Magnesium and Hypertension in Old Age
Abstract
1. Introduction
2. Magnesium Metabolism, Dietary Sources, and Requirements
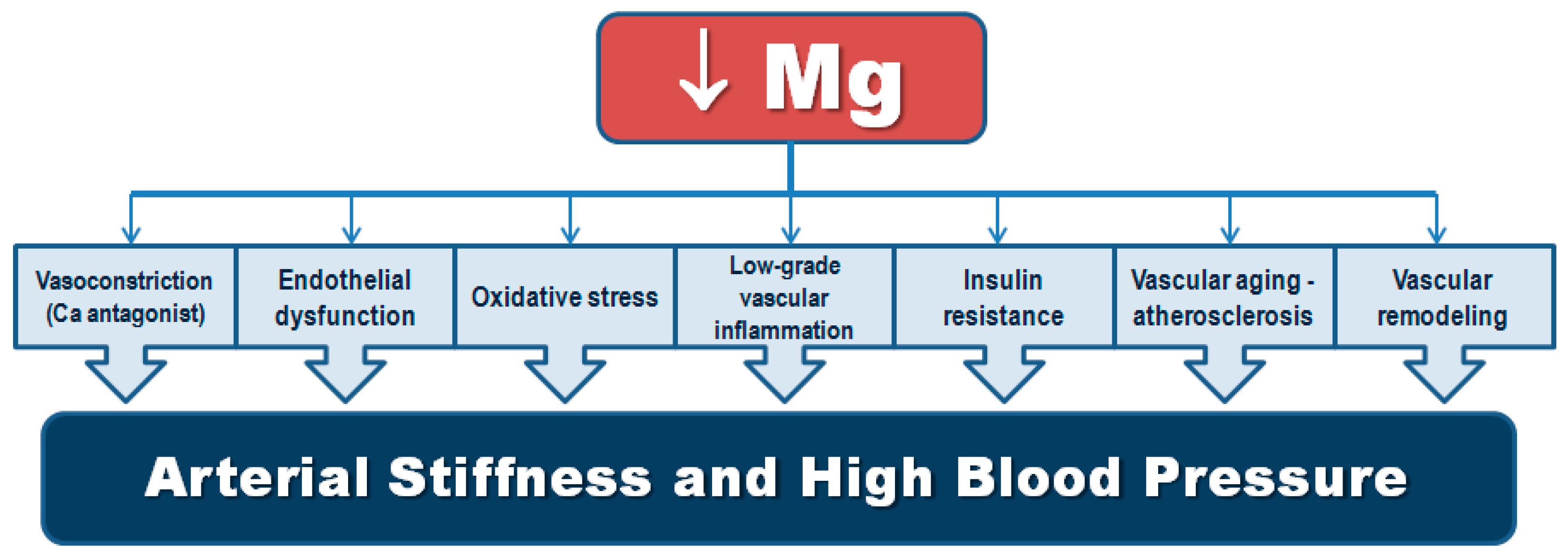
3. Mechanistic Insights on the Relationship of Magnesium and Hypertension
3.1. Regulation of Vascular Tone and Contraction
3.1.1. Magnesium as a Calcium Antagonist
3.1.2. Magnesium and Endothelial Function
3.1.3. Magnesium and the Renin-Angiotensin-Aldosterone System (RAAS)
3.1.4. Magnesium and Catecholamines
3.1.5. Magnesium and Vascular Calcification
3.2. Magnesium, Insulin Action, Diabetes, and Cardiometabolic Syndrome
3.3. Magnesium, Oxidative Stress and Chronic Inflammation
4. Hypertension in Old Age and Magnesium Deficit—Two Frequent Coexisting Conditions
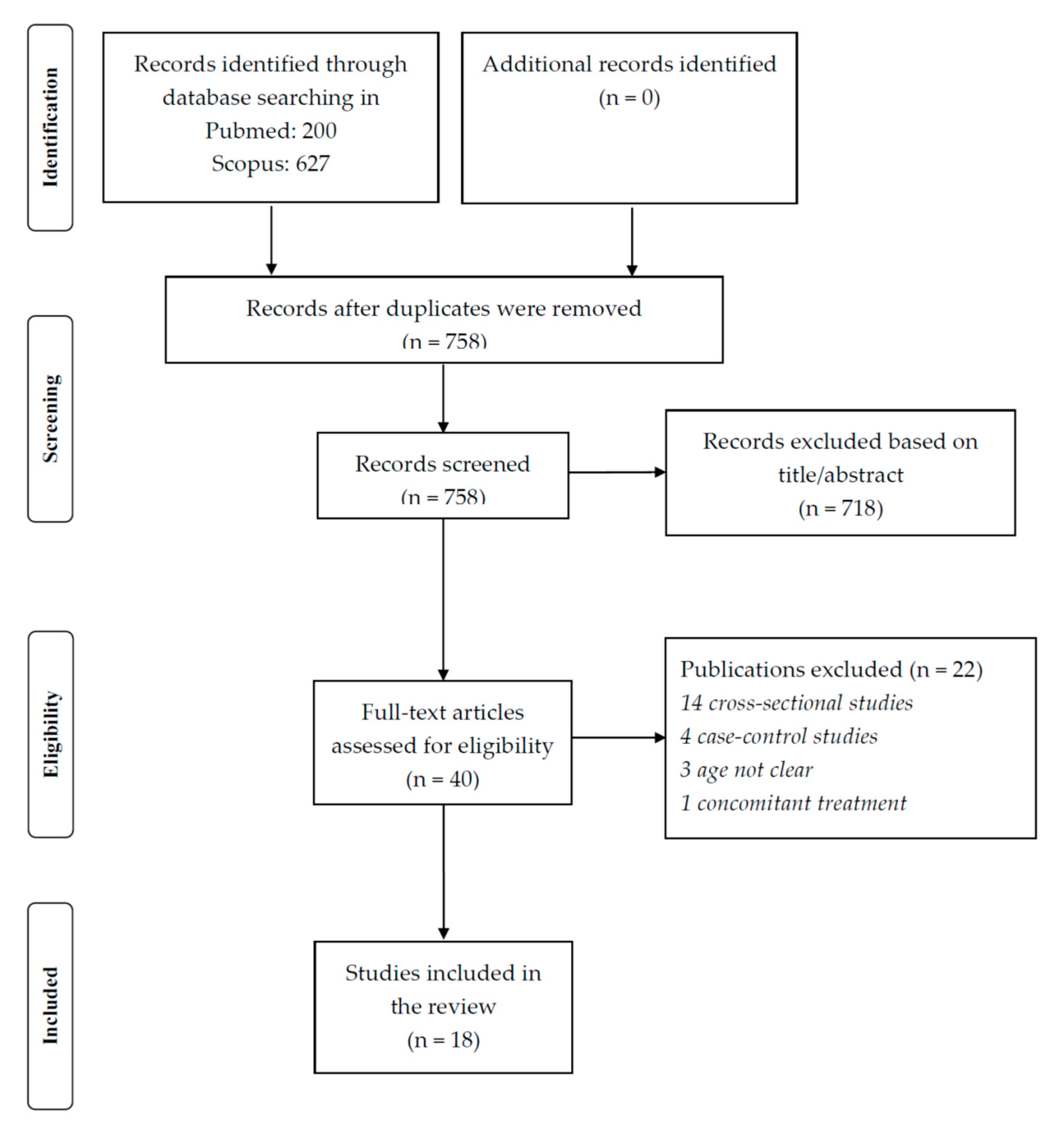
5. Methods
6. Available Evidence of the Effects of Dietary and Supplemental Magnesium on Hypertension
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ebel, H.; Günther, T.; Günther, H.E.T. Magnesium metabolism: A review. Clin. Chem. Lab. Med. 1980, 18, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Altman, T.; Dreher, K.; Fulcher, C.A.; Subhraveti, P.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The metacyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2011, 40, D742–D753. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo’, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Blackfan, K.; Hamilton, B. Uremia in acute glomerular nephritis: The cause and treatment in children. Med. Surg. J. 1925, 193, 617–628. [Google Scholar]
- Resnick, L.M.; Laragh, J.H.; Sealey, J.E.; Alderman, M.H. Divalent cations in essential hypertension. relations between serum ionized calcium, magnesium, and plasma renin activity. N. Engl. J. Med. 1983, 309, 888–891. [Google Scholar] [CrossRef]
- Resnick, L.M.; Gupta, R.K.; Laragh, J.H. Intracellular free magnesium in erythrocytes of essential hypertension: Relation to blood pressure and serum divalent cations. Proc. Natl. Acad. Sci. USA 1984, 81, 6511–6515. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Resnick, L.M. Magnesium metabolism in hypertension and type 2 diabetes mellitus. Am. J. Ther. 2007, 14, 375–385. [Google Scholar] [CrossRef]
- Villa-Bellosta, R. Impact of magnesium: Calcium ratio on calcification of the aortic wall. PLoS ONE 2017, 12, e0178872. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.; Ligia, J.; Galioto, A.; Pineo, A.; Belvedere, M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes. Res. 2010, 23, 131–137. [Google Scholar]
- Shechter, M.; Sharir, M.; Labrador, M.J.P.; Forrester, J.; Silver, B.; Merz, C.N.B. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef]
- Song, Y.; Li, T.Y.; Van Dam, R.M.; Manson, J.E.; Hu, F.B. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am. J. Clin. Nutr. 2007, 85, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Resnick, L.M.; Militianu, D.; Cunnings, A.J.; Pipe, J.G.; Evelhoch, J.L.; Soulen, R.L. Direct magnetic resonance determination of aortic distensibility in essential hypertension: Relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension 1997, 30, 654–659. [Google Scholar] [CrossRef]
- Soave, P.; Conti, G.; Costa, R.; Arcangeli, A. Magnesium and anaesthesia. Curr. Drug Targets 2009, 10, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes Res. 2009, 22, 235–246. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Ellis, T. Magnesium intake and serum C-reactive protein levels in children. Magnes. Res. 2007, 20, 32–36. [Google Scholar]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global burden of hypertension and systolic blood pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory (GHO) Data. Blood Pressure. 2018. Available online: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence/en/ (accessed on 26 October 2020).
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.-P.; Hering, D.; et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; Song, Y.; Rosanoff, A.; Shechter, M.; He, K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Gobbo, D.; Liana, C.; Zhang, W.; Rosanoff, A.; Wang, J.; Song, Y. Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.S.; Weekes, J.; Carpenter, L.W. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The effect of electrolytes on blood pressure: A brief summary of meta-analyses. Nutrients 2019, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H. Dietary magnesium intake in a national sample of U.S. adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Rosanoff, A.; Dai, Q.; Shapses, S.A. Essential nutrient interactions: Does low or suboptimal magnesium status interact with vitamin D and/or calcium status? Adv. Nutr. 2016, 7, 25–43. [Google Scholar] [CrossRef]
- National Institutes of Health, Magnesium, National Institutes of Health, Bethesda, Maryland, USA, 2018. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 24 October 2020).
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2019, 59, 263–272. [Google Scholar] [CrossRef]
- Department of Health and Human Services. US Department of Agriculture (2015) 2015–2020 Dietary Guidelines for Americans, 8th ed.; Department of Health and Human Services: Washington, DC, USA, 2020.
- Van Leer, E.M.; Seidell, J.C.; Kromhout, D. Dietary calcium, potassium, magnesium and blood pressure in the Netherlands. Int. J. Epidemiol. 1995, 24, 1117–1123. [Google Scholar] [CrossRef]
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The aric study. J. Clin. Epidemiol. 1995, 48, 927–940. [Google Scholar] [CrossRef]
- Kesteloot, H.; Joossens, J.V. Relationship of dietary sodium, potassium, calcium, and magnesium with blood pressure. Belgian interuniversity research on nutrition and health. Hypertension 1988, 12, 594–599. [Google Scholar] [CrossRef]
- Witteman, J.C.; Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Sacks, F.M.; Speizer, F.E.; Rosner, B.; Hennekens, C.H. A prospective study of nutritional factors and hypertension among US women. Circulation 1989, 80, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Hennekens, C.; Willett, W.C.; Sacks, F.; Rosner, B.; Manson, J.; Witteman, J.; Stampfer, M.J. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996, 27, 1065–1072. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.L.; Colditz, G.A.; Rosner, B.A.; Willett, W.C.; Sacks, F.; Stampfer, M.J. A prospective study of nutritional factors and hypertension among US men. Circulation 1992, 86, 1475–1484. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 113, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sesso, H.D.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am. J. Cardiol. 2006, 98, 1616–1621. [Google Scholar] [CrossRef]
- Peacock, J.M.; Folsom, A.R.; Arnett, D.K.; Eckfeldt, J.H.; Szklo, M. Relationship of serum and dietary magnesium to incident hypertension: The Atherosclerosis Risk in Communities (ARIC) Study. Ann. Epidemiol. 1999, 9, 159–165. [Google Scholar] [CrossRef]
- Charlton, E.; Steyn, K.; Levitt, N.S.; Zulu, J.V.; Jonathan, D.; Veldman, F.J.; Nel, J.H. Diet and blood pressure in South Africa: Intake of foods containing sodium, potassium, calcium, and magnesium in three ethnic groups. Nutrition 2005, 21, 39–50. [Google Scholar] [CrossRef]
- Townsend, M.S.; Fulgoni, V.L., 3rd; Stern, J.S.; Adu-Afarwuah, S.; McCarron, D.A. Low mineral intake is associated with high systolic blood pressure in the Third and Fourth National Health and Nutrition Examination Surveys: Could we all be right? Am. J. Hypertens. 2005, 18, 261–269. [Google Scholar] [CrossRef][Green Version]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Miller, E.R., 3rd; Guallar, E.; Singh, V.K.; Appel, L.J.; Klag, M.J. The effect of magnesium supplementation on blood pressure: A meta-analysis of randomized clinical trials. Am. J. Hypertens. 2002, 15, 691–696. [Google Scholar] [CrossRef]
- Quamme, G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008, 24, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Shils, M.E. Experimental production of magnesium deficiency in man*. Ann. N. Y. Acad. Sci. 1969, 162, 847–855. [Google Scholar] [CrossRef]
- Quamme, G.A. Renal magnesium handling: New insights in understanding old problems. Kidney Int. 1997, 52, 1180–1195. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 2007, 458, 40–47. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; Sui, Z.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: Evidence from a nationally representative cross-sectional study. BMJ Open 2019, 9, e029544. [Google Scholar] [CrossRef]
- Martinez Steele, E.; Baraldi, L.G.; Louzada, M.L.; Moubarac, J.C.; Mozafarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, e009892. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diaseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 26 October 2020).
- Rico-Campà, A.; AMartínez-González, M.; Alvarez-Alvarez, I.; Mendonça, R.D.D.; De La Fuente-Arrillaga, C.; Gómez-Donoso, C.; Bes-Rastrollo, M. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 2019, 365, l1949. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; AHu, E.; Rebholz, C.M. Ultra-processed food intake and mortality in the USA: Results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. 2019, 22, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Eicher-Miller, H.A.; Fulgoni, V.L., III; Keast, D.R. Contributions of processed foods to dietary intake in the US from 2003–2008: A report of the food and nutrition science solutions joint task force of the academy of nutrition and dietetics, american society for nutrition, institute of food technologists, and international food information council. J. Nutr. 2012, 142, 2065–2072. [Google Scholar]
- Slimani, N.; Deharveng, G.; Southgate, D.A.T.; Biessy, C.; Chajès, V.; Van Bakel, M.M.E.; Boutron-Ruault, M.C.; McTaggart, A.; Grioni, S.; Verkaik-Kloosterman, J.; et al. Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European Prospective Investigation into Cancer and Nutrition study. Eur. J. Clin. Nutr. 2009, 63, S206–S225. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Bes-Rastrollo, M.; Román-Viñas, B.; Pfrimer, K.; Sánchez-Villegas, A.; Martínez-González, M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009, 101, S21–S28. [Google Scholar] [CrossRef]
- Altura, B.M. Magnesium ions and contraction of vascular smooth muscles: Relationship to some vascular diseases. Fed. Proc. 1981, 40, 2672–2679. [Google Scholar]
- Altura, B.M.; Gebrewold, A.; Ising, H.; Gunther, T. Magnesium deficiency and hypertension: Correlation between magnesium-deficient diets and microcirculatory changes in situ. Science 1984, 223, 1315–1317. [Google Scholar] [CrossRef]
- Turlapaty, P.; Altura, B. Magnesium deficiency produces spasms of coronary arteries: Relationship to etiology of sudden death ischemic heart disease. Science 1980, 208, 198–200. [Google Scholar] [CrossRef]
- Machado, A.R.D.C.; Umbelino, B.; Correia, M.L.; Neves, M.F. Magnesium and vascular changes in hypertension. Int. J. Hypertens. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Agus, Z.S.; Kelepouris, E.; Dukes, I.; Morad, M. Cytosolic magnesium modulates calcium channel activity in mammalian ventricular cells. Am. J. Physiol. Physiol. 1989, 256, C452–C455. [Google Scholar] [CrossRef] [PubMed]
- Louvet, L.; Bazin, D.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS ONE 2015, 10, e0115342. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014, 22, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The Role of Magnesium in Hypertension and Cardiovascular Disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef]
- Belin, R.J.; He, K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes. Res. 2007, 20, 107–129. [Google Scholar]
- Altura, B.M.; Altura, B.T. Cardiovascular risk factors and magnesium: Relationships to atherosclerosis, ischemic heart disease and hypertension. Magnes. Trace Elements 1991, 10, 182–192. [Google Scholar]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Satake, K.; Lee, J.-D.; Shimizu, H.; Uzui, H.; Mitsuke, Y.; Yue, H.; Ueda, T. Effects of magnesium on prostacyclin synthesis and intracellular free calcium concentration in vascular cells. Magnes. Res. 2004, 17, 20–27. [Google Scholar]
- Soltani, N.; Keshavarz, M.; Sohanaki, H.; Asl, S.Z.; Dehpour, A.R. Relaxatory effect of magnesium on mesenteric vascular beds differs from normal and streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2005, 508, 177–181. [Google Scholar] [CrossRef]
- Laurant, P.; Berthelot, A. Endothelin-1-induced contraction in isolated aortae from normotensive and DOCA-salt hypertensive rats: Effect of magnesium. Br. J. Pharmacol. 1996, 119, 1367–1374. [Google Scholar] [CrossRef]
- Ferrè, S.; Baldoli, E.; Leidi, M.; Maier, J.A. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2011, 122, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.C.A.A.; Klein, M.R.S.T.; Da Cunha, M.R.; Mattos, S.D.S.; Nogueira, L.D.P.; De Paula, T.; Corrêa, F.M.; Oigman, W.; Neves, M.F. Effects of Oral Magnesium Supplementation on Vascular Function: A Systematic Review and Meta-analysis of Randomized Controlled Trials. High Blood Press. Cardiovasc. Prev. 2019, 27, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Laurant, P.; Dalle, M.; Berthelot, A.; Rayssiguier, Y. Time-course of the change in blood pressure level in magnesium-deficient Wistar rats. Br. J. Nutr. 1999, 82, 243–251. [Google Scholar] [CrossRef]
- Cantin, M. Relationship of juxtaglomerular apparatus and adrenal cortex to biochemical and extracellular fluid volume changes in magnesium deficiency. Lab. Investig. 1970, 22, 558–568. [Google Scholar]
- Nadler, J.L.; Buchanan, T.; Natarajan, R.; Antonipillai, I.; Bergman, R.; Rude, R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension 1993, 21, 1024–1029. [Google Scholar] [CrossRef]
- DeLalio, L.J.; Sved, A.F.; Stocker, S.D. Sympathetic nervous system contributions to hypertension: Updates and therapeutic relevance. Can. J. Cardiol. 2020, 36, 712–720. [Google Scholar] [CrossRef]
- James, M.M.F.M. Use of magnesium sulphate in the anaesthetic management of phaeochromocytoma: A review of 17 anaesthetics. Br. J. Anaesth. 1989, 62, 616–623. [Google Scholar] [CrossRef]
- James, M.F.M.; Cronje, L. Pheochromocytoma Crisis: The Use of Magnesium Sulfate. Anesthesia Analg. 2004, 99, 680–686. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.; Beer, R.E.; Esser, J.D. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth. Analg. 1989, 68, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Torshin, I.I.; Gromova, O.A.; Gusev, E.I. Mechanisms of antistress and antidepressive effects of magnesium and pyridoxine. Zhurnal Nevrol. i psikhiatrii im. S.S. Korsakova 2009, 109, 107–111. [Google Scholar]
- Caddell, J.; Kupiecki, R.; Proxmire, D.L.; Satoh, P.; Hutchinson, B. Plasma catecholamines in acute magnesium deficiency in weanling rats. J. Nutr. 1986, 116, 1896–1901. [Google Scholar] [CrossRef]
- Shimosawa, T.; Takano, K.; Ando, K.; Fujita, T. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension 2004, 44, 897–902. [Google Scholar] [CrossRef]
- Greenwood, J.; Nygard, B.; Brickey, D. Effectiveness of intravenous magnesium sulfate to attenuate hemodynamic changes in laparoscopic surgery: A systematic review and meta-analysis. JBI Evid. Synth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.S.; Shanahan, C.M. Vascular calcification and hypertension: Cause and effect. Ann. Med. 2012, 44, S85–S92. [Google Scholar] [CrossRef]
- Gorgels, T.G.M.F.; Waarsing, J.H.; De Wolf, A.; Brink, J.B.T.; Loves, W.J.P.; Bergen, A.A.B. Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med. 2010, 88, 467–475. [Google Scholar] [CrossRef]
- Turgut, F.H.; Kanbay, M.; Metin, M.R.; Uz, E.; Akcay, A.; Covic, A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int. Urol. Nephrol. 2008, 40, 1075–1082. [Google Scholar] [CrossRef]
- Louvet, L.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transplant. 2012, 28, 869–878. [Google Scholar] [CrossRef]
- Kircelli, F.; Peter, M.E.; Ok, E.S.; Celenk, F.G.; Yilmaz, M.; Steppan, S.; Asci, G.; Passlick-Deetjen, J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol. Dial. Transplant. 2011, 27, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, A.; Guerrero, F.; Martinez-Moreno, J.M.; Madueno, J.A.; Herencia, C.; Peralta, A.; Almaden, Y.; Lopez, I.; Aguilera-Tejero, E.; Gundlach, K.; et al. Magnesium inhibits Wnt/beta-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS ONE 2014, 9, e89525. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; O’Donnell, C.J.; Jacques, P.F.; Meigs, J.B.; Hoffmann, U.; McKeown, N.M. Magnesium intake is inversely associated with coronary artery calcification: The Framingham Heart Study. JACC Cardiovasc. Imaging 2014, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Bangalore, S.; Benetos, A.; Davis, A.M.; Michos, E.D.; Muntner, P.; Rossing, P.; Zoungas, S.; Bakris, G. Diabetes and hypertension: A position statement by the american diabetes association. Diabetes Care 2017, 40, 1273–1284. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Gonzalez-Villalpando, C.; Meigs, J.B.; Ferrannini, E. Hypertension and diabetes mellitus: Coprediction and time trajectories. Hypertension 2018, 71, 422–428. [Google Scholar] [CrossRef]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L. Ligia, J. Magnesium intake in the pathophysiology and treatment of the cardiometabolic syndrome: Where are we in 2006? J. CardioMetabolic Syndr. 2006, 1, 356–357. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol. 2002, 39, 209–213. [Google Scholar] [CrossRef]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium Intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older, U.S. Women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef]
- Corica, F.; Corsonello, C.P.A.R.A.I.A.; Ientile, R.; Cucinotta, D.; Di Benedetto, A.; Perticone, F.; Dominguez, L.J.; Barbagallo, M. Serum Ionized Magnesium Levels in Relation to Metabolic Syndrome in Type 2 Diabetic Patients. J. Am. Coll. Nutr. 2006, 25, 210–215. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes. 2015, 6, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Mather, H.; Levin, G. Magnesium status in diabetes. Lancet 1979, 313, 924. [Google Scholar] [CrossRef]
- Schnack, C.; Bauer, I.; Pregant, P.; Hopmeier, P.; Schernthaner, G. Hypomagnesaemia in Type 2 (non-insulin-dependent) diabetes mellitus is not corrected by improvement of long-term metabolic control. Diabetologia 1992, 35, 77–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Resnick, L.M.; Barbagallo, M.; Gupta, R.K.; Laragh, J.H. Ionic basis of hypertension in diabetes mellitus. Role of hyperglycemia. Am. J. Hypertens. 1993, 6, 413–417. [Google Scholar] [CrossRef]
- Resnick, L.M.; Altura, B.T.; Gupta, R.K.; Laragh, J.H.; Alderman, M.H. Intracellular and extracellular magnesium depletion in Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993, 36, 767–770. [Google Scholar] [CrossRef]
- Barbagallo, M.; Di Bella, G.; Brucato, V.; D’Angelo, D.; Damiani, P.; Monteverde, A.; Belvedere, M.; Dominguez, L.J. Serum ionized magnesium in diabetic older persons. Metabolism 2014, 63, 502–509. [Google Scholar] [CrossRef]
- Wälti, M.K.; Zimmermann, M.B.; Walczyk, T.; Spinas, G.A.; Hurrell, R.F. Measurement of magnesium absorption and retention in type 2 diabetic patients with the use of stable isotopes. Am. J. Clin. Nutr. 2003, 78, 448–453. [Google Scholar] [CrossRef]
- McNair, P.; Christensen, M.S.; Christiansen, C.; Madsbad, S.; Transbøl, I. Renal hypomagnesaemia in human diabetes mellitus: Its relation to glucose homeostasis. Eur. J. Clin. Investig. 1982, 12, 81–85. [Google Scholar] [CrossRef]
- Djurhuus, M.; Skøtt, P.; Hother-Nielsen, O.; Klitgaard, N.; Beck-Nielsen, H. Insulin increases renal magnesium excretion: A possible cause of magnesium depletion in hyperinsulinaemic states. Diabet. Med. 1995, 12, 664–669. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A. Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar]
- Atchley, D.W.; Loeb, R.F.; Richards, D.W.; Benedict, E.M.; Driscoll, M.E. On diabetic acidosis: A detailed study of electrolyte balances following the withdrawal and reestablishment of insulin therapy. J. Clin. Investig. 1933, 12, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Aikaws, J.K. Effect of glucose and insulin on magnesium metabolism in rabbits. A study with Mg28. Proc. Soc. Exp. Biol. Med. 1960, 103, 363–366. [Google Scholar] [CrossRef]
- Mandon, B.; Siga, E.; Chabardes, D.; Firsov, D.; Roinel, N.; De Rouffignac, C. Insulin stimulates Na+, Cl−, Ca2+, and Mg2+ transports in TAL of mouse nephron: Cross-potentiation with AVP. Am. J. Physiol. Physiol. 1993, 265, F361–F369. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-J.; Ritchie, G.; Bapty, B.W.; Kerstan, D.; Quamme, G.A. Insulin stimulates Mg2+ uptake in mouse distal convoluted tubule cells. Am. J. Physiol. Content 1999, 277, F907–F913. [Google Scholar] [CrossRef]
- Nair, A.V.; Hocher, B.; Verkaart, S.; Van Zeeland, F.; Pfab, T.; Slowinski, T.; Chen, Y.-P.; Schlingmann, K.P.; Schaller, A.; Gallati, S.; et al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc. Natl. Acad. Sci. USA 2012, 109, 11324–11329. [Google Scholar] [CrossRef]
- Hruby, A.; Ngwa, J.S.; Renström, F.; Wojczynski, M.K.; Ganna, A.; Hallmans, G.; Houston, D.K.; Jacques, P.F.; Kanoni, S.; Lehtimäki, T.; et al. Higher magnesium intake is associated with lower fasting glucose and insulin, with no evidence of interaction with select genetic loci, in a meta-analysis of 15 charge consortium studies. J. Nutr. 2013, 143, 345–353. [Google Scholar] [CrossRef]
- Lee, C.-T.; Lien, Y.-H.; Lai, L.-W.; Chen, J.-B.; Lin, C.-R.; Chen, H.-C. Increased renal calcium and magnesium transporter abundance in streptozotocin-induced diabetes mellitus. Kidney Int. 2006, 69, 1786–1791. [Google Scholar] [CrossRef]
- Takayanagi, K.; Shimizu, T.; Tayama, Y.; Ikari, A.; Anzai, N.; Iwashita, T.; Asakura, J.; Hayashi, K.; Mitarai, T.; Hasegawa, H. Downregulation of transient receptor potential M6 channels as a cause of hypermagnesiuric hypomagnesemia in obese type 2 diabetic rats. Am. J. Physiol. Physiol. 2015, 308, F1386–F1397. [Google Scholar] [CrossRef]
- Groenestege, W.M.T.; Hoenderop, J.G.; Heuvel, L.V.D.; Knoers, N.; Bindels, R.J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 2006, 17, 1035–1043. [Google Scholar] [CrossRef]
- Chávez-Canales, M.; Arroyo, J.P.; Ko, B.; Vázquez, N.; Bautista, R.; Castañeda-Bueno, M.; Bobadilla, N.A.; Hoover, R.S.; Gamba, G. Insulin increases the functional activity of the renal NaCl cotransporter. J. Hypertens. 2013, 31, 303–311. [Google Scholar] [CrossRef]
- Komers, R.; Rogers, S.; Oyama, T.T.; Xu, B.; Yang, C.-L.; McCormick, J.; Ellison, D.H. Enhanced phosphorylation of Na+–Cl− co-transporter in experimental metabolic syndrome: Role of insulin. Clin. Sci. 2012, 123, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Sohara, E.; Nomura, N.; Chiga, M.; Alessi, D.R.; Rai, T.; Sasaki, S.; Uchida, S. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension 2012, 60, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Sohara, E.; Rai, T.; Yang, S.-S.; Ohta, A.; Naito, S.; Chiga, M.; Nomura, N.; Lin, S.-H.; Vandewalle, A.; Ohta, E.; et al. Acute insulin stimulation induces phosphorylation of the Na-Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS ONE 2011, 6, e24277. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 2003, 27, 59–65. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.; Ligia, J. Magnesium and aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef]
- Suarez, A.; Pulido, N.; Casla, A.; Casanova, B.; Arrieta, F.J.; Rovira, A. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia 1995, 38, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Matsunobu, S.; Terashima, Y.; Senshu, T.; Sano, H.; Itoh, H. Insulin secretion and glucose uptake in hypomagnesemic sheep fed a low magnesium, high potassium diet. J. Nutr. Biochem. 1990, 1, 167–171. [Google Scholar] [CrossRef]
- Balon, T.W.; Gu, J.L.; Tokuyama, Y.; Jasman, A.P.; Nadler, J.L. Magnesium supplementation reduces development of diabetes in a rat model of spontaneous NIDDM. Am. J. Physiol. Content 1995, 269, 745–752. [Google Scholar] [CrossRef]
- Fung, T.T.; Manson, J.E.; Solomon, C.G.; Liu, S.; Willett, W.C.; Hu, F.B. The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J. Am. Coll. Nutr. 2003, 22, 533–538. [Google Scholar] [CrossRef]
- Humphries, S.; Kushner, H.; Falkner, B. Low dietary magnesium is associated with insulin resistance in a sample of young, nondiabetic Black Americans. Am. J. Hypertens. 1999, 12, 747–756. [Google Scholar] [CrossRef]
- Von Ehrlich, B.; Barbagallo, M.; Classen, H.G.; Guerrero-Romero, F.; Mooren, F.C.; Rodriguez-Moran, M.; Vierling, W.; Vormann, J.; Kisters, K. The significance of magnesium in insulin resistance, metabolic syndrome and diabetes—Recommendations of the association of magnesium research. V. |die bedeutung von magnesium für insulinresistenz, metabolisches sindrom un diabetes mellitus—Empfehlungen der gesellschaft für magnesium forschung e.V. Diabetol. Stoffwechs. 2014, 9, 96–100. [Google Scholar]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Dustan, H.P. Irvine Page lecture. Legacies of Irvine, H. Page. J. Hypertens. Suppl. 1990, 8, S29–S34. [Google Scholar] [PubMed]
- Harrison, D.G. The Mosaic Theory revisited: Common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J. Am. Soc. Hypertens 2013, 7, 68–74. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nat. Cell Biol. 1993, 362, 801–809. [Google Scholar] [CrossRef]
- Barrows, I.R.; Ramezani, A.; Raj, D.S. Inflammation, immunity, and oxidative stress in hypertension—Partners in crime? Adv. Chronic Kidney Dis. 2019, 26, 122–130. [Google Scholar] [CrossRef]
- Carbone, F.; Elia, E.; Casula, M.; Bonaventura, A.; Liberale, L.; Bertolotto, M.; Artom, N.; Minetti, S.; Dallegri, F.; Contini, P.; et al. Baseline hs-CRP predicts hypertension remission in metabolic syndrome. Eur. J. Clin. Investig. 2019, 49, e13128. [Google Scholar] [CrossRef]
- Schüler, R.; Efentakis, P.; Wild, J.; Lagrange, J.; Garlapati, V.; Molitor, M.; Kossmann, S.; Oelze, M.; Stamm, P.; Li, H.; et al. T cell-derived IL-17A induces vascular dysfunction via perivascular fibrosis formation and dysregulation of ·NO/cGMP signaling. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Tomiyama, H.; Shiina, K.; Matsumoto-Nakano, C.; Ninomiya, T.; Komatsu, S.; Kimura, K.; Chikamori, T.; Yamashina, A. The Contribution of Inflammation to the Development of Hypertension Mediated by Increased Arterial Stiffness. J. Am. Heart Assoc. 2017, 6, e005729. [Google Scholar] [CrossRef]
- Kramer, J.H.; Mak, I.T.; Phillips, T.M.; Weglicki, W.B. Dietary Magnesium Intake Influences Circulating Pro-Inflammatory Neuropeptide Levels and Loss of Myocardial Tolerance to Postischemic Stress. Exp. Biol. Med. 2003, 228, 665–673. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J.A.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Malpuech-Brugère, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.-P.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2000, 1501, 91–98. [Google Scholar] [CrossRef]
- Galland, L. Magnesium and immune function: An overview. Magnesium 1988, 7, 290–299. [Google Scholar] [PubMed]
- Bussière, F.; Tridon, A.; Zimowska, W.; Mazur, A.; Rayssiguier, Y. Increase in complement component C3 is an early response to experimental magnesium deficiency in rats. Life Sci. 2003, 73, 499–507. [Google Scholar] [CrossRef]
- Maier, J.A.; Malpuech-Brugere, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta 2004, 1689, 13–21. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Bermudez-Peña, C.; Rodríguez-Morán, M. Severe hypomagnesemia and low-grade inflammation in metabolic syndrome. Magnes. Res. 2011, 24, 45–53. [Google Scholar] [CrossRef]
- Kim, D.J.; Xun, P.; Liu, K.; Loria, C.; Yokota, K.; Jacobs, D.R.; He, K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 2010, 33, 2604–2610. [Google Scholar] [CrossRef]
- Konstari, S.; Sares-Jäske, L.; Heliövaara, M.; Rissanen, H.; Knekt, P.; Arokoski, J.; Sundvall, J.; Karppinen, J. Dietary magnesium intake, serum high sensitivity C-reactive protein and the risk of incident knee osteoarthritis leading to hospitalization—A cohort study of 4953 Finns. PLoS ONE 2019, 14, e0214064. [Google Scholar] [CrossRef]
- Weglicki, W.B.; Mak, I.T.; Kramer, J.H.; Dickens, B.F.; Cassidy, M.M.; Stafford, R.E.; Phillips, T.M. Role of free radicals and substance P in magnesium deficiency. Cardiovasc. Res. 1996, 31, 677–682. [Google Scholar] [CrossRef]
- Kolisek, M.; Launay, P.; Beck, A.; Sponder, G.; Serafini, N.; Brenkus, M.; Froschauer-Neuhauser, E.; Martens, H.; Fleig, A.; Schweigel, M. SLC41A1 is a novel mammalian Mg2+carrier. J. Biol. Chem. 2008, 283, 16235–16247. [Google Scholar] [CrossRef]
- Yamanaka, R.; Tabata, S.; Shindo, Y.; Hotta, K.; Suzuki, K.; Soga, T.; Oka, K. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016, 6, 30027. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Smorodchenko, A.; Aschenbach, J.R.; Kolisek, M.; Sponder, G. Solute carrier 41A3 encodes for a mitochondrial Mg(2+) efflux system. Sci. Rep. 2016, 6, 27999. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jeong, E.-M.; Liu, H.; Xie, A.; So, E.Y.; Shi, G.; Jeong, G.E.; Zhou, A.; Dudley, J.S.C. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Xie, A.; Kang, G.-J.; Feng, F.; Zhou, X.; Zhao, Y.; Dudley, S.C. Magnesium deficiency causes reversible diastolic and systolic cardiomyopathy. Biophys. J. 2020, 118, 245. [Google Scholar] [CrossRef]
- Gout, E.; Rébeillé, F.; Douce, R.; Bligny, R. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. USA 2014, 111, E4560–E4567. [Google Scholar] [CrossRef]
- Panov, A.; Scarpa, A. Mg2+Control of Respiration in Isolated Rat Liver Mitochondria†. Biochemistry 1996, 35, 12849–12856. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.; Moreno-Sánchez, R.; Rodriguez-Zavala, J.S. Modulation of oxidative phosphorylation by Mg2+ in rat heart mitochondria. J. Biol. Chem. 1998, 273, 7850–7855. [Google Scholar] [CrossRef]
- Kramer, J.H.; Mišík, V.; Weglicki, W.B. Magnesium-deficiency potentiates free radical production associated with postischemic injury to rat hearts: Vitamin E affords protection. Free Radic. Biol. Med. 1994, 16, 713–723. [Google Scholar] [CrossRef]
- Morais, J.B.; Severo, J.S.; Santos, L.R.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.; do Nascimento Marreiro, D. Role of magnesium in oxidative stress in individuals with obesity. Biol. Trace. Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Calviello, G.; Ricci, P.; Lauro, L.; Palozza, P.; Cittadini, A. Mg deficiency induces mineral content changes and oxidative stress in rats. Biochem. Mol. Biol. Int. 1994, 32, 903–911. [Google Scholar]
- Shah, N.C.; Liu, J.-P.; Iqbal, J.; Hussain, M.; Jiang, X.-C.; Li, Z.; Li, Y.; Zheng, T.; Li, W.; Sica, A.C.; et al. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: Relevance to de novo synthesis of ceramide, serum Mg2+ and atherogenesis. Int. J. Clin. Exp. Med. 2011, 4, 103–118. [Google Scholar] [PubMed]
- Kumar, B.P.; Shivakumar, K. Depressed antioxidant defense in rat heart in experimental magnesium deficiency implications for the pathogenesis of myocardial lesions. Biol. Trace Element Res. 1997, 60, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Racay, P. Effect of magnesium on calcium-induced depolarisation of mitochondrial transmembrane potential. Cell Biol. Int. 2008, 32, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Blomeyer, C.A.; Bazil, J.N.; Stowe, D.F.; Dash, R.K.; Camara, A.K. Mg2+ differentially regulates two modes of mitochondrial Ca2+ uptake in isolated cardiac mitochondria: Implications for mitochondrial Ca2+ sequestration. J. Bioenerg. Biomembr. 2016, 48, 175–188. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, X.; Yan, P.; Han, Y.; Sun, S.; Wu, K.-C.; Fan, D. Human mitochondrial Mrs2 protein promotes multidrug resistance in gastric cancer cells by regulating p27, cyclin D1 expression and cytochrome C release. Cancer Biol. Ther. 2009, 8, 607–614. [Google Scholar] [CrossRef]
- Salvi, M.; Bozac, A.; Toninello, A. Gliotoxin induces Mg2+ efflux from intact brain mitochondria. Neurochem. Int. 2004, 45, 759–764. [Google Scholar] [CrossRef]
- Sponder, G.; Abdulhanan, N.; Fröhlich, N.; Mastrototaro, L.; Aschenbach, J.R.; Röntgen, M.; Pilchova, I.; Cibulka, M.; Racay, P.; Kolisek, M. Overexpression of Na+/Mg2+ exchanger SLC41A1 attenuates pro-survival signaling. Oncotarget 2017, 9, 5084–5104. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Dołowy, K.; Szewczyk, A. Matrix Mg2+regulates mitochondrial ATP-dependent potassium channel from heart. FEBS Lett. 2005, 579, 1625–1632. [Google Scholar] [CrossRef]
- Beavis, A.D.; Powers, M.F. On the regulation of the mitochondrial inner membrane anion channel by magnesium and protons. J. Biol. Chem. 1989, 264, 17148–17155. [Google Scholar]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Gorgoglione, V.; Laraspata, D.; La Piana, G.; Marzulli, D.; Lofrumento, N.E. Protective effect of magnesium and potassium ions on the permeability of the external mitochondrial membrane. Arch. Biochem. Biophys. 2007, 461, 13–23. [Google Scholar] [CrossRef] [PubMed]
- La Piana, G.; Gorgoglione, V.; Laraspata, D.; Marzulli, D.; Lofrumento, N.E. Effect of magnesium ions on the activity of the cytosolic NADH/cytochrome c electron transport system. FEBS J. 2008, 275, 6168–6179. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-W.; Na Shin, J.; Ko, K.H.; Cha, J.H.; Park, J.Y.; Lee, B.R.; Yun, C.-W.; Kim, Y.M.; Seol, D.-W.; Kim, D.-W.; et al. The Molecular Mechanism of Noxa-induced Mitochondrial Dysfunction in p53-Mediated Cell Death. J. Biol. Chem. 2003, 278, 48292–48299. [Google Scholar] [CrossRef]
- Sharikabad, M.N.; Ostbye, K.M.; Brors, O. Increased [Mg2+]o reduces Ca2+ influx and disruption of mitochondrial membrane potential during reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2113–H2123. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Hsieh, Y.L.; Ju, D.T.; Lin, C.C.; Kuo, C.H.; Liou, Y.-F.; Ho, T.-J.; Tsai, C.-H.; Lin, J.-Y. Attenuation of magnesium sulfate on CoCl2—Induced cell death by activating ERK1/2/MAPK and inhibiting HIF-1alpha via mitochondrial apoptotic signaling suppression in a neuronal cell line. Chin. J. Physiol. 2015, 58, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Albertini, A.; Curello, S.; Ceconi, C.; Di Lisa, F.; Raddino, R.; Visioli, O. Myocardial recovery during post-ischaemic reperfusion: Effects of nifedipine, calcium and magnesium. J. Mol. Cell. Cardiol. 1986, 18, 487–498. [Google Scholar] [CrossRef]
- Boelens, A.D.; Pradhan, R.K.; Blomeyer, C.A.; Camara, A.K.S.; Dash, R.K.; Stowe, D.F. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake–independent respiration and redox state in cardiac isolated mitochondria. J. Bioenerg. Biomembr. 2013, 45, 203–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Wang, J.; Yue, J.; Wang, Y.; Yang, C.; Cui, Q. High magnesium prevents matrix vesicle-mediated mineralization in human bone marrow-derived mesenchymal stem cells via mitochondrial pathway and autophagy. Cell Biol. Int. 2018, 42, 205–215. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Weglicki, W.B.; Bloom, S.; Cassidy, M.M.; Freedman, A.M.; Atrakchi, A.H.; Dickens, B.F. Antioxidants and the cardiomyopathy of Mg-deficiency. Am. J. Cardiovasc. Pathol. 1992, 4, 210–215. [Google Scholar]
- AlGhatrif, M.; Wang, M.; Fedorova, O.V.; Bagrov, A.Y.; Lakatta, E.G. The pressure of aging. Med. Clin. N. Am. 2017, 101, 81–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vasan, R.S.; Beiser, A.; Seshadri, S.; Larson, M.G.; Kannel, W.B.; D’Agostino, R.B.; Levy, D. Residual lifetime risk for developing hypertension in middle-aged women and men: The framingham heart study. JAMA 2002, 287, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part III: Cellular and molecular clues to heart and arterial aging. Circulation 2003, 107, 490–497. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003, 107, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A "set up" for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Levy, B.I.; Struijker-Boudier, H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003, 107, 2864–2869. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Lacourciere, Y.; Ouellet, J.P.; Izzo, J.L., Jr.; Neutel, J.; Kerwin, L.J.; Block, A.J.; Pfeffer, M.A. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: The role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 2003, 108, 1592–1598. [Google Scholar] [CrossRef]
- Lakatta, E.G. The reality of aging viewed from the arterial wall. Artery Res. 2013, 7, 73–80. [Google Scholar] [CrossRef]
- Laurant, P.; Hayoz, D.; Brunner, H.; Berthelot, A. Dietary magnesium intake can affect mechanical properties of rat carotid artery. Br. J. Nutr. 2000, 84, 757–764. [Google Scholar] [CrossRef]
- Adrian, M.; Chanut, E.; Laurant, P.; Gaume, V.; Berthelot, A. A long-term moderate magnesium-deficient diet aggravates cardiovascular risks associated with aging and increases mortality in rats. J. Hypertens. 2008, 26, 44–52. [Google Scholar] [CrossRef]
- Hirohama, D.; Fujita, T. Evaluation of the pathophysiological mechanisms of salt-sensitive hypertension. Hypertens. Res. 2019, 42, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Resnick, L.M.; Gupta, R.K.; DiFabio, B.; Barbagallo, M.; Mann, S.; Marion, R.; Laragh, J.H. Intracellular ionic consequences of dietary salt loading in essential hypertension. Relation to blood pressure and effects of calcium channel blockade. J. Clin. Investig. 1994, 94, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Hosseini, J.M.; Ruddel, M.E.; Elin, R.J. Blood magnesium parameters do not differ with age. J. Am. Coll. Nutr. 1990, 9, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Midtvedt, K.; Dolva, L.Ø.; Norseth, J.; Kjekshus, J. The magnesium loading test: Reference values in healthy subjects. Scand. J. Clin. Lab. Investig. 1994, 54, 23–31. [Google Scholar] [CrossRef]
- Barbagallo, M.; Gupta, R.K.; Dominguez, L.J.; Resnick, L.M. Cellular ionic alterations with age: Relation to hypertension and diabetes. J. Am. Geriatr. Soc. 2000, 48, 1111–1116. [Google Scholar] [CrossRef]
- Galan, P.; Preziosi, P.; Durlach, V.; Valeix, P.; Ribas, L.; Bouzid, D.; Favier, A.; Hercberg, S. Dietary magnesium intake in a French adult population. Magnes. Res. 1997, 10, 321–328. [Google Scholar]
- Dominguez, L.; Ligia, J.; Barbagallo, M. The multidomain nature of malnutrition in older persons. J. Am. Med. Dir. Assoc. 2017, 18, 908–912. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia, weight loss, and frailty. J. Am. Med. Dir. Assoc. 2010, 11, 225–228. [Google Scholar] [CrossRef]
- O’Shea, E.; Trawley, S.; Manning, E.; Barrett, A.; Browne, V.; Timmons, S. Malnutrition in hospitalised older adults: A multicentre observational study of prevalence, associations and outcomes. J. Nutr. Health Aging 2016, 21, 830–836. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.; Ms, R.P.S.A.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in Older Adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef]
- Thomas, A.J.; Bunker, V.W.; Sodha, N.; Clayton, B.E. Calcium, magnesium and phosphorus status of elderly inpatients: Dietary intake, metabolic balance studies and biochemical status. Br. J. Nutr. 1989, 62, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Löwik, M.R.; Van Dokkum, W.; Kistemaker, C.; Schaafsma, G.; Ockhuizen, T. Body composition, health status and urinary magnesium excretion among elderly people (Dutch Nutrition Surveillance System). Magnes. Res. 1993, 6, 223–232. [Google Scholar] [PubMed]
- Costello, R.B.; Moser-Veillon, P.B. A review of magnesium intake in the elderly. A cause for concern? Magnes. Res. 1992, 5, 61–67. [Google Scholar] [PubMed]
- McIntosh, W.; Kubena, K.S.; Walker, J.; Smith, D.; Landmann, W.A. The relationship between beliefs about nutrition and dietary practices of the elderly. J. Am. Diet. Assoc. 1990, 90, 671–676. [Google Scholar]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.D.; Johnson, L.K. Magnesium requirements: New estimations for men and women by cross-sectional statistical analyses of metabolic magnesium balance data. Am. J. Clin. Nutr. 2006, 84, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Gámez, C.; Artacho, R.; Ruiz-López, M.-D.; Navarro, M.; Puerta, A.; López, M. Serum concentration and dietary intake of Mg and Ca in institutionalized elderly people. Sci. Total Environ. 1997, 203, 245–251. [Google Scholar] [CrossRef]
- Lipski, P.S.; Torrance, A.; Kelly, P.J.; James, O.F.W. A study of nutritional deficits of long-stay geriatric patients. Age Ageing 1993, 22, 244–255. [Google Scholar] [CrossRef]
- Aghdassi, E.; McArthur, M.; Liu, B.; McGeer, A.; Simor, A.; Allard, J.P.; McGeer, A. Dietary intake of elderly living in Toronto long-term care facilities: Comparison to the dietary reference intake. Rejuvenation Res. 2007, 10, 301–310. [Google Scholar] [CrossRef]
- Iuliano-Burns, S.; Olden, A.; Woods, J. Meeting the nutritional needs of elderly residents in aged-care: Are we doing enough? J. Nutr. Health Aging 2013, 17, 503–508. [Google Scholar] [CrossRef]
- Lengyel, C.O.; Whiting, S.J.; Zello, G.A. Nutrient inadequacies among elderly residents of long-term care facilities. Can. J. Diet. Pract. Res. 2008, 69, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lammes, E.; Törner, A.; Akner, G. Nutrient density and variation in nutrient intake with changing energy intake in multimorbid nursing home residents. J. Hum. Nutr. Diet. 2009, 22, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.P. Magnesium and trace elements in the elderly: Intake, status and recommendations. J. Nutr. Health Aging 2002, 6, 147–153. [Google Scholar] [PubMed]
- Coudray, C.; Feillet-Coudray, C.; Rambeau, M.; Tressol, J.C.; Gueux, E.; Mazur, A.; Rayssiguier, Y. The effect of aging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: A stable isotope study. J. Trace Elements Med. Biol. 2006, 20, 73–81. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Adverse cardiovascular and blood pressure effects of drug-induced hypomagnesemia. Expert Opin. Drug Saf. 2019, 19, 59–67. [Google Scholar] [CrossRef]
- Hansen, B.-A.; Bruserud, Ø. Hypomagnesemia in critically ill patients. J. Intensive Care 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Almoussa, M.; Goertzen, A.; Brauckmann, S.; Fauser, B.; Zimmermann, C.W. Posterior reversible encephalopathy syndrome due to hypomagnesemia: A case report and literature review. Case Rep. Med. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Koiwai, K.; Takemi, Y.; Hayashi, F.; Ogata, H.; Matsumoto, S.; Ozawa, K.; Machado, P.P.; Monteiro, C.A. Consumption of ultra-processed foods decreases the quality of the overall diet of middle-aged Japanese adults. Public Health Nutr. 2019, 22, 2999–3008. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazıcı, M.A.; Tutus, Y.; Ozturk, L. Glyphosate reduced seed and leaf concentrations of calcium, manganese, magnesium, and iron in non-glyphosate resistant soybean. Eur. J. Agron. 2009, 31, 114–119. [Google Scholar] [CrossRef]
- Griffiths, A.M.; Cook, D.M.; Eggett, D.L.; Christensen, M.J. A retail market study of organic and conventional potatoes (Solanum tuberosum): Mineral content and nutritional implications. Int. J. Food Sci. Nutr. 2012, 63, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.O.; Nicolson, D.; Campbell, F.; Cook, J.V.; Beyer, F.R.; Ford, G.A.; Mason, J. Magnesium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. 2006, CD004640. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Plesset, M.R. Oral magnesium supplements decrease high blood pressure (SBP > 155mmHg) in hypertensive subjects on anti-hypertensive medications: A targeted meta-analysis. Magnes. Res. 2013, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xun, P.; Tang, Q.; Cai, W.; He, K. Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Altman, D.; Carroli, G.; Duley, L.; Farrell, B.; Moodley, J.; Neilson, J.; Smith, D.; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet 2002, 359, 1877–1890. [Google Scholar]
- Fishel Bartal, M.; Sibai, B.M. Eclampsia in the 21(st) century. Am. J. Obstet. Gynecol. 2020, S0002-9378(20)31128-5. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Liu, Z.; Chen, J.; Tan, H.; Liang, Y.; Chen, D. Posterior reversible encephalopathy syndrome in preeclampsia and eclampsia: The role of hypomagnesemia. Seizure 2020, 76, 12–16. [Google Scholar] [CrossRef]
- Pollock, W.; Peek, M.J.; Wang, A.; Li, Z.; Ellwood, D.; Homer, C.; Pulver, L.J.; McLintock, C.; Vaughan, G.; Knight, M.; et al. Eclampsia in Australia and New Zealand: A prospective population-based study. Aust. N. Z. J. Obstet. Gynaecol. 2019, 60, 533–540. [Google Scholar] [CrossRef]
- Winkler, A.W.; Smith, P.K.; Hoff, H.E. Intravenous magnesium sulfate in the treatment of nephritic convulsions in adults. J. Clin. Investig. 1942, 21, 207–216. [Google Scholar] [CrossRef]
- Joffres, M.R.; Reed, D.M.; Yano, K. Relationship of magnesium intake and other dietary factors to blood pressure: The Honolulu heart study. Am. J. Clin. Nutr. 1987, 45, 469–475. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Pünsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 59, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Busch, V.; Van Stel, H.F.; Schrijvers, A.J.P.; De Leeuw, J.R.J. Clustering of health-related behaviors, health outcomes and demographics in Dutch adolescents: A cross-sectional study. BMC Public Health 2013, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
| Food | Serving | Magnesium (mg) |
|---|---|---|
| Cereal all bran | ½ cup | 112 |
| Cereal oat bran | ½ cup dry | 96 |
| Brown rice, medium-grain, cooked | 1 cup | 86 |
| Fish, mackerel, cooked | 3 ounces | 82 |
| Spinach, frozen, chopped, cooked | ½ cup | 78 |
| Almonds | 1 ounce (23 almonds) | 77 |
| Swiss chard, chopped, cooked | ½ cup | 75 |
| Lima beans, large, cooked | ½ cup | 63 |
| Cereal, shredded wheat | 2 biscuits | 61 |
| Peanuts | 1 ounce | 48 |
| Molasses, blackstrap | 1 tablespoon | 48 |
| Hazelnuts | 1 ounce (21 hazelnuts) | 46 |
| Walnuts | 1 ounce (14 walnuts) | 44 |
| Okra, frozen, cooked | ½ cup | 37 |
| Milk, 1% fat | 8 fluid ounces | 34 |
| Banana | 1 medium | 32 |
|
|
| Authors/Country | Year | N. of Trials or Prospective Cohort Studies | N. of Participants/Cases | Study Characteristics | Magnesium Dose | Duration of Follow-up or Trials | Summary of Results |
|---|---|---|---|---|---|---|---|
| Witteman et al. USA [36] | 1989 | - | 58,218/3275 | Prospective cohort | - | 4 years | For women with high intakes of magnesium vs. low intakes, the RR of hypertension was 0.65 (95% CI, 0.53–0.80). |
| Ascherio et al. USA [38] | 1992 | - | 30,681/1248 | Prospective cohort | - | 4 years | Among male health professionals, dietary magnesium was significantly associated with lower risk of hypertension after adjustment for age, relative weight, alcohol consumption, and energy intake. |
| Ascherio et al. USA [37] | 1996 | - | 41,541/2526 | Prospective cohort | - | 4 years | Among women who did not report hypertension during follow-up, magnesium was significantly inversely associated with self-reported systolic and diastolic BP, after adjusting for age, BMI, alcohol consumption, and energy intake. Dietary magnesium was not significantly associated with risk of hypertension, after adjusting for age, BMI, alcohol, and energy intake. |
| Peacock et al. USA [41] | 1999 | - | 7731/1577 | Prospective cohort | - | 6 years | Significant trend for the association of serum magnesium and incident hypertension in women, after adjustment for age, race, and other risk factors (p trend = 0.01) but not in men (p trend = 0.16). No association between dietary magnesium intake and incident hypertension. |
| Townsend et al. USA [43] | 2005 | - | 10,033/1045 in NHANES III 2311/299 in NHANES IV | Two waves national survey | - | Similar intakes of magnesium and other minerals in hypertensive and non-hypertensive participants in both surveys. The pattern of significantly lower mineral intake (potassium + calcium + magnesium) emerged as unique to persons with isolated systolic hypertension in both waves. | |
| He et al. USA [39] | 2006 | - | 4637/608 MS | Prospective cohort | - | 15 years | Magnesium intake was inversely associated with incidence of metabolic syndrome after adjustment for major lifestyle and dietary variables and baseline status of each component of the metabolic syndrome. The inverse associations were not modified by gender and race. Magnesium intake was also inversely related with individual component of the metabolic syndrome. |
| Song et al. USA [40] | 2006 | - | 28,349/8544 | Prospective cohort | - | 9.8 years | Among women, magnesium intake was inversely associated with the risk of hypertension (p for trend < 0.0001 of magnesium quintiles). This inverse association was attenuated but remained significant after further adjustment for known risk factors (p for trend = 0.03). Similar associations were observed for women who never smoked and reported no history of high cholesterol or diabetes at baseline. |
| Jee et al. Korea, USA [45] | 2002 | 20 (14 in hypertensives) | 1220 | Meta-analysis of interventional studies | 10–40 mmol/d | 3–24 wks | Apparent dose-dependent effect of magnesium on BP, with reductions of 4.3 mm Hg in systolic BP and of 2.3 mm Hg in diastolic BP for each 10 mmol/d increase in magnesium dose. Limiting the analysis to the 14 trials in hypertensives, for each 10 mmol/d of magnesium SBP was reduced by 3.3 mm Hg and DBP by 2.3 mm Hg. |
| Dickinson et al. UK [226] | 2006 | 12 | 545 | Cochrane review- Meta-analysis of RCTs | 10–40 mmol/d | 8–26 wks | On average, people receiving magnesium achieved slightly but significantly lower DBP (mean difference: −2.2 mmHg). Poor quality and heterogeneity of the trials. None of the studies reported any serious side effects. |
| Kass et al. UK [24] | 2012 | 22 | 1173 | Meta-analysis of interventional studies | 120–973 mg/d | 3–24 wks | Small but significant reduction in SBP of 3–4 mm Hg and DBP of 2–3 mm Hg, with greater increased in trials with crossover design and magnesium dose >370 mg/d. |
| Rosanoff et al. USA [227] | 2013 | 7 | 135 | Meta-analysis of interventional studies | 10.5–18.5 mmol/d | 6–17 wks | Significant mean reduction in SBP (mean −18.7 mmHg) and DBP (mean −10.9 mmHg) in hypertensives on continuous anti-hypertensive medication for at least six months, with no more than a two-week washout, and mean starting SPB > 155 mmHg. |
| Zhang et al. USA, China, Canada, Japan [23] | 2016 | 34 | 2028 | Meta-analysis of RCTs | 238–960 mg/d | 3 wks to 6 months | Significant reduction in SBP (mean −2.0 mmHg) and DBP (mean −1.78 mmHg) accompanied by 0.05 mmol/L rise in serum magnesium vs. placebo. Greater BP reduction found in trials with high quality or low dropout rate. |
| Dibaba et al. USA, Israel [22] | 2017 | 11 | 543 | Meta-analysis of RCTs | 365–450 mg/d | 1–6 months | Significant decrease in BP: mean reduction of 4.18 mm Hg in SBP and 2.27 mm Hg in DBP in participants with insulin resistance, prediabetes, or other noncommunicable chronic diseases. |
| Verma et al. India [44] | 2017 | 28 (19 trials included for HTN analyses, 4 in hypertensives) | 1694 | Meta-analysis of RCTs | 300–1006 mg/d | 4–24 wks | Significant reduction in SBP (weighted mean difference = −3.056 mmHg) with greater beneficial effect in diabetic patients with hypomagnesaemia. High heterogeneity of the trials. In meta-regression, elemental magnesium dose was inversely DBP (p < 0.001). |
| Han et al. China, Sweden, USA, Norway [21] | 2017 | 9 | 180,566/20,119 | Meta-analysis of prospective cohort studies | - | 4–15 years | Inverse association between dietary magnesium intake and the risk of hypertension. A 100 mg/d increment in magnesium intake was associated with a 5% reduction in the risk of hypertension. The association of serum magnesium concentration with the risk of hypertension was marginally significant. |
| Wu et al. China, USA [228] | 2017 | 11 (3 on HTN) | Total: 38,808/4437 HTN: 14,876/3149 | Meta-analysis of prospective cohort studies | - | 6–8 years | Comparing highest vs. lowest category of circulating magnesium concentration, the pooled RR was 0.91 (95% CI 0.80, 1.02) for incident hypertension. Every 0.1 mmol/L increment in circulating magnesium levels was associated with 4% (RR 0.96; 95% CI: 0.94, 0.99) reduction in hypertension incidence. |
| Ikbal et al. Austria [25] | 2019 | 8 (5 of RCTs, 3 of observational studies) | RCTs: 135–1694 | Summary of meta-analyses | 120–1006 mg/d | RCTs: 3–24 wks; observational studies: 4–15 years | The summary showed SBP reductions in the range of −0.2 and −18.7 mmHg, and DBP reductions between −0.3 and −10.9 mmHg. The meta-analysis [227] showing the largest effect, included a small sample of treated hypertensive patients, which probably responded highly to magnesium. When omitting this meta-analysis, the BP lowering effects of magnesium were attenuated to a low to moderate level. Observational studies showed a lower risk for hypertension with increasing magnesium intake or higher circulating magnesium levels. |
| Veronese et al. Italy, UK, Australia, Spain [31] | 2020 | 16 meta-analyses | RCTs: 2262 participants in 34 RCTs; Observational studies: 180,566/20119 | Umbrella review of systematic reviews and meta-analyses | 120–1006 mg/d | RCTs: 3–24 wks; observational studies: 4–15 years | High class evidence for the association of diastolic blood pressure and magnesium in intervention studies with magnesium supplementation vs. placebo and moderate class evidence for systolic blood pressure. Large heterogeneity found for this outcome. The evidence was suggestive for the association of a higher dietary magnesium intake with a lower risk of stroke in observational studies. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2021, 13, 139. https://doi.org/10.3390/nu13010139
Dominguez LJ, Veronese N, Barbagallo M. Magnesium and Hypertension in Old Age. Nutrients. 2021; 13(1):139. https://doi.org/10.3390/nu13010139
Chicago/Turabian StyleDominguez, Ligia J., Nicola Veronese, and Mario Barbagallo. 2021. "Magnesium and Hypertension in Old Age" Nutrients 13, no. 1: 139. https://doi.org/10.3390/nu13010139
APA StyleDominguez, L. J., Veronese, N., & Barbagallo, M. (2021). Magnesium and Hypertension in Old Age. Nutrients, 13(1), 139. https://doi.org/10.3390/nu13010139







