Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America
Abstract
1. Introduction
2. Mushrooms Native to North America
3. Medicinal Properties of Mushrooms Native to North America
3.1. Anti-Bacterial and Anti-Viral Activities
3.2. Anti-Proliferative Activity
3.2.1. Anti-Proliferative Activity In-Vitro
3.2.2. Anti-Proliferative/Anti-Cancer Activity in Animal Models
3.2.3. Anti-Proliferative/Anti-Cancer Activity in Humans
3.3. Anti-Inflammatory Activity
3.3.1. Anti-Inflammatory Activity In-Vitro
3.3.2. Anti-Inflammatory Activity in Animal Models
3.3.3. Anti-Inflammatory Activity in Clinical Studies
3.4. Immuno-Stimulatory Activity
3.4.1. Immuno-Stimulatory Activity In-Vitro
3.4.2. Immuno-Stimulatory Activity in Animal Models
3.4.3. Immuno-Stimulatory Activity in Clinical Studies
3.5. Anti-Oxidant Activity
3.5.1. Anti-Oxidant Activity In-Vitro
3.5.2. Anti-Oxidant Activity in Animal Models
3.6. Anti-Fungal Activity
3.7. Other Bioactivities
4. Bioactive Compounds from Mushrooms Native to North America
4.1. Large Molecular Weight Compounds
4.2. Small Molecules
5. Mushrooms from North America as a Source for Drug Discovery
6. Edibility of Mushrooms Native to North America
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, S.T.; Hayes, W.A. (Eds.) The Biology and Cultivation of Edible Mushrooms; Academic Press Inc.: New York, NY, USA, 1978; p. 19. [Google Scholar]
- Hawksworth, D.L.; Lücking, R. Chapter 4, Fungal diversity revisited: 2.2 to 3.8 million species. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 79–95. [Google Scholar]
- Hawksworth, D.L. Mushrooms: The extent of the unexplored potential. Int. J. Med. Mushrooms. 2001, 3, 1–5. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Chang, S.T. Global impact of edible and medicinal mushrooms on human welfare in the 21st century: Non green revolution. Int. J. Med. Mushrooms 1999, 1, 1–7. [Google Scholar] [CrossRef]
- Reshetnikov, S.V.; Tan, K.K. Higher Basidiomycota as a source of antitumor and immunostimulating polysaccharides. Int. J. Med. Mushrooms 2001, 3, 1–34. [Google Scholar] [CrossRef]
- Van Griensven, L.J. Culinary-medicinal mushrooms: Must action be taken? Int. J. Med. Mushrooms 2009, 11, 281–286. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives. Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef]
- Ivanova, T.S.; Krupodorova, T.A.; Barshteyn, V.Y.; Artamonova, A.B.; Shlyakhovenko, V.A. Anticancer substances of mushroom origin. Exp. Oncol. 2014, 36, 58–66. [Google Scholar]
- Panda, M.K.; Paul, M.; Singdevsachan, S.K.; Tayung, K.; Das, S.K.; Thatoi, H. Promising anticancer therapeutics from mushrooms: Current findings and future perceptions. Curr. Pharm. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ejike, U.C.; Chan, C.J.; Okechukwu, P.N.; Lim, R.L.Y. New advances and potentials of fungal immunomodulatory proteins for therapeutic purposes. Crit. Rev. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Burk, W.R. Puffball usages among North American Indians. J. Ethnobiol. 1983, 3, 55–62. [Google Scholar]
- Blanchette, R.A.; Compton, B.D.; Turner, N.J.; Gilbertson, R.L. Nineteenth century shaman grave guardians are carved Fomitopsis officinalis sporophores. Mycologia 1992, 84, 119–124. [Google Scholar] [CrossRef]
- Song, X.; Gaascht, F.; Schmidt-Dannert, C.; Salomon, C.E. Discovery of antifungal and biofilm preventative compounds from mycelial cultures of a unique North American Hericium sp. fungus. Molecules 2020, 25, 963. [Google Scholar] [CrossRef]
- Javed, S.; Li, W.M.; Zeb, M.; Yaqoob, A.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Cheung, P.C.K.; Payne, G.W.; Lee, C.H. Anti-inflammatory activity of the wild mushroom, Echinodontium tinctorium, in RAW264. 7 macrophage cells and mouse microcirculation. Molecules 2019, 24, 3509. [Google Scholar] [CrossRef]
- Shao, D.; Tang, S.; Healy, R.A.; Imerman, P.M.; Schrunk, D.E.; Rumbeiha, W.K. A novel orellanine containing mushroom Cortinarius armillatus. Toxicon 2016, 114, 65–74. [Google Scholar] [CrossRef]
- Buvall, L.; Hedman, H.; Khramova, A.; Najar, D.; Bergwall, L.; Ebefors, K.; Sihlbom, C.; Lundstam, S.; Herrmann, A.; Wallentin, H.; et al. Orellanine specifically targets renal clear cell carcinoma. Oncotarget 2017, 8, 91085–91098. [Google Scholar] [CrossRef]
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.A.; Monte, A. Antibacterial compounds from mushrooms I: A lanostane-type triterpene and prenylphenol derivatives from Jahnoporus hirtus and Albatrellus flettii and their activities against Bacillus cereus and Enterococcus faecalis. Planta Med. 2010, 76, 182–185. [Google Scholar] [CrossRef]
- Yaqoob, A.; Li, W.M.; Liu, V.; Wang, C.; Mackedenski, S.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Reimer, K.; Lee, C.H. Grifolin, neogrifolin and confluentin from the terricolous polypore Albatrellus flettii suppress KRAS expression in human colon cancer cells. PLoS ONE 2020, 15, e0231948. [Google Scholar] [CrossRef]
- Deo, G.S.; Khatra, J.; Buttar, S.; Li, W.M.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Reimer, K.; Lee, C.H. Antiproliferative, immuno-stimulatory, and anti-inflammatory activities of extracts derived from mushrooms collected in Haida Gwaii, British Columbia (Canada). Int. J. Med. Mushrooms 2019, 21, 629–643. [Google Scholar] [CrossRef]
- Smith, A.; Javed, S.; Barad, A.; Myhre, V.; Li, W.M.; Reimer, K.; Massicotte, H.B.; Tackaberry, L.E.; Payne, G.W.; Egger, K.N.; et al. Growth-inhibitory and immunomodulatory activities of wild mushrooms from North-Central British Columbia (Canada). Int. J. Med. Mushrooms 2017, 19, 485–497. [Google Scholar] [CrossRef]
- Stanikunaite, R.; Trappe, J.M.; Khan, S.I.; Ross, S.A. Evaluation of therapeutic activity of hypogeous ascomycetes and basidiomycetes from North America. Int. J. Med. Mushrooms 2007, 9, 7–14. [Google Scholar] [CrossRef]
- Hassan, F.; Ni, S.; Becker, T.L.; Kinstedt, C.M.; Abdul-Samad, J.L.; Actis, L.A.; Kennedy, M.A. Evaluation of the antibacterial activity of 75 mushrooms collected in the vicinity of Oxford, Ohio (USA). Int. J. Med. Mushrooms 2019, 21, 131–141. [Google Scholar] [CrossRef]
- Gu, Y.H.; Leonard, J. In vitro effects on proliferation, apoptosis and colony inhibition in ER-dependent and ER-independent human breast cancer cells by selected mushroom species. Oncol. Rep. 2006, 15, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, K.; Yang, G.; Xia, C.; Polston, J.E.; Li, G.; Li, S.; Lin, Z.; Yang, L.J.; Bruner, S.D.; et al. Cytotoxic protein from the mushroom Coprinus comatus possesses a unique mode for glycan binding and specificity. Proc. Natl. Acad. Sci. USA 2017, 114, 8980–8985. [Google Scholar] [CrossRef] [PubMed]
- Stanikunaite, R.; Khan, S.I.; Trappe, J.M.; Ross, S.A. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phytother. Res. 2009, 23, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Winkler, A.L.; Schwan, W.R.; Volk, T.J.; Rott, M.; Monte, A. Antibacterial compounds from mushrooms II: Lanostane triterpenoids and an ergostane steroid with activity against Bacillus cereus isolated from Fomitopsis pinicola. Planta Med. 2010, 76, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Sanchez, M.; Boutin, Y.; Angers, P.; Gosselin, A.; Tweddell, R.J. A bioactive (1→3)-, (1→4)-β-d-glucan from Collybia dryophila and other mushrooms. Mycologia 2006, 98, 180–185. [Google Scholar] [CrossRef]
- Pacheco-Sánchez, M.; Boutin, Y.; Angers, P.; Gosselin, A.; Tweddell, R.J. Inhibitory effect of CDP, a polysaccharide extracted from the mushroom Collybia dryophila, on nitric oxide synthase expression and nitric oxide production in macrophages. Eur. J. Pharmacol. 2007, 555, 61–66. [Google Scholar] [CrossRef]
- Shideler, S.; Reckseidler-Zenteno, S.; Treu, R.; Lewenza, S. Membrane damage-responsive biosensors for the discovery of antimicrobials from Lenzites betulina and Haploporus odorus. In Proceedings of the 2017 URSCA, Calgary, Alberta, 7–8 April 2017; Volume 3. [Google Scholar]
- Javed, S.; Mitchell, K.; Sidsworth, D.; Sellers, S.L.; Reutens-Hernandez, J.; Massicotte, H.B.; Egger, K.N.; Payne, G.W.; Lee, C.H. Inonotus obliquus attenuates histamine-induced microvascular inflammation. PLoS ONE 2019, 14, e0220776. [Google Scholar] [CrossRef] [PubMed]
- Van, Q.; Nayak, B.N.; Reimer, M.; Jones, P.J.H.; Fulcher, R.G.; Rempel, C.B. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J. Ethnopharmacol. 2009, 125, 487–493. [Google Scholar] [CrossRef]
- Schwan, W.R.; Dunek, C.; Gebhardt, M.; Engelbrecht, K.; Klett, T.; Monte, A.; Toce, J.; Rott, M.; Volk, T.J.; Lipuma, J.L.; et al. Screening a mushroom extract library for activity against Acinetobacter baumannii and Burkholderia cepacia and the identification of a compound with anti-Burkholderia activity. Annals Clin. Microbiol. Antimicrob. 2010, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Barad, A.; Mackedenski, S.; Li, W.M.; Li, X.J.; Lim, B.C.C.; Rashid, F.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Reimer, K.; et al. Anti-proliferative activity of a purified polysaccharide isolated from the basidiomycete fungus Paxillus involutus. Carbohydr. Polym. 2018, 181, 923–930. [Google Scholar] [CrossRef]
- Pineda-Alegría, J.A.; Sánchez-Vázquez, J.E.; González-Cortazar, M.; Zamilpa, A.; López-Arellano, M.E.; Cuevas-Padilla, E.J.; Mendoza-de-Gives, P.; Aguilar-Marcelino, L. The edible mushroom Pleurotus djamor produces metabolites with lethal activity against the parasitic nematode Haemonchus contortus. J. Med. Food 2017, 20, 1184–1192. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Martínez-Carrera, D.; Morales, P.; Sobal, M.; Escudero, H.; Meneses, M.E.; Avila-Nava, A.; Castillo, I.; Bonilla, M. Comparative study of antioxidant and antibacterial properties of the edible mushrooms Pleurotus levis, P. ostreatus, P. pulmonarius and P. tuber-regium. Int. J. Food Sci. 2018, 53, 1316–1330. [Google Scholar] [CrossRef]
- Zhang, B.B.; Cheung, P.C. Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuber-regium by submerged fermentation. J. Agric. Food Chem. 2011, 59, 1210–1216. [Google Scholar] [CrossRef]
- Liu, X.T.; Schwan, W.R.; Volk, T.J.; Rott, M.; Liu, M.; Huang, P.; Liu, Z.; Wang, Y.; Zitomer, N.C.; Sleger, C.; et al. Antibacterial spirobisnaphthalenes from the North American cup fungus Urnula craterium. J. Nat. Prod. 2012, 75, 1534–1538. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms in human clinical studies. Part I. Anticancer, oncoimmunological, and immunomodulatory activities: A review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Council of Canadian Academies. When Antibiotics Fail: The Expert Panel on the Potential Socio-Economic Impacts of Antimicrobial Resistance in Canada; Council of Canadian Academies: Ottawa, ON, USA, 2019. [Google Scholar]
- Strachan, C.R.; Davies, J. The whys and wherefores of antibiotic resistance. Cold Spring Harb. Perspect. Med. 2017, 7, a025171. [Google Scholar] [CrossRef]
- Gould, I.M.; Gunasekara, C.; Khan, A. Antibacterials in the pipeline and perspectives for the near future. Curr. Opin. Pharmacol. 2019, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.; Dias, J.F.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial cuparene-type sesquiterpenes, enokipodins C and D, from a mycelial culture of flammulina v elutipes. J. Nat. Prod. 2001, 64, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Beattie, K.D.; Rouf, R.; Gander, L.; May, T.W.; Ratkowsky, D.; Donner, C.D.; Gill, M.; Tiralongo, E. Antibacterial metabolites from Australian macrofungi from the genus Cortinarius. Phytochemistry 2010, 71, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.; Dumitrache-Anghel, C.N.; Backhaus, J.; Christie, G.; Cross, R.F.; Lonergan, G.T.; Baker, W.L. A case for caution in assessing the antibiotic activity of extracts of culinary-medicinal Shiitake mushroom [Lentinus edodes (Berk.) Singer] (Agaricomycetideae). Int. J. Med. Mushrooms 2003, 5, 1–6. [Google Scholar] [CrossRef]
- Centko, R.M.; Ramon-Garcia, S.; Taylor, T.; Patrick, B.O.; Thompson, C.J.; Miao, V.P.; Andersen, R.J. Ramariolides A-D, antimycobacterial butenolides isolated from the mushroom Ramaria cystidiophora. J. Nat. Prod. 2012, 75, 2178–2182. [Google Scholar] [CrossRef]
- Stamets, P.E.; Naeger, N.L.; Evans, J.D.; Han, J.O.; Hopkins, B.K.; Lopez, D.; Moershel, H.M.; Naily, R.; Sumerlin, D.; Taylor, A.W.; et al. Extracts from polypore mushroom mycelia reduce viruses in honey bees. Sci. Rep. 2018, 8, 13936. [Google Scholar] [CrossRef]
- Maness, L.; Sneed, N.; Hardy, B.; Yu, J.; Ahmedna, M.; Goktepe, I. Anti-proliferative effect of Pleurotus tuberregium against colon and cervical cancer cells. J. Med. Plants Res. 2011, 5, 6650–6655. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel medicinal mushroom blend as a promising supplement in integrative oncology: A multi-tiered study using 4T1 triple-negative mouse breast cancer model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, C.S.; Hung, M.H.; Chen, S.; Wang, W.; Tai, C.J.; Lu, C.L. The effect of mushroom beta-glucans from solid culture of Ganoderma lucidum on inhibition of the primary tumor metastasis. Evid. Based Complementary Altern. Med. 2014, 2014, 1–7. [Google Scholar]
- Masuda, Y.; Inoue, M.; Miyata, A.; Mizuno, S.; Nanba, H. Maitake β-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int. Immunopharmacol. 2009, 9, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Lu, F.H.; Su, Y.C.; Ou, H.Y.; Hung, H.C.; Wu, J.S.; Yang, Y.C.; Chang, C.J. In vivo and in vitro anti-tumor effects of fungal extracts. Molecules 2014, 19, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Nam, S.H.; Friedman, M. Hericium erinaceus (Lion’s Mane) mushroom extracts inhibit metastasis of cancer cells to the lung in CT-26 colon cancer-transplanted mice. J. Agric. Food Chem. 2013, 61, 4898–4904. [Google Scholar] [CrossRef]
- Lu, C.C.; Huang, W.S.; Lee, K.F.; Lee, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, L.Y.; Lee, B.O.; Teng, C.C.; Shen, C.H.; et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J. Funct. Foods 2016, 21, 474–484. [Google Scholar] [CrossRef]
- Diling, C.; Chaoqun, Z.; Jian, Y.; Jian, L.; Jiyan, S.; Yishen, X.; Guoxiao, L. Immunomodulatory activities of a fungal protein extracted from Hericium erinaceus through regulating the gut microbiota. Front. Immunol. 2017, 8, 666. [Google Scholar] [CrossRef]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Zhu, J.X.; Chen, S.; Hu, G.Y. Anti-tumor effects of proteoglycan from Phellinus linteus by immunomodulating and inhibiting Reg IV/EGFR/Akt signaling pathway in colorectal carcinoma. Int. J. Biol. Macromol. 2011, 48, 511–517. [Google Scholar] [CrossRef]
- Youn, M.J.; Kim, J.K.; Park, S.Y.; Kim, Y.; Park, C.; Kim, E.S.; Park, K.I.; So, H.S.; Park, R. Potential anticancer properties of the water extract of Inonotus obliquus by induction of apoptosis in melanoma B16-F10 cells. J. Ethnopharmacol. 2009, 121, 221–228. [Google Scholar] [CrossRef]
- Chung, M.J.; Chung, C.K.; Jeong, Y.; Ham, S.S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 2010, 4, 177–182. [Google Scholar] [CrossRef]
- Nakata, T.; Yamada, T.; Taji, S.; Ohishi, H.; Wada, S.; Tokuda, H.; Sakuma, K.; Tanaka, R. Structure determination of inonotsuoxides A and B and in vivo anti-tumor promoting activity of inotodiol from the sclerotia of Inonotus obliquus. Bioorg. Med. Chem. 2007, 15, 257–264. [Google Scholar] [CrossRef]
- Nomura, M.; Takahashi, T.; Uesugi, A.; Tanaka, R.; Kobayashi, S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008, 28, 2691–2696. [Google Scholar] [PubMed]
- Awadasseid, A.; Hou, J.; Gamallat, Y.; Xueqi, S.; Eugene, K.D.; Musa Hago, A.; Bamba, D.; Meyiah, A.; Gift, C.; Xin, Y. Purification, characterization and antitumor activity of a novel glucan from the fruiting bodies of Coriolus versicolor. PLoS ONE 2017, 12, e0171270. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dai, X.; Chen, G.; Ye, J.; Zhou, S. A randomized, placebo-controlled, multicenter study of Ganoderma lucidum (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) polysaccharides (Ganopoly®) in patients with advanced lung cancer. Int. J. Med. Mushrooms 2003, 5, 1–4. [Google Scholar] [CrossRef]
- Huang, M.; Gao, Y.; Tang, W.; Dai, X.; Gao, H.; Chen, G.; Ye, J.; Chan, E.; Zhou, S. Immune responses to water-soluble Ling Zhi mushroom Ganoderma lucidum (W. Curt.: Fr.) P. Karst. polysaccharides in patients with advanced colorectal cancer. Int. J. Med. Mushrooms 2005, 7, 525–538. [Google Scholar] [CrossRef]
- Deng, G.; Smith-Jones, H.L.; Seidman, A.D.; Fornier, M.; D’Andrea, G.; Wesa, K.; Cunningham-Rundles, S.; Yeung, K.S.; Vickers, A.; Cassileth, B.R. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients. J. Clin. Oncol. 2008, 26, 3024. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Xie, X.; Holman, C.D.A.J. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int. J. Cancer 2009, 124, 1404–1408. [Google Scholar] [CrossRef]
- Hara, M.; Hanaoka, T.; Kobayashi, M.; Otani, T.; Adachi, H.Y.; Montani, A.; Natsukawa, S.; Shaura, K.; Koizumi, Y.; Kasuga, Y.; et al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr. Cancer 2003, 46, 138–147. [Google Scholar] [CrossRef]
- Hazama, S.; Watanabe, S.; Ohashi, M.; Yagi, M.; Suzuki, M.; Matsuda, K.; Yamamoto, T.; Suga, Y.; Suga, T.; Nakazawa, S.; et al. Efficacy of orally administered superfine dispersed lentinan (β-1, 3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res. 2009, 29, 2611–2617. [Google Scholar]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef]
- Du, B.; Zhu, F.; Xu, B. An insight into the anti-inflammatory properties of edible and medicinal mushrooms. J. Func. Foods 2018, 47, 334–342. [Google Scholar] [CrossRef]
- Ooi, V.E.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Enshasy, H.A.E.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotech. 2014, 31, 668–677. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotech. 2014, 26, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Persin, Z.; Stana-Kleinschek, K.; Foster, T.J.; van Dam, J.E.G.; Boeriu, C.G.; Navard, P. Challenges and opportunities in polysaccharides research and technology: The EPNOE views for the next decade in the areas of materials, food and health care. Carbohydr. Polym. 2011, 84, 22–32. [Google Scholar] [CrossRef]
- Lin, Z.B.; Zhang, H.N. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sinica 2004, 25, 1387–1395. [Google Scholar]
- Jakopovic, B.; Oršolić, N.; Kraljević Pavelić, S. Antitumor, Immunomodulatory and Antiangiogenic Efficacy of Medicinal Mushroom Extract Mixtures in Advanced Colorectal Cancer Animal Model. Molecules 2020, 25, 5005. [Google Scholar] [CrossRef]
- Ullah, M.I.; Akhtar, M.; Awais, M.M.; Anwar, M.I.; Khaliq, K. Evaluation of immunostimulatory and immunotherapeutic effects of tropical mushroom (Lentinus edodes) against eimeriasis in chicken. Trop. Anim. Health Prod. 2018, 50, 97–104. [Google Scholar] [CrossRef]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef]
- Yurkiv, B.; Wasser, S.P.; Nevo, E.D.; Sybirna, N.O. Antioxidant effects of medicinal mushrooms Agaricus brasiliensis and Ganoderma lucidum (higher Basidiomycetes): Evidence from animal studies. Int. J. Med. Mushrooms 2015, 17, 943–955. [Google Scholar] [CrossRef]
- Vitak, T.Y.; Wasser, S.P.; Nevo, E.D.; Sybirna, N.O. Enzymatic system of antioxidant protection of erythrocytes in diabetic rats treated with medicinal mushrooms Agaricus brasiliensis and Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 697–708. [Google Scholar] [CrossRef]
- Yeh, M.Y.; Ko, W.C.; Lin, L.Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). Biomed. Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antifungal activity of mushroom (basidiomycetes) extracts and isolated compounds. Curr. Top. Med. Chem. 2013, 13, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Vaz, J.A.; Vasconcelos, M.H.; Martins, A. Compounds from wild mushrooms with antitumor potential. Anti-Cancer Agents Med. Chem. 2010, 10, 424–436. [Google Scholar] [CrossRef]
- Zhang, M.M.; Qiao, Y.; Ang, E.L.; Zhao, H. Using natural products for drug discovery: The impact of the genomics era. Expert Opin. Drug Disc. 2017, 12, 475–487. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Dejong, C.A.; Rees, P.N.; Johnstone, C.W.; Li, H.; Webster, A.L.; Wyatt, M.A.; Magarvey, N.A. Genomes to natural products: Prediction Informatics for secondary metabolomes (PRISM). Nucleic Acids Res. 2015, 43, 9645–9662. [Google Scholar] [CrossRef]
- Skellam, E. Strategies for engineering natural product biosynthesis in fungi. Trends Biotech. 2019, 37, 416–427. [Google Scholar] [CrossRef]
- Almeida, H.; Tsang, A.; Diallo, A.B. Towards accurate identification of biosynthetic gene clusters in fungi. F1000 Research. In Proceedings of the ISMB/ECCB, Basel, Switzerland, 21–25 July 2019. [Google Scholar]
- California Fungi: Gymnopus dryophilus—MycoWeb. Available online: https://www.mykoweb.com/CAF/species/Gymnopus_dryophilus.html (accessed on 18 November 2020).
- Marasmius oreades (Bolton) Fr.—Fairy Ring Champignon. Available online: https://www.first-nature.com/fungi/marasmius-oreades.php (accessed on 18 November 2020).
- Ehlers, T.; Hobby, T. The chanterelle mushroom harvest on northern Vancouver Island, British Columbia: Factors relating to successful commercial development. BC J. Ecosyst. Manag. 2010, 11, 72–83. [Google Scholar]
- Kuo, M.; Dewsbury, D.R.; O’ Donnell, K.; Carter, A.M.; Rehner, S.A.; Moore, J.D.; Moncalvo, J.-M.; Canfield, S.A.; Stephenson, S.L.; Methven, A.S.; et al. Taxonomic revision of true morels (Morchella) in Canada and the United States. Mycologia 2012, 104, 1159–1177. [Google Scholar] [CrossRef]
- Laperriere, G.; Desgagne-Penix, I.; Germain, H. DNA distribution and metabolite profile of wild edible lobster mushroom (Hypomyces lactifluorum/Russula brevipes). Genome 2018, 61, 329–336. [Google Scholar] [CrossRef]
- Antkowiak, W.Z.; Gessner, W.P. The structure of orellanine and orelline. Tetrahedon Lett. 1979, 20, 1931–1934. [Google Scholar] [CrossRef]

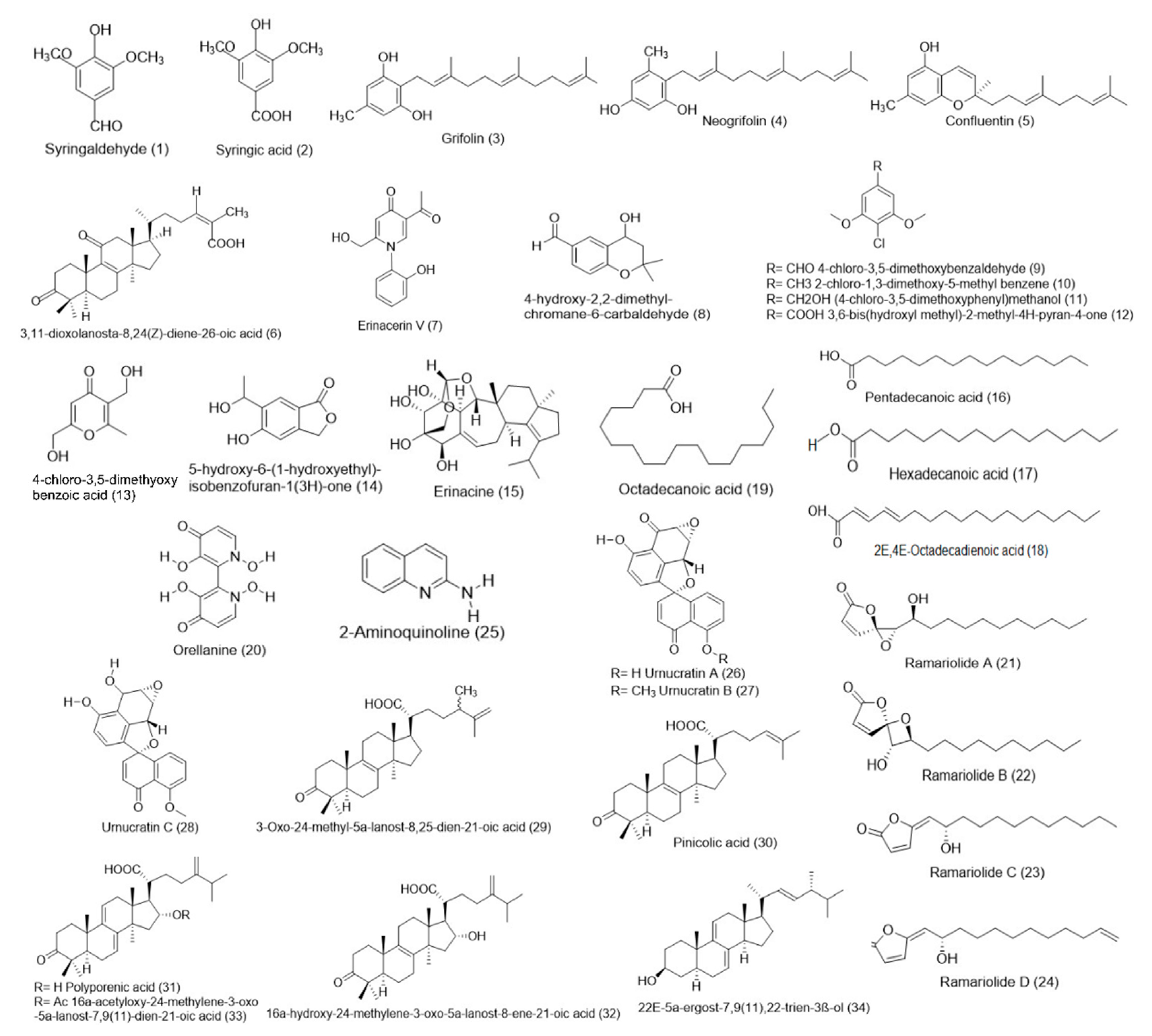
| Mushroom Species | Origin | Bioactivity | Bioactive Component 1 or Extraction Solvent | Active Dose 2 |
|---|---|---|---|---|
| Albatrellus flettii | La Crosse, Wisconsin | Antimicrobial [21] | Grifolin (3) Neogrifolin (4) Confluentin (5) | MIC = 10 μg/mL (3), 20 μg/mL (4,5) on B. cereus. MIC = 0.5 μg/mL (3,4), 1 μg/mL (5) on E. faecalis |
| Albatrellus flettii | Smithers, BC | Anti-proliferative [22] | Grifolin (3) Neogrifolin (4) Confluentin (5) | IC50 = 27.4 μM (3), 24.3 μM (4), 25.9 μM (5) on HeLa. IC50 = 35.4 μM (3), 34.6 μM (4), 33.5 μM (5) on SW480. IC50 = 30.7 μM (3), 30.1 μM (4), 25.8 μM (5) on HT29. |
| Amanita augusta | Haida Gwaii, BC | Anti-proliferative [23] | 50% methanol (a) and 5% NaOH (b) | IC50 = 0.7 mg/mL (a), 0.55 mg/mL (b) |
| Amanita augusta | Haida Gwaii, BC | Immuno-stimulatory [23] | Water | 1 mg/mL |
| Amanita augusta | Haida Gwaii, BC | Anti-inflammatory [23] | 5% NaOH | 1 mg/mL |
| Amanita muscaria | Prince George, BC | Anti-proliferative [24] | 80% ethanol (a) and 50% methanol (b) | IC50 = 0.2 mg/mL (a) (+++) IC50 = 0.6 mg/mL (b) (++) |
| Amanita muscaria | Prince George, BC | Immuno-stimulatory [24] | 2% ammonium oxalate | 1 mg/mL (+) |
| Astraeus pteridis | Linn County, Oregon | Antituberculosis [25] | 95% ethanol | IC50 = < 20 μg/mL (+++) |
| Auricularia fuscosuccinea | Oxford, Ohio | Antimicrobial [26] | Water | Against B. subtilis (+++), P. aeruginosa, S. epidermidis (MIC = 1 mg/mL), P. fluorescens, M. luteus (++) |
| Barssia oregonensis | Clackamas, Oregon | Antituberculosis [25] | 95% ethanol | IC50 = 20–50 μg/mL (++) |
| Cantharellus cibarius | Haida Gwaii, BC | Anti-proliferative [23] | 80% ethanol | 0.1 mg/mL |
| Cantharellus cibarius | Haida Gwaii, BC | Weak immuno-stimulatory [23] | Water | 1 mg/mL |
| Cantharellus cibarius | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol, 50% methanol, water and 5% NaOH | 1 mg/mL |
| Chroogomphus tomentosus | Haida Gwaii, BC | Anti-proliferative (a), immuno-stimulatory (b) [23] | 80% ethanol (a) and water (b) | 0.2 mg/mL (a) (++), 1 mg/mL (b) (++) |
| Chroogomphus tomentosus | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a) and 50% methanol (b) | 1 mg/mL (a,b) (+++) |
| Clavulina cinerea | Haida Gwaii, BC | Anti-proliferative [23] | 80% ethanol | (++) |
| Coprinellus sp. | Seattle, WA, USA | Anti-proliferative [27] | Water | IC50 = 40 μg/mL on MDA-MB-231 cells (+++), 120 μg/mL on MCF-7 cells (+), 150 μg/mL on BT-20 cells (+) |
| Coprinus comatus | Seattle, WA, USA | Anti-proliferative | Water (a) [27], Protein (b) [28] | IC50 = 400 μg/mL on MDA-MB-231 cells (a), 450 μg/mL on MCF-7 cells (a), 10 μM (b) |
| Cortinarius armillatus | Massachusetts | Anti-proliferative [19] | Orellanine | 20 μg/mL clear cell RCC (a), 40 μM (10 mg/L) intraperitoneal injection via PD solution (b) |
| Echinodontium tinctorium | Smithers and Terrace, BC | Anti-inflammatory [18] | Polysaccharide AIPetinc | 1 mg/mL |
| Elaphomyces granulatus | Oregon and Bonner County, Idaho | Anti-inflammatory [25,29] | Syringaldehyde (1), Syringic acid (2), and 95% ethanol (a) | IC50 = 3.5 μg/mL (1) (19.23 μM) (+) IC50 = 0.4 μg/mL (2) (2.02 μM) (+++) 50 μg/mL (a) (+++) |
| Elaphomyces granulatus | Oregon and Bonner County, Idaho | Antioxidant [29] | Syringic acid (2) and 95% ethanol | IC50 = 0.7 μg/mL (2) (+++) Extract IC50 = 41 μg/mL |
| Elaphomyces muricatus | Benton County, Oregon | Anti-inflammatory (a), antioxidant (b), antituberculosis (c) [25] | 95% ethanol (a,c), 70% ethanol (b) | 50 μg/mL (a) (++), IC50 ≥ 50 μg/mL (a) (+), IC50 ≥ 50 μg/mL (a) (+) |
| Flammulina velutipes | Seattle, WA, USA | Anti-proliferative [27] | Water | IC50 = 30 μg/mL on BT-20 cells (+++), 75 μg/mL on MDA-MB-231 cells (++), 150 μg/mL on MCF-7 cells (+) |
| Fomes fomentarius | Prince George, BC | Anti-proliferative [24] | 80% ethanol (a) and 50% methanol (b) | 0.5 mg/mL (a) (+++), 0.5 mg/mL (b) (+++) |
| Fomes fomentarius | Prince George, BC | Immuno-stimulatory [24] | Water (a) and 2% ammonium oxalate (b) | 1 mg/mL (a) (+), 1 mg/mL (b) (++) |
| Fomitopsis pinicola | Oregon, USA | Antimicrobial [30] | 3-Oxo-24-methyl-5α-lanost-8,25-dien-21-oic acid (30), pinicolic acid (31), polyporenic acid (32), 16α-hydroxy-24-methylene-3-oxo-5α-lanost-8-ene-21-oic acid (33), 16α-acetyloxy-24-methylene-3-oxo-5α-lanost-7,9(11)-dien-21-oic acid (34), 22E-5α-ergost-7,9(11),22-trien-3β-ol (35) | MIC = 32 μg/mL (30) (++), 16 μg/mL (31) (++), 32 μg/mL (32) (++), 32 μg/mL (33) (++), 128 μg/mL (34) (+), 64 μg/mL (35) (++) against B. cereus |
| Ganoderma applanatum | Terrace, BC and Oxford, Ohio | Anti-proliferative [24] | 80% ethanol (a) and water (b) | 0.5 mg/mL (a) (++), 1 mg/mL (b) (+) |
| Ganoderma applanatum | Terrace, BC and Oxford, Ohio | Anti-inflammatory [24] | 80% ethanol (a) and 50% methanol (b) | 1 mg/mL (a) (+++), 1 mg/mL (b) (+++) |
| Ganoderma applanatum | Terrace, BC and Oxford, Ohio | Immuno-stimulatory [24] | Water(a), 5% NaOH (b), and 2% ammonium oxalate (c) | 1 mg/mL (a) (+++), 1 mg/mL (b) (+), 1 mg/mL (c) (+) |
| Ganoderma applanatum | Terrace, BC and Oxford, Ohio | Antimicrobial [26] | Water | Against P. aeruginosa, P. fluorescens, B. subtilis, S. epidermidis, (MIC = 100 mg/mL (isolate 1), 10 mg/mL (isolate 2), and M. luteus (+++) |
| Ganoderma lucidum | Oxford, Ohio | Antimicrobial [26] | Water | MIC = 0.1 mg/mL (+++) against S. epidermidis |
| Ganoderma tsugae | Haida Gwaii, BC | Anti-proliferative (a), immuno-stimulatory (b) [23] | 80% ethanol (a) and water (b) | (a) (++),1 mg/mL (b) (+++) |
| Ganoderma tsugae | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a) and 5% NaOH (b) | 1 mg/mL (a) (+++), 1 mg/mL (b) (++) |
| Gautieria monticola | Benton County, Oregon | Antioxidant [25] | 70% ethanol | IC50 = > 50 μg/mL (+) |
| Geopora clausa | Inyo Country, California | Antioxidant (a), anti-proliferative [25] | 70% ethanol | IC50 = 20–50 μg/mL (a) (++) |
| Guepina helvelloides | Haida Gwaii, BC | Anti-proliferative (a), immuno-stimulatory (b) [23] | 80% ethanol (a) and water (b) | (a) (++),1 mg/mL (b) (+++) |
| Guepina helvelloides | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a), 50% methanol (b), and 5% NaOH (c) | |
| Gymnopus dryophilus | Quebec | Anti-inflammatory [31,32] | CDP polysaccharide | 400 and 800 μg/mL |
| Gyromitra esculenta | Prince George, BC | Anti-proliferative [24] | 80% ethanol, 50% methanol | 1 mg/mL (+) |
| Gyromitra esculenta | Prince George, BC | Immuno-stimulatory [24] | 80% ethanol, 50% methanol, water | 1 mg/mL (++) |
| Haploporus odorus | Study conducted in Calgary, Canada | Antimicrobial [33] | Extracts | - |
| Hericium corralloides | Prince George, BC | Immuno-stimulatory [24] | 50% methanol (a), water (b), 5% NaOH (c) | 1 mg/mL (a) (++), 1 mg/mL (b) (++), 1 mg/mL (c) (+) |
| Hericium corralloides | Prince George, BC | Anti-inflammatory [24] | 80% ethanol | 1 mg/mL (+) |
| Hericium sp. | Minnesota | Antifungal [17] | 2-chloro-1,3-dimethoxy-5-methyl benzene (10) | MIC = 31.3–62.5 μg/mL against C. albicans and C. neoformans |
| Hericium sp. | Minnesota | Antifungal (a), antibacterial (b) [17] | Ethyl acetate, acetone, methanol | MIC = 250 μg/mL against C. albicans and C. neoformans (a), MIC > 500 μg/mL) against S. aureus (b) |
| Hydnellum sp. | Prince George, BC | Anti-proliferative (a), immuno-stimulatory (b) [24] | 80% ethanol, 50% methanol, water | 1 mg/mL (a) (++), 1 mg/mL (b) (+) |
| Hydnum repandum | Haida Gwaii, BC | Anti-proliferative (a), anti-inflammatory (b & c) [23] | 80% ethanol (a) (b) and 50% methanol (c) | 0.6 mg/mL (a) (++), 1 mg/mL (b) (+++), 1 mg/mL (c) (++) |
| Hygrophoropsis aurantiaca | Haida Gwaii, BC | Anti-proliferative, [23] | 50% methanol, water and 5% NaOH | (+) |
| Hygrophoropsis aurantiaca | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol, 50% methanol, and water | 1 mg/mL (+++) |
| Hymenogaster subalpinus | Benton County, Oregon | Anti-inflammatory (a), antituberculosis (b) [25] | 95% ethanol | 50 μg/mL (a) (++), IC50 = < 20 μg/mL (b) (+++) |
| Hymenopellis furfuracea | Oxford, Ohio | Antimicrobial [26] | Water | Against P. fluorescens, M. luteus (++), B. subtilis, S. episdermidis (+) |
| Hypholoma fasciculare | Haida Gwaii, BC | Anti-proliferative [23] | 80% ethanol (a) and 50% methanol (b) | 0.2 mg/mL (a) (++), 0.1 mg/mL (b) (++) |
| Hypholoma fasciculare | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol, 50% methanol, and water | 1 mg/mL (+++) |
| Inocybe sp. | Haida Gwaii, BC | Anti-proliferative, [23] | 80% ethanol | (++) |
| Inocybe sp. | Haida Gwaii, BC | Immuno-stimulatory [23] | 50% methanol and water | 1 mg/mL (++) |
| Inocybe sp. | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a) and 5% NaOH (b) | 1 mg/mL (a) (+++), 1 mg/mL (b) (+) |
| Inonotus obliquus | Manitoba & Prince George, BC | Anti-inflammatory [34,35] | 50% methanol | 0.25 μg/μL (a), 1 μg/μL in vivo (b) |
| Jahnoporus hirtus | USA | Antimicrobial [21] | 3,11-Dioxolanosta-8,24(Z)-diene-26-oic acid (6) | 40 μg/mL (B. cereus), 32 μg/mL (E. faecalis) |
| Laetiporus conifericola | Haida Gwaii, BC | Anti-proliferative (a), immuno-stimulatory (b), anti-inflammatory (c) [23] | 80% ethanol (a,c) and water (b) | (++), 1 mg/mL (b) (++), 1 mg/mL (c) (+++) |
| Laetiporus sulphureus | Oxford, Ohio | Antimicrobial [26] | Water | MIC = 0.1 mg/mL (+++) against S. epidermidis |
| Lentinellus subaustralis | Oxford, Ohio | Antimicrobial [26] | Water | Against P. aeruginosa, and B. subtilis (+), P. fluorescens, S. epidermidis (MIC = 10 mg/mL), and M. luteus |
| Lentinus edodes | Quebec | Anti-inflammatory [31] | CDP-like polysaccharide | 50 μg/mL (++) |
| Leucogaster rubescens | Pend Oreille County, Oregon | Antioxidant [25] | 95% ethanol | IC50 = 20–50 μg/mL (++) |
| Leucocybe connata | Prince George, BC | Anti-proliferative (a), anti-inflammatory (b) [24] | 5% NaOH (a) and 80% ethanol (b) | 1 mg/mL (a) (++), 1 mg/mL (b) (+++) |
| Leucocybe connata | Prince George, BC | Immuno-stimulatory [24] | Water, 5% NaOH | 1 mg/mL (++) |
| Leucopaxillus albissimus | USA | Antimicrobial [36] | 2-Aminoquinoline | MIC = 8–65 μg/mL against multidrug resistant clinical isolates (++), 128 μg/mL against A. baumannio (+) |
| Marasmius oreades | Quebec | Anti-inflammatory [31] | CDP-like polysaccharide | 50 μg/mL (++) |
| Melanogaster tuberiformis | Lane County, Oregon | Antituberculosis (a), anti-inflammatory (b), antioxidant (c) [25] | 95% ethanol | IC50 = < 20 μg/mL (a) (+++), 50 μg/mL (b) (++), IC50 = > 50 μg/mL (c) (+) |
| Paxillus involutus | Prince George, BC | Anti-proliferative [37] | GIPinv Polysaccharide | IC50 = 0.05 mg/mL (+++) on HeLa,0.04 mg/mL (++) on MCF-7 |
| Phellinopsis conchata | Oxford, Ohio | Antimicrobial [26] | Water | MIC = 1 mg/mL against S. epidermidis (++) |
| Phellinus conchatus | Oxford, Ohio | Antimicrobial [26] | Water | MIC = 100 mg/mL against S. epidermidis (+++) |
| Phellinus igniarius | Terrace, BC | Anti-proliferative [24] | 80% ethanol (a), water (b) | 0.2 mg/mL (a) (+++), 1 mg/mL (b) (+) |
| Phellinus igniarius | Terrace, BC | Anti-inflammatory [24] | 80% ethanol | 1 mg/mL (+++) |
| Phellinus nigricans | Terrace, BC | Anti-proliferative [24] | 80% ethanol (a), water (b) | 1 mg/mL (a) (+), 1 mg/mL (b) (+) |
| Phellinus nigricans | Terrace, BC | Immuno-stimulatory (a,b), anti-inflammatory (c) [24] | Water (a), 5% NaOH (b), and 50% methanol (c) | 1 mg/mL (a) (+), 1 mg/mL (b) (+++), 1 mg/mL (c) (+++) |
| Phellodon atratus | Haida Gwaii, BC | Anti-proliferative, immuno-stimulatory (b & c) [23] | 80% ethanol (a), 50% methanol (b) and water (c) | (a) (+), 1 mg/mL (b,c) (+) |
| Phellodon atratus | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a) and 5% NaOH (b) | 1 mg/mL (a) (+++), 1 mg/mL (b) (++) |
| Pholiota terrestris | Oxford, Ohio | Antimicrobial [26] | Water | Against S. epidermidis, P. fluorescens, and M. luteus (++) |
| Piptoporus betulinus | Prince George, BC | Anti-proliferative [24] | 80% ethanol (a) and water (b) | 0.1 mg/mL (a) (+++), 0.2 mg/mL (b) (+) |
| Piptoporus betulinus | Prince George, BC | Anti-inflammatory [24] | 80% ethanol (a), 50% methanol (b) and water (c) | 1 mg/mL (a–c) |
| Pleurotus djamor | Study from Mexico | Anthelmintic activity [38] | Hydro-alcoholic extracts, Pentadecanoic, hexadecanoic, octadecadienoic, octadecanoic acid | 40 mg/mL |
| Pleurotus levis | Mexico | Antimicrobial (a) (+++), antioxidant (b) (+++) [39] | Water and alcohol | MIC = 3.33 μg/mL against B. subtilis, 13.32 μg/mL against S. agalactiae (a), 26.64 μg/mL against S. aureus, EC-50 = 0.52 μg/mL (b) (DPPH assay) |
| Pleurotus ostreatus | USA & Haida Gwaii, BC | Anti-proliferative (a), anti-inflammatory (b) [23] | 80% ethanol, 50% methanol | (a) (++), 1 mg/mL (b) (+++) |
| Pleurotus ostreatus | USA & Haida Gwaii, BC | Immuno-stimulatory [23] | Water | 1 mg/mL (++) |
| Pleurotus ostreatus | USA & Haida Gwaii, BC | Antioxidant (a), Antimicrobial (b) [39] | Water and alcohol | EC50 = 1.05 μg/mL (a) (++) (DPPH assay), MIC = 7.83 μg/mL (b) (+) against S. agalactiae |
| Pleurotus tuber-regium | Olympia, WA | Anti-proliferative (a) [40], antimicrobial (b) [39] | Polysaccharide (a), and water and alcohol (b) | MIC = 6.03 μg/mL (b) (++) against S. agalactiae |
| Polyporus badius | Oxford, Ohio | Antimicrobial [26] | Water | (+) |
| Polyporus squamosus | Oxford, Ohio | Antimicrobial [26] | Water | (MIC = 10 mg/mL (isolate 1&2), 100 mg/mL (isolate 3), and M. luteus |
| Pseudoinonotus dryadeus | Oxford, Ohio | Antimicrobial [26] | 100% methanol | (++) |
| Pyrofomes demidoffi | Oxford, Ohio | Antimicrobial [26] | Water | MIC = 1 mg/mL against S. epidermidis (+++) |
| Ramaria cystidiophora | Haida Gwaii, BC | Anti-proliferative [23] | 80% ethanol (a), 50% methanol (b) | 0.1 mg/mL (a) (+++), 0.8 mg/mL (b) (++) |
| Ramaria cystidiophora | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a), 50% methanol (b), water (c) and 5% NaOH (d) | 1 mg/mL (a-d) (+++) |
| Ramaria cystidiophora | Vancouver, BC | Antimicrobial [26] | Ramariolide A (22) | MIC = 8 μg/mL against M. smegmatis, 64−128 μg/mL against M. tuberculosis |
| Rhizopogon couchii | Lebanon State Forest, New Jersey | Anti-inflammatory, antioxidant, antituberculosis [25] | 95% ethanol | 50 g/mL (a) (++), IC50 = 20–50 μg/mL (b) (++), IC50 = 20–50 μg/mL (b) (++) |
| Rhizopogon nigrescens | Lebanon State Forest, New Jersey | Anti-inflammatory (a), antioxidant (b) [25] | 95% ethanol | 50 g/mL (a) (+++), IC50 = > 50 μg/mL (b) (+) |
| Rhizopogon pedicellus | Pend Oreille County, Oregon | Antioxidant (a), antituberculosis (b) [25] | 95% ethanol (a),70% ethanol (b) | IC50 = 20–50 μg/mL (a) (++), IC50 ≥ 50 μg/mL (b) (+) |
| Rhizopogon subareolatus | Lewis County, Washington | Antimalarial [25] | 95% ethanol (+) | 15.9 μg/mL (+) |
| Rhizopogon subaustralis | Lebanon State Forest, New Jersey | Anti-inflammatory (a), antioxidant (b) [25] | 95% ethanol | IC50 ≥ 50 μg/mL (a) (+), 50 μg/mL (b) (+++) |
| Rhizopogon subgelatinosus | Jackson County, Oregon | Anti-inflammatory (a), anti-proliferative [25] | 95% ethanol | 50 μg/mL (a) |
| Russula paludosa | Haida Gwaii, BC | Anti-proliferative [23] | 80% ethanol (a), 50% methanol (b), water (c), and 5% NaOH (d) | (a–d) (+) |
| Russula paludosa | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a), 50% methanol (b), water (c) | 1 mg/mL (a–c) (+++) |
| Scleroderma laeve | Lebanon State Forest, New Jersey | Anti-inflammatory (a), antioxidant(b), antituberculosis (c) [25] | 95% ethanol (+++) | 50 μg/mL (a), IC50 = < 20 μg/mL (b), IC50 = < 20 μg/mL (c) |
| Stereum hirsutum | Oxford, Ohio | Antimicrobial [26] | Water | Against B. subtilis, S. epidermidis, and P. fluorescens (+) |
| Trametes versicolor | Oxford, Ohio | Antimicrobial [26] | Water | MIC = 10 mg/mL against S. epidermidis (+++) |
| Trichaptum abietinum | Prince George, BC | Anti-proliferative [24] | 80% ethanol (a) and water (b) | 0.2 mg/mL (a) (++), 0.2 mg/mL (b) (+) |
| Trichaptum abietinum | Prince George, BC | Immuno-stimulatory (a), anti-inflammatory (b) [24] | 50% methanol (a) and 5% NaOH (b) | 1 mg/mL (a) (+++), 1 mg/mL (b) |
| Tricholomopsis rutilans | Haida Gwaii, BC | Anti-proliferative [23] | 80% ethanol | (++) |
| Tricholomopsis rutilans | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a), 50% methanol (b) and water (c) | 1 mg/mL (a–c) (+++) |
| Tyromyces chioneus | Haida Gwaii, BC | Anti-proliferative (a), immuno-stimulatory (b) [23] | 80% ethanol (a) and water (b) | (a) (+), 1 mg/mL (b) (++) |
| Tyromyces chioneus | Haida Gwaii, BC | Anti-inflammatory [23] | 80% ethanol (a) and m50% ethanol (b) | 1 mg/mL (a) (+++), 1 mg/mL (b) (++) |
| Urnula craterium | La Crosse, Wisconsin | Antimicrobial [41] | Urnucratin A (27) Urnucratin B (28) (++) Urnucratin C (29) (+++) | (27) MIC = 2 μg/mL (+) (methicillin-resistant S aureus), 1 μg/mL (vancomycin-resistant E. faecium), 0.5 μg/mL (S. pyogenes) |
| Types | Bioactive Compound | References | Mushrooms |
|---|---|---|---|
| Small molecules | Syringaldehyde (1) | [29] | E. granulatus |
| Syringic acid (2) | [29] | E. granulatus | |
| Grifolin (3) | [21,22] | A. flettii | |
| Neogrifolin (4) | [21,22] | A. flettii | |
| Confluentin (5) | [21,22] | A. flettii | |
| 3,11-Dioxolanosta-8,24(Z)-diene-26-oic acid 1 (6) | [21] | J. hirtus | |
| Erinacerin V 1 (7) | [17] | Hericium sp. | |
| 4-Hydroxy-2,2-dimethylchromane-6-carbaldehyde 1 (8) | [17] | Hericium sp. | |
| 4-Chloro-3,5-dimethoxybenzaldehyde (9) | [17] | Hericium sp. | |
| 2-Chloro-1,3-dimethoxy-5-methyl benzene (10) | [17] | Hericium sp. | |
| 4-Chloro-3,5-dimethoxyphenylmethanol (11) | [17] | Hericium sp. | |
| 3,6-Bis(hydroxyl methyl)-2-methyl-4H-pyran-4-one (12) | [17] | Hericium sp. | |
| 4-Chloro-3,5-dimethoxybenzoic acid (13) | [17] | Hericium sp. | |
| 5-Hydroxy-6-(1-hydroxyethyl)isobenzofuran-1(3H)-one (14) | Hericium sp. | ||
| Erinacine (15) | [17] | Hericium sp. | |
| Pentadecanoic acid (16), Hexadecanoic acid (17), Octadecadienoic acid (18), Octadecanoic acid (19) | [38] | P. djamar | |
| Orellanine (3,3′,4,4′-tetrahydroxy-2,2′-bipyridine-1,1′-dioxide) (20) | [19] | C. armillatus | |
| Ramariolide A 1 (21) | [50] | R. cystidiophora | |
| Ramariolide B 1 (22) | [50] | R. cystidiophora | |
| Ramariolide C 1 (23) | [50] | R. cystidiophora | |
| Ramariolide D 1 (24) | [50] | R. cystidiophora | |
| 2-Aminoquinoline (25) | [36] | L. albissimus | |
| Urnucratin A 1 (26) | [41] | U. craterium | |
| Urnucratin B 1 (27) | [41] | U. craterium | |
| Urnucratin C 1 (28) | [41] | U. craterium | |
| 3-Oxo-24-methyl-5α-lanost-8,25-dien-21-oic acid 1 (29) | [30] | F. pinicola | |
| Pinicolic acid (30) | [30] | F. pinicola | |
| Polyporenic acid (31) | [30] | F. pinicola | |
| 16α-Hydroxy-24-methylene-3-oxo-5α-lanost-8-ene-21-oic acid (32) | [30] | F. pinicola | |
| 16α-Acetyloxy-24-methylene-3-oxo-5α-lanost-7,9(11)-dien-21-oic acid (33) | [30] | F. pinicola | |
| 22E-5α-Ergost-7,9(11),22-trien-3β-ol (34) | [30] | F. pinicola | |
| Large molecules | GIPinv 1 | [37] | P. involutus |
| CDP 1 | [31,32] | G. dryophilus | |
| AlPetinc 1 | [18] | E. tinctorium | |
| CDP-like polysaccharide 1 | [31] | L. edodes | |
| CDP-like polysaccharide 1 | [31] | M. oreades | |
| EPS 1 | [52] | P. tuber-regium | |
| Y3 1 | [28] | C. comatus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeb, M.; Lee, C.H. Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules 2021, 26, 251. https://doi.org/10.3390/molecules26020251
Zeb M, Lee CH. Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules. 2021; 26(2):251. https://doi.org/10.3390/molecules26020251
Chicago/Turabian StyleZeb, Mehreen, and Chow H. Lee. 2021. "Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America" Molecules 26, no. 2: 251. https://doi.org/10.3390/molecules26020251
APA StyleZeb, M., & Lee, C. H. (2021). Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules, 26(2), 251. https://doi.org/10.3390/molecules26020251







