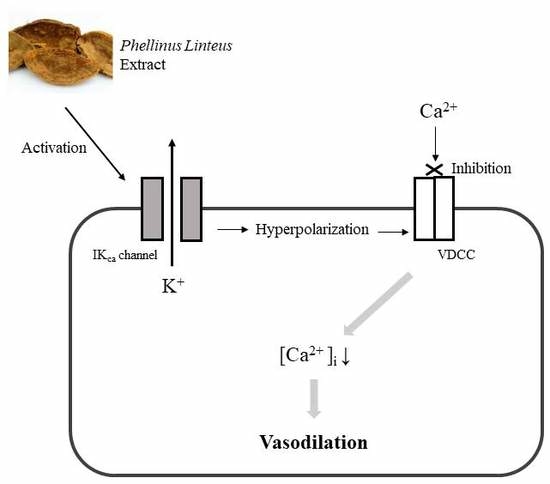
Vasodilatory Effect of Phellinus linteus Extract in Rat Mesenteric Arteries
Abstract
1. Introduction
2. Results
2.1. Gas Chromatograms of the Compounds in Phellinus linteus Extract
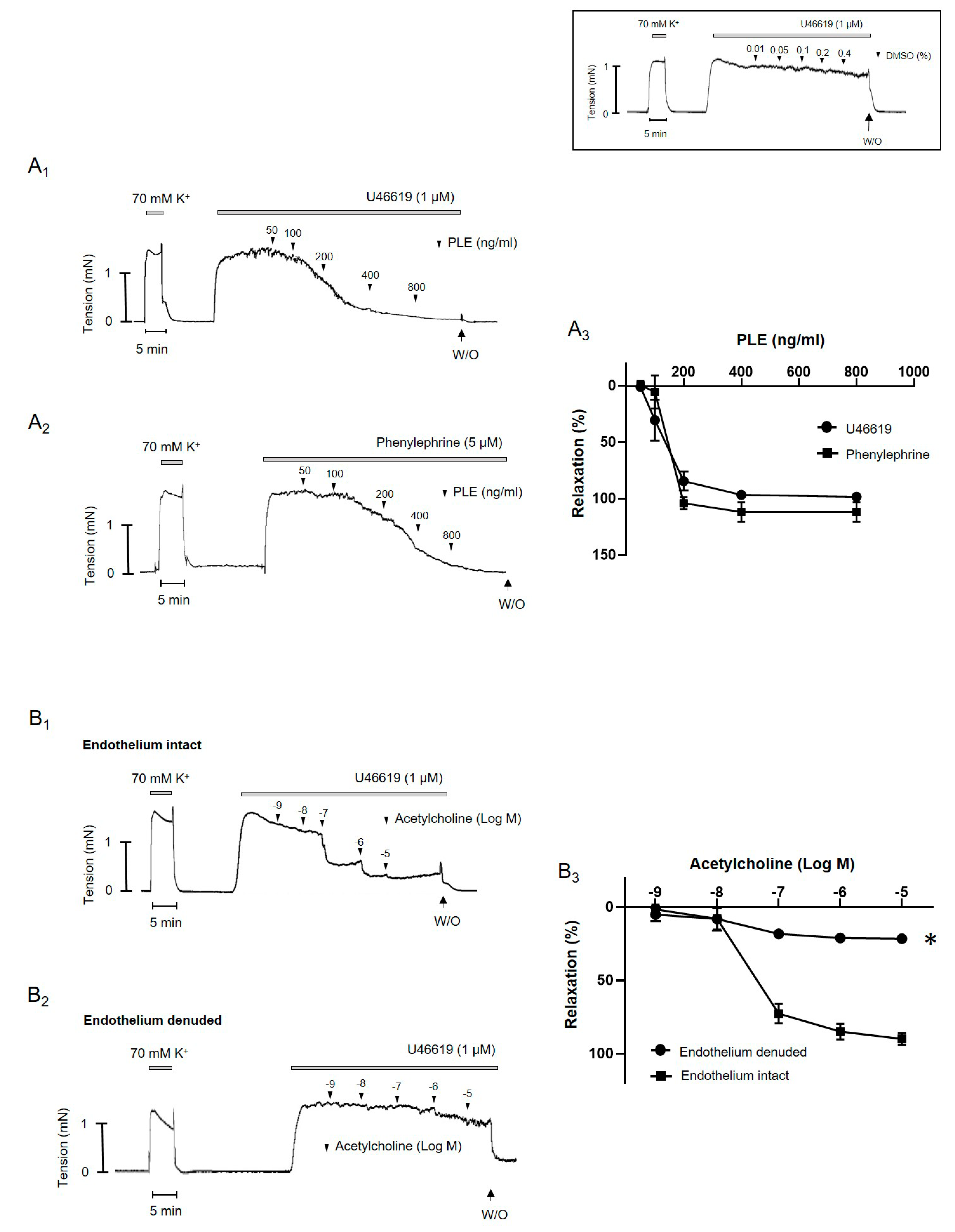
2.2. Effect of Phellinus linteus Extract on Agonist-Induced Contraction in Mesenteric Arteries of Rats
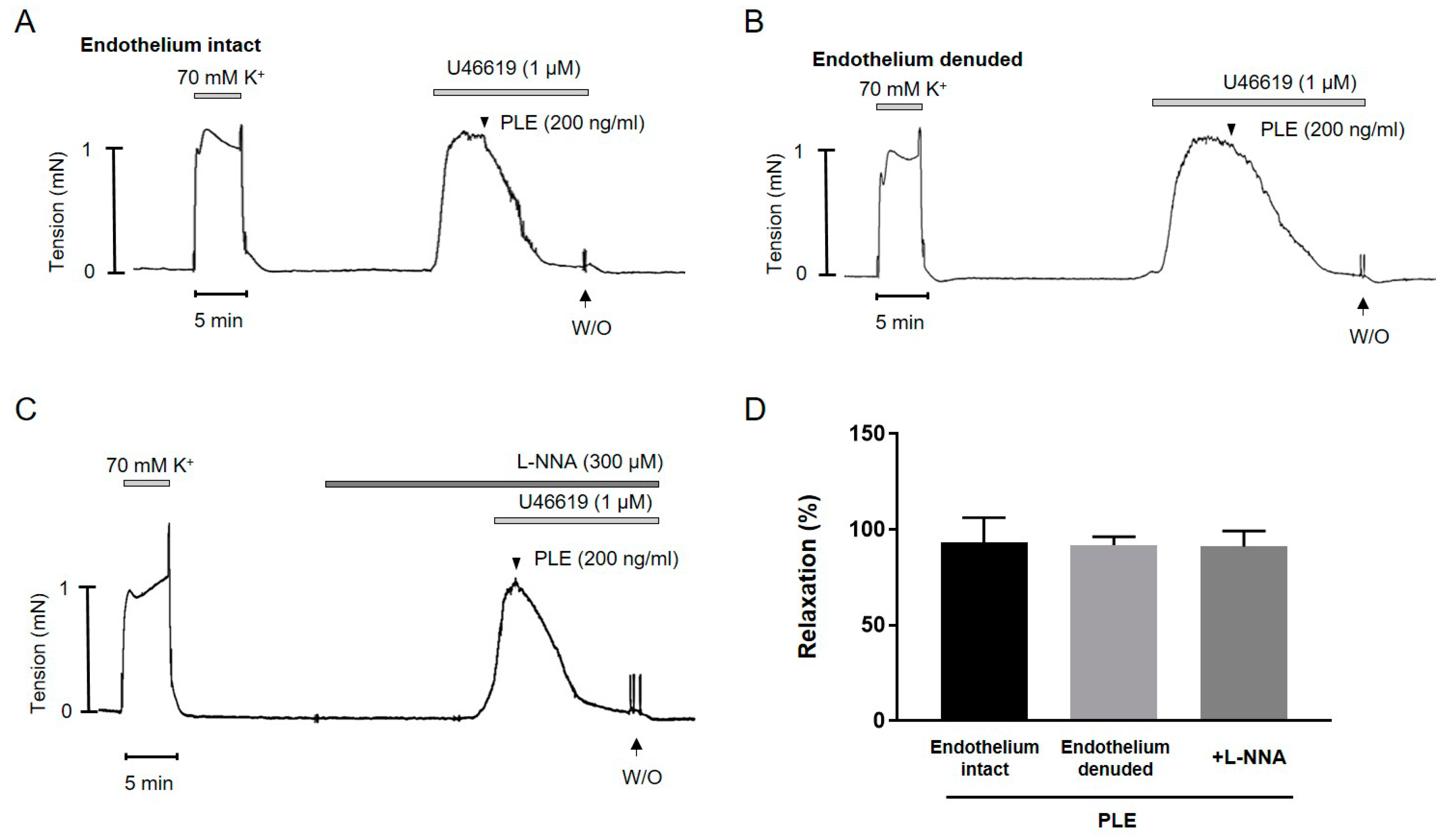
2.3. Phellinus linteus Extract-Induced Endothelium-Independent Relaxation
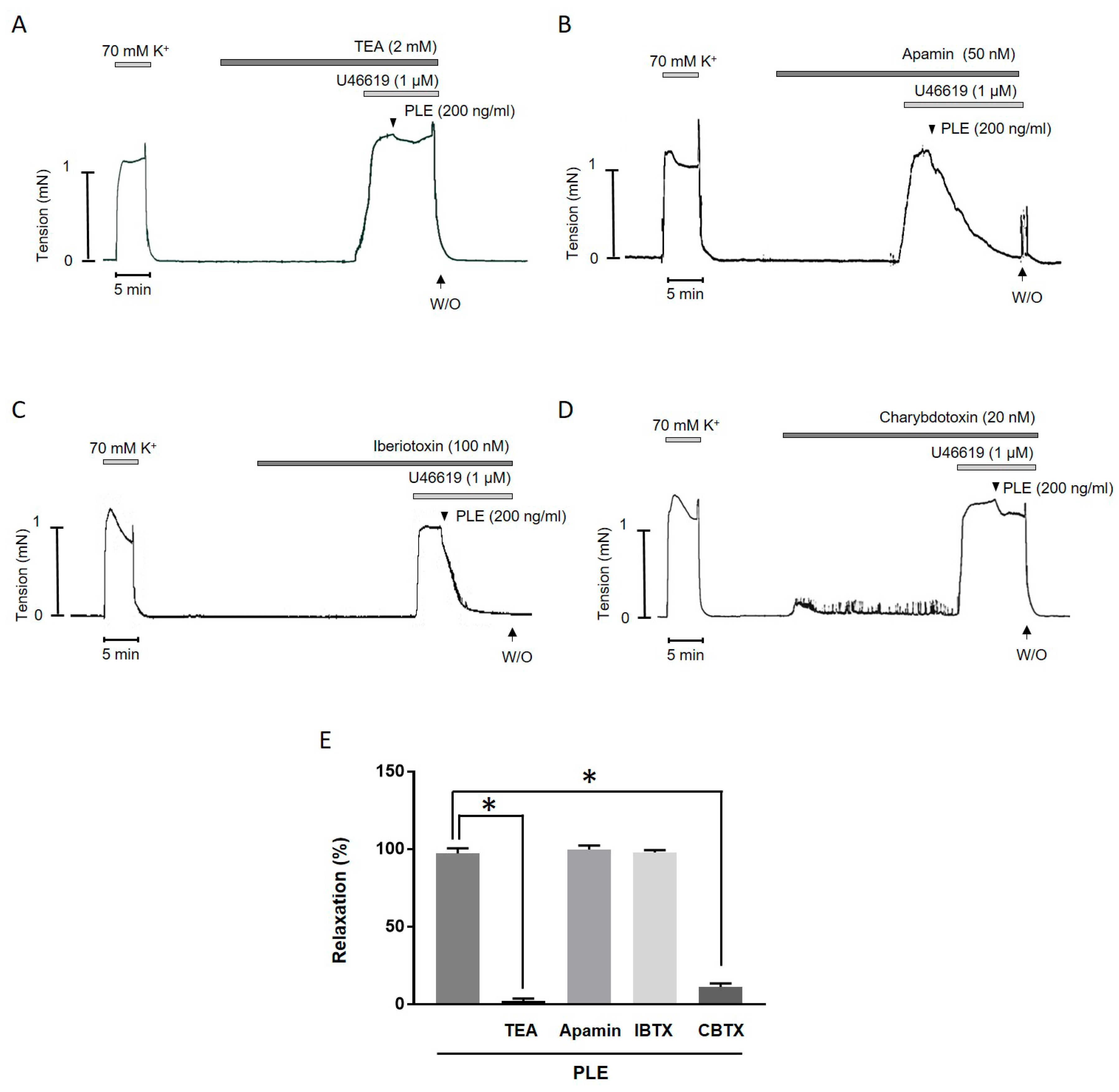
2.4. Inhibition of Phellinus linteus Extract-Induced Relaxation by K+ Channel Blockers
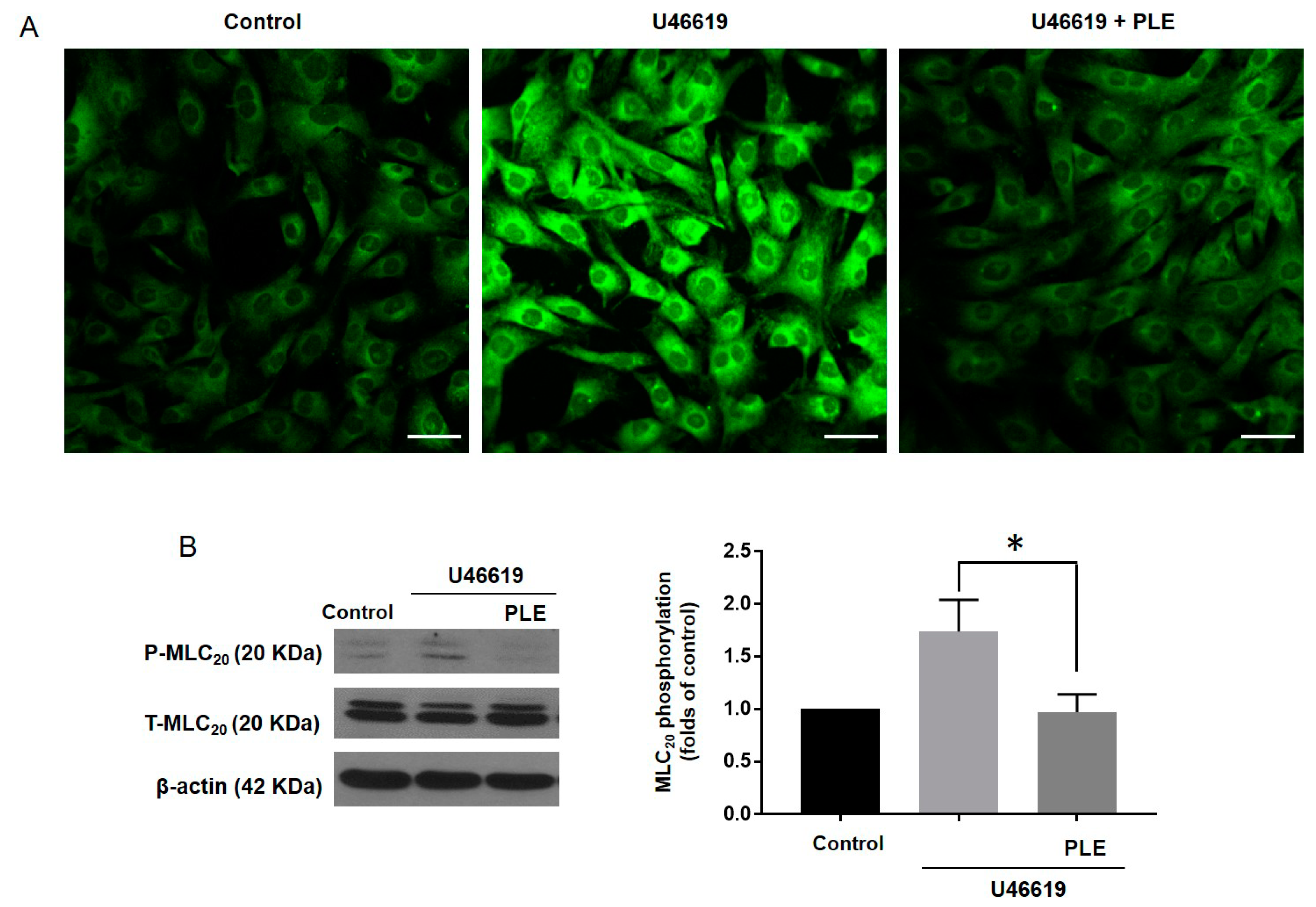
2.5. Effect of Phellinus linteus Extract on the Membrane Potential and Phosphorylation of 20 KDa Myosin Light Chain (MLC20)
3. Discussion
4. Methods
4.1. Phellinus linteus Extract Preparation
4.2. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis
4.3. Tissue Preparation
4.4. Isometric Tension Recording
4.5. Isolation and Culture of Vascular Smooth Muscle cells (VSMCs)
4.6. Western Blot Analysis
4.7. Determination of Membrane Potential of Vascular Smooth Muscle Cells by Confocal Microscopy
4.8. Drugs
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.; Reesink, K.D.; A Kroon, A.; Schurgers, L.J. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef]
- Brayden, J.E. POTASSIUM CHANNELS IN VASCULAR SMOOTH MUSCLE. Clin. Exp. Pharmacol. Physiol. 1996, 23, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.; Werner, M.E.; Brayden, J.E.; Nelson, M.T. Calcium-Activated Potassium Channels and the Regulation of Vascular Tone. Physiology 2006, 21, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, T.; Miao, H.; Zhao, Y.-Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia 2016, 113, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lin, Q.; Guo, T.; Yang, T.; Zhou, W.; Deng, X.; Yan, J.-K.; Luo, Y.; Ju, M.; Luo, F. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohydr. Polym. 2018, 200, 487–497. [Google Scholar] [CrossRef]
- Kim, B.-C.; Jeon, W.-K.; Hong, H.-Y.; Jeon, K.-B.; Hahn, J.-H.; Kim, Y.-M.; Numazawa, S.; Yosida, T.; Park, E.-H.; Lim, C.-J. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCδ/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J. Ethnopharmacol. 2007, 113, 240–247. [Google Scholar] [CrossRef]
- Mei, Y.; Zhu, H.; Hu, Q.; Liu, Y.; Zhao, S.; Peng, N.; Liang, Y. A novel polysaccharide from mycelia of cultured Phellinus linteus displays antitumor activity through apoptosis. Carbohydr. Polym. 2015, 124, 90–97. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, G.; Fan, L.; Zhao, M. A proteomic analysis of mushroom polysaccharide-treated HepG2 cells. Sci. Rep. 2016, 6, 23565. [Google Scholar] [CrossRef]
- Wang, H.; Wu, G.; Park, H.-J.; Jiang, P.P.; Sit, W.-H.; Van Griensven, L.J.; Wan, J.M. Protective effect of Phellinus linteus polysaccharide extracts against thioacetamide-induced liver fibrosis in rats: A proteomics analysis. Chin. Med. 2012, 7, 23. [Google Scholar] [CrossRef]
- Huang, S.C.; Wang, P.W.; Kuo, P.C.; Hung, H.Y.; Pan, T.L. Hepatoprotective Principles and Other Chemical Constituents from the Mycelium of Phellinus linteus. Molecules 2018, 23, 1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, J.S.; Kim, J.Y.; Park, S.-K.; Kim, H.S.; Lee, Y.J.; Yun, J.; Hong, J.T.; Kim, Y.; Han, S.-B. Evaluation of antidiabetic activity of polysaccharide isolated from Phellinus linteus in non-obese diabetic mouse. Int. Immunopharmacol. 2010, 10, 72–78. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, J.S.; Song, J.E.; Sim, Y.C.; Ha, S.-J.; Hong, E.K. Cytoprotective Effect of Hispidin against Palmitate-Induced Lipotoxicity in C2C12 Myotubes. Molecules 2015, 20, 5456–5467. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Cho, S.; Seo, J.Y.; Lee, H.B.; Park, Y.I. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutr. Res. 2016, 36, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Van Griensven, L.J.L.D.; Verhoeven, H.A. Phellinus linteus polysaccharide extracts increase the mitochondrial membrane potential and cause apoptotic death of THP-1 monocytes. Chin. Med. 2013, 8, 25. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, Y.H.; Shin, E.K.; Kim, D.H.; Lim, S.S.; Lee, J.-Y.; Kim, J.-K. Anti-angiogenic activity of methanol extract of Phellinus linteus and its fractions. J. Ethnopharmacol. 2010, 131, 56–62. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, S.-H.; Sa, J.-H.; Jin, C.; Lim, C.-J.; Park, E.-H. Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J. Ethnopharmacol. 2003, 88, 113–116. [Google Scholar] [CrossRef]
- Shon, Y.-H.; Nam, K.-S. Inhibition of cytochrome P450 isozymes in rat liver microsomes by polysaccharides derived from Phellinus linteus. Biotechnol. Lett. 2003, 25, 167–172. [Google Scholar] [CrossRef]
- Fan, G.; Jian, D.; Sun, M.; Zhan, Y.; Sun, F. Endogenous and Exogenous Calcium Involved in the Betulin Production from Submerged Culture of Phellinus linteus Induced by Hydrogen Sulfide. Appl. Biochem. Biotechnol. 2015, 178, 594–603. [Google Scholar] [CrossRef]
- Nam, S.W.; Baek, J.T.; Kang, S.B.; Lee, D.S.; Kim, J.I.; Cho, S.H.; Park, S.-H.; Han, J.-Y.; Ahn, B.M.; Kim, J.K.; et al. A case of the hepatocellular carcinoma during the pregnancy and metastasis to the left atrium. Korean J. Hepatol. 2005, 11, 381–385. [Google Scholar]
- Liu, Y.; Wang, C.; Li, J.; Mei, Y.; Liang, Y. Hypoglycemic and Hypolipidemic Effects of Phellinus Linteus Mycelial Extract from Solid-State Culture in A Rat Model of Type 2 Diabetes. Nutrients 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Pomposiello, S.I.; Alva, M.; Wilde, D.W.; Carretero, O.A. Linoleic acid induces relaxation and hyperpolarization of the pig coronary artery. Hypertension 1998, 31, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv. Pharmacol. 2016, 78, 89–144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wen, J.; Wang, N.; Wang, C.; Xu, Q.; Yang, Y. Ion Channels and Vascular Diseases. Arter. Thromb. Vasc. Boil. 2019, 39, e146–e156. [Google Scholar] [CrossRef]
- Kshatri, A.; González-Hernández, A.J.; Giraldez, T. Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System. Front. Mol. Neurosci. 2018, 11, 258. [Google Scholar] [CrossRef]
- Bi, D.; Toyama, K.; Lemaître, V.; Takai, J.; Fan, F.; Jenkins, D.P.; Wulff, H.; Gutterman, D.D.; Park, F.; Miura, H. The Intermediate Conductance Calcium-activated Potassium Channel KCa3.1 Regulates Vascular Smooth Muscle Cell Proliferation via Controlling Calcium-dependent Signaling. J. Biol. Chem. 2013, 288, 15843–15853. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Kroigaard, C.; Misfeldt, M.; Bek, T.; Simonsen, U. Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br. J. Pharmacol. 2010, 160, 1496–1508. [Google Scholar] [CrossRef]
- Hermann, A.; Erxleben, C. Charybdotoxin selectively blocks small Ca-activated K channels in Aplysia neurons. J. Gen. Physiol. 1987, 90, 27–47. [Google Scholar] [CrossRef]
- Sánchez-Carranza, O.; Torres-Rodríguez, P.; Darszon, A.; Treviño, C.L.; López-González, I. Pharmacology of hSlo3 channels and their contribution in the capacitation-associated hyperpolarization of human sperm. Biochem. Biophys. Res. Commun. 2015, 466, 554–559. [Google Scholar] [CrossRef]
- Logsdon, N.J.; Kang, J.; Togo, J.A.; Christian, E.P.; Aiyar, J. A Novel Gene, hKCa4, Encodes the Calcium-activated Potassium Channel in Human T Lymphocytes. J. Boil. Chem. 1997, 272, 32723–32726. [Google Scholar] [CrossRef]
- Anderson, A.J.; Harvey, A.L.; Rowan, E.G.; Strong, P.N. Effects of charybdotoxin, a blocker of Ca2+ -activated K + channels, on motor nerve terminals. Br. J. Pharmacol. 1988, 95, 1329–1335. [Google Scholar] [CrossRef]
- Ip, K.; Sobieszek, A.; Solomon, D.; Jiao, Y.; Paré, P.; Seow, C. Physical Integrity of Smooth Muscle Myosin Filaments is Enhanced by Phosphorylation of the Regulatory Myosin Light Chain. Cell. Physiol. Biochem. 2007, 20, 649–658. [Google Scholar] [CrossRef]
- Deng, M.; Ding, W.; Min, X.; Xia, Y. MLCK-independent phosphorylation of MLC20 and its regulation by MAP kinase pathway in human bladder smooth muscle cells. Cytoskeleton 2010, 68, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, G.M.; Tao, T.; Wei, L.S.; Pan, Y.; Zhu, M.S. Isometric Contractility Measurement of the Mouse Mesenteric Artery Using Wire Myography. J. Vis. Exp. 2018, 20, e58064. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Kwon, Y.; Byeon, S.; Haam, C.E.; Lee, Y.-H. AdipoRon, adiponectin receptor agonist, improves vascular function in the mesenteric arteries of type 2 diabetic mice. PLoS ONE 2020, 15, e0230227. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Ahn, D.S.; Lee, Y.-H. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc. Res. 2008, 82, 324–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
Sample Availability: Not available. |
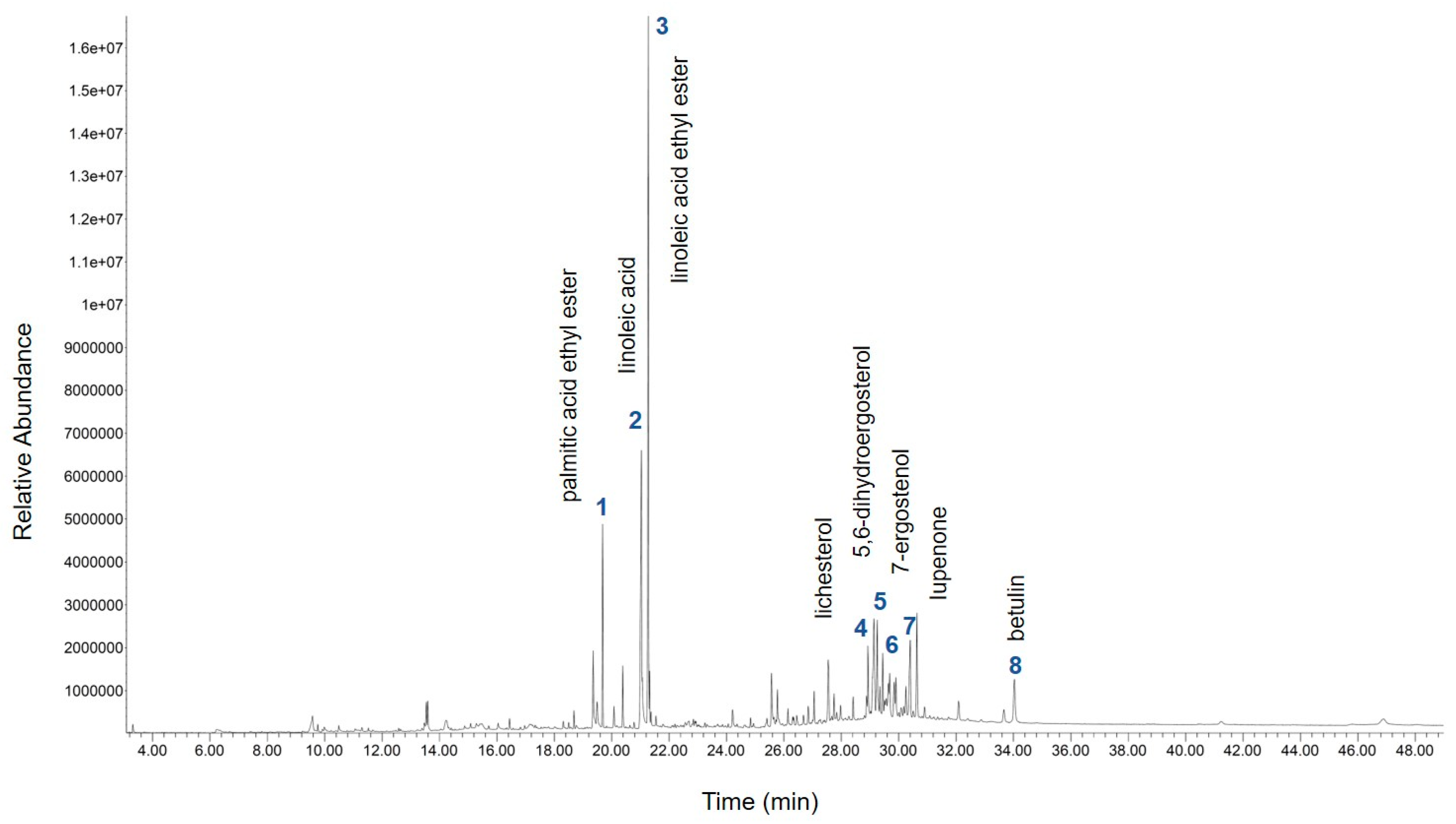
| PK | RT (min) | Tentative Compounds | Total (%) |
|---|---|---|---|
| 1 | 19.68 | palmitic acid ethyl ester | 3.48 |
| 2 | 21.03 | linoleic acid | 10.39 |
| 3 | 21.27 | linoleic acid ethyl ester | 15.86 |
| 4 | 28.93 | lichesterol | 2.59 |
| 5 | 29.25 | 5,6-dihydroergosterol | 3.26 |
| 6 | 29.69 | 7-ergostenol | 2.80 |
| 7 | 30.40 | lupenone | 3.24 |
| 8 | 34.03 | betulin | 2.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.; Haam, C.E.; Byeon, S.; Choi, S.J.; Shin, D.-H.; Choi, S.-K.; Lee, Y.-H. Vasodilatory Effect of Phellinus linteus Extract in Rat Mesenteric Arteries. Molecules 2020, 25, 3160. https://doi.org/10.3390/molecules25143160
Kwon Y, Haam CE, Byeon S, Choi SJ, Shin D-H, Choi S-K, Lee Y-H. Vasodilatory Effect of Phellinus linteus Extract in Rat Mesenteric Arteries. Molecules. 2020; 25(14):3160. https://doi.org/10.3390/molecules25143160
Chicago/Turabian StyleKwon, Youngin, Chae Eun Haam, Seonhee Byeon, Soo Jung Choi, Dong-Hoon Shin, Soo-Kyoung Choi, and Young-Ho Lee. 2020. "Vasodilatory Effect of Phellinus linteus Extract in Rat Mesenteric Arteries" Molecules 25, no. 14: 3160. https://doi.org/10.3390/molecules25143160
APA StyleKwon, Y., Haam, C. E., Byeon, S., Choi, S. J., Shin, D.-H., Choi, S.-K., & Lee, Y.-H. (2020). Vasodilatory Effect of Phellinus linteus Extract in Rat Mesenteric Arteries. Molecules, 25(14), 3160. https://doi.org/10.3390/molecules25143160