Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents
Abstract
1. Introduction
2. Use of Artemisia Species as Food, Spices, Condiments and Beverages
3. Nutritional Value of Artemisia Species
4. Adverse Effects Reported to Artemisia Species and Some Constituents
5. Therapeutic Uses of Artemisia Species Based on Clinical Trials
6. Some Sesquiterpene Lactones Constituents of Artemisia Species with High Clinical Relevance
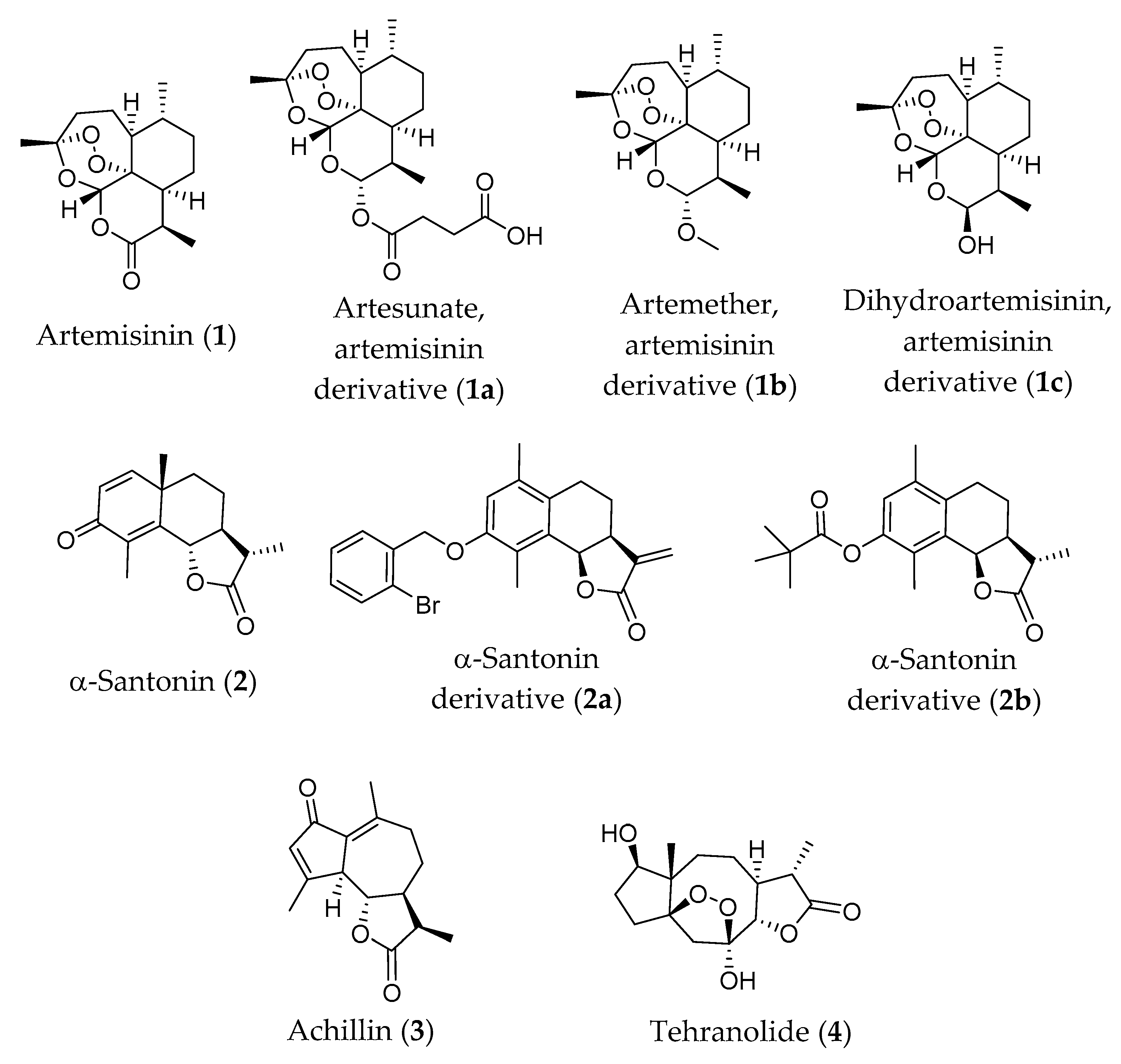
6.1. Artemisinin and Its Derivatives
6.1.1. Antiparasitic Activity In Vivo and Clinical Trials
6.1.2. Antitumor Activity In Vivo and Clinical Trials
6.2. α-Santonin and Its Derivatives
6.3. Achillin
6.4. Tehranolide
7. Hotpoint Research: Artemisia Species and Its Constituents as Strategy to Treat COVID-19 Infection
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Plant List, a Working List of All Plant Species. Available online: http://www.theplantlist.org/tpl1.1/search?q=Artemisia (accessed on 24 June 2020).
- Vallès, J.; Garcia, S.; Hidalgo, O.; Martín, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, genome evolution, biotechnological issues and research including applied perspectives in Artemisia (Asteraceae). In Advances in Botanical Research; Kader, J.-C., Delseny, M., Eds.; Academic Press: Burlington, NJ, USA, 2011; Volume 60, pp. 349–419. ISBN 978-0-12-385851-1. [Google Scholar]
- Hussain, A.; Potter, D.; Kim, S.; Hayat, M.Q.; Bokhari, S.A. Molecular phylogeny of Artemisia (Asteraceae-Anthemideae) with emphasis on undescribed taxa from Gilgit-Baltistan (Pakistan) based on nrDNA (ITS and ETS) and cpDNA (psbA-trnH) sequences. Plant Ecol. Evol. 2019, 152, 507–520. [Google Scholar] [CrossRef]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A promising plant for the treatment of cancer. Bioorg. Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the History of Medicine and Its Possible Contemporary Applications Substantiated by Phytochemical and Pharmacological Studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef] [PubMed]
- Verloove, F.; Andeweg, R. Artemisia princeps L. (Asteraceae), an overlooked invasive Far Eastern weed in Western Europe. Gorteria 2020, 42, 1–18. [Google Scholar]
- Boršić, I.; Milović, M.; Dujmović, I.; Bogdanović, S.; Cigić, P.; Rešetnik, I.; Nikolić, T.; Mitić, B. Preliminary check-list of invasive alien plant species (IAS) in Croatia. Nat. Croat. 2008, 17, 55–71. [Google Scholar]
- Nadeem, M.; Shinwari, Z.K.; Qaiser, M. Screening of folk remedies by genus Artemisia based on ethnomedicinal surveys and traditional knowledge of native communities of Pakistan. Pak. J. Bot. 2013, 45, 111–117. [Google Scholar]
- Sadiq, A.; Hayat, M.Q.; Ashraf, M. Ethnopharmacology of Artemisia annua L.: A review. In Artemisia Annua—Pharmacology and Biotechnology; Aftab, T., Ferreira, J., Khan, M., Naeem, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Vouillamoz, J.F.; Carlen, C.; Taglialatela-Scafati, O.; Pollastro, F.; Appendino, G. The génépi Artemisia species. Ethnopharmacology, cultivation, phytochemistry, and bioactivity. Fitoterapia 2015, 106, 231–241. [Google Scholar] [CrossRef]
- Dib, I.; Angenot, L.; Mihamou, A.; Ziyyat, A.; Tits, M. Artemisia campestris L.: Ethnomedicinal, phytochemical and pharmacological review. J. Herb. Med. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Ahuja, A.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Ethnopharmacological properties of Artemisia asiatica: A comprehensive review. J. Ethnopharmacol. 2018, 220, 117–128. [Google Scholar] [CrossRef]
- Abiri, R.; Silva, A.L.M.; De Mesquita, L.S.S.; De Mesquita, J.W.C.; Atabaki, N.; De Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef]
- Kumar, A.; Aswal, S.; Semwal, R.B.; Chauhan, A.; Semwal, D.K. Insights on the pharmacological, phytochemical and ethnobotanical aspects of Artemisia roxburghiana: A rather less explored but therapeutically important species of lower Himalayas. Phytochem. Rev. 2019, 18, 199–214. [Google Scholar] [CrossRef]
- Du Toit, A.; Van Der Kooy, F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnopharmacol. 2019, 244, 112127. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Rakoto, M.L.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B.-L. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Beshbishy, A.M.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Liu, N.; Van Der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? S. Afr. J. Bot. 2009, 75, 185–195. [Google Scholar] [CrossRef]
- Semenya, S.S.; Maroyi, A. Ethnobotanical study of curative plants used by traditional healers to treat rhinitis in the Limpopo Province, South Africa. Afr. Health Sci. 2018, 18, 1076–1087. [Google Scholar] [CrossRef]
- Native American Ethnobotany. Available online: http://naeb.brit.org/uses/search/?string=Artemisia+dracunculus (accessed on 14 October 2020).
- Nabimeybodi, R.; Zareshahi, R.; Tansaz, M.; Dastjerdi, M.V.; Hajimehdipoor, H. Scientific Evaluation of Medicinal Plants Used for the Treatment of Cervicitis (Qorohe- Rahem) in Iranian Traditional Medicine. Iran. J. Pharm. Res. 2019, 18, 1884–1901. [Google Scholar]
- Native American Ethnobotany. Available online: http://naeb.brit.org/uses/search/?string=Artemisia+vulgaris (accessed on 14 October 2020).
- Xiao, B.; Wang, J.-H.; Zhou, C.-Y.; Chen, J.-M.; Zhang, N.; Zhao, N.; Han, X.-Y.; Niu, Y.-X.; Feng, Y.-B.; Du, G.-H. Ethno-medicinal study of Artemisia ordosica Krasch. (traditional Chinese/Mongolian medicine) extracts for the treatment of allergic rhinitis and nasosinusitis. J. Ethnopharmacol. 2020, 248, 112262. [Google Scholar] [CrossRef]
- Xiao, B.; Bai, J.J.; Qi, L.; Lu, L.S.; Tian, X.R.; Yin, J.; Su, Y.X. Research progress on resource distribution, chemical components, and pharmacological activities of Artemisia ordosica Krasch. J. Chin. Pharm. 2016, 13, 1862–1864. [Google Scholar]
- Native American Ethnobotany. Available online: http://naeb.brit.org/uses/search/?string=Artemisia+tripartita (accessed on 14 October 2020).
- Calderone, V.; Martinotti, E.; Baragatti, B.; Breschi, M.C.; Morelli, I. Vascular effects of aqueous crude extracts of Artemisia verlotorum Lamotte (Compositae): In vivo and in vitro pharmacological studies in rats. Phytother. Res. 1999, 13, 645–648. [Google Scholar] [CrossRef]
- Hussain, A. Distribution and Molecular Phylogeny of Artemisia Plants from Gilgit-Baltistan, Pakistan. Ph.D. Thesis, University of International Islamic University Islamabad, Islamabad, Pakistan, March 2019; pp. 69–70, Reg. No. 31-FBAS/PHDBT/F14. Available online: http://prr.hec.gov.pk/jspui/handle/123456789/11070 (accessed on 15 October 2020).
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; WHO Press: Geneva, Switzerland, 2019; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Miller, F.G.; Emanuel, E.J.; Rosenstein, D.L.; Straus, S.E. Ethical Issues Concerning Research in Complementary and Alternative Medicine. JAMA 2004, 291, 599. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Ernst, E.; Colquhoun, D.; Sampson, W. ‘Complementary & Alternative Medicine’ (CAM): Ethical and policy issues. Bioethics 2016, 30, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.J.A.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Turi, C.E.; Shipley, P.R.; Murch, S.J. North American Artemisia species from the subgenus Tridentatae (Sagebrush): A phytochemical, botanical and pharmacological review. Phytochemistry 2014, 98, 9–26. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Xia, J.-X.; Tang, L. Chemical constituents, biological activities and clinical applications of Artemisia rupestris. Zhongguo Zhongyao Zazhi 2017, 42, 4565–4573. [Google Scholar]
- Kefale, A.T.; Dabe, N.E. Antidiabetic effects of artemisia species: A systematic review. Anc. Sci. Life 2017, 36, 175–181. [Google Scholar] [CrossRef]
- Yalçinkaya, E.; Özgüç, S.; Aydinalp, A.; Zeybek, U. The importance of Artemisia annua L. in the anticancer activity research. Ank. Univ. Eczacilik Fak. Derg. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Gondwe, M.; Mpalala, A.; Zongo, L.; Kamadyaapa, D.; Ndebia, E.; Sewani-Rusike, C.; Shauli, M.; Iputo, J. Investigation of anti-inflammatory and antinociceptive effects of aqueous extracts of Artemisia afra in wistar rats. Asian J. Pharm. Clin. Res. 2018, 11, 190–193. [Google Scholar] [CrossRef][Green Version]
- Koyuncu, I. Evaluation of anticancer, antioxidant activity and phenolic compounds of Artemisia absinthium L. extract. Cell. Mol. Biol. 2018, 6, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.J.A.; Del Olmo, L.M.B.; Ticona, L.A.; Benito, P.B. The Artemisia L. genus: A review of bioactive sesquiterpene lactones. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 37, Chapter 2; pp. 43–65. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.H.H.; El-Sayed, M.A.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 2010, 4, 1–25. [Google Scholar]
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive Compounds and Health Benefits of Artemisia Species. Nat. Prod. Commun. 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Gruessner, B.M.; Cornet-Vernet, L.; Desrosiers, M.R.; Lutgen, P.; Towler, M.J.; Weathers, P.J. It is not just artemisinin: Artemisia sp. for treating diseases including malaria and schistosomiasis. Phytochem. Rev. 2019, 18, 1509–1527. [Google Scholar] [CrossRef]
- Song, X.; Wen, X.; He, J.; Wang, J.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Dib, I.; El Alaoui-Faris, F.E. Artemisia campestris L.: Review on taxonomical aspects, cytogeography, biological activities and bioactive compounds. Biomed. Pharmacother. 2019, 109, 1884–1906. [Google Scholar] [CrossRef]
- Liu, S.-J.; Liao, Z.-X.; Tang, Z.-S.; Cui, C.-L.; Liu, H.-B.; Liang, Y.-N.; Zhang, Y.; Shi, H.-X.; Liu, Y.-R. Phytochemicals and biological activities of Artemisia sieversiana. Phytochem. Rev. 2017, 16, 441–460. [Google Scholar] [CrossRef]
- Koul, B.; Khatri, T. The Artemisia genus: Panacea to several maladies. In Bioactive Natural Products in Drug Discovery; Singh, J., Meshram, V., Gupta, M., Eds.; Springer: Singapore, 2020; pp. 3–95. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, J. Phytochemistry and bioactivities of sesquiterpenoids from the Artemisia species. J. Chin. Pharm. Sci. 2017, 26, 317–334. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, D.-B.; Li, M.-J.; Liu, X.-H.; Wang, H.-Q. Studies on flavonoid constituents from herbs of Artemisia ordosica II. Zhongguo Zhongyao Zazhi 2006, 31, 1959–1961. [Google Scholar] [PubMed]
- Nurbek, S.; Murata, T.; Suganuma, K.; Ishikawa, Y.; Buyankhishig, B.; Kikuchi, T.; Byambajav, T.; Davaapurev, B.-O.; Sasaki, K.; Batkhuu, J. Isolation and evaluation of trypanocidal activity of sesquiterpenoids, flavonoids, and lignans in Artemisia sieversiana collected in Mongolia. J. Nat. Med. 2020, 74, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-M.; Dong, X.; Ma, Y.-N.; Wu, Z.H.; Yan, Y.-M.; Cheng, Y.-X. Antifungal coumarins and lignans from Artemisia annua. Fitoterapia 2019, 134, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Labruzzo, A.; Cantrell, C.L.; Carrubba, A.; Ali, A.; Wedge, D.E.; Duke, S.O. Phytotoxic Lignans from Artemisia arborescens. Nat. Prod. Commun. 2018, 13, 237–240. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, J.-H.; Hao, J.-S.; Xu, Y.-H. Structure Elucidation of a New Lignan Glycoside from Artemisia ordosica. Chem. Nat. Compd. 2019, 55, 1007–1009. [Google Scholar] [CrossRef]
- Rashid, M.U.; Alamzeb, M.; Ali, S.; Ullah, Z.; Shah, Z.A.; Naz, I.; Khan, M.R. The chemistry and pharmacology of alkaloids and allied nitrogen compounds from Artemisia species: A review. Phytother. Res. 2019, 33, 2661–2684. [Google Scholar] [CrossRef]
- Giang, P.M.; Tran, T.T.N.; Phan, T.S.; Otsuka, H.; Matsunami, K. Two new sesquiterpene lactones and other chemical constituents of Artemisia roxbughiana. Biochem. Syst. Ecol. 2012, 45, 115–119. [Google Scholar] [CrossRef]
- Megdiche-Ksouri, W.; Trabelsi, N.; Mkadmini, K.; Bourgou, S.; Noumi, A.; Snoussi, M.; Barbria, R.; Tebourbi, O.; Ksouri, R. Artemisia campestris phenolic compounds have antioxidant and antimicrobial activity. Ind. Crop. Prod. 2015, 63, 104–113. [Google Scholar] [CrossRef]
- Souhila, T.; Zohra, B.F.; Tahar, H.S. Identification and quantification of phenolic compounds of Artemisia herba-alba at three harvest time by HPLC–ESI–Q-TOF–MS. Int. J. Food Prop. 2019, 22, 843–852. [Google Scholar] [CrossRef]
- Seidemann, J. World Spice Plants: Economic Usage, Botany, Taxonomy; Springer: Berlin/Heidelberg, Germany, 2005; ISBN 978-3-540-22279-8. [Google Scholar]
- Allen, G. The Herbalist in the Kitchen; University of Illinois Press: Champaign, IL, USA, 2010; ISBN 025209039X. [Google Scholar]
- Vaughan, J.; Geissler, C. The New Oxford Book of Food Plants, 2nd ed.; Oxford University Press: Oxford, UK, 2009; ISBN 0191609498. [Google Scholar]
- Kains, M.G. Culinary Herbs: Their Cultivation, Harvesting, Curing and Uses; Orange Judd Company: New York, NY, USA, 1912. [Google Scholar]
- Fern, K. Plants for a Future: Edible & Useful Plants for a Healthier World; Permanent Publications: East Meon, UK, 2000; ISBN 9781856230117. [Google Scholar]
- Parada, M.; Carrió, E.; Vallès, J. Ethnobotany of food plants in the alt empordà region (Catalonia, Iberian peninsula). J. Appl. Bot. Food Qual. 2011, 84, 11–25. [Google Scholar]
- Wright, C.W. Artemisia. Medicinal and Aromatic Plants—Industrial Profiles; Taylor & Francis Ltd.: London, UK, 2003; ISBN 0203303067. [Google Scholar]
- Amidon, C.; Barnett, R.; Cathers, J.; Chambers, B.; Hamilton, L.; Kellett, A.; Kennel, E.; Montowski, J.; Thomas, M.A.; Watson, B. Artemisia: An Essential Guide from The Herb Society of America; The Herb Society of America: Kirtland, OH, USA, 2014. [Google Scholar]
- Densmore, F. How Indians Use Wild Plants for Food, Medicine, & Crafts; Dover Publications: Mineola, NY, USA, 1974; ISBN 0486406709. [Google Scholar]
- Mladenova, O. Grapes and Wine in the Balkans: An Ethno-Linguistic Study; Harrassowitz Verlag: Wiesbaden, Germany, 1998; ISBN 978-3447040372. [Google Scholar]
- Koul, B.; Taak, P.; Kumar, A.; Khatri, T.; Sanyal, I. The Artemisia Genus: A Review on Traditional Uses, Phytochemical Constituents, Pharmacological Properties and Germplasm Conservation. J. Glycom. Lipidom. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.L. Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation; Springer: New York, NY, USA, 2014; ISBN 9781493914920. [Google Scholar]
- Tonutti, I.; Liddle, P. Aromatic plants in alcoholic beverages: A review. Flavour Fragr. J. 2010, 25, 341–350. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, E.C.; Lim, H.J.; Choi, S.J.; Kim, C.R.; Suh, S.H.; Kim, C.J.; Park, G.G.; Park, C.S.; Kim, H.K.; et al. Characterization of nutritional composition, antioxidative capacity, and sensory attributes of Seomae Mugwort, a native Korean variety of Artemisia argyi H. Lév. & Vaniot. J. Anal. Methods Chem. 2015, 2015, 916346. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seyler, B.C.; Ticktin, T.; Zeng, Y.; Ayu, K. An ethnobotanical survey of wild edible plants used by the Yi people of Liangshan Prefecture, Sichuan Province, China. J. Ethnobiol. Ethnomedicine 2020, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky, E. Food Plants of the North American Indians; U.S. Dept. of Agriculture: Washington, DC, USA, 1936.
- Moerman, D.E. Native American Food Plants: An Ethnobotanical Dictionary; Timber Press Inc.: Portland, OR, USA, 2010; ISBN 9781604691894. [Google Scholar]
- de Santayana, M.P.; Morales, R. Manzanillas ibéricas: Historia y usos tradicionales. Rev. Fitoter. 2006, 6, 143–153. [Google Scholar]
- Bezza, L.; Mannarino, A.; Fattarsi, K.; Mikail, C.; Abou, L.; Hadji-Minaglou, F.; Kaloustian, J. Composition chimique de l’huile essentielle d’Artemisia herba-alba provenant de la région de Biskra (Algérie). Phytothérapie 2010, 8, 277–281. [Google Scholar] [CrossRef]
- Kunkel, G. Plants for Human Consumption: An Annotated Checklist of the Edible Phanerogams and Ferns; Koeltz Scientific Books: Koenigatein, West Germany, 1984; ISBN 9783874292160. [Google Scholar]
- Sanmi, S.; McCabe, S.; Satoko, I. Chado the Way of Tea: A Japanese Tea Master’s Almanac; Tuttle Publishing: North Clarendon, VT, USA, 2005; ISBN 0804837163. [Google Scholar]
- Xing, X.H.; Zhang, Z.M.; Hu, X.Z.; Wu, R.Q.; Xu, C. Antidiabetic effects of Artemisia sphaerocephala Krasch. gum, a novel food additive in China, on streptozotocin-induced type 2 diabetic rats. J. Ethnopharmacol. 2009, 125, 410–416. [Google Scholar] [CrossRef]
- Boggia, L.; Pignata, G.; Sgorbini, B.; Colombo, M.L.; Marengo, A.; Casale, M.; Nicola, S.; Bicchi, C.; Rubiolo, P. Artemisia umbelliformis Lam. and Génépi Liqueur: Volatile Profile as Diagnostic Marker for Geographic Origin and to Predict Liqueur Safety. J. Agric. Food Chem. 2017, 65, 2849–2856. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. Culinary Herbs and Spices of the World; The University of Chicago Press: Chicago, IL, USA, 2013; ISBN 9780226091839. [Google Scholar]
- Judžentiene, A. Wormwood (Artemisia absinthium L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier Academic Press: London, UK, 2016; pp. 849–856. ISBN 9780124166448. [Google Scholar]
- Morata, A.; Vaquero, C.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Suárez-Lepe, J.A. Technology of vermouth wines. In Alcoholic Beverages: Volume 7: The Science of Beverages; Elsevier Woodhead Publishing: Duxford, UK, 2019; pp. 35–63. ISBN 9780128152690. [Google Scholar]
- How Prepare Peiln. Available online: https://www.bgfermer.bg/Article/4834261 (accessed on 10 August 2020).
- Vin de Pelin—Preparare, Administrare, Indicații Terapeutice|LaTAIFAS. Available online: https://lataifas.ro/retete-naturiste/vinuri-medicinale-retete-naturiste/22852/vin-de-pelin-preparare-indicatii-terapeutice/# (accessed on 10 August 2020).
- Arnold, W.N. Absinthe. Sci. Am. 1989, 260, 112–117. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kröner, L.U. Absinthe—A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef]
- Veretnova, O.Y.; Gulenkova, G.S.; Chepeleva, G.G.; Fedchenko, E.A.; Rybakova, G.R. Rationale and methods of the use of Artemisia absinthium L., Ledum palustre L. and Tanacetum vulgare L. for food purposes. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 421. [Google Scholar]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Teixeira, M.C.; Brodelius, M. Fatty acids profile of selected Artemisia spp. plants: Health promotion. LWT Food Sci. Technol. 2011, 44, 293–298. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Aksu-Kalmuk, N. Achene fatty acid composition in the tribe anthemideae (Asteraceae). Rom. Biotechnol. Lett. 2016, 21, 11576–11584. [Google Scholar]
- Boufennara, S.; Lopez, S.; Bousseboua, H.; Bodas, R.; Bouazza, L. Chemical composition and digestibility of some browse plant species collected from Algerian arid rangelands. Span. J. Agric. Res. 2012, 10, 88. [Google Scholar] [CrossRef]
- Olson, K.A.; Murray, M.G.; Fuller, T.K. Vegetation Composition and Nutritional Quality of Forage for Gazelles in Eastern Mongolia. Rangel. Ecol. Manag. 2010, 63, 593–598. [Google Scholar] [CrossRef]
- Al-Masri, M. Nutritive evaluation of some native range plants and their nutritional and anti-nutritional components. J. Appl. Anim. Res. 2013, 41, 427–431. [Google Scholar] [CrossRef]
- Bouazza, L.; Boufennara, S.; Bensaada, M.; Zeraib, A.; Rahal, K.; Saro, C.; Ranilla, M.J.; López, S. In vitro screening of Algerian steppe browse plants for digestibility, rumen fermentation profile and methane mitigation. Agrofor. Syst. 2020, 94, 1433–1443. [Google Scholar] [CrossRef]
- Randalova, T.E.; Dylenova, E.P.; Renchenbyamba, S.; Zhigzhitzhapova, S.V.; Radnaeva, L.D.; Taraskin, V.V. The composition of fatty acids isolated from plants of Absinthium section of floras of Buryatia and Mongolia. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019; Volume 320, p. 012057. [Google Scholar]
- Tsybikova, S.Z.; Randalova, T.E.; Radnaeva, L.D. Fatty acid composition of Artemisia santolinifolia Turcz. ex Bess. of flora of Buryatia. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019; Volume 320, p. 012058. [Google Scholar]
- Towhidi, A.; Saberifar, T.; Dirandeh, E. Nutritive value of some herbage for dromedary camels in the central arid zone of Iran. Trop. Anim. Heal. Prod. 2011, 43, 617–622. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, Y.; Nie, Y.; Yang, N.; Yang, X. Hypoglycemic and hepatoprotective effects of polysaccharides from Artemisia sphaerocephala Krasch seeds. Int. J. Biol. Macromol. 2014, 69, 296–306. [Google Scholar] [CrossRef]
- Davies, K.G.; Bates, J.D.; Johnson, D.D.; Nafus, A.M. Influence of Mowing Artemisia tridentata ssp. wyomingensis on Winter Habitat for Wildlife. Environ. Manag. 2009, 44, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Mataix, J. Fatty acid composition of nuts—implications for cardiovascular health. Br. J. Nutr. 2006, 96, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.-M.; Dumont, O. Dietary oleic acid not used during brain development and in adult in rat, in contrast with sciatic nerve. Neurosci. Lett. 2003, 336, 180–184. [Google Scholar] [CrossRef]
- Innis, S.M. Fatty acids and early human development. Early Hum. Dev. 2007, 83, 761–766. [Google Scholar] [CrossRef]
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 39–103. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Kakar, M.U.; Kakar, I.U.; Mehboob, M.Z.; Zada, S.; Soomro, H.; Umair, M.; Iqbal, I.; Umer, M.; Shaheen, S.; Syed, S.F.; et al. A review on polysaccharides from Artemisia sphaerocephala Krasch seeds, their extraction, modification, structure, and applications. Carbohydr. Polym. 2021, 252, 117113. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Miao, X.; Chen, X.; Nan, S.; Fu, H. Genome-Scale Transcriptome Analysis of the Desert Shrub Artemisia sphaerocephala. PLoS ONE 2016, 11, e0154300. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–88. [Google Scholar] [CrossRef]
- Fellows, P.J. Properties of food and principles of processing. In Food Processing Technology; Elsevier: Cambridge, UK, 2017; pp. 3–200. [Google Scholar]
- Stach, A.; García-Mozo, H.; Prieto-Baena, J.C.; Czarnecka-Operacz, M.; Jenerowicz, D.; Silny, W.; Galán, C. Prevalence of Artemisia species pollinosis in western Poland: Impact of climate change on aerobiological trends, 1995–2004. J. Investig. Allergol. Clin. Immunol. 2007, 17, 39–47. [Google Scholar] [PubMed]
- Cristofori, A.; Bucher, E.; Rossi, M.; Cristofolini, F.; Kofler, V.; Prosser, F.; Gottardini, E. The late flowering of invasive species contributes to the increase of Artemisia allergenic pollen in autumn: An analysis of 25 years of aerobiological data (1995–2019) in Trentino-Alto Adige (Northern Italy). Aerobiologia (Bologna) 2020, 36, 669–682. [Google Scholar] [CrossRef]
- D’Amato, G.; Spieksma, F.T.M. Allergenic pollen in europe. Grana 1991, 30, 67–70. [Google Scholar] [CrossRef]
- D’Amato, G.; Spieksma, F.T.M.; Liccardi, G.; Jäger, S.; Russo, M.; Nikkels, H.; Wuthrich, B.; Bonini, S.; Kontou-Fili, K. Pollen-related allergy in Europe. Allergy 1998, 53, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Sun, J.-L.; Yin, J.; Li, Z. Artemisia Allergy Research in China. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Gao, Z.; Fu, W.-Y.; Sun, Y.; Gao, B.; Wang, H.-Y.; Liu, M.; Luo, F.-M.; Zhou, X.; Jin, J.; Zhao, L.; et al. Artemisia pollen allergy in China: Component-resolved diagnosis reveals allergic asthma patients have significant multiple allergen sensitization. Allergy 2019, 74, 284–293. [Google Scholar] [CrossRef]
- Brandys, J.; Grimsoen, A.; Nilsen, B.M.; Smestad Paulsen, B.; Park, H.S.; Hong, C.S. Cross-reactivity between pollen extracts from six Artemisia species. Planta Med. 1993, 59, 221–228. [Google Scholar] [CrossRef]
- Hirschwehr, R.; Heppner, C.; Spitzauer, S.; Sperr, W.R.; Valent, P.; Bergerd, U.; Horak, F.; Jäger, S.; Kraft, D.; Valenta, R. Identification of common allergenic structures in mugwort and ragweed pollen. J. Allergy Clin. Immunol. 1998, 101, 196–206. [Google Scholar] [CrossRef]
- Ortiz, J.C.G.; Martin, P.C.; Lopez-Asunsolo, A. Allergy to foods in patients monosensitized to Artemisia pollen. Allergy 1996, 51, 927–931. [Google Scholar] [CrossRef]
- Oteros, J.; Bartusel, E.; Alessandrini, F.; Núñez, A.; Moreno, D.A.; Behrendt, H.; Schmidt-Weber, C.; Traidl-Hoffmann, C.; Buters, J. Artemisia pollen is the main vector for airborne endotoxin. J. Allergy Clin. Immunol. 2019, 143, 369–377. [Google Scholar] [CrossRef]
- Grewling, Ł.; Bogawski, P.; Kostecki, Ł.; Nowak, M.; Szymańska, A.; Frątczak, A. Atmospheric exposure to the major Artemisia pollen allergen (Art v 1): Seasonality, impact of weather, and clinical implications. Sci. Total Environ. 2020, 713, 136611. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants and Herbs of Eastern and Central North America; Peterson, R.T., Ed.; Houghton Mifflin Harcourt: Boston, MA, USA, 2000; ISBN 0395988144, 9780395988145. [Google Scholar]
- Czygan, F.-C. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis; Wichtl, M., Bisset, N.G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0849319617, 9780849319617. [Google Scholar]
- Paulsen, E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Derm. 2017, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Derm. 2002, 47, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kurz, G.; Rapaport, M.J. External/internal allergy to plants (Artemisia). Contact Derm. 1979, 5, 407–408. [Google Scholar] [CrossRef]
- Wu, P.; He, Y.; Zeng, Z.; Yang, Z.; Li, Y. Allergic contact dermatitis by Artemisia: Report of two cases. Contact Derm. 2020, 83, 31–32. [Google Scholar] [CrossRef]
- Mitchell, J.C.; Dupuis, G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br. J. Dermatol. 1971, 84, 139–150. [Google Scholar] [CrossRef]
- Haw, S.; Cho, H.-R.; Lee, M.-H. Allergic contact dermatitis associated with mugwort (Artemisia vulgaris). Contact Derm. 2010, 62, 61–63. [Google Scholar] [CrossRef]
- Zeller, W.; de Gols, M.; Hausen, B.M. The sensitizing capacity of Compositae plants—VI: Guinea pig sensitization experiments with ornamental plants and weeds using different methods. Arch. Dermatol. Res. 1984, 277, 28–35. [Google Scholar] [CrossRef]
- Park, Y.M. Relationship between sensitization to outdoor aeroallergen and month of birth. Pediatr. Allergy Respir. Dis. 2016, 15, 257–262. [Google Scholar] [CrossRef]
- Lundh, K.; Hindsén, M.; Gruvberger, B.; Möller, H.; Svensson, Å.; Bruze, M. Contact allergy to herbal teas derived from Asteraceae plants. Contact Derm. 2006, 54, 196–201. [Google Scholar] [CrossRef]
- Amorim, M.H.R.; Gil Da Costa, R.M.; Lopes, C.; Bastos, M.M.S.M. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Denisow-Pietrzyk, M.; Pietrzyk, Ł.; Denisow, B. Asteraceae species as potential environmental factors of allergy. Environ. Sci. Pollut. Res. 2019, 26, 6290–6300. [Google Scholar] [CrossRef] [PubMed]
- Weisbord, S.D.; Soule, J.B.; Kimmel, P.L. Poison on line—Acute renal failure caused by oil of wormwood purchased through the internet. N. Engl. J. Med. 1997, 337, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Padosch, S.A.; Lachenmeier, D.W.; Kröner, L.U. Absinthism: A fictitious 19th century syndrome with present impact. Subst. Abus. Treat. Prev. Policy 2006, 1, 14. [Google Scholar] [CrossRef][Green Version]
- Lachenmeier, D.W.; Uebelacker, M. Risk assessment of thujone in foods and medicines containing sage and wormwood—Evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 2010, 58, 437–443. [Google Scholar] [CrossRef]
- European Parliament and Council. Available online: https://eur-lex.europa.eu/eli/reg/2008/1334/oj (accessed on 12 October 2020).
- European Commission Health and Consumer Protection Directorate-General. Opinion of the Scientific Committee on Food on Thujone. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/fs_food-improvement-agents_flavourings-out162.pdf (accessed on 14 October 2020).
- Community Herbal Monograph on Artemisia absinthium L., Herba. EMA/HMPC/234463/2008. 2009. Available online: http://golbid.com/wp-content/uploads/2017/09/artemisia-absinthium.pdf (accessed on 12 October 2020).
- De Boer, H.J.; Cotingting, C. Medicinal plants for women’s healthcare in southeast Asia: A meta-analysis of their traditional use, chemical constituents, and pharmacology. J. Ethnopharmacol. 2014, 151, 747–767. [Google Scholar] [CrossRef]
- Almasad, M.M.; Qazan, W.S.; Daradka, H. Reproductive toxic effects of Artemisia herba alba ingestion in female Spague-dawley rats. Pak. J. Biol. Sci. 2007, 10, 3158–3161. [Google Scholar] [CrossRef][Green Version]
- Abolaji, A.O.; Eteng, M.U.; Ebong, P.E.; Brisibe, E.A.; Dar, A.; Kabir, N.; Choudhary, M.I. A safety assessment of the antimalarial herb Artemisia annua during pregnancy in wistar rats. Phyther. Res. 2013, 27, 647–654. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Eteng, M.U.; Ebong, P.E.; Dar, A.; Farombi, E.O.; Choudhary, M.I. Artemisia annua as a possible contraceptive agent: A clue from mammalian rat model. Nat. Prod. Res. 2014, 28, 2342–2346. [Google Scholar] [CrossRef]
- Laadraoui, J.; Aboufatima, R.; El Gabbas, Z.; Ferehan, H.; Bezza, K.; Ait Laaradia, M.; Marhoume, F.; Wakrim, E.M.; Chait, A. Effect of Artemisia herba-alba consumption during pregnancy on fertility, morphological and behaviors of mice offspring. J. Ethnopharmacol. 2018, 226, 105–110. [Google Scholar] [CrossRef]
- Oliaee, D.; Boroushaki, M.T.; Oliaee, N.; Ghorbani, A. Evaluation of cytotoxicity and antifertility effect of Artemisia kopetdaghensis. Adv. Pharmacol. Sci. 2014, 2014, 745760. [Google Scholar] [CrossRef] [PubMed]
- Rabl, W.; Katzgraber, F.; Steinlechner, M. Camphor ingestion for abortion (case report). Forensic Sci. Int. 1997, 89, 137–140. [Google Scholar] [CrossRef]
- Gomes, C.; Boareto, A.C.; Dalsenter, P.R. Clinical and non-clinical safety of artemisinin derivatives in pregnancy. Reprod. Toxicol. 2016, 65, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Taylor, T.E.; Kamchonwongpaisan, S. Artemisinin and the antimalarial endoperoxides: From herbal remedy to targeted chemotherapy. Microbiol. Rev. 1996, 60, 301–315. [Google Scholar] [CrossRef]
- Alkadi, H.O. Antimalarial drug toxicity: A review. Chemotherapy 2007, 53, 385–391. [Google Scholar] [CrossRef]
- Adjuik, M.; Babiker, A.; Garner, P.; Olliaro, P.; Taylor, W.; White, N.; International Artemisinin Study Group. Artesunate combinations for treatment of malaria: Meta-analysis. Lancet 2004, 363, 9–17. [Google Scholar] [CrossRef]
- Staedke, S.G.; Jagannathan, P.; Yeka, A.; Bukirwa, H.; Banek, K.; Maiteki-Sebuguzi, C.; Clark, T.D.; Nzarubara, B.; Njama-Meya, D.; Mpimbaza, A.; et al. Monitoring antimalarial safety and tolerability in clinical trials: A case study from Uganda. Malar. J. 2008, 7, 107. [Google Scholar] [CrossRef]
- Ribeiro, I.R.; Olliaro, P. Safety of artemisinin and its derivatives a review of published and unpublished clinical trials. Med. Trop. 1998, 58, 50–53. [Google Scholar]
- Efferth, T.; Kaina, B. Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 2010, 40, 405–421. [Google Scholar] [CrossRef]
- Saeed, M.; Breuer, E.; Hegazi, M.A.; Efferth, T. Retrospective study of small pet tumors treated with Artemisia annua and iron. Int. J. Oncol. 2020, 56, 123–138. [Google Scholar] [CrossRef]
- Hunt, S.; Stebbings, S.; McNamara, D. An open-label six-month extension study to investigate the safety and efficacy of an extract of Artemisia annua for managing pain, stiffness and functional limitation associated with osteoarthritis of the hip and knee. N. Z. Med. J. 2016, 129, 97–102. [Google Scholar] [PubMed]
- Stebbings, S.; Beattie, E.; McNamara, D.; Hunt, S. A pilot randomized, placebo-controlled clinical trial to investigate the efficacy and safety of an extract of Artemisia annua administered over 12 weeks, for managing pain, stiffness, and functional limitation associated with osteoarthritis of the hip and knee. Clin. Rheumatol. 2016, 35, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Daddy, N.B.; Kalisya, L.M.; Bagire, P.G.; Watt, R.L.; Towler, M.J.; Weathers, P.J. Artemisia annua dried leaf tablets treated malaria resistant to ACT and i.v. artesunate: Case reports. Phytomedicine 2017, 32, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Mendez, V.M.; Puebla-Perez, A.M.; Sanchez-Pena, M.J.; Gonzalez-Ortiz, L.J.; Martinez-Abundis, E.; Gonzalez-Ortiz, M. Effect of Artemisia dracunculus administration on glycemic control, insulin sensitivity, and insulin secretion in patients with impaired glucose tolerance. J. Med. Food. 2016, 19, 481–485. [Google Scholar] [CrossRef]
- Choi, J.Y.; Shin, S.K.; Jeon, S.M.; Jeong, T.; Baek, N.I.; Chung, H.G.; Lee, K.T.; Lee, M.K.; Choi, M.S. Dose–response study of sajabalssuk ethanol extract from Artemisia princeps Pampanini on blood glucose in subjects with impaired fasting glucose or mild type 2 diabetes. J. Med. Food. 2011, 14, 101–107. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, M.; Zhai, X.; Huang, Y.; Khalid, A.; Malik, A.; Shah, P.; Karim, S.; Azhar, S.; Hou, X. Effect of Gymnema sylvestre, Citrullus colocynthis and Artemisia absinthium on blood glucose and lipid profile in diabetic human. Acta Pol. Pharm. 2015, 72, 981–985. [Google Scholar]
- Basiri, Z.; Zeraati, F.; Esna-Ashari, F.; Mohammadi, F.; Razzaghi, K.; Araghchian, M.; Moradkhani, S. Topical effects of Artemisia absinthium ointment and liniment in comparison with piroxicam gel in patients with knee joint osteoarthritis: A randomized double-blind controlled trial. Iran. J. Med. Sci. 2017, 42, 524–531. [Google Scholar]
- Hatziieremia, S.; Gray, A.I.; Ferro, V.A.; Paul, A.; Plein, R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signaling pathways in monocytes/macro- phages. Br. J. Pharmacol. 2006, 149, 188–198. [Google Scholar] [CrossRef]
- Krebs, S.; Omer, T.N.; Omer, B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease–A controlled clinical trial. Phytomedicine 2010, 17, 305–309. [Google Scholar] [CrossRef]
- Ogawa, R.; Hyakusoku, H.; Ogawa, N.; Nakao, C. Effectiveness of mugwort lotion for the treatment of post-burn hypertrophic scars. JPARS 2008, 61, 210–212. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Lv, W.-L.; Li, J.-M.; Zhang, T.-T.; Zhou, W.-H.; Zhang, Q.; Wang, J.-C.; Wang, Q.-N.; Zhang, R.X.; Zhao, X.; et al. Efficacy and safety of Yin Qi San Huang antiviral decoction in chronic hepatitis B: Study protocol for a randomized, placebo controlled, double-blinded trial. Trials 2020, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Wang, X.; Wei, Q.; Zhao, C.; Xing, Z.; Zhang, Q.; Meng, J.; Zhang, S.; Zhou, H.; Mak, R.; et al. Artemisia Annua sublingual immunotherapy for seasonal allergic rhinitis: A multicenter, randomized trial. WAO J. 2020, 13, 100458. [Google Scholar] [CrossRef] [PubMed]
- Remberg, P.; Björk, L.; Hedner, T.; Sterner, O. Characteristics, clinical effect profile and tolerability of a nasal spray preparation of Artemisia abrotanum L. for allergic rhinitis. Phytomedicine 2004, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Munyangi, J.; Cornet-Vernet, L.; Idumbo, M.; Lu, C.; Lutgen, P.; Perronne, C.; Ngombe, N.; Bianga, J.; Mupenda, B.; Lalukala, P.; et al. Artemisia annua and Artemisia afra tea infusions vs. artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial. Phytomedicine 2019, 57, 49–56. [Google Scholar] [CrossRef]
- Munyangi, J.; Cornet-Vernet, L.; Idumbo, M.; Lud, C.; Lutgen, P.; Perronne, C.; Ngombe, N.; Bianga, J.; Mupenda, B.; Lalukala, P.; et al. Effect of Artemisia annua and Artemisia afra tea infusions on schistosomiasis in a large clinical trial. Phytomedicine 2018, 51, 233–240. [Google Scholar] [CrossRef]
- Gillibert, A.; Stephane, J.; Yves, H.; Xavier, A.; Jordi, L.; Erice, C.; Gaudart Jean, G. TEMPORARY REMOVAL: Comment on A. annua and A. afra infusions vs. Artesunate-amodiaquine (ASAQ) in treating Plasmodium falciparum malaria in a large scale, double blind, randomized clinical trial. Phytomedicine 2019, 59, 152981. [Google Scholar] [CrossRef]
- Farage, M.A. The prevalence of sensitive skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef]
- Zyad, A.; Tilaoui, M.; Jaafari, A.; Oukerrou, M.A.; Mouse, H.A. More insights into the pharmacological effects of artemisinin. Phytother. Res. 2017, 32, 1–14. [Google Scholar] [CrossRef]
- Yu, J.; Wang, G.; Jiang, N. Study on the repairing effect of cosmetics containing Artemisia annua on sensitive skin. J. Cosmet. Dermatol. 2020, 10, 8–19. [Google Scholar] [CrossRef][Green Version]
- El-Askarya, H.I.; Mohamed, S.S.; El-Gohari, H.M.A.; Ezzata, S.M.; Meselhya, M.R. Quinic acid derivatives from Artemisia annua L. leaves; biological activities and seasonal variation. S. Afr. J. Bot. 2020, 128, 200–208. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.B.; Muñoz-Sanchez, A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Martino, V.S. Overview. In Sesquiterpene Lactones: Advances in their Chemistry and Biological Aspects; Sülsen, V.P., Martino, V.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar] [CrossRef]
- Moujir, L.M.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of sesquiterpene lactones: A review of some potential success cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Tu, Y. From Artemisia annua L. to Artemisinins: The Discovery and Development of Artemisinins and Antimalarial Agents; Academic Press: London, UK, 2017; 468p, ISBN 9780128116562. [Google Scholar]
- Letchmanan, K.; Shen, S.; Ng, W.K.; Tan, R.B. Application of transglycosylated stevia and hesperidin as drug carriers to enhance biopharmaceutical properties of poorly-soluble artemisinin. Colloids Surf. B Biointerfaces 2018, 161, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Malaria. In Antibiotics and Chemotherapy, 9th ed.; Elsevier: Philadelphia, PA, USA, 2010; pp. 809–822. [Google Scholar] [CrossRef]
- Konstat-Korzenny, E.; Ascencio-Aragón, J.A.; Niezen-Lugo, S.; Vázquez-López, R. Artemisinin and its synthetic derivatives as a possible therapy for cancer. Med. Sci. 2018, 6, 19. [Google Scholar] [CrossRef]
- Li, Y. Artemisinin and derivatives pharmacodynamics, toxicology, pharmacokinetics, mechanism of action, resistance, and immune regulation. In Artemisinin-Based and Other Antimalarials; Li, G., Li, Y., Li, Z., Zeng, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 5; pp. 197–351. [Google Scholar] [CrossRef]
- Saeed, M.; Krishna, S.; Greten, H.J.; Kremsner, P.G.; Efferth, T. Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res. 2016, 110, 216–226. [Google Scholar] [CrossRef]
- Pérez del Villar, L.; Burguillo, F.J.; López-Abán, J.; Muro, A. Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS ONE 2012, 7, e45867. [Google Scholar] [CrossRef]
- Naß, J.; Efferth, T. The activity of Artemisia spp. and their constituents against Trypanosomiasis. Phytomedicine 2018, 47, 184–191. [Google Scholar] [CrossRef]
- Charlie-Silva, I.; Fernandes Fraceto, L.; Ferreira Silva de Melo, N. Progress in nano-drug delivery of artemisinin and its derivatives: Towards to use in immunomodulatory approaches. Artif. Cells Nanomed. Biotechnol. 2018, 4, S611–S620. [Google Scholar] [CrossRef]
- Hou, L.; Huang, H. Immune suppressive properties of artemisinin family drugs. Pharmacol. Ther. 2016, 166, 123–127. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Wong, Y.K.; Li, Y.; Liao, F.; Jiang, T.; Tu, Y.Y. Artemisinin, the magic drug discovered from Traditional Chinese Medicine. Engineering 2019, 5, 32–39. [Google Scholar] [CrossRef]
- Ajeigbe, K.O.; Emikpe, B.O.; Olaleye, S.B. Effects of artemisinin, with or without lumefantrine and amodiaquine on gastric ulcer healing in rat. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, F.M.; Kaboutari, J.; Zendehdel, M.; Javdani, M. The antinociceptive effect of artemisinin on the inflammatory pain and role of GABAergic and opioidergic systems. Korean J. Pain 2019, 32, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Mao, X.; Sun, Y.; Cui, H. Antibacterial mechanism of artemisinin/beta-cyclodextrins against methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2018, 118, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.H.J.; Tan, D.W.S.; Wong, F.W.S.; Tran, T. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol. Res. 2020, 158, 1049012. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Du, H.; Chang, C.; Li, X. Artemisinin reduces atherosclerosis in apolipoprotein E deficient mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40 (Suppl. 1), A346. [Google Scholar] [CrossRef]
- Jiang, W.; Cen, Y.; Song, Y.; Li, P.; Qin, R.; Liu, C.; Zhao, Y.; Zheng, J.; Zhou, H. Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine 2016, 23, 1259–1266. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Kaur, G. Pharmacological and analytical aspects of artemisinin for malaria: Advances and challenges. Asian Pac. J. Trop. Med. 2019, 12, 339–346. [Google Scholar] [CrossRef]
- Rudrapal, M.; Chetia, D. Endoperoxide antimalarials: Development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold. Drug Des. Devel. Ther. 2016, 10, 3575–3590. [Google Scholar] [CrossRef]
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their growing importance in medicine. Trends Pharmacol. Sci. 2008, 29, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, D.; Goswami, A.; Saikia, P.P.; Barua, N.C.; Rao, P.G. Artemisinin and its derivatives: A novel class of anti-malarial and anti-cancer agents. Chem. Soc. Rev. 2010, 39, 435–454. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A. The molecular mechanism of action of artemisinin—The debate continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Ganguly, S.; Saha, P.; Chatterjee, M. Efficacy of artemisinin in experimental visceral leishmaniasis. Int. J. Antimicrob. Agents 2010, 36, 43–49. [Google Scholar] [CrossRef]
- WHO. The World Malaria Report 2018; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/malaria/publications/world-malaria-report-2018/en/ (accessed on 26 October 2020).
- Muangphrom, P.; Seki, H.; Fukushima, E.O.; Muranaka, T. Artemisinin-based antimalarial research: Application of biotechnology to the production of artemisinin, its mode of action, and the mechanism of resistance of Plasmodium Parasites. J. Nat. Med. 2016, 70, 318–334. [Google Scholar] [CrossRef]
- Li, Q.; Weina, P. Artesunate: The best drug in the treatment of severe and complicated malaria. Pharmaceuticals 2010, 3, 2322–2332. [Google Scholar] [CrossRef]
- Noubiap, J.J. Shifting from quinine to artesunate as first-line treatment of severe malaria in children and adults: Saving more lives. J. Infect. Public Health. 2014, 7, 407–412. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Fanello, C.I.; Hendriksen, I.C.E.; Gomes, E.; Seni, A.; Chhaganlal, K.D.; Bojang, K.; Olaosebikan, R.; Anunobi, N.; AQUAMAT Group; et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open-label, randomised trial. Lancet 2010, 376, 1647–1657. [Google Scholar] [CrossRef]
- Ferrari, G.F.; Ntuku, H.M.; Burri, C.; Tshefu, A.K.; Duparc, S.; Hugo, P.; Mitembo, D.K.; Rossi, A.; Ngwala, P.L.; Luwawu, J.N.; et al. An operational comparative study of quinine and artesunate for the treatment of severe malaria in hospitals and health centres in the Democratic Republic of Congo: The MATIAS study. Malar. J. 2015, 14, 226. [Google Scholar] [CrossRef]
- Warsame, M.; Gyapong, M.; Mpeka, B.; Rodrigues, A.; Singlovic, J.; Babiker, A.; Mworozi, E.; Agyepong, I.; Ansah, E.; Azairwe, R.; et al. Pre-referral rectal artesunate treatment by community- based treatment providers in Ghana, Guinea-Bissau, Tanzania, and Uganda (Study 18): A cluster-randomized trial. Clin. Infect. Dis. 2016, 63, 312–321. [Google Scholar] [CrossRef]
- Dobaño, C.; Nhabomba, A.J.; Manaca, M.N.; Berthoud, T.; Aguilar, R.; Quintó, L.; Barbosa, A.; Rodríguez, M.H.; Jiménez, A.; Groves, P.L.; et al. A Balanced Proinflammatory and Regulatory Cytokine Signature in Young African Children Is Associated With Lower Risk of Clinical Malaria. Clin. Infect. Dis. 2019, 69, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Manna, S.; Saha, B.; Hati, A.K.; Roy, S. Novel pfkelch13 gene polymorphism associates with artemisinin resistance in Eastern India. Clin. Infect. Dis. 2019, 69, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Kannan, D.; Yadav, N.; Ahmad, S.; Namdev, P.; Bhattacharjee, S.; Lochab, B.; Singh, A. Pre-clinical study of iron oxide nanoparticles fortified artesunate for efficient targeting of malarial parasite. EBioMedicine 2019, 45, 261–277. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/docs/default-source/documents/publications/gmp/guidelines-for-the-treatment-of-malaria-eng.pdf (accessed on 26 October 2020).
- Pull, L.; Lupoglazoff, J.M.; Beardmore, M.; Michel, J.F.; Buffet, P.; Bouchaud, O.; Siriez, J.Y. Artenimol–piperaquine in children with uncomplicated imported falciparum malaria: Experience from a prospective cohort. Malar. J. 2019, 18, 419. [Google Scholar] [CrossRef]
- Leblanc, C.; Vasse, C.; Minodier, P.; Mornand, P.; Naudin, J.; Quinet, B.; Siriez, J.Y.; Sorged, F.; de Suremain, N.; Thellier, M.; et al. Management and prevention of imported malaria in children. Update of the French guidelines. Med. Mal. Infect. 2020, 50, 127–140. [Google Scholar] [CrossRef]
- Lingani, M.; Bonkian, L.N.; Yerbanga, I.; Kazienga, A.; Valéa, I.; Sorgho, H.; Ouédraogo, J.B.; Mens, P.F.; Schallig, H.D.F.H.; Ravinetto, R.; et al. In vivo/ex vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine as first-line treatment for uncomplicated falciparum malaria in children: An open label randomized controlled trial in Burkina Faso. Malar. J. 2020, 19, 8–13. [Google Scholar] [CrossRef]
- Banda, C.G.; Chaponda, M.; Mukaka, M.; Mulenga, M.; Hachizovu, S.; Kabuya, J.B.; Mulenga, J.; Sikalima, J.; Kalilani-Phiri, L.; Terlouw, D.J.; et al. Efficacy and safety of artemether–lumefantrine as treatment for Plasmodium falciparum uncomplicated malaria in adult patients on efavirenz-based antiretroviral therapy in Zambia: An open label non-randomized interventional trial. Malar. J. 2019, 18, 180. [Google Scholar] [CrossRef]
- Commons, R.J.; Simpson, J.A.; Thriemer, K.; Abreha, T.; Adam, I.; Anstey, N.M.; Assefa, A.; Awab, G.R.; Baird, K.J.; Barber, B.E.; et al. The efficacy of dihydroartemisinin-piperaquine and artemether-lumefantrine with and without primaquine on Plasmodium vivax recurrence: A systematic review and individual patient data meta-analysis. PLoS Med. 2019, 16, 1002928. [Google Scholar] [CrossRef]
- Ballard, S.-B.; Salinger, A.; Arguin, P.M.; Desai, M.; Tan, K.R. Mphc Updated CDC Recommendations for Using Artemether-Lumefantrine for the Treatment of Uncomplicated Malaria in Pregnant Women in the United States. Morb. Mortal. Wkly. Rep. 2018, 67, 424–431. [Google Scholar] [CrossRef]
- D’Alessandro, U.; Hill, J.; Tarning, J.; Pell, C.; Webster, J.; Gutman, J.; Sevene, E. Treatment of uncomplicated and severe malaria during pregnancy. Lancet Infect. Dis. 2018, 18, e133–e146. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, N.; Saliba, A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, G.; Zhang, S.; Wang, D.; Prabha, P.S.; Zuo, Z. Antitumor Research on Artemisinin and Its Bioactive Derivatives. Nat. Prod. Bioprospect. 2018, 8, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [CrossRef] [PubMed]
- Dell’Eva, R.; Pfeffer, U.; Vene, R.; Anfosso, L.; Forlani, A.; Albini, A.; Efferth, T. Inhibition of angiogenesis in vivo and growth of Kaposi’s sarcoma xenograft tumors by the anti-malarial artesunate. Biochem. Pharmacol. 2004, 68, 2359–2366. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.; Wei, M. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef]
- Efferth, T. Artemisinin–Second career as anticancer drug? World J. Tradit. Chin. Med. 2015, 1, 2–25. [Google Scholar] [CrossRef]
- Bhaw-Luximon, A.; Jhurry, D. Artemisinin and its derivatives in cancer therapy: Status of progress, mechanism of action, and future perspectives. Cancer Chemother. Pharmacol. 2017, 79, 451–466. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.-M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef]
- Von Hagens, C.; Walter-Sack, I.; Goeckenjan, M.; Osburg, J.; Storch-Hagenlocher, B.; Sertel, S.; Elsässer, M.; Remppis, B.A.; Edler, L.; Munzinger, J.; et al. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res. Treat. 2017, 164, 359–369. [Google Scholar] [CrossRef]
- Trimble, C.L.; Levinson, K.; Maldonado, L.; Donovan, M.J.; Clark, K.T.; Fu, J.; Shay, M.E.; Sauter, M.E.; Sanders, S.A.; Frantz, P.S.; et al. A first-in-human proof-of-concept trial of intravaginal artesunate to treat cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol. Oncol. 2020, 157, 188–194. [Google Scholar] [CrossRef]
- Burlec, A.F.; Cioancă, O.; Enache, L.C.; Hăncianu, M. Promising biological activities of sesquiterpene lactones. Med. Surg. J. 2017, 121, 645–652. [Google Scholar]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.L.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. EBioMedicine 2015, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Panwar, V.K. Case report of a pituitary macroadenoma treated with artemether. Integr. Cancer Ther. 2006, 5, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.H.; Adoubi, I.; Comoe, K.; de Cnodder, T.; Jansen, N.; Tschulakow, A.; Efferth, T. First study of oral artenimol-R in advanced cervical cancer: Clinical benefit, tolerability and tumor markers. Anticancer Res. 2011, 31, 4417–4422. [Google Scholar]
- Wang, S.J.; Gao, Y.; Chen, H.; Kong, R.; Jiang, H.C.; Pan, S.H.; Xue, D.-B.; Bai, X.-W.; Sun, B. Dihydroartemisinin inactivates NF-κB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett. 2010, 293, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Tilaoui, M.; Mouse, H.A.; Jaafari, A.; Zyad, A. Differential effect of artemisinin against cancer cell lines. Nat. Prod. Bioprospect. 2014, 4, 189–196. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yu, S.-P.; Miao, L.-Y.; Huanh, X.-Y.; Zhang, X.-P.; Zhu, Y.-P.; Xia, X.-H. Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: A randomized controlled trial. Chin. J. Integr. Med. 2008, 6, 134–138. [Google Scholar] [CrossRef]
- Liu, W.M.; Gravett, A.M.; Dalgleish, A.G. The antimalarial agent artesunate possesses anticancer properties that can be enhanced by combination strategies. Int. J. Cancer 2011, 128, 1471–1480. [Google Scholar] [CrossRef]
- Beck, J.; Schwarzer, A.; Gläser, D.; Mügge, L.-O.; Uhlig, J.; Heyn, S.; Kragl, B.; Mohren, M.; Hoffmann, F.A.; Lange, T.; et al. Lenalidomide in combination with bendamustine and prednisolone in relapsed/refractory multiple myeloma: Results of a phase 2 clinical trial (OSHO-#077). J. Cancer Res. Clin. Oncol. 2017, 143, 2545–2553. [Google Scholar] [CrossRef]
- Singh, N.P.; Verma, K.B. Case report of a laryngeal squamous cell carcinoma treated with artesunate. Arch. Oncol. 2002, 10, 279–280. [Google Scholar] [CrossRef]
- Berger, T.G.; Dieckmann, D.; Efferth, T.; Schultz, E.S.; Funk, J.O.; Baur, A.; Schuler, G. Artesunate in the treatment of metastatic uveal melanoma--first experiences. Oncol. Rep. 2005, 14, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, C.; Chen, P.; Cheng, B.; Cheng, Y. RACKI induces chemotherapy resistance in esophageal carcinoma by upregulating the PI3K/AKT pathway and Bcl-2 expression. Oncol. Targets Ther. 2018, 11, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Reungpatthanaphong, P.; Mankhetkorn, S. Modulation of multidrug resistance by artemisinin, artesunate and dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines. Biol. Pharm. Bull. 2002, 25, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Kahler, M. Ueber einen neuen Stoff im Semen Cinae. Arch. Pharm. 2006, 34, 318–319. [Google Scholar] [CrossRef]
- Sharma, S.; Anand, N. Natural Products. In Pharmacochemistry Library Approaches to Design and Synthesis of Antiparasitic Drugs; Sharma, S., Anand, N., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 25, Chapter 3; pp. 71–123. [Google Scholar] [CrossRef]
- Marshall, J.A.; Wuts, P.G.M. Stereocontrolled total synthesis of alpha- and beta-santonin. J. Org. Chem. 1978, 43, 1086–1089. [Google Scholar] [CrossRef]
- Birladeanu, L. The stories of santonin and santonic acid. Angew. Chem. Int. Ed. 2003, 42, 1202–1208. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Moss, G.P.; Whittle, J.A. Investigations on the biosynthesis of steroids and terpenoids. Part I A preliminary study of the biosynthesis of santonin. J. Chem. Soc. C 1968, 1813–1818. [Google Scholar] [CrossRef]
- De Kraker, J.W.; Franssen, M.C.; Dalm, M.C.; de Groot, A.; Bouwmeester, H.J. Biosynthesis of germacrene A carboxylic acid in chicory roots. Demonstration of a cytochrome P450 (+)-germacrene a hydroxylase and NADP+-dependent sesquiterpenoid dehydrogenase(s) involved in sesquiterpene lactone biosynthesis. Plant Physiol. 2001, 125, 1930–1940. [Google Scholar] [CrossRef]
- Wynn, S.G.; Fougère, B.J. Veterinary herbal medicine: A systems-based approach. In Veterinary Herbal Medicine; Wynn, S.G., Fougère, B.J., Eds.; Mosby: Saint Louis, MO, USA, 2007; Chapter 20; pp. 291–409. ISBN 978-0-323-02998-8. [Google Scholar]
- Sakipova, Z.; Wong, N.S.H.; Bekezhanova, T.; Sadykova; Shukirbekova, A.; Boylan, F. Quantification of santonin in eight species of Artemisia from Kazakhstan by means of HPLC-UV: Method development and validation. PLoS ONE 2017, 12, 173714. [Google Scholar] [CrossRef]
- Yang, D.-Z.; Du, L.-D.; Lu, Y. Santonin. In Natural Small Molecule Drugs from Plants; Du, G.-H., Ed.; PMPH Springer: Singapore, 2018; pp. 619–624. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Zheng, Q. Development and application of anthelminthic drugs in China. Acta Trop. 2019, 200, 105181. [Google Scholar] [CrossRef]
- Xiao, S.-H. Progress in anthelmintic agent study since the founding of the People’s Republic of China and current challenges. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2009, 27, 383–389. [Google Scholar] [PubMed]
- Yu, C.; Ai, D.; Lin, R.; Cheng, S. Effects of toxic β-glucosides on carbohydrate metabolism in cotton bollworm, Helicoverpa armigera (Hübner). Arch. Insect Biochem. Physiol. 2019, 100, e21526. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, A. Control of insect pests in crop plants and stored food grains using plant saponins: A review. LWT 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Wang, J.; Su, S.; Zhang, S.; Zhai, S.; Sheng, R.; Wu, W.; Guo, R. Structure-activity relationship and synthetic methodologies of α-santonin derivatives with diverse bioactivities: A mini-review. Eur. J. Med. Chem. 2019, 175, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Song, J.H.; Choi, B.G.; Kim, H.-J.; Kim, T.S. Chemical modification of santonin into a diacetoxy acetal form confers the ability to induce differentiation of human promyelocytic leukemia cells via the down-regulation of NF-kappaB DNA binding activity. J. Biol. Chem. 2006, 281, 13117–13125. [Google Scholar] [CrossRef] [PubMed]
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.D.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of α-santonin. Eur. J. Med. Chem. 2013, 63, 279–289. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Acharjee, N. A Molecular electron density theory study of the chemoselectivity, regioselectivity, and diastereofacial selectivity in the synthesis of an anticancer spiroisoxazoline derived from α-santonin. Molecules 2019, 24, 832. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Yu, Z.; Cheng, Z.; Yuan, H.; Zhao, Z.; Wu, G.; Xie, N.; Yuan, X.; Sun, Q.; et al. Synthesis and biological evaluation of α-santonin derivatives as anti-hepatoma agents. Eur. J. Med. Chem. 2018, 149, 90–97. [Google Scholar] [CrossRef]
- Chinthakindi, P.K.; Singh, J.; Gupta, S.; Nargotra, A.; Mahajan, P.; Kaul, A.; Ahmed, Z.; Koul, S.; Sangwan, P.L. Synthesis of α-santonin derivatives for diminutive effect on T and B-cell proliferation and their structure activity relationships. Eur. J. Med. Chem. 2017, 127, 1047–1058. [Google Scholar] [CrossRef]
- Quach, H.T.; Kondo, T.; Watanabe, M.; Tamura, R.; Yajima, Y.; Sayama, S.; Ando, M.; Kataoka, T. Eudesmane-type sesquiterpene lactones inhibit nuclear translocation of the nuclear factor κB subunit RelB in response to a lymphotoxin β stimulation. Biol. Pharm. Bull. 2017, 40, 1669–1677. [Google Scholar] [CrossRef]
- Coricello, A.; El-Magboub, A.; Luna, M.; Ferrario, A.; Haworth, I.S.; Gomer, C.J.; Aiello, F.; Adams, J.D. Rational drug design and synthesis of new α-santonin derivatives as potential COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Filomena, P.; Luca, F.; Ian, H.; Matteo, B.; Asma, E.; Angela, F.; Charles, G.; Francesca, A.; David, A.J. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, 1366. [Google Scholar] [CrossRef]
- Chen, H.; Wu, G.; Gao, S.; Guo, R.; Zhao, Z.; Yuan, H.; Liu, S.; Wu, J.; Lu, X.; Yuan, X.; et al. Discovery of potent small-molecule inhibitors of ubiquitin-conjugating enzyme UbcH5c from α-santonin derivatives. J. Med. Chem. 2017, 60, 6828–6852. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.; Schiopu, A.; Wood, K.J. Role of T cells in graft rejection and transplantation tolerance. Expert Rev. Clin. Immunol. 2010, 6, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Dangroo, N.A.; Singh, J.; Gupta, N.; Singh, S.; Kaul, A.; Khuroo, M.A.; Sangwan, P.L. T- and B-cell immunosuppressive activity of novel α-santonin analogs with humoral and cellular immune response in Balb/c mice. MedChemComm 2017, 8, 211–219. [Google Scholar] [CrossRef]
- White, E.H.; Winter, R.E.K. Natural products from Achillea lanulosa. Tetrahedron Lett. 1963, 4, 137–140. [Google Scholar] [CrossRef]
- White, E.H.; Marx, J.N. The synthesis and stereochemistry of deacetoxymatricarin and achillin. J. Am. Chem. Soc. 1967, 89, 5511–5513. [Google Scholar] [CrossRef]
- Kim, Y.S.; Bahn, K.N.; Hah, C.K.; Gang, H.I.; Ha, Y.L. Inhibition of 7,12-dimethylbenz[a]anthracene induced mouse skin carcinogenesis by Artemisia capillaris. J. Food Sci. 2008, 73, T16–T20. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Hou, P.; Wen, G.; Gao, Y. Physiological responses to allelopathy of aquatic stem and leaf extract of Artemisia frigida in seedling of several pasture plants. Acta Ecol. Sin. 2010, 30, 2197–2204. [Google Scholar]
- Kang, T.H.; Pae, H.O.; Jeong, S.J.; Yoo, J.C.; Choi, B.M.; Jun, C.D.; Chung, H.T.; Miyamoto, T.; Higuchi, R.; Kim, Y.C. Scopoletin: An inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med. 1999, 65, 400–403. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Anaya-Eugenio, G.; Pérez-Vásquez, A.; Martínez, A.L.; Mata, R. Quantitative analysis and pharmacological effects of Artemisia ludoviciana aqueous extract and compounds. Nat. Prod. Comm. 2017, 12, 1531–1534. [Google Scholar] [CrossRef]
- Ho, C.; Choi, E.J.; Yoo, G.S.; Kim, K.-M.; Ryu, S.Y. Desacetylmatricarin, an anti-allergic component from Taraxacum platycarpum. Planta Med. 1998, 64, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, A.M.; Yusufoglu, H.S.; Salkini, M.A.A.; Alam, A. New cytotoxic sesquiterpene lactones from Anthemis scrobicularis. J. Asian Nat. Prod. Res. 2014, 16, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Vera, W.J.; Gonzalez, N.C. Synthesis of terpenoid compounds from α-santonin. Tetrahedron 1993, 49, 4761–4788. [Google Scholar] [CrossRef]
- Blust, M.H.; Hopkins, T.L. Gustatory responses of a specialist and a generalist grasshopper to terpenoids of Artemisia ludoviciana. Entomol. Exp. Appl. 1987, 45, 37–46. [Google Scholar] [CrossRef]
- Sanchez-Carranza, J.N.; González-Maya, L.; Razo-Hernández, R.S.; Salas-Vidal, E.; Nolasco-Quintana, N.Y.; Clemente-Soto, A.F.; García-Arizmendi, L.; Sánchez-Ramos, M.; Marquina, S.; Alvarez, L. Achillin increases chemosensitivity to paclitaxel, overcoming resistance and enhancing apoptosis in human hepatocellular carcinoma cell line resistant to paclitaxel (Hep3B/PTX). Pharmaceutics 2019, 11, 512. [Google Scholar] [CrossRef]
- Castro, V.; Murillo, R.; Klaas, C.A.; Meunier, C.; Mora, G.; Pahl, H.L.; Merfort, I. Inhibition of the transcription factor NF-κB by sesquiterpene lactones from Podachaenium eminens 1. Planta Med. 2000, 66, 591–595. [Google Scholar] [CrossRef]
- Zapata-Martínez, J.; Sánchez-Toranzo, G.; Chaín, F.; Catalán, C.A.N.; Bühler, M.I. Effect of guaianolides in the meiosis reinitiation of amphibian oocytes. Zygote 2017, 25, 10–16. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Lou, J.; Yang, X.-G.; Lu, Y.-Q.; Lu, S.-S.; Lu, K.-H. Effect of the meiotic inhibitor cilostamide on resumption of meiosis and cytoskeletal distribution in buffalo oocytes. Anim. Reprod. Sci. 2016, 174, 37–44. [Google Scholar] [CrossRef]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Chávez-Silva, F.; Navarrete-Vázquez, G.; León-Rivera, I.; Perea-Arango, I.; Villalobos-Molina, R.; Ibarra-Barajas, M. Tracheal relaxation through calcium channel blockade of Achillea millefolium hexanic extract and its main bioactive compounds. J. Ethnopharmacol. 2020, 253, 112643. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Sigari, H.; Jakupovic, J.; Grenz, M. A sesquiterpene lactone from Artemisia diffusa. Phytochemistry 1989, 28, 2723–2725. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Vahedi, M. Malaria parasites, traditional medicinal plants and artediffusin (tehranolide) as a new candidate of antimalaria agent. J. Biological. Act. Prod. Nat. 2012, 2, 200–217. [Google Scholar] [CrossRef]
- Barrero, A.F.; Rosales, A.; Cuerva, J.M.; Oltra, J.E. Unified synthesis of eudesmanolides, combining biomimetic strategies with homogeneous catalysis and free-radical chemistry. Org. Lett. 2003, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Patrushev, S.S.; Shakirov, M.M.; Shults, E.E. Synthetic transformations of sesquiterpene lactones 9. Synthesis of 13-(pyridinyl)eudesmanolides. Chem. Heterocycl. Comp. 2016, 52, 165–171. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Nahrevanian, H.; Kazemi, M. A new antimalarial agent; effect of extracts of Artemisia diffusa against Plasmodium berghei. Pharmacogn. Mag. 2009, 5, 1–7. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Nahrevanian, H.; Kazemi, M. Tehranolide, a sesquiterpene lactone with an endoperoxide group that probably has the same effect as the antimalarial agent artemisinin. Planta Med. 2009, 75, PD1. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Nahrevanian, H.; Kazemi, M. Isolation of artediffusin (tehranolide) as a new antimalarial agent. Asian J. Chem. 2011, 23, 4810–4814. [Google Scholar]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular mechanisms of drug resistance in Plasmodium falciparum malaria. Ann. Rev. Microbiol. 2020, 74, 431–454. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Faridchehr, A.; Bakhtiyar, M. Sesquiterpene Lactones of Iranian Compositae Family (Astraceae); Their Chemical Constituents and Anti-plasmodial Properties of Tehranolide (A Review). Orient. J. Chem. 2017, 33, 2188–2197. [Google Scholar] [CrossRef]
- Noori, S.; Taghikhani, M.; Hassan, Z.M.; Al-Lameh, A.; Mostafaei, A. Tehranolide could shift the immune response towards Th1 and modulate the intra-tumor infiltrated T regulatory cells. Iran. J. Immunol. 2009, 6, 216–224. [Google Scholar]
- Noori, S.; Taghikhani, M.; Hassan, Z.M.; Allameha, A.; Mostafaei, A. Tehranolide molecule modulates the immune response, reduce regulatory T cell and inhibits tumor growth in vivo. Mol. Immunol. 2010, 47, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Hassan, Z.M. Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic. Biol. Med. 2012, 52, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Hassan, Z.M. Tehranolide inhibits cell proliferation via calmodulin inhibition, PDE, and PKA activation. Tumour Biol. 2014, 35, 257–264. [Google Scholar] [CrossRef][Green Version]
- You, W.; Henneberg, M. Cancer incidence increasing globally: The role of relaxed natural selection. Evol. Appl. 2017, 11, 140–152. [Google Scholar] [CrossRef]
- Sohrabi, C.; Alsa, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Haq, F.U.; Roman, M.; Ahmad, K.; Rahman, S.U.; Shah, S.; Suleman, N.; Ullah, S.; Ahmad, I.; Ullah, W. Artemisia annua: Trials are needed for COVID-19. Phytother. Res. 2020, 34, 2423–2424. [Google Scholar] [CrossRef]
- Zhuang, W.; Fan, Z.; Chu, Y.; Wang, H.; Yang, Y.; Wu, L.; Sun, N.; Sun, G.; Shen, Y.; Lin, X.; et al. Chinese patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front. Pharmacol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhou, L.; Zhou, X.; Xie, B.; Zhang, W.; Sun, J. Prevention and treatment of COVID-19 using Traditional Chinese Medicine: A review. Phytomedicine 2020, in press. [Google Scholar] [CrossRef]
- Efferth, T.; Romero, M.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Wang, C.; Xuan, X.; Yao, W.; Huang, G.; Jin, J. Anti-profibrotic effects of artesunate on bleomycin-induced pulmonary fibrosis in Sprague Dawley rats. Mol. Med. Rep. 2015, 12, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Chen, C.; Zhang, H.-Q.; Guo, H.-Y.; Wang, H.; Wang, L.; Zhang, X.; Hua, S.-N.; Yu, J.; Xiao, P.-G.; et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005, 67, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data. Phytother. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.; Seibert, I.; Klimkait, T.; Van der Kooy, F. Ethnopharmacology in overdrive: The remarkable anti-HIV activity of Artemisia annua. J. Ethnopharmacol. 2012, 141, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Karamoddini, M.K.; Emami, S.A.; Ghannad, M.S.; Sani, E.A.; Sahebkar, A. Antiviral activities of aerial subsets of Artemisia species against herpes simplex virus type 1 (HSV1) in vitro. Asian Biomed. 2011, 5, 63–68. [Google Scholar] [CrossRef]
- Rolta, R.; Salaria, D.; Kumar, V.; Sourirajan, A.; Dev, K. Phytocompounds of Rheum emodi, Thymus serpyllum and Artemisia annua inhibit COVID-19 binding to ACE2 receptor: In silico approach. Res. Sq. 2020, in press. [Google Scholar] [CrossRef]
- Kapepula, P.M.; Kabengele, J.K.; Kingombe, M.; Van Bambeke, F.; Tulkens, P.M.; Sadiki, A.; Decloedt, E.H.; Zumla, A.; Tiberi, S.; Suleman, F.; et al. Artemisia spp. derivatives for COVID-19 treatment: Anecdotal use, political hype, treatment potential, challenges, and road map to randomized clinical trials. Am. J. Trop. Med. Hyg. 2020, 103, 960–964. [Google Scholar] [CrossRef]
| Species | Common Name | Distribution * | Edible Part | Use | Ref. |
|---|---|---|---|---|---|
| Artemisia abrotanum L. | Southernwood | S. Europe | Young shoots | Flavoring cakes, salads and vinegars; herb tea | [60,61,62,63,64,65] |
| A. absinthium | Mugwort, common wormwood, absinthe | Europe, Asia | Herb | Spice; flavoring beer, wine, vermouth, absinthe, liquors and aperitifs; pelinkovac | [60,61,62,65,66,67,68,69,70,71,72] |
| A. afra | African wormwood | Africa | Herb | Flavoring; preparation of vermouth; as a tea | [60,61,62,63,64,65,66,67] |
| Artemisia alba Turra (syn. A. camphorata Vill.) | Camphor absinthe | S. Europe, C. Europe, N.W. Africa | Herb | Spice and flavoring | [60] |
| A. annua | Qing Hao, Sweet sagewort | S.E. Europe to W. Asia. | Leaves | Essential oil in the leaves is used as a flavoring in spirits such as vermouth; as a vegetable | [67] |
| Artemisia arborescens (Vaill.) L. | Silver wormwood | N. Africa, S. Europe | Herb | Spice added to the green tea prepared by Moroccans | [60,65,67] |
| Artemisia argyi H. Lév. & Vaniot | Aicao, Gaiyou, Seomae mugwort | N. Asia, N. Europe, N. America | Leaves, buds, herb | As a tea or other forms of food supplements; dried leaves as a flavoring and colorant for the Chinese dish Qingtua | [46,73] |
| Artemisia balchanorum Krasch. | Turkmenia | Herb | Spice; potherb | [60] | |
| Artemisia capillaris Thunb. | Yin Chen Hao | E. Asia—China, Japan, Korea | Leaves, stems, shoots | Soaked and boiled eaten as food supplements in times of famine | [67,74] |
| Artemisia carvifolia Buch.-Ham. ex Roxb. | E. Asia—China, Japan, Himalayas | Leaves | Flavoring for tea and coffee; Young plants—cooked in the spring | [67] | |
| Artemisia dracunculoides Pursh. | Russian Tarragon, Tarragon, French Tarragon | N. America. N. Europe. N. Asia—Siberia | Leaves, seeds | Leaves—raw in salads; The N. American Indians bake the leaves between hot stones and then eat them with salt water; Seed—raw or cooked as an oily texture. | [62,67,68,75] |
| A. dracunculus | Tarragon, French Tarragon | S. Europe to W. Asia. | Leaves, young shoots | Leaves—raw or used as a flavoring in soups, oily foods, salads, vinegar, etc.; The young shoots can also be cooked and used as a potherb | [60,62,63,66,67,70,76] |
| Artemisia frigida Willd. | Fringed Wormwood, Prairie sagewort | N. America, N. Asia. | Leaves | The leaves are used by the Hopi Indians as a flavoring for sweet corn | [68,76] |
| A. genipi. | Genepi, black wormwart, black wormwood, génépi noir | S. Europe | Leaves, flower heads | Spice, flavoring for liqueurs | [10,60,61,66,67] |
| Artemisia glacialis L. | Glacier wormwood | C. Europe | Herb, flower heads | Flavoring in vermouth and liqueurs | [10,60,61,67] |
| Artemisia granatensis Boiss. | Spain | Herb | Herb tea | [77] | |
| Artemisia herba-alba Asso | Africa, Mediterranean area | Herb | Herb tea; Flavoring tea and coffee | [78] | |
| Artemisia indica Willd. | E. Asia—China, Japan, India. | Leaves | Young leaves—cooked and eaten with barley; the leaves pounded with steamed rice dumplings to give a flavor and coloring | [60,70] | |
| Artemisia japonica Thunb. | E. Asia—China, Japan, Korea. | Young leaves | Raw as a vegetable or cooked | [70] | |
| Artemisia keiskeana Miq. | E. Asia—China, Japan, Korea, E. Russia. | Leaves, shoot tips | Cooked | [67] | |
| Artemisia ludoviciana Nutt. | White Sage, Louisiana Sage, Prairie Sage, Western Mugwort | N. America | Leaves, flowering heads | Flavoring or garnish for sauces, gravies, etc.; Used like absinthe; herb tea | [60,61,67,75,76] |
| Artemisia maritima L. | Sea Wormwood | Europe, E. Asia, C. Asia. | Leaves | Spice; flavoring in some Danish schnapps, beer and liqueurs | [60,61,67] |
| Artemisia montana (Nakai.) Pamp. | E. Asia—China, Japan. | Leaves | Young leaves—cooked; herb tea | [79] | |
| Artemisia pallens Wall. ex DC. | Davana | N.E. India, Thailand | Herb | Spice; flavoring for cakes, pastries, candy, chewing gum, ice cream, beverages, tobacco; for production of essential oil (davana oil) | [60,61,67] |
| Artemisia pontica L. | Roman wormwood; Small absinthe | S.E. Europe to Siberia, C. Asia | Leaves, herb | Spice, flavoring, like A. absinthium | [60,61,66,67] |
| A. princeps | Mugwort mochi, Yomogi | E. Asia—China, Japan, Korea. | Leaves, young seedlings | Raw or cooked in salads and soups; for flavoring and coloring of rice dumplings (‘mochi’) | [60,67,80] |
| Artemisia schmidtiana Maxim. | Sagebrush, Silvermound, Wormwood, Mugwort | E. Asia—Japan. | Stems | Cooked; for flavoring and coloring of rice dumplings (‘mochi’) | [78,80] |
| Artemisia sphaerocephala Krasch. | China | Seed | Seed powder added to noodles and other traditional Chinese foods to improve sensory qualities such as elasticity and chewing quality | [81] | |
| Artemisia tilesii Ledeb. | Wormwood, Tilesius’ wormwood | E. Asia, N.W. America. | Leaves, shoots | The fresh shoots are peeled and eaten, usually with oil; Flavoring rice dumplings | [67] |
| Artemisia tridentata Nutt. | Sage Brush, Big sagebrush, Bonneville big sagebrush | N. America | Leaves, seeds | Leaves—cooked, as a condiment and to make a tea with sage-like flavor; Seed—can be roasted then ground into a powder and mixed with water or eaten raw | [61,75,76] |
| A. umbelliformis (syn. A. mutellina Vill.) | Alpine Wormwood | Europe—Alps, N. Apennines | Herb, leaves, flower heads | As a condiment; preparation of a tea and a liqueur, often with the addition of absinthe | [10,60,66,67,82] |
| Artemisia vallesiaca All. | Alpine Wormwood, Valais wormwood | Europe—N. Italy, Switzerland, S. E. France | Herb | Flavoring for liqueurs; product of santonin | [10,60,66] |
| A. vulgaris | Mugwort, Common wormwood, Felon Herb, Chrysanthemum Weed, Wild Wormwood | Temperate regions of Europe and Asia | Leaves, young shoots, flowering tops | Flavoring fatty foods; to give color and flavor to rice dumplings (‘mochi’); as a potherb; flavoring in beer and liqueurs | [60,61,66,67,70,71,80] |
| Artemisia wrightii A. Gray. | N. America | Leaves, seeds | Raw or cooked—an oily texture; Seed—ground with water, made into balls and steamed | [75] |
| Plant Species | Plant Part | Nutrient Composition * | Ref. |
|---|---|---|---|
| A. absinthium | Oil cake ** | Sugars (9.4%) | [90] |
| A. annua | Leaves | Protein (27.1%); TAA (27.6%), EAA (16.1%), NEAA (11.5%); Crude fat (8.34%); Minerals: K (26.3 mg/g DM), Ca (11.5 mg/g DM), Mg (7.1 mg/g DM), P (7.1 mg/g DM), S (3.9 mg/g DM), Fe (0.2 mg/g DM), Mn (0.2 mg/g DM), Zn (0.06 mg/g DM); Vitamin A (<0.3 μg/100 g DM); Vitamin E (22.63 mg/kg) | [91] |
| Inflorescence | Protein (18.4%); Crude fat (10.5%); TAA (18.3%), EAA (10.14%), NEAA (8.11%); Minerals: K (24.6 mg/g DM), Ca (4.4 mg/g DM), Mg (2.3 mg/g DM), P (3.4 mg/g DM), S (4.6 mg/g DM), Fe (0.2 mg/g DM), Mn (0.3 mg/g DM), Zn (0.06 mg/g DM); Vitamin A (<0.3 μg/100 g DM); Vitamin E (19.38 mg/kg) | ||
| Stems | Protein (10.7%); Crude fat (2.60%); TAA (10.3%), EAA (5.91%), NEAA (4.38%); Minerals: K (13.3 mg/g DM), Ca (0.9 mg/g DM), Mg (0.9 mg/g DM), P (0.7 mg/g DM), S (0.5 mg/g DM), Fe (0.7 mg/g DM), Mn (0.02 mg/g DM), Zn (0.08 mg/g DM); Vitamin A (<0.3 μg/100 g DM); Vitamin E (1.19 mg/kg) | ||
| Roots | Protein (8.23%); Crude fat (2.13%); TAA (8.01%), EAA (4.34%), NEAA (3.66%); Minerals: K (11.1 mg/g DM), Ca (11.5 mg/g DM), Mg (7.1 mg/g DM), P (7.1 mg/g DM), S (3.9 mg/g DM), Fe (0.2 mg/g DM), Mn (0.2 mg/g DM), Zn (0.06 mg/g DM) Vitamin A (<0.3 μg/100 g DM); Vitamin E (1.36 mg/kg) | ||
| Leaves | Protein (24.37 mg/100 g); Crude fat (6.07%); TFA (4.19 mg/g FW), SFA (22.9%), UFA (77.1%), MUFA (8.4%), PUFA (68.7%) Carbohydrates (8%); Fibre (14.2%); Vitamins: Tocopherol (2.74%) | [92,93] | |
| Achene | Lipids: SFA (29.21%), UFA (70.87%), MUFA (13.99%), PUFA (56.88%) | [94] | |
| A. arborescens | Leaves | Lipids: TFA (3.31 mg/g FW), SFA (47.4%), UFA (52.6%), MUFA (16.3%), PUFA (36.3%) | [92] |
| A. argyi | Leaves | Protein (22.0 mg/g FW); Free amino acids: EAA (3.71 mg/g DW), NEAA (2.42 mg/g DW), FAA (6.13 mg/g DW); Total lipid (24.7 mg/g FW); SFA (40.8%), MUFA (7.1%), PUFA (52.1%); Total carbohydrates (52.3 mg/g FW); Dietary fiber (39.9 mg/g FW); Minerals: K (74.22 mg/100 g FW), Ca (14.74 mg/100 g FW), Mg (36.64 mg/100 g FW), Zn (0.89 mg/100 g FW), Cu (0.13 mg/100 g FW), Mn (0.76 mg/100 g FW), Fe (3.15 mg/100 g FW); Vitamin C (total ascorbic acid) 2.09 mg/g DW | [46,73] |
| A. austriaca Jacq. | Achene | Lipids: SFA (47.43%), UFA (49.02%), MUFA (9.65%), PUFA (39.37%) | [94] |
| A. campestris L. | Leaves | Lipids: TFA (10.22 mg/g FW), SFA (21.0%), UFA (79.0%), MUFA (3.6%), PUFA (75.3%) | [92] |
| Aerial | Crude protein (115 mg/g DM) | [95] | |
| A. camphorata Vill. | Leaves | Lipids: TFA (14.82 mg/g FW), SFA (37.4%), UFA (62.6%), MUFA (8.3%), PUFA (54.3%) | [92] |
| A capilaris | Leaves | Lipids: TFA (6.01 mg/g FW), SFA (16.0%), UFA (84.0%), MUFA (4.7%), PUFA (79.4%) | [92] |
| A. frigida | Aerial | Crude protein (17.9%); Minerals: K (18.34 mg/g DM), Ca (7.46 mg/g DM),P (2.54 mg/g DM), Mg (2.17 mg/g DM), Cu (1.1 mg/100 g DM), Mn (24 mg/100 g DM), Fe (20.0 mg/100 g DM) Zn (1.9 mg/100 g DM); Na (5 mg/100 g DM) | [96] |
| A. glacialis | Leaves | Lipids: TFA (8.95 mg/g FW), SFA (21.6%), UFA (78.4%), MUFA (6.8%), PUFA (71.6%) | [92] |
| A. gmellini Weber ex Stechm. | Leaves | Lipids: TFA (14.11 mg/g FW), SFA (25.5%), UFA (74.5%), MUFA (4.5%), PUFA (70.0%) | [92] |
| A. herba-alba | Aerial | Crude protein (103.4–153.6 mg/g DM); Crude fibre (407.9 mg/g DM) | [95,97,98] |
| A. jacutica Drobow | Leaves | Lipids: SFA (61.21–68.12%), UFA (31.88–38.79%) | [99] |
| A. ludoviciana | Leaves | Lipids: TFA (14.28 mg/g FW), SFA (19.6%), UFA (80.4%), MUFA (5.3%), PUFA (75.1%) | [92] |
| A. macrocephala Jaq. ex Bess | Leaves | Lipids: UFA (50.80–65.22%), SFA (34.78–49.20%). | [99] |
| A. oleandica (Besser) Krasch | Leaves | Lipids: TFA (9.84 mg/g FW), SFA (17.6%), UFA (82.4%), MUFA (4.7%), PUFA (77.7%) | [92] |
| A. princeps | Leaves | Lipids: TFA (6.49 mg/g FW), SFA (20.2%), UFA (79.8%), MUFA (5.7%), PUFA (74.1%) | [92] |
| Leaves | Lipids: SFA (27.5%), MUFA (35.1%), PUFA (37.4%); Free amino acids: EAA (3.19 mg/g DW), NEAA (2.42 mg/g DW), FAA (5.61 mg/g DW); Vitamin C (total ascorbic acid) 1.01 mg/g DW; | [73] | |
| A. santolinifolia Turcz. ex Bess | Leaves | Lipids: SFA (51.8–65.02%), PFA (9.74–44.14%), MFA (4.06–30.85%) | [100] |
| A. santonicum L. | Achene | Lipids: SFA (43.70%), UFA (56.33%), MUFA (8.26%), PUFA (48.07%) | [94] |
| A. sieberi Besser | Aerial | Crude protein (55 mg/g DM); Crude fiber (484 mg/g DM); Minerals: K (13.1 mg/g DM), Ca (15.9 mg/g DM),P (2.5 mg/g DM), Mg (1.8 mg/g DM), Cu (1.37 mg/100 g DM), Mn (2.26 mg/100 g DM), Fe (20.0 mg/100 g DM) Zn (21.2 mg/100 g DM) | [101] |
| A. sieversiana Ehrh. ex Willd | Leaves | Lipids: UFA (64.11–73.23%), SFA (26.77–35.89%) | [99] |
| A. sphaerocephala | Seed | Carbohydrate (73%) | [102] |
| A. stelleriana Bess | Leaves | Lipids: TFA (17.78 mg/g FW), SFA (70.2%), UFA (29.8%), MUFA (1.3%), PUFA (28.4%) | [92] |
| A. tridentata subsp. wyomingensis Beetle & A.L.Young | Leaves | Crude protein (15.7%) | [103] |
| A. vallesiaca | Leaves | Lipids: TFA (5.27 mg/g FW), SFA (17.1%), UFA (82.9%), MUFA (9.3%), PUFA (73.6%) | [92] |
| A. vulgaris | Leaves | Lipids: TFA (13.32 mg/g FW), SFA (15.2%), UFA (84.8%), MUFA (3.7%), PUFA (81.1%) | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, A.; Moujir, L.M.; Sousa, P.M.C.; Seca, A.M.L. Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods 2021, 10, 65. https://doi.org/10.3390/foods10010065
Trendafilova A, Moujir LM, Sousa PMC, Seca AML. Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods. 2021; 10(1):65. https://doi.org/10.3390/foods10010065
Chicago/Turabian StyleTrendafilova, Antoaneta, Laila M. Moujir, Pedro M. C. Sousa, and Ana M. L. Seca. 2021. "Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents" Foods 10, no. 1: 65. https://doi.org/10.3390/foods10010065
APA StyleTrendafilova, A., Moujir, L. M., Sousa, P. M. C., & Seca, A. M. L. (2021). Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods, 10(1), 65. https://doi.org/10.3390/foods10010065