jauhiainen, J.; Straková, P.; Heinonsalo, J.; Laiho, R.; Kusin, K.; Limin, S.; Vasander, H. Deforested and drained tropical peatland sites show poorer peat substrate quality and lower microbial biomass and activity than unmanaged swamp forest. Soil Biol. Biochem. 2018, 123, 229–241. [Google Scholar] [CrossRef]
- Domínguez, E.; Nelson, B.; Muñoz-Escobar, C. Efectos de la extracción de turba sobre la composición y estructura de una turbera de Sphagnum explotada y abandonada hace 20 años, Chile. An. Inst. Patagon. 2011, 40, 37–45. [Google Scholar] [CrossRef]
- Miguel, E. Manuales de Desarrollo Sostenible: 2. Conservación y Restauración de Turberas; Fundación Santander Central Hispano: Madrid, Spain, 2011. [Google Scholar]
- Pratap-Singh, D.; Bahadur-Singh, H.; Prabha, R. Microbial inoculants in sustainable agricultural productivity. In Functional Applications; Springer: New Delhi, India, 2016; Volume 2, p. 301. [Google Scholar]
- Zaller, J.G. Vermicompost as a substitute for peat in potting media: Effects on germination, biomass allocation, yields and fruit quality of three tomato varieties. Sci. Hortic. 2007, 112, 191–199. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochar’s derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crop. Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of Miscanthus as growing substrate in soilless cultivation of vegetables (tomatoes, cucumbers) and subsequent direct combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Deppe, N.A.; Palmquist, D.E.; Berhow, M.A. Extracted sweet corn tassels as a renewable alternative to peat in greenhouse substrates. Ind. Crop. Prod. 2011, 33, 514–517. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Bustamante, M.A.; Ben Amara, M.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.L.; Gascó, G.; Plaza, C.; Paz-Ferreiro, J.; Méndez, A. Hydrochars from biosolids and urban wastes as substitute materials for peat. Land Degrad. Dev. 2017, 28, 2268–2276. [Google Scholar] [CrossRef]
- Robert, D.W.; Brian, E.J.; Michael, C.B.; Jake, F.B. The landscape performance of annual bedding plants grown in pine tree substrate. HortTechnology 2009, 19, 78–82. [Google Scholar] [CrossRef]
- Ramos Del Carmen, M. Evaluación de un Proceso de Biorremediación de Lodos Urbanos y Agroindustriales. Ph.D. Thesis, Benemérita Universidad Autónoma de Puebla, Puebla, Mexico, 2019. [Google Scholar]
- Parameswaran, B. Sugarcane bagasse. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Candido, R.G.; Gonçalves, A.R. Evaluation of two different applications for cellulose isolated from sugarcane bagasse in a biorefinery concept. Ind. Crop. Prod. 2019, 142, 111616. [Google Scholar] [CrossRef]
- Dantas, G.A.; Legey, L.F.L.; Mazzone, A. Energy from sugarcane bagasse in Brazil: An assessment of the productivity and cost of different technological routes. Renew. Sustain. Energy Rev. 2013, 21, 356–364. [Google Scholar] [CrossRef]
- Ortiz, P.S.; de Oliveira, S., Jr. Exergy analysis of pretreatment processes of bioethanol production based on sugarcane bagasse. Energy 2014, 76, 130–138. [Google Scholar] [CrossRef]
- Tanwar, D.A.; Aggarwal, A. Sugarcane bagasse: A novel substrate for mass multiplication of Funneliformis mosseae with onion as host. J. Cent. Eur Agric. 2013, 14, 1502–1511. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenerg. 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Janke, L.; Leite, A.; Nikolausz, M.; Schmidt, T.; Liebetrau, J.; Nelles, M.; Stinner, W. Biogas production from sugarcane waste: Assessment on kinetic challenges for process designing. Int. J. Mol. Sci. 2015, 16, 20685–20703. [Google Scholar] [CrossRef]
- Leite, A.F.; Janke, L.; Harms, H.; Zang, J.W.; Fonseca-Zang, W.A.; Stinner, W.; Nikolausz, M. Assessment of the variations in characteristics and methane potential of major waste products from the Brazilian bioethanol industry along an operating season. Energ. Fuel. 2015, 29, 4022–4029. [Google Scholar] [CrossRef]
- López González, L.M.; Pereda Reyes, I.; Dewulf, J.; Budde, J.; Heiermann, M.; Vervaeren, H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour. Technol. 2014, 169, 284–290. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, R.; Rios-Leal, E.; Martinez-Toledo, Á.; Ramos-Morales, F.R.; Cruz-Sánchez, J.S.; Cuevas-Díaz, M.d.C. Uso de cachaza y bagazo de caña de azúcar en la remoción de hidrocarburos en suelo contaminado. Rev. Int. Contam. Ambient. 2011, 27, 31–39. [Google Scholar]
- Seleiman, M.F.; Kheir, A.M.S. Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere 2018, 193, 538–546. [Google Scholar] [CrossRef]
- Bento, L.R.; Castro, A.J.R.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Melo, C.A. Release of nutrients and organic carbon in different soil types from hydrochar obtained using sugarcane bagasse and vinasse. Geoderma 2019, 334, 24–32. [Google Scholar] [CrossRef]
- Hasan, H.; Dang, L.; Khabbaz, H.; Fatahi, B.; Terzaghi, S. Remediation of expansive soils using agricultural waste bagasse ash. Procedia Eng. 2016, 143, 1368–1375. [Google Scholar] [CrossRef]
- Lyra, G.P.; dos Santos, V.; De Santis, B.C.; Rivaben, R.R.; Fischer, C.; Pallone, E.M.d.J.A.; Rossignolo, J.A. Reuse of sugarcane bagasse ash to produce a lightweight aggregate using microwave oven sintering. Const. Build. Mater. 2019, 222, 222–228. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Muruganandam, L.; Srinivasan, K. An overview of the influences of mechanical and chemical processing on sugarcane bagasse ash characterization as a supplementary cementitious material. J. Clean. Prod. 2019, 245, 118854. [Google Scholar] [CrossRef]
- Hernández-Olivares, F.; Elizabeth Medina-Alvarado, R.; Burneo-Valdivieso, X.E.; Rodrigo Zúñiga-Suárez, A. Short sugarcane bagasse fibers cementitious composites for building construction. Const. Build. Mater. 2020, 247, 118451. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019; Available online: https://www.aoac.org/aoac_prod_imis/AOAC/Publications/Official_Methods_of_Analysis/AOAC_Member/Pubs/OMA/AOAC_Official_Methods_of_Analysis.aspx (accessed on 19 January 2021).
- Gonçalves, A.R.; Moriya, R.Y.; Oliveira, L.R.M.; Saad, M.B.W. Xylanase recycling for the economical biobleaching of sugarcane bagasse and straw pulps. Enzym. Microb. Technol. 2008, 43, 157–163. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and organic growing media have distinct community structure, stability and functionality in soilless culture systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef]
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- Flogeac, K.; Guillon, E.; Aplincourt, M.; Marceau, E.; Stievano, L.; Beaunier, P.; Frapart, Y.-M. Characterization of soil particles by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR) and transmission electron microscopy (TEM). Agron. Sustain. Dev. 2005, 25, 345–353. [Google Scholar] [CrossRef]
- Cui, L.; Wei, X.; Chang, G.; Huang, X.; Han, D. Structure and saccharification of sugarcane bagasse pretreated with acid coupled alkaline. In Proceedings of the International Symposium on Mechanical Engineering and Material Science (ISMEMS 2017), Suzhou, China, 17–19 November 2017. [Google Scholar]
- Kamath, S.R.; Proctor, A. Silica gel from rice hull ash: Preparation and characterization. Cereal Chem. 1998, 75, 484–487. [Google Scholar] [CrossRef]
- James, J. Sugarcane press mud modification of expansive soil stabilized at optimum lime content: Strength, mineralogy and microstructural investigation. J. Rock Mech. Geotech. Eng. 2020, 12, 395–402. [Google Scholar] [CrossRef]
- Munasir; Triwikantoro; Zainuri, M.; Darminto. Syntheis of SiO2 containing quartz and cristobalite phases from silica sands. Mater. Sci-Poland. 2015, 33, 47–55. [Google Scholar] [CrossRef]
- Bar-Tal, A.; Saha, U.K.; Raviv, M.; Tuller, M. Chapter 7—Inorganic and synthetic organic components of soilless culture and potting mixtures. In Soilless Culture, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier: Boston, MA, USA, 2019; pp. 259–301. [Google Scholar]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Chem. Phys. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Wong Sak Hoi, L.; Martincigh, B.S. Sugar cane plant fibres: Separation and characterisation. Ind. Crop Prod. 2013, 47, 1–12. [Google Scholar] [CrossRef]
- Toledo-Jaldin, H.P.; Sánchez-Mendieta, V.; Blanco-Flores, A.; López-Téllez, G.; Vilchis-Nestor, A.R.; Martín-Hernández, O. Low-cost sugarcane bagasse and peanut shell magnetic-composites applied in the removal of carbofuran and iprodione pesticides. Environ. Sci. Pollut. Res. 2020, 27, 7872–7885. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Tailoring chemical, physical, and morphological properties of sugarcane bagasse cellulose nanocrystals via phosphorylation method. J. Nat. Fibers. 2019. [Google Scholar] [CrossRef]
- Gharachorloo, M.; Ghasemi Afshar, P.; Honarvar, M.; Eshratabadi, P.; Bazyar, B. Advances in environmental biology investigation of the physico-chemical properties of press mud: A sugar industry waste. Adv. Environ. Biol. 2014, 8, 1053–1058. [Google Scholar]
- Van der Marel, H.W.; Beutelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and their Admixtures; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Castaldelli, V.; Akasaki, J.; Melges, J.L.P.; Tashima, M.; Soriano, L.; Borrachero, M.; Monzo, J.; Payá, J. Use of Slag/Sugar Cane Bagasse Ash (SCBA) blends in the production of alkali-activated materials. Materials 2013, 6, 3108–3127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Tripathi, S.; Balomajumder, C. Characterization of pressmud: A sugar industry waste. Fuel 2011, 90, 389–394. [Google Scholar] [CrossRef]
- Ribeiro, D.; Morelli, M. Effect of calcination temperature on the pozzolanic activity of Brazilian Sugar Cane Bagasse Ash (SCBA). Mater. Res. 2014, 17, 974–981. [Google Scholar] [CrossRef]
- Maldonado-García, M.A.; Hernández-Toledo, U.I.; Montes-García, P.; Valdez-Tamez, P.L. The influence of untreated sugarcane bagasse ash on the microstructural and mechanical properties of mortars. Mater. Construcción 2018, 68, e148. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Zereffa, E. Summary on adsorption and photocatalysis for pollutant remediation: Mini review. J. Encap. Adsorp. Sci. 2018, 08, 225–255. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Saifullah, M.; Mollick, A.K.M.; Hossain, M.; Halim, G.M.A.; Asao, T. Influence of soilless culture substrate on improvement of yield and produce quality of horticultural crops. In Soilless Culture-Use of Substrates for the Production of Quality Horticultural Crop; InTech: Rijeka, Croatia, 2015; pp. 1–32. [Google Scholar]
- Ming, D.; Allen, E. Use of natural zeolites in agronomy, horticulture and environmental soil remediation. Rev. Mineral. Geochem. 2001, 45, 619–654. [Google Scholar] [CrossRef]
- Albino, V.S.; Peixoto, J.R.; Caetano Junior, V.; Vilela, M.S. Rootstock performance for cherry tomato production under organic, greenhouse production system. Hortic. Bras. 2018, 36, 130–135. [Google Scholar] [CrossRef]
- Borgognone, D.; Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Schwarz, D. Effect of nitrogen form and nutrient solution pH on growth and mineral composition of self-grafted and grafted tomatoes. Sci. Hortic. 2013, 149, 61–69. [Google Scholar] [CrossRef]
- Dyśko, J.; Kaniszewski, S.; Kowalczyk, W. The effect of nutrient solution PH on phosphorus availability in soilless culture of tomato. J. Elem. 2008, 13, 189–198. [Google Scholar]
- Dyśko, J.; Kowalczyk, W.; Kaniszewski, S. The influence of pH of nutrient solution on yield and nutritional status of tomato plants grown in soilless culture system. Veg. Crops Res. Bull. 2009, 70, 59–69. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of rhamnolipid and initial compost particle size on the two-stage composting of green waste. Bioresour. Technol. 2014, 163, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Improving green waste composting by addition of sugarcane bagasse and exhausted grape marc. Bioresour. Technol. 2016, 218, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.A.; Said-Pullicino, D.; Agulló, E.; Andreu, J.; Paredes, C.; Moral, R. Application of winery and distillery waste composts to a Jumilla (SE Spain) vineyard: Effects on the characteristics of a calcareous sandy-loam soil. Agric. Ecosyst. Environ. 2011, 140, 80–87. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, D.; Singh, B.L.; Kumar, U.; Shweta. Composting of sugar-cane waste by-products through treatment with microorganisms and subsequent vermicomposting. Bioresour. Technol. 2010, 101, 6707–6711. [Google Scholar] [CrossRef]
- Teixeira, D.L.; Matos, A.T.d.; Melo, E.d.C. Resistance to forced airflow through layers of composting organic material. J. Waste Manag. 2015, 36, 57–62. [Google Scholar] [CrossRef]
- Tanwar, A.; Aggarwal, A.; Yadav, A.; Parkash, V. Screening and selection of efficient host and sugarcane bagasse as substrate for mass multiplication of Funneliformis mosseae. Biol. Agric. Hortic. J. 2013, 29, 107–117. [Google Scholar] [CrossRef]
- Meunchang, S.; Panichsakpatana, S.; Weaver, R.W. Co-composting of filter cake and bagasse; by-products from a sugar mill. Bioresour. Technol. 2005, 96, 437–442. [Google Scholar] [CrossRef]
- Webber, C.; White, P., Jr.; Spaunhorst, D.; Petrie, E. Impact of sugarcane bagasse ash as an amendment on the physical properties, nutrient content and seedling growth of a certified organic greenhouse growing media. J. Agric. Sci. 2017, 9, 1. [Google Scholar] [CrossRef]
- Webber, C.; White, P.; Petrie, E.; Shrefler, J.; Taylor, M. Sugarcane bagasse ash as a seedling growth media component. J. Agric. Sci. 2015, 8, 1. [Google Scholar] [CrossRef]
- Cifuentes, R.; de León, R.; Porres, C.; Rolz, C. Windrow composting of waste sugar cane and press mud mixtures. Sugar Tech 2013, 15, 406–411. [Google Scholar] [CrossRef]
- Saleh-e-In, M.; Yeasmin, S.; Paul, B.; Ahsan, M.; Rahman, M.; Roy, S. Chemical studies on press mud: A sugar industries waste in Bangladesh. Sugar Tech. 2012, 14, 109–118. [Google Scholar] [CrossRef]
- Negim, O.; Mustafa, A.; Fouad, H. Effect of pressmud, as an organic fertilizer, on some soil properties, growth of tomato plant and infestation of tuta absluta under saline irrigation water. J. Soil Sci. Agric. Eng. 2016, 7, 557–563. [Google Scholar] [CrossRef]
- Samuel, J.D.; Marta Camps, A.; Peter, A.B.; Jason, J.W. Closing the loop: Use of biochar produced from tomato crop green waste as a substrate for soilless, hydroponic tomato production. HortScience 2015, 50, 1572–1581. [Google Scholar] [CrossRef]
- Almanza-Merchán, P.J.; Arévalo, Y.A.; Cely, R.G.E.; Pinzón, E.H.; Serrano, C.P.A. Fruit growth characterization of the tomato (Solanum lycopersicum L.) hybrid Ichiban’grown under cover. Agron. Colomb. 2016, 34, 155–162. [Google Scholar] [CrossRef]
- López González, L.M.; Pereda Reyes, I.; Pedraza Garciga, J.; Barrera, E.L.; Romero Romero, O. Energetic, economic and environmental assessment for the anaerobic digestion of pretreated and codigested press mud. J. Waste Manag. 2020, 102, 249–259. [Google Scholar] [CrossRef]
- Mendieta, O.; Castro, L.; Rodríguez, J.; Escalante, H. Synergistic effect of sugarcane scum as an accelerant co-substrate on anaerobic co-digestion with agricultural crop residues from non-centrifugal cane sugar agribusiness sector. Bioresour. Techn. 2020, 303, 122957. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.Y.; Cifuentes, B.; Cobo, M. Controlling sugarcane press-mud fermentation to increase bioethanol steam reforming for hydrogen production. J. Waste Manag. 2019, 98, 1–13. [Google Scholar] [CrossRef]
- Karwal, M.; Kaushik, A. Co-composting and vermicomposting of coal fly-ash with press mud: Changes in nutrients, micro-nutrients and enzyme activities. J. Environ. Technol. Innov. 2020, 18, 100708. [Google Scholar] [CrossRef]
- Khan, M.Y.; Haque, M.M.; Molla, A.H.; Rahman, M.M.; Alam, M.Z. Antioxidant compounds and minerals in tomatoes by Trichoderma-enriched biofertilizer and their relationship with the soil environments. J. Integr. Agr. 2017, 16, 691–703. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).