Exploring the Potentialities of Photoinduced Glycation to Steer Protein Functionalities: The Study Case of Freeze-Dried Egg White Proteins/Carbohydrates Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. UV-C Light Treatments
2.4. Irradiance
2.5. Color
2.6. Solubility
2.7. HPLC Gel-Permeation Analysis
2.8. Functional Properties
2.8.1. Critical Concentration
2.8.2. Particle Size
2.8.3. Apparent Viscosity
2.8.4. Gelling Temperature
2.8.5. Foam Ability and Stability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Browning Development
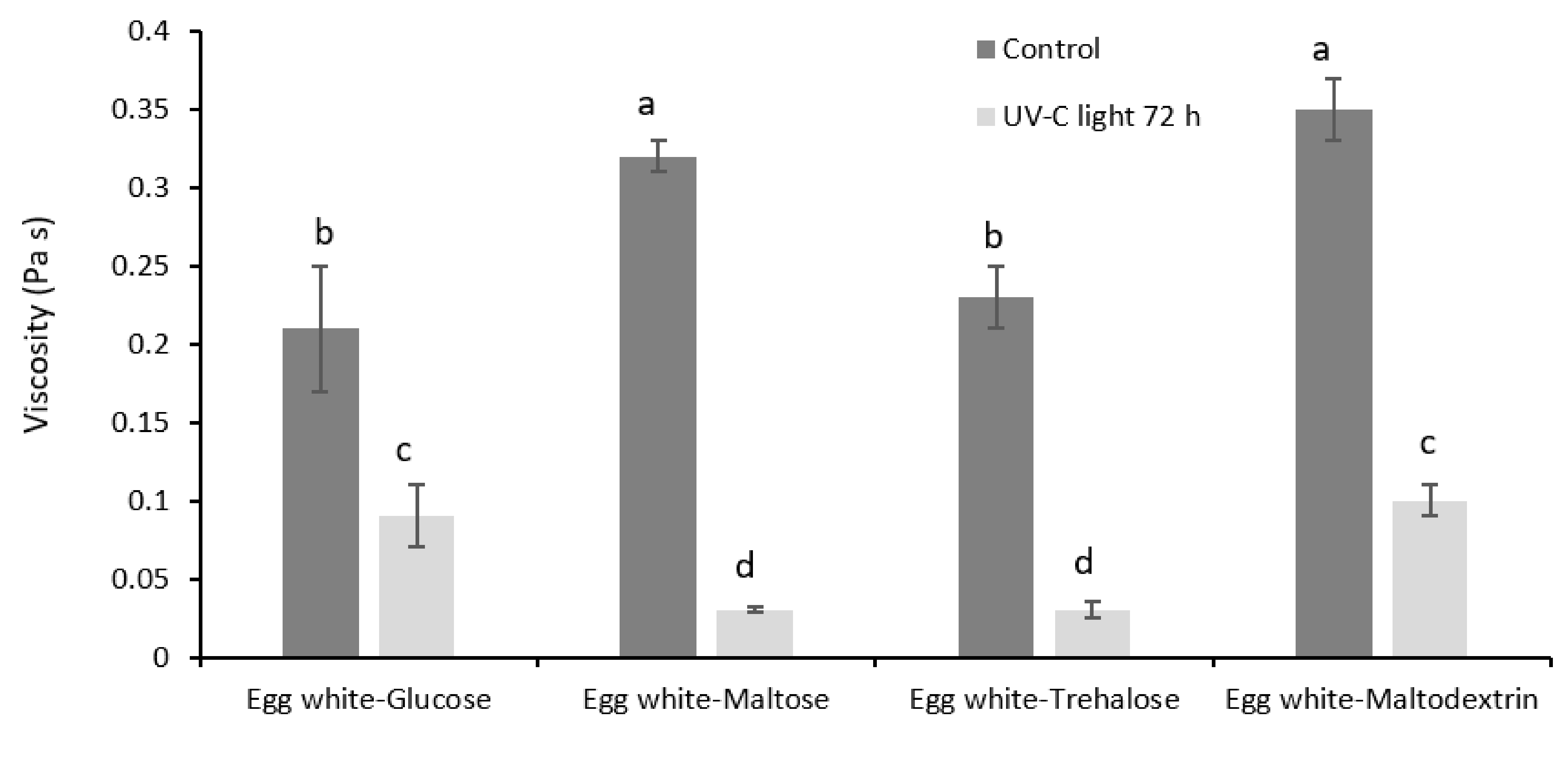
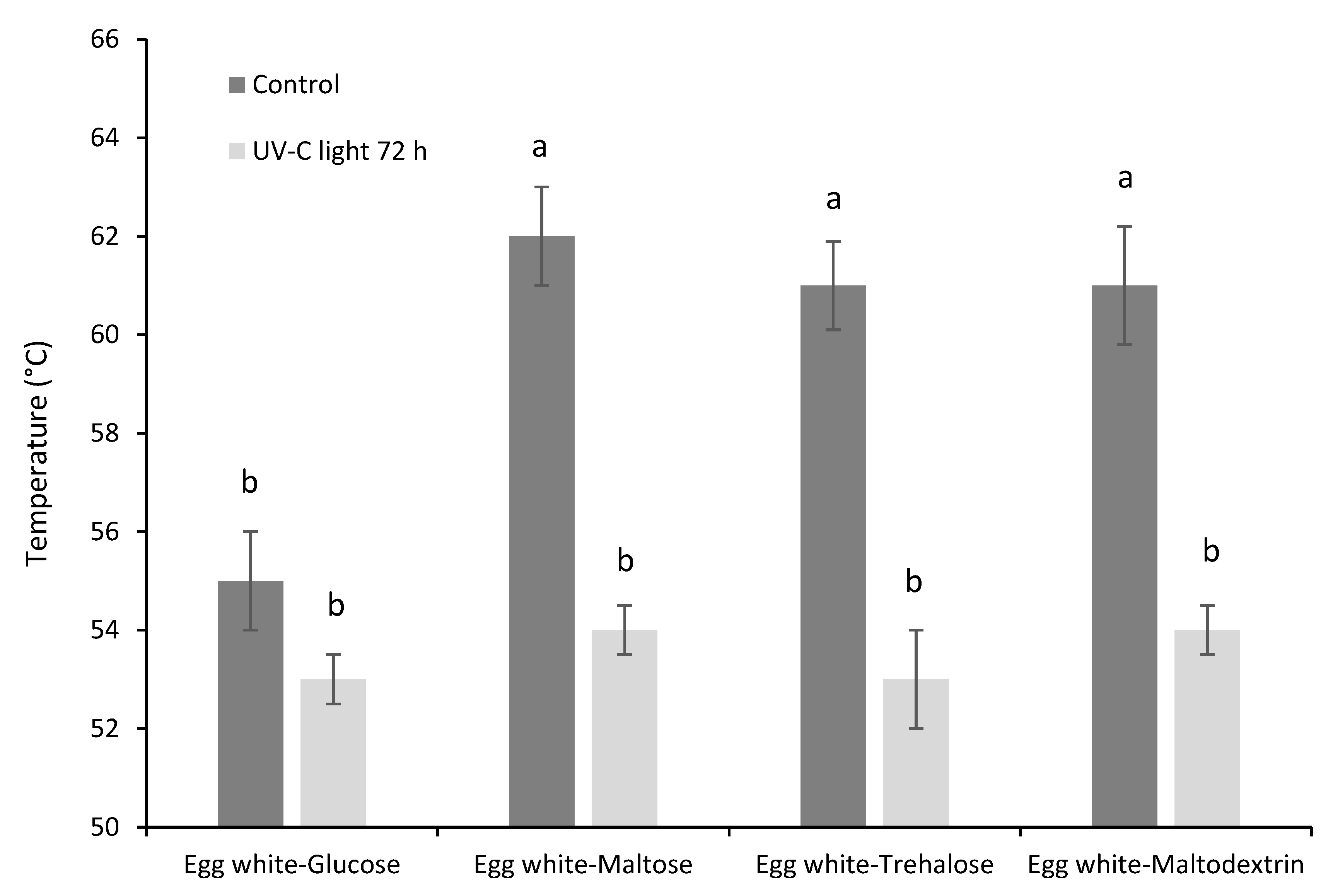
3.2. Functional Properties
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Gray, H.B.; Winkler, J.R. Electron transfer in proteins. Annu. Rev. Biochem. 1996, 65, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L. Photo-Induced Modification of Food Protein Structure and Functionality. Food Eng. Rev. 2015, 7, 346–356. [Google Scholar] [CrossRef]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef]
- Tomihata, K.; Burczak, K.; Shiraki, K.; Ikada, Y. Cross-linking and biodegradation of native and denatured collagen. Polym. Biol. Biomed. Signif. 1994, 540, 275–286. [Google Scholar]
- Rhim, J.W.; Gennadios, A.; Fu, D.; Weller, C.L.; Hanna, M. A Properties of ultraviolet irradiated protein films. LWT Food Sci. Technol. 1999, 32, 129–133. [Google Scholar] [CrossRef]
- Kuan, Y.-H.; Bhat, T.; Karim, A.A. Emulsifying and foaming properties of ultraviolet-irradiated egg white protein and sodium caseinate. J. Agric. Food. Chem. 2011, 59, 4111–4118. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Ultraviolet irradiation improves gel strength of fish gelatin. Food Chem. 2009, 113, 1160–1164. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zheng, Y.R.; Wang, D.F.; Liu, Z.M.; Deng, Y. Structural and oxidative modifications, and antioxidant activity of β-lactoglobulin treated by ultraviolet light-C and ultrasound. Int. J. Food Prop. 2017, 20, S3289–S3300. [Google Scholar] [CrossRef]
- Manzocco, L.; Panozzo, A.; Nicoli, M.C. Effect of ultraviolet processing on selected properties of egg white. Food Chem. 2012, 135, 522–527. [Google Scholar] [CrossRef]
- Mendes de Souza, P.; Müller, A.; Beniaich, A.; Mayer Miebach, E.; Oehlke, K.; Stahl, M.; Greiner, R.; Fernández, A. Functional properties and nutritional composition of liquid egg products treated in a coiled tube UV-C reactor. Inn. Food Sci. Emerg. Technol. 2015, 32, 156–164. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Tomasik, P. Molecular distribution and pasting properties of UV-irradiated corn starches. Starch 1999, 51, 126–131. [Google Scholar] [CrossRef]
- Vatanasuchart, N.; Naivikul, O.; Charoenrein, S.; Sriroth, K. Molecular properties of cassava starch modified with different UV irradiations to enhance baking expansion. Carbohyd. Polym. 2005, 61, 80–87. [Google Scholar] [CrossRef]
- Follain, N.; Joly, C.; Dole, P.; Bliard, C. Properties of starch based blends. Part 2. Influence of poly vinyl alcohol addition and photocrosslinking on starch based materials mechanical properties. Carbohyd. Polym. 2005, 60, 185–192. [Google Scholar] [CrossRef]
- Lee, J.S.; Kumar, R.N.; Rozman, H.D.; Azemi, B.M.N. Pasting, swelling and solubility properties of UV initiated starch-graft-poly(AA). Food Chem. 2005, 91, 203–221. [Google Scholar] [CrossRef]
- Delvill, J.; Joly, C.; Dole, P.; Bliard, C. Influence of photocrosslinking on the retrogradation of wheat starch based films. Carbohyd. Polym. 2003, 53, 373–381. [Google Scholar] [CrossRef]
- Gennadios, A.; Rhim, J.W.; Weller, C.L.; Hanna, M.A. Ultraviolet radiation affects physical and molecular properties of soy protein films. J. Food Sci. 1998, 63, 225–228. [Google Scholar] [CrossRef]
- Ohan, M.P.; Weadock, K.S.; Dunn, M.G. Synergistic effects of glucose and ultraviolet irradiation on the physical properties of collagen. J. Biomed. Mater. Res. 2002, 60, 384–391. [Google Scholar] [CrossRef]
- Manzocco, L.; Maifreni, M.; Anese, M.; Munari, M.; Bartolomeoli, I.; Zanardi, S.; Suman, M.; Nicoli, M.C. Effect of pulsed light on safety and quality of fresh egg pasta. Food Bioprocess Technol. 2014, 7, 197–1980. [Google Scholar] [CrossRef]
- Guan, J.-J.; Qiu, A.-Y.; Liu, X.-Y.; Hua, Y.-F.; Ma, Y.-H. Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chem. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.H.; Leonard, M.L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- He, Z.J.; Tong, C.Y.; Sheng, L.; Ma, M.H.; Cai, Z.X. Monitoring Glycation-induced structural and biofunctional changes in chicken immunoglobulin y by different monosaccharides. Poult. Sci. 2016, 95, 2715–2723. [Google Scholar] [CrossRef]
- Xu, L.; Gong, Y.S.; Gern, J.E.; Ikeda, S.; Lucey, J.A. Glycation of whey protein with dextrans of different molar mass: Effect on immunoglobulin E-binding capacity with blood sera obtained from patients with cow milk protein allergy. J. Dairy Sci. 2018, 101, 6823–6834. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, D.; Wang, Q.; Li, W.R.; Cai, J.; Li, S.Y.; Lamikanra, O.; Qin, X.G. Maillard-Reaction-Functionalized Egg Ovalbumin Stabilizes Oil Nanoemulsions. J. Agric. Food Chem. 2018, 66, 4251–4258. [Google Scholar] [CrossRef]
- Ai, M.; Xiao, N.; Jian, A. Molecular structural modification of duck egg white protein conjugates with monosaccharides for improving emulsifying capacity. Food Hydrocoll. 2021, 111, 106271. [Google Scholar] [CrossRef]
- Raikos, V.; Campbell, L.; Euston, S.R. Effects of sucrose and sodium chloride on foaming properties of egg white proteins. Food Res. Int. 2007, 40, 347–355. [Google Scholar] [CrossRef]
- Aguilar, K.; Garvin, A.; Azuara, E.; Ibarz, A. Modelling of 5-hydroxymethylfurfural photo-degradation by UV irradiation. Influence of temperature and pH. Food Res. Int. 2015, 71, 165–173. [Google Scholar] [CrossRef]
- Ros-Polski, V.; Popovic, V.; Koutchma, T. Effect of ultraviolet-C light treatment on Hydroxymethylfurfural (5-HMF) content in high fructose corn syrup (HFCS) and model syrups. J. Food Eng. 2016, 179, 78–87. [Google Scholar] [CrossRef]
- Davies, M.J.; Truscott, R.J.W. Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B Biol. 2001, 63, 114–125. [Google Scholar] [CrossRef]
- Manzocco, L.; Quarta, B.; Dri, A. Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innov. Food Sci. Emerg. Technol. 2009, 10, 506–511. [Google Scholar] [CrossRef]
- Manzocco, L.; Panozzo, A.; Nicoli, M.C. Inactivation of polyphenoloxidase by pulsed light. J. Food Sci. 2013, 78, E1183–E1187. [Google Scholar] [CrossRef]
- Bhaskar, A.J.; Yang, W.; Marshall, M.R.; Reyes-De-Corcuera, J.I.; Rababah, T.M. Nonthermal inactivation of soy (Glycine max Sp.) lypoxygenase by pulsed ultraviolet light. J. Food Sci. 2014, 79, C8–C18. [Google Scholar]
- Lapasin, R.; Pricl, S. Rheology of Industry Polysaccharides: Theory and Applications; Blackie Academic & Professional: Glasgow, Poland, 1995. [Google Scholar]
- Manzocco, L.; Nicoli, M.C. Self crowding as a determinant of egg white photostability. Food Biophys. 2015, 10, 155–161. [Google Scholar] [CrossRef]
- Kuan, Y.-H.; Bhat, R.; Patras, A.; Karim, A.A. Radiation processing of food proteins: A review on the recent developments. Trends Food Sci. Technol. 2013, 30, 105–120. [Google Scholar] [CrossRef]
- Harding, S.E. The intrinsic viscosity of biological molecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog. Biophys. Mol. Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef]
- Xie, J.; Qin, M.; Cao, Y.; Wang, W. Mechanistic insight of photo-induced aggregation of chicken egg white lysozyme: The interplay between hydrophobic interactions and formation of intermolecular disulfide bonds. Proteins 2011, 79, 2505–2516. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilagyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic stabilization of charged colloidal particles with adsorbed polyelectrolytes of opposite charge. Langmuir 2010, 26, 15109–15111. [Google Scholar] [CrossRef]
| ΔE* | Ovalbumin Reduction (%) | |||||
|---|---|---|---|---|---|---|
| 4 h | 24 h | 72 h | 4 h | 24 h | 72 h | |
| Egg white | 8.29 | 17.79 | 19.41 | 54.2 | 75.4 | 85.5 |
| Glucose | 2.15 | 4.71 | 5.41 | - | - | - |
| Maltose | 4.40 | 5.27 | 2.09 | - | - | - |
| Trehalose | 7.28 | 10.43 | 11.72 | - | - | - |
| Maltodextrin | 0.84 | 1.17 | 3.76 | - | - | - |
| Egg white–Glucose | 15.36 | 23.18 | 24.35 | 28.5 | 53.6 | 65.6 |
| Egg white–Maltose | 10.91 | 20.97 | 24.86 | 46.2 | 50.1 | 64.3 |
| Egg white–Trehalose | 12.00 | 20.32 | 24.74 | 47.6 | 63.2 | 72.1 |
| Egg white–Maltodextrin | 11.30 | 19.71 | 22.07 | 67.1 | 72.6 | 75.3 |
| Mass (%) | ||
|---|---|---|
| Control | UV-C Light 72 h | |
| Egg white | 70 ± 2 | 17 ± 1 |
| Egg white–Glucose | 80 ± 1 | 63 ± 1 |
| Egg white–Maltose | 91 ± 1 | 52 ± 2 |
| Egg white–Trehalose | 95 ± 5 | 52 ± 2 |
| Egg white–Maltodextrin | 81 ± 1 | 43 ± 3 |
| Sample | Egg White | Egg White–Glucose | Egg White–Threalose | Egg White–Maltose | Egg White–Maltodextrin | |
|---|---|---|---|---|---|---|
| Control | Critical Concentration (g/L) | 14 | 1667 | 667 | 130 | 67 |
| Population I (nm) | 193.6 ± 134.3 (16.1) | 62.4 ± 23.9 (17.9) | 71.4 ± 37.3 (21.3) | 63.9 ± 71.0 (36.8) | 94.7 ± 57.6 (15.7) | |
| Population II (nm) | 4733.9 ± 3305.5 (83.9) | 4347.3 ± 1310.2 (82.1) | 5517.7 ± 1832.4 (78.7) | 5198.0 ± 3958.4 (63.2) | 8161.7 ± 13.0 (84.3) | |
| UV-C light 72 h | Critical Concentration (g/L) | 1200 | 2023 | 2488 | 547 | 670 |
| Population I (nm) | 202.4 ± 220.8 (33.1) | 170.2 ± 27.7 (86.2) | 108.7 ± 66.5 (31.9) | 167.3 ± 21.4 (45.1) | 11.5 ± 10.1 (7.5) | |
| Population II (nm) | 7265.0 ± 2745.1 (69.9) | 2738.9 ± 577.9 (13.8) | 4941.8 ± 3706.3 (68.1) | 2968.6 ± 1161.4 (55.0) | 360.8 ± 319.3 (92.5) |
| Foam Ability (%) | Foam Stability (%) | |||
|---|---|---|---|---|
| Control | UV-C Light 72 h | Control | UV-C Light 72 h | |
| Egg white–Glucose | 41.0 ± 0.0 | 30.6 ± 1.6 | 77.8 ± 0.0 | 86.8 ± 1.6 |
| Egg white–Maltose | 40.7 ± 0.4 | 35.9 ± 0.7 | 78.2 ± 0.6 | 88.6 ± 1.6 |
| Egg white–Trehalose | 45.8 ± 0.6 | 35.9 ± 0.7 | 89.1 ± 1.2 | 88.6. ± 1.6 |
| Egg white–Maltodextrin | 35.8 ± 0.6 | 50.2 ± 0.3 | 75.9 ± 1.3 | 91.1 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzocco, L.; Plazzotta, S.; Calligaris, S. Exploring the Potentialities of Photoinduced Glycation to Steer Protein Functionalities: The Study Case of Freeze-Dried Egg White Proteins/Carbohydrates Mixtures. Foods 2021, 10, 26. https://doi.org/10.3390/foods10010026
Manzocco L, Plazzotta S, Calligaris S. Exploring the Potentialities of Photoinduced Glycation to Steer Protein Functionalities: The Study Case of Freeze-Dried Egg White Proteins/Carbohydrates Mixtures. Foods. 2021; 10(1):26. https://doi.org/10.3390/foods10010026
Chicago/Turabian StyleManzocco, Lara, Stella Plazzotta, and Sonia Calligaris. 2021. "Exploring the Potentialities of Photoinduced Glycation to Steer Protein Functionalities: The Study Case of Freeze-Dried Egg White Proteins/Carbohydrates Mixtures" Foods 10, no. 1: 26. https://doi.org/10.3390/foods10010026
APA StyleManzocco, L., Plazzotta, S., & Calligaris, S. (2021). Exploring the Potentialities of Photoinduced Glycation to Steer Protein Functionalities: The Study Case of Freeze-Dried Egg White Proteins/Carbohydrates Mixtures. Foods, 10(1), 26. https://doi.org/10.3390/foods10010026





