Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality
Abstract
1. Introduction
2. Phenomenology of Ultrasound Waves
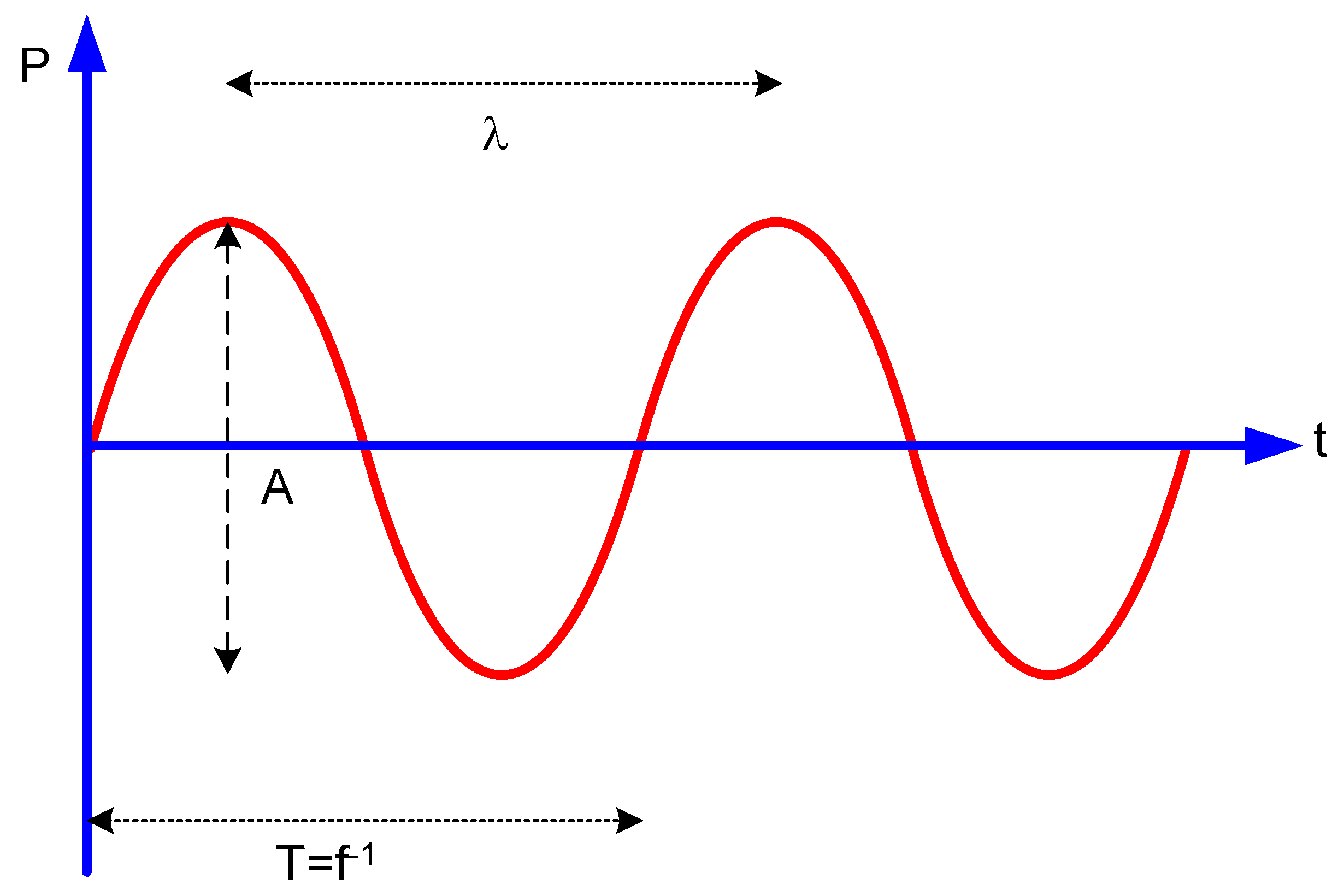
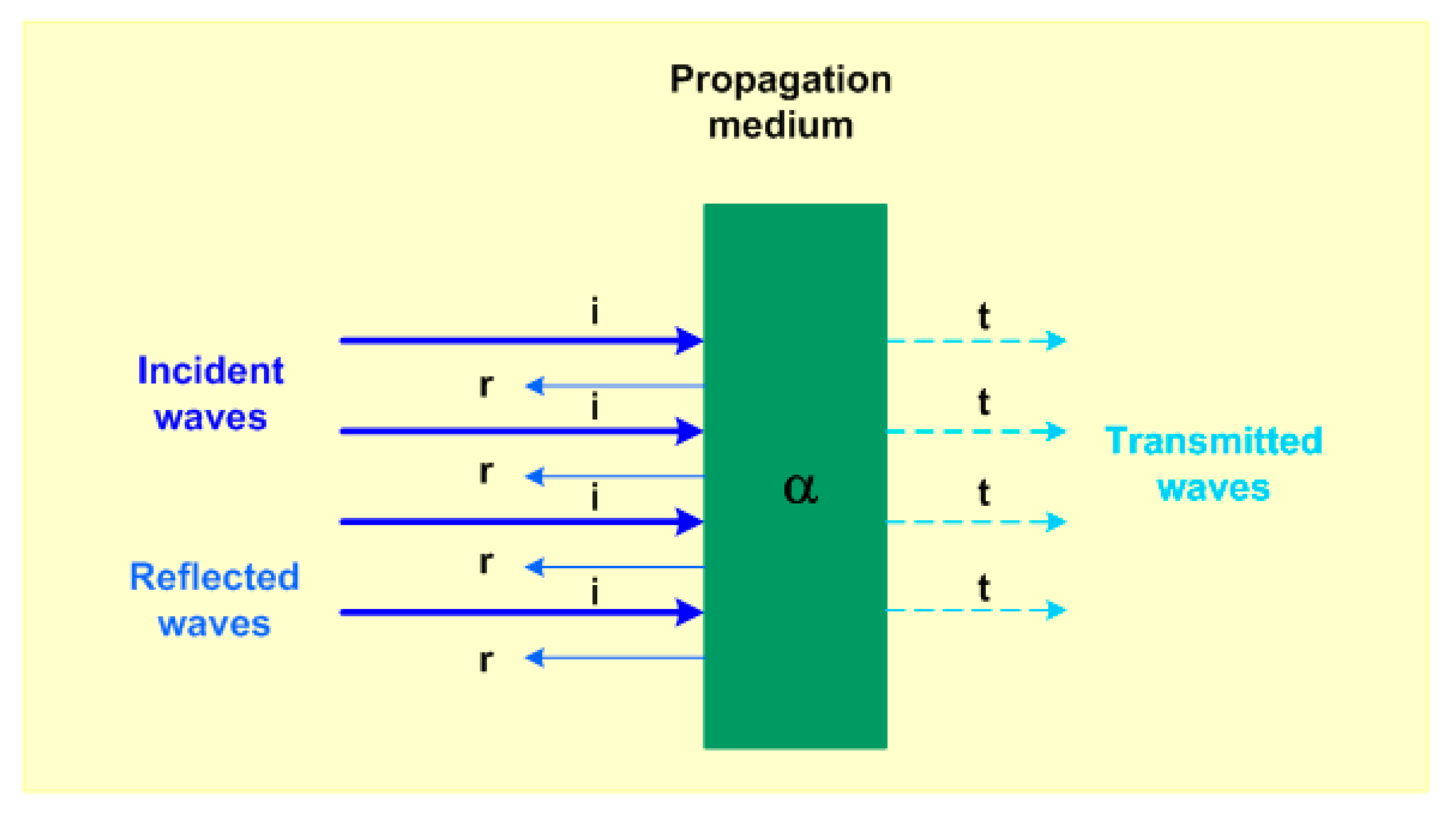
2.1. Acoustic Waves
2.2. Ultrasounds
2.3. Piezoelectric Materials
2.4. Low-Intensity Ultrasounds
2.5. High-Intensity Ultrasounds
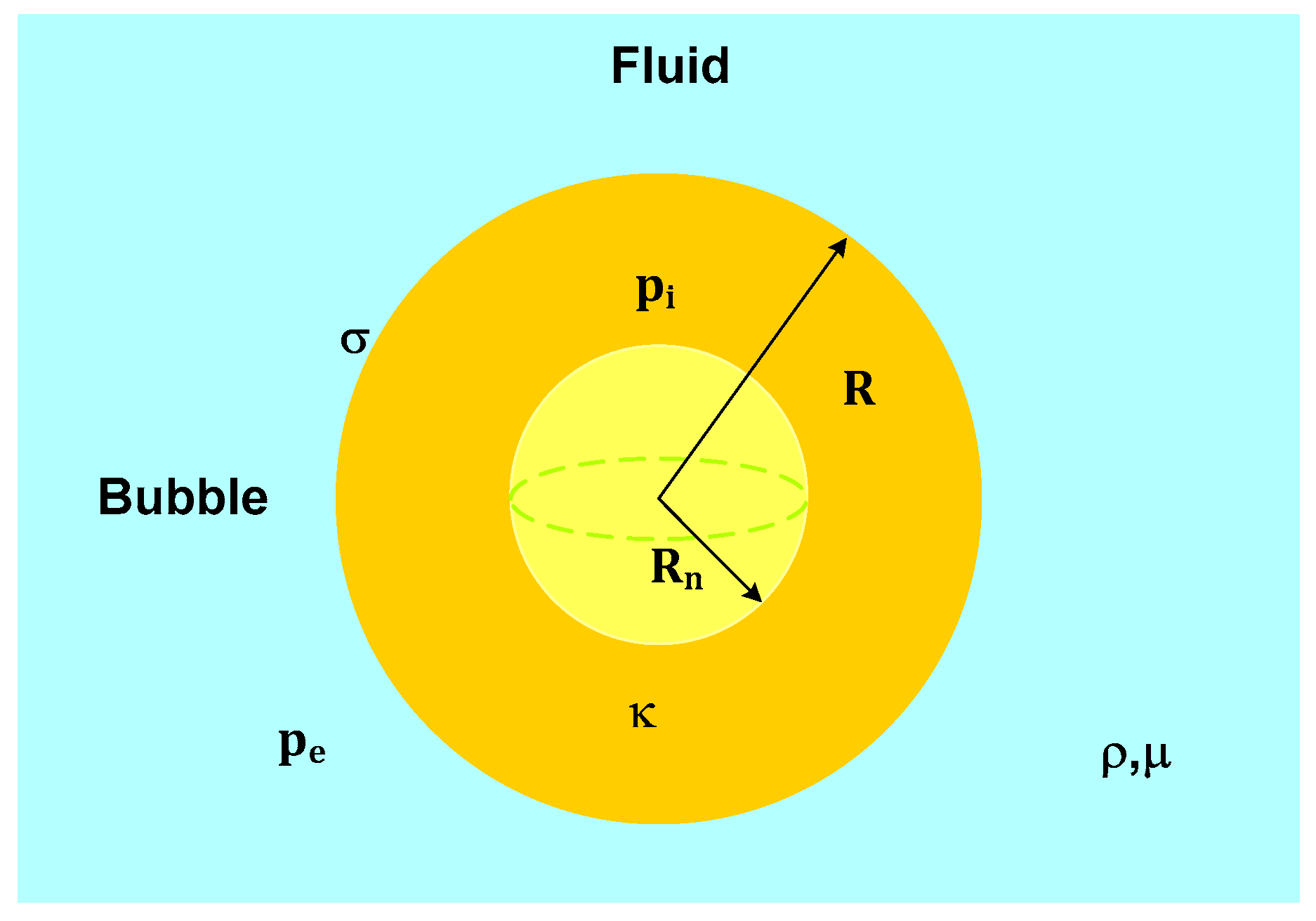
2.6. Acoustic Cavitation
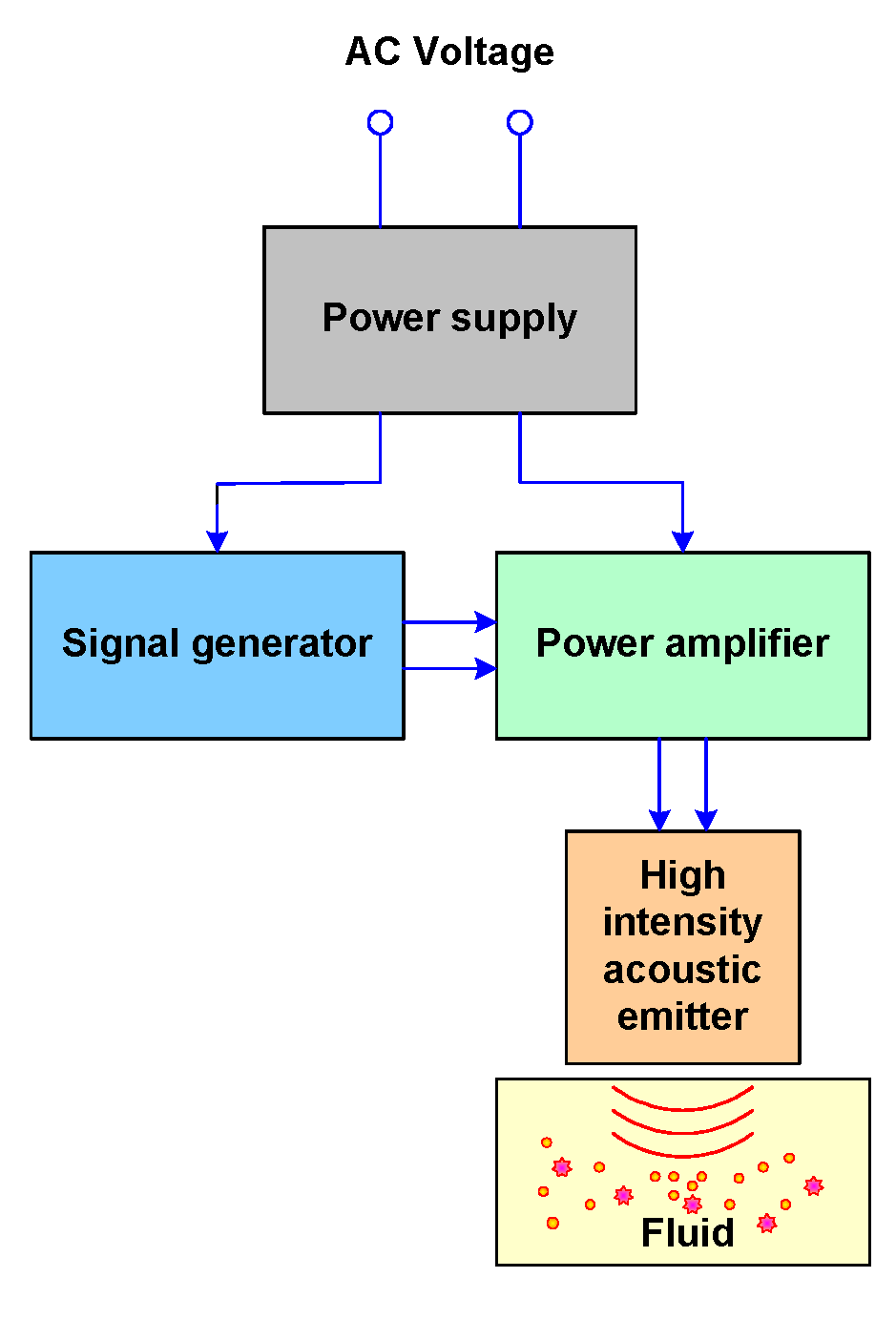
2.7. High-Intensity Ultrasound Equipment
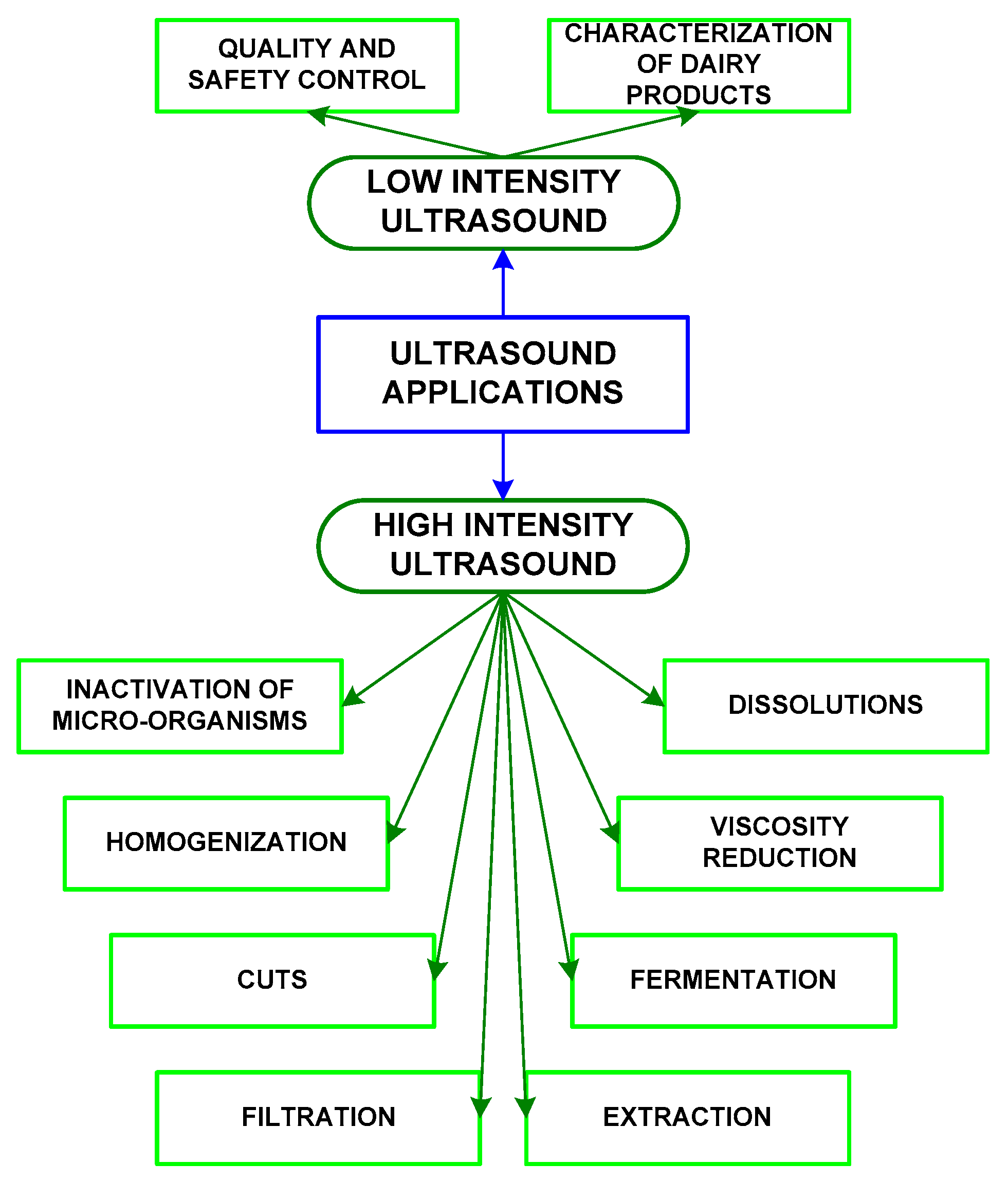
3. Ultrasounds Applied to Dairy Products
3.1. Low-Intensity Ultrasoundsin Dairy Production
3.2. High-Intensity Ultrasoundsin Dairy Production
3.2.1. Fermentation Process
3.2.2. Microbial Inactivation
3.2.3. Foam
3.2.4. Homogenization
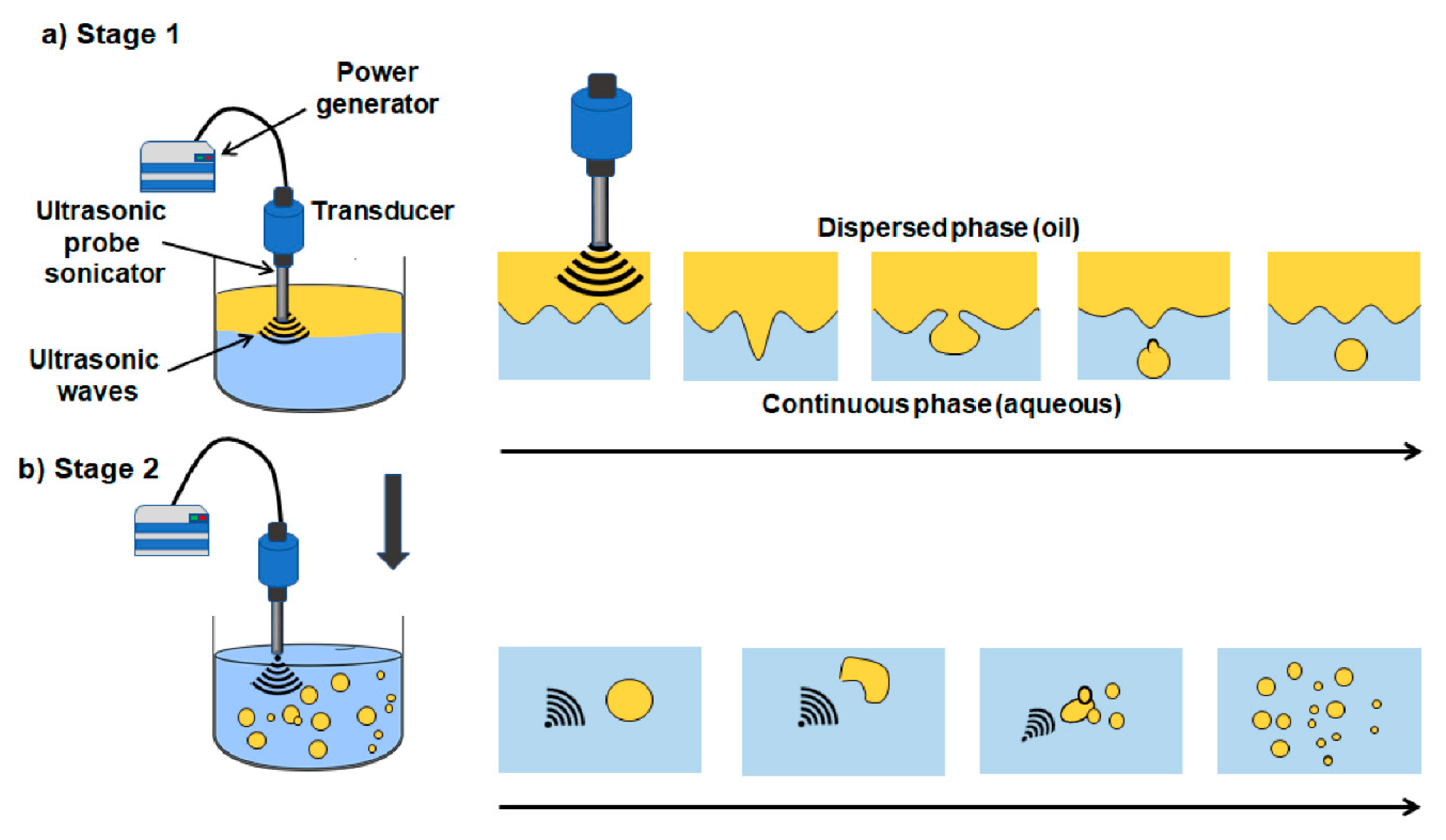
3.2.5. Emulsification
3.2.6. Crystallization
3.2.7. Milk Proteins
3.2.8. Cheese Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent Advances in Ultrasound Application as a Novel Technique in Analysis, Processing and Quality Control of Fruits, Juices and Dairy Products Industries: A Review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Juliano, P.; Kutter, A.; Cheng, L.; Swiergon, P.; Mawson, R.; Augustin, M. Enhanced Creaming of Milk Fat Globules in Milk Emulsions by the Application of Ultrasound and Detection by Means of Optical Methods. Ultrason. Sonochem. 2011, 18, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Annand, C.; Grewell, D.; Talbert, J.N.; Raman, D.R.; Clark, S. Batch Thermosonication for the Reduction of Plasmin Activity in Skim Milk. J. Food Process. Preserv. 2018, 42, e13616. [Google Scholar] [CrossRef]
- Dahroud, B.D.; Mokarram, R.R.; Khiabani, M.S.; Hamishehkar, H.; Bialvaei, A.Z.; Yousefi, M.; Kafil, H.S. Low Intensity Ultrasound Increases the Fermentation Efficiency of Lactobacillus Casei Subsp. Casei ATTC 39392. Int. J. Biol. Macromol. 2016, 86, 462–467. [Google Scholar] [CrossRef]
- Gholamhosseinpour, A.; Hashemi, S.M.B.; Jahromi, L.R.; Sourki, A.H. Conventional Heating, Ultrasound and Microwave Treatments of Milk: Fermentation Efficiency and Biological Activities. Int. Dairy J. 2020, 110, 104809. [Google Scholar] [CrossRef]
- Abesinghe, A.; Islam, N.; Vidanarachchi, J.; Prakash, S.; Silva, K.; Karim, M. Effects of Ultrasound on the Fermentation Profile of Fermented Milk Products Incorporated with Lactic Acid Bacteria. Int. Dairy J. 2019, 90, 1–14. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, Y.; Dai, C.; Sun, L.; Ma, H.; He, R. Stimulation of Low Intensity Ultrasound on Fermentation of Skim Milk Medium for Yield of Yoghurt Peptides by Lactobacillus Paracasei. Ultrason. Sonochem. 2019, 51, 315–324. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; AshokKumar, M.; Freitas, M.Q.; Cruz, A.G. High-Intensity Ultrasound: A Novel Technology for the Development of Probiotic and Prebiotic Dairy Products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Du, L.; Zhang, X.; Yang, W.; Zhang, H. Effect of Ultrasound Assisted Heating on Structure and Antioxidant Activity of Whey Protein Peptide Grafted with Galactose. LWT 2019, 109, 130–136. [Google Scholar] [CrossRef]
- Hernández-Falcón, T.A.; Monter-Arciniega, A.; Cruz-Cansino, N.D.S.; Alanís-García, E.; Rodríguez-Serrano, G.M.; Castañeda-Ovando, A.; García-Garibay, M.; Ramírez-Moreno, E.; Jaimez-Ordaz, J. Effect of Thermoultrasound on Aflatoxin m1 Levels, Physicochemical and Microbiological Properties of Milk During Storage. Ultrason. Sonochem. 2018, 48, 396–403. [Google Scholar] [CrossRef]
- Monteiro, S.H.; Silva, E.K.; Guimarães, J.T.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. High-Intensity Ultrasound Energy Density: How Different Modes of Application Influence the Quality Parameters of a Dairy Beverage. Ultrason. Sonochem. 2020, 63, 104928. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.H.; Silva, E.K.; Alvarenga, V.O.; Moraes, J.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.; Sant’Ana, A.S.; Meireles, M.A.D.A.; Cruz, A.G. Effects of Ultrasound Energy Density on the Non-Thermal Pasteurization of Chocolate Milk Beverage. Ultrason. Sonochem. 2018, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Potoroko, I.; Kalinina, I.; Botvinnikova, V.; Krasulya, O.; Fatkullin, R.; Bagale, U.; Sonawane, S. Ultrasound Effects Based on Simulation of Milk Processing Properties. Ultrason. Sonochem. 2018, 48, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, O.; Yilmaz, Y.; Gokce, O.; Ertan, K. Effect of Ultrasound Power on Physicochemical and Rheological Properties of Yoghurt Drink Produced with Thermosonicated Milk. Emir. J. Food Agric. 2016, 28, 235. [Google Scholar] [CrossRef]
- Ragab, E.S.; Zhang, S.; Pang, X.; Lu, J.; Nassar, K.S.; Yang, B.; Obaroakpo, U.J.; Jiaping, L. Ultrasound Improves the Rheological Properties and Microstructure of Rennet-Induced Gel from Goat Milk. Int. Dairy J. 2020, 104, 104642. [Google Scholar] [CrossRef]
- Scudino, H.; Silva, E.K.; Gomes, A.; Guimarães, J.T.; Cunha, R.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Ultrasound Stabilization of Raw Milk: Microbial and Enzymatic Inactivation, Physicochemical Properties and Kinetic Stability. Ultrason. Sonochem. 2020, 67, 105185. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Alvarenga, V.O.; Costa, A.L.R.; Cunha, R.L.; Sant’Ana, A.S.; Freitas, M.Q.; Meireles, M.A.D.A.; Cruz, A.G. Physicochemical Changes and Microbial Inactivation After High-Intensity Ultrasound Processing of Prebiotic Whey Beverage Applying Different Ultrasonic Power Levels. Ultrason. Sonochem. 2018, 44, 251–260. [Google Scholar] [CrossRef]
- Jalilzadeh, A.; Hesari, J.; Peighambardoust, S.; Javidipour, I. The Effect of Ultrasound Treatment on Microbial and Physicochemical Properties of Iranian Ultrafiltered Feta-Type Cheese. J. Dairy Sci. 2018, 101, 5809–5820. [Google Scholar] [CrossRef]
- Marchesini, G.; Fasolato, L.; Novelli, E.; Balzan, S.; Contiero, B.; Montemurro, F.; Andrighetto, I.; Segato, S. Ultrasonic Inactivation of Microorganisms: A Compromise Between Lethal Capacity and Sensory Quality of Milk. Innov. Food Sci. Emerg. Technol. 2015, 29, 215–221. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimarães, J.T.; Bevilacqua, A.; Corbo, M.R.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.; et al. Ultrasound Processing of Fresh and Frozen Semi-Skimmed Sheep Milk and Its Effects on Microbiological and Physical-Chemical Quality. Ultrason. Sonochem. 2019, 51, 241–248. [Google Scholar] [CrossRef]
- Popova, N.; Potoroko, I.; Kretova, Y.; Ruskina, A.; Tsirulnichenko, L.; Kalinina, I. Effect of Ultrasonic Treatment on the Dissolution of Milk Solids during the Reconstitution of Skim Milk Powder. Agron. Res. 2019, 17, 1414–1423. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Q.; Wang, Y.; Fu, L. Effect of Ultrasound Treatment on Allergenicity Reduction of Milk Casein via Colloid Formation. J. Agric. Food Chem. 2020, 68, 4678–4686. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, F.; Juhl, P.M. Fundamentals of General Linear Acoustics; John Wiley & Sons: Honoken, NJ, USA, 2013. [Google Scholar]
- Sfakianakis, P.; Topakas, E.; Tzia, C. Comparative Study on High-Intensity Ultrasound and Pressure Milk Homogenization: Effect on the Kinetics of Yogurt Fermentation Process. Food Bioprocess Technol. 2014, 8, 548–557. [Google Scholar] [CrossRef]
- Körzendörfer, A.; Nöbel, S.; Hinrichs, J. Particle Formation Induced by Sonication during Yogurt Fermentation–Impact of Exopolysaccharide-Producing Starter Cultures on Physical Properties. Food Res. Int. 2017, 97, 170–177. [Google Scholar] [CrossRef]
- Nöbel, S.; Ross, N.-L.; Protte, K.; Körzendörfer, A.; Hitzmann, B.; Hinrichs, J. Microgel Particle Formation in Yogurt as Influenced by Sonication During Fermentation. J. Food Eng. 2016, 180, 29–38. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalın, A.S. New Approach for Yoghurt and Ice Cream Production: High-Intensity Ultrasound. Trends Food Sci. Technol. 2019, 86, 392–398. [Google Scholar] [CrossRef]
- Gholamhosseinpour, A.; Hashemi, S.M.B. Ultrasound Pretreatment of Fermented Milk Containing Probiotic Lactobacillus Plantarum AF1: Carbohydrate Metabolism and Antioxidant Activity. J. Food Process. Eng. 2018, 42, e12930. [Google Scholar] [CrossRef]
- Amiri, A.; Mousakhani-Ganjeh, A.; Torbati, S.; Ghaffarinejhad, G.; Kenari, R.E. Impact of High-Intensity Ultrasound Duration and Intensity on the Structural Properties of Whipped Cream. Int. Dairy J. 2018, 78, 152–158. [Google Scholar] [CrossRef]
- PrabhuZantye, T.; Khaire, R.A.; Gogate, P.R. Enhancing the Recovery of Whey Proteins Based on Application of Ultrasound in Ultrafiltration and Spray Drying. Ultrason. Sonochem. 2019, 55, 125–134. [Google Scholar] [CrossRef]
- Lee, J.; Martini, S. Modifying the Physical Properties of Butter Using High-Intensity Ultrasound. J. Dairy Sci. 2019, 102, 1918–1926. [Google Scholar] [CrossRef]
- Pitt, W.G.; Ross, S.A. Ultrasound Increases the Rate of Bacterial Cell Growth. Biotechnol. Prog. 2003, 19, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-Y.; Benner, L.C.; Clark, S.D. Neither Thermosonication nor Cold Sonication Is Better Than Pasteurization for Milk Shelf Life. J. Dairy Sci. 2019, 102, 3965–3977. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, S.; Sunkesula, V.; Kumar, R.; Shah, K. Emerging Applications of Ultrasonication and Cavitation in Dairy Industry: A Review. Cogent Food Agric. 2018, 4, 1549187. [Google Scholar] [CrossRef]
- Schroeder, M.; Rossing, T.D.; Dunn, F.; Hartmann, W.M.; Campbell, D.M.; Fletcher, N.H. Springer Handbook of Acoustics, 1st ed.; Springer Publishing Company, Incorporated: New York, NY, USA, 2007. [Google Scholar]
- Hecht, E. Óptica, 3rd ed.; Addison Wesley Iberoamericana: Madrid, Spain, 2000. [Google Scholar]
- Kinsler, L.E.; Frey, A.R.; Coppens, A.B.; Sanders, J.V. Fundamentals of Acoustics; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar]
- Alves-Pereira, M.; Castelobranco, N.; Branco, N.A.C. Vibroacoustic Disease: Biological Effects of Infrasound and Low-Frequency Noise Explained by Mechanotransduction Cellular Signalling. Prog. Biophys. Mol. Biol. 2007, 93, 256–279. [Google Scholar] [CrossRef]
- Leighton, T. What is Ultrasound? Prog. Biophys. Mol. Biol. 2007, 93, 3–83. [Google Scholar] [CrossRef]
- Pai, F.; Huang, G. Theory and Design of Acoustic Metamaterials; SPIE Press: Paris, France, 2015; Volume PM260. [Google Scholar]
- Farnell, W.G.W. Types and Properties of Surface. In Topics in Applied Physics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1978; pp. 13–60. [Google Scholar]
- Arnau, V.A. Piezoelectric Transducers and Applications, 2nd ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Szabo, T.L.; Lewin, P.A. Piezoelectric Materials for Imaging. J. Ultrasound Med. 2007, 26, 283–288. [Google Scholar] [CrossRef][Green Version]
- Giraud, F.; Giraud-Audine, C. Piezoelectric Actuators: Vector Control Method, Basic, Modeling and Mechatronic Design of Ultrasonic Devices, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Nye, J.F.; Lindsay, R.B. Physical Properties of Crystals: Their Representation by Tensors and Matrices. Phys. Today 1957, 10, 26. [Google Scholar] [CrossRef]
- Franc, J.-P.; Michel, J.-M. Fluid Mechanics and Its Applications. In Fundamentals of Cavitation; Kluwer Academic Publishers: Dordrecht, Germany, 2005; Volume 76. [Google Scholar]
- Crum, L.A. Sonoluminescence, Sonochemistry, and Sonophysics. J. Acoust. Soc. Am. 1994, 95, 559. [Google Scholar] [CrossRef]
- Crum, L.A. Comments on the Evolving Field of Sonochemistry by a Cavitation Physicist. Ultrason. Sonochem. 1995, 2, S147–S152. [Google Scholar] [CrossRef]
- Weissler, A. Sonochemistry: The Production of Chemical Changes with Sound Waves. J. Acoust. Soc. Am. 1953, 25, 651–657. [Google Scholar] [CrossRef]
- Suslick, K.S. Mechanochemistry and Sonochemistry: Concluding Remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L., VIII. On the Pressure Developed in a Liquid During the Collapse of a Spherical Cavity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1917, 34, 94–98. [Google Scholar] [CrossRef]
- Leighton, T. The Acoustic Bubble; Elsevier Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Gilmore, F.R. The Growth or Collapse of a Spherical Bubble in a Viscous Compressible Liquid Nd, 26-Calif. Inst. Tech Eng. Rep. 1952, 26, 1–40. [Google Scholar]
- Keller, J.B.; Miksis, M. Bubble Oscillations of Large Amplitude. J. Acoust. Soc. Am. 1980, 68, 628–633. [Google Scholar] [CrossRef]
- Suslick, K.S.; Flannigan, D.J. Inside a Collapsing Bubble: Sonoluminescence and the Conditions During Cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef]
- Flint, E.B.; Suslick, K.S. The Temperature of Cavitation. Science. 1991, 253, 1397–1399. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T. Physics of Bubble Oscillations. Rep. Prog. Phys. 2010, 73, 106501. [Google Scholar] [CrossRef]
- An, Y. Formulation of Multibubble Cavitation. Phys. Rev. E 2011, 83, 066313. [Google Scholar] [CrossRef]
- Landau, L.; Lifshitz, E. Fluid Mechanics, 2nd ed.; Pergamon Press: Oxford, UK, 1987; Volume 6. [Google Scholar]
- Zlatev, Z.; Pehlivanova, T.; Dimitrova, A.; Baycheva, S.; Taneva, I.; Keremidchieva, K. Development of an Ultrasonic Device for Quality Evaluation of Yogurt. Eng. Rev. 2018, 38, 279–287. [Google Scholar] [CrossRef]
- Elvira, L.; Rodríguez, J.; Lynnworth, L.C. Sound Speed and Density Characterization of Milk Adulterated with Melamine. J. Acoust. Soc. Am. 2009, 125, EL177. [Google Scholar] [CrossRef]
- Kudryashov, E.; Hunt, N.; Arikainen, E.; Buckin, V. Monitoring of Acidified Milk Gel Formation by Ultrasonic Shear Wave Measurements. High-Frequency Viscoelastic Moduli of Milk and Acidified Milk Gel. J. Dairy Sci. 2001, 84, 375–388. [Google Scholar] [CrossRef]
- Lee, H.O.; Luan, H.; Daut, D.G. Use of an Ultrasonic Technique to Evaluate the Rheological Properties of Cheese and Dough. J. Food Eng. 1992, 16, 127–150. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ay, C. Milk Coagulation Cut-Time Determination Using Ultrasonics. J. Food Process. Eng. 1996, 19, 63–73. [Google Scholar] [CrossRef]
- Benedito, J.; Carcel, J.; Gonzalez, R.; Mulet, A. Application of Low Intensity Ultrasonics to Cheese Manufacturing Processes. Ultrasonics 2002, 40, 19–23. [Google Scholar] [CrossRef]
- Benedito, J.; Simal, S.; Clemente, G.; Mulet, A. Manchego Cheese Texture Evaluation by Ultrasonics and Surface Probes. Int. Dairy J. 2006, 16, 431–438. [Google Scholar] [CrossRef]
- Corredig, M.; Alexander, M.; Dalgleish, D.G. The Application of Ultrasonic Spectroscopy to the Study of the Gelation of Milk Components. Food Res. Int. 2004, 37, 557–565. [Google Scholar] [CrossRef]
- Bakkali, F.; Moudden, A.; Faiz, B.; Amghar, A.; Mazé, G.; De Espinosa, F.M.; Akhnak, M. Ultrasonic Measurement of Milk Coagulation Time. Meas. Sci. Technol. 2001, 12, 2154–2159. [Google Scholar] [CrossRef]
- Benedito, J.; Cárcel, J.A.; Clemente, G.; Mulet, A. Cheese Maturity Assessment Using Ultrasonics. J. Dairy Sci. 2000, 83, 248–254. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Verespej, E.; Alexander, M.; Corredig, M. The Ultrasonic Properties of Skim Milk Related to the Release of Calcium from Casein Micelles During Acidification. Int. Dairy J. 2005, 15, 1105–1112. [Google Scholar] [CrossRef]
- Ogasawara, H.; Mizutani, K.; Ohbuchi, T.; Nakamura, T. Acoustical Experiment of Yogurt Fermentation Process. Ultrasonics 2006, 44, e727–e730. [Google Scholar] [CrossRef]
- Elvira, L.; Sampedro, L.; De Espinosa, F.M.; Matesanz, J.; Gómez-Ullate, Y.; Resa, P.; Echevarría, F.; Iglesias, J. Eight-Channel Ultrasonic Device for Non-Invasive Quality Evaluation in Packed Milk. Ultrasonics 2006, 45, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Izbaim, D.; Faiz, B.; Moudden, A.; Malainine, M.; Aboudaoud, I. Ultrasonic Characterization of Yogurt Fermentation Process. In Proceedings of the Acoustics 2012 Conference, Société Françaised’Acoustique, Nantes, France, 23–27 April 2012. [Google Scholar]
- Ehssein, C.; Serfaty, S.; Griesmar, P.; Le Huerou, J.-Y.; Caplain, E.; Martínez, L.; Wilkie-Chancellier, N.; Gindre, M. Ultrasonic Monitoring of Yoghurt Formation by Using at-Cut Quartz: Lighting of Casein Micelles Interactions Process During the Acidification. Ultrasonics 2006, 44, e875–e879. [Google Scholar] [CrossRef]
- Pais, V.F.; Veríssimo, M.I.S.; Oliveira, J.; Gomes, M.T.S. Using Acoustic Wave Sensors to Follow Milk Coagulation and to Separate the Cheeses According to the Milk Origin. Sens. Actuators B Chem. 2015, 207, 1121–1128. [Google Scholar] [CrossRef]
- Ay, C.; Gunasekaran, S. Ultrasonic Attenuation Measurements for Estimating Milk Coagulation Time. Trans. ASAE 1994, 37, 857–862. [Google Scholar] [CrossRef]
- Nassar, G.; Nongaillard, B.; Noël, Y. Study by Ultrasound of the Impact of Technological Parameters Changes in the Milk Gelation Process. J. Food Eng. 2004, 63, 229–236. [Google Scholar] [CrossRef]
- Dwyer, C.; Donnelly, L.; Buckin, V. Ultrasonic Analysis of Rennet-Induced Pre-Gelation and Gelation Processes in Milk. J. Dairy Res. 2005, 72, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bulca, S.; Kulozik, U. A Comparison of Low-Intensity Ultrasound and Oscillating Rheology to Assess the Renneting Properties of Casein Solutions after UHT Heat Pre-Treatment. Int. Dairy J. 2007, 17, 50–58. [Google Scholar] [CrossRef]
- Koc, A.B.; Ozer, B. Nondestructive Monitoring of Renetted Whole Milk during Cheese Manufacturing. Food Res. Int. 2008, 41, 745–750. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and Food Technology: A Review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Chandrapala, J.; Leong, T. Ultrasonic Processing for Dairy Applications: Recent Advances. Food Eng. Rev. 2014, 7, 143–158. [Google Scholar] [CrossRef]
- Higuera-Barraza, O.; Del Toro-Sanchez, C.; Ruiz-Cruz, S.; Marquez-Ríos, E. Effects of High-Energy Ultrasound on the Functional Properties of Proteins. Ultrason. Sonochem. 2016, 31, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Gajendragadkar, C.N.; Gogate, P.R. Intensified Recovery of Valuable Products from Whey by Use of Ultrasound in Processing Steps–A Review. Ultrason. Sonochem. 2016, 32, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.; Juliano, P.; Knoerzer, K. Advances in Ultrasonic and Megasonic Processing of Foods. Food Eng. Rev. 2017, 9, 237–256. [Google Scholar] [CrossRef]
- Paniwnyk, L. Applications of Ultrasound in Processing of Liquid Foods: A Review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound Technology for Food Fermentation Applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Shokri, S.; Shekarforoush, S.S.; Hosseinzadeh, S. Stimulatory Effects of Low Intensity Ultrasound on the Growth Kinetics and Metabolic Activity of Lactococcus Lactis Subsp. Lactis. Process. Biochem. 2020, 89, 1–8. [Google Scholar] [CrossRef]
- AshokKumar, M.; Bhaskaracharya, R.; Kentish, S.; Lee, J.; Palmer, M.; Zisu, B. The Ultrasonic Processing of Dairy Products–An Overview. Dairy Sci. Technol. 2010, 90, 147–168. [Google Scholar] [CrossRef]
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. A Comparison of Selected Quality Characteristics of Yoghurts Prepared From Thermosonicated and Conventionally Heated Milks. Food Chem. 2010, 119, 1108–1113. [Google Scholar] [CrossRef]
- Abesinghe, A.; Vidanarachchi, J.; Islam, N.; Prakash, S.; Silva, K.; Bhandari, B.; Karim, M. Effects of Ultrasonication on the Physicochemical Properties of Milk Fat Globules of Bubalus Bubalis (Water Buffalo) Under Processing Conditions: A Comparison with Shear-Homogenization. Innov. Food Sci. Emerg. Technol. 2020, 59, 102237. [Google Scholar] [CrossRef]
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. The Effect of Thermosonication of Milk on Selected Physicochemical and Microstructural Properties of Yoghurt Gels during Fermentation. Food Chem. 2009, 114, 905–911. [Google Scholar] [CrossRef]
- Körzendörfer, A.; Schäfer, J.; Hinrichs, J.; Nöbel, S. Power Ultrasound as a Tool to Improve the Processability of Protein-Enriched Fermented Milk Gels for Greek Yogurt Manufacture. J. Dairy Sci. 2019, 102, 7826–7837. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.J.; Silk, T.M.; Wu, J.; Guo, M. Inactivation of Microorganisms in Milk and Apple Cider Treated with Ultrasound. J. Food Prot. 2006, 69, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Chouliara, E.; Georgogianni, K.; Kanellopoulou, N.; Kontominas, M.G. Effect of Ultrasonication on Microbiological, Chemical and Sensory Properties of Raw, Thermized and Pasteurized Milk. Int. Dairy J. 2010, 20, 307–313. [Google Scholar] [CrossRef]
- Gera, N.; Doores, S. Kinetics and Mechanism of Bacterial Inactivation by Ultrasound Waves and Sonoprotective Effect of Milk Components. J. Food Sci. 2011, 76, M111–M119. [Google Scholar] [CrossRef]
- Geciova, J.; Bury, D.; Jelen, P. Methods for Disruption of Microbial Cells for Potential Use in the Dairy Industry—A Review. Int. Dairy J. 2002, 12, 541–553. [Google Scholar] [CrossRef]
- Lee, H.; Zhou, B.; Liang, W.; Feng, H.; Martin, S.E. Inactivation of Escherichia Coli Cells with Sonication, Manosonication, Thermosonication, and Manothermosonication: Microbial Responses and Kinetics Modeling. J. Food Eng. 2009, 93, 354–364. [Google Scholar] [CrossRef]
- Gao, S.; Hemar, Y.; Lewis, G.D.; AshokKumar, M. Inactivation of Enterobacter Aerogenes in Reconstituted Skim Milk by High- and Low-Frequency Ultrasound. Ultrason. Sonochem. 2014, 21, 2099–2106. [Google Scholar] [CrossRef]
- Doulah, M.S. Mechanism of Disintegration of Biological Cells in Ultrasonic Cavitation. Biotechnol. Bioeng. 1977, 19, 649–660. [Google Scholar] [CrossRef]
- Shamila-Syuhada, A.K.; Chuah, L.-O.; Wan-Nadiah, W.A.; Cheng, L.H.; Alkarkhi, A.F.; Effarizah, M.E.; Ali, G.R.R. Inactivation of Microbiota and Selected Spoilage and Pathogenic Bacteria in Milk by Combinations of Ultrasound, Hydrogen Peroxide, and Active Lactoperoxidase System. Int. Dairy J. 2016, 61, 120–125. [Google Scholar] [CrossRef]
- Monsen, T.; Lövgren, E.; Widerström, M.; Wallinder, L. In Vitro Effect of Ultrasound on Bacteria and Suggested Protocol for Sonication and Diagnosis of Prosthetic Infections. J. Clin. Microbiol. 2009, 47, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Van Hekken, D.; Renye, J.; Bucci, A.; Tomasula, P.M. Characterization of the Physical, Microbiological, and Chemical Properties of Sonicated Raw Bovine Milk. J. Dairy Sci. 2019, 102, 6928–6942. [Google Scholar] [CrossRef] [PubMed]
- Feliu, J.X.; Cubarsi, R.; Villaverde, A. Optimized Release of Recombinant Proteins by Ultrasonication of E. Coli Cells. Biotechnol. Bioeng. 1998, 58, 536–540. [Google Scholar] [CrossRef]
- Kuboi, R.; Umakoshi, H.; Takagi, N.; Komasawa, I. Optimal Disruption Methods for the Selective Recovery of B-Galactosidase from E. Coli. J. Ferment. Bioeng. 1995, 79, 335–341. [Google Scholar] [CrossRef]
- Evelyn, E.; Silva, F.V. Thermosonication Versus Thermal Processing of Skim Milk and Beef Slurry: Modeling the Inactivation Kinetics of Psychrotrophic Bacillus Cereus Spores. Food Res. Int. 2015, 67, 67–74. [Google Scholar] [CrossRef]
- Frank, J.F.; Yousef, A.E. Chapter 08Tests for Groups of Microorganisms. In Standard Methods for the Examination of Dairy Products; Marshall, R.T., Ed.; American Public Health Association: Washington, DC, USA, 2004; pp. 271–286. [Google Scholar]
- Villamiel, M.; Verdurmen, R.; Jong, P.D. Degassing of Milk by High-Intensity Ultrasound. Milchwissenschaft 2000, 55, 123–125. [Google Scholar]
- Riera, E.; Gallego-Juárez, J.A.; Mason, T.J. Airborne Ultrasound for the Precipitation of Smokes and Powders and the Destruction of Foams. Ultrason. Sonochem. 2005, 13, 107–116. [Google Scholar] [CrossRef]
- Aslan, D.; Dogan, M. The Influence of Ultrasound on the Stability of Dairy-Based, Emulsifier-Free Emulsions: Rheological and Morphological Aspect. Eur. Food Res. Technol. 2017, 244, 409–421. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.; Gamlath, C.J.; Martin, G.J.; AshokKumar, M. Effects of High Pressure, Microwave and Ultrasound Processing on Proteins and Enzyme Activity in Dairy Systems—A Review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Shen, X.; Shao, S.; Guo, M. Ultrasound-Induced Changes in Physical and Functional Properties of Whey Proteins. Int. J. Food Sci. Technol. 2016, 52, 381–388. [Google Scholar] [CrossRef]
- Bjørnø, L. Ultrasound in the Food Industry. In Ultrasonics International 91; Clayton, L., Ed.; Butterworth-Heinemann: Le Touquet, France, 1991; pp. 23–29. [Google Scholar]
- Paniwnyk, L. Application of Ultrasound. In Emerging Technologies for Food Processing; Sun, D.W., Ed.; Elsevier: London, UK, 2014; pp. 271–291. [Google Scholar]
- Chandrapala, J.; Martin, G.; Zisu, B.; Kentish, S.E.; AshokKumar, M. The Effect of Ultrasound on Casein Micelle Integrity. J. Dairy Sci. 2012, 95, 6882–6890. [Google Scholar] [CrossRef] [PubMed]
- Crudo, D.; Bosco, V.; Cavaglià, G.; Mantegna, S.; Battaglia, L.; Cravotto, G. Process Intensification in the Food Industry: Hydrodynamic and Acoustic Cavitation in Fresh Milk Treatment. Agric. Food Ind. Tech 2014, 25, 55–59. [Google Scholar]
- Bermúdez-Aguirre, D.; Mawson, R.; Barbosa-Cánovas, G. Microstructure of Fat Globules in Whole Milk after Thermosonication Treatment. J. Food Sci. 2008, 73, E325–E332. [Google Scholar] [CrossRef] [PubMed]
- Ertugay, M.F.; Şengül, M.; Şengül, M. Effect of Ultrasound Treatment on Milk Homogenisation and Particle Size Distribution of Fat. Turk. J. Vet. Anim. Sci. 2004, 28, 303–308. [Google Scholar]
- Perdih, T.S.; Zupanc, M.; Dular, M. Revision of the Mechanisms Behind Oil-Water (O/W) Emulsion Preparation by Ultrasound and Cavitation. Ultrason. Sonochem. 2019, 51, 298–304. [Google Scholar] [CrossRef]
- Plüisch, C.S.; Wittemann, A. Assembly of Nanoparticles into “Colloidal Molecules”: Toward Complex and yet Defined Colloids with Exiting Perspectives. In Advances in Colloid Science; Rahman, M.M., Asiri, A.M., Eds.; IntechOpen: Rijeka, Croatia, 2016; p. 11. [Google Scholar]
- Mullin, J.W. Crystallization, 4th ed.; Mullin, J.W., Ed.; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar]
- Zheng, L.; Sun, D.-W. Innovative Applications of Power Ultrasound during Food Freezing Processes—A Review. Trends Food Sci. Technol. 2006, 17, 16–23. [Google Scholar] [CrossRef]
- Bund, R.; Pandit, A. Sonocrystallization: Effect on Lactose Recovery and Crystal Habit. Ultrason. Sonochem. 2007, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. Developments in Ultrasound—Non-Medical. Prog. Biophys. Mol. Biol. 2007, 93, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Luque de castro, M.; Priego-Capote, F. Ultrasound-Assisted Crystallization (Sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Acton, E.; Morris, G.J. Method and Apparatus for the Control of Solidification in Liquids. Worldw. Pat. WO 1992, 92, 20420. [Google Scholar]
- Martini, S.; Suzuki, A.H.; Hartel, R.W. Effect of High Intensity Ultrasound on Crystallization Behavior of Anhydrous Milk Fat. J. Am. Oil Chem. Soc. 2008, 85, 621–628. [Google Scholar] [CrossRef]
- AshokKumar, M.; Lee, J.; Zisu, B.; Bhaskarcharya, R.; Palmer, M.; Kentish, S.E. Hot Topic: Sonication Increases the Heat Stability of Whey Proteins. J. Dairy Sci. 2009, 92, 5353–5356. [Google Scholar] [CrossRef]
- Krešić, G.; Lelas, V.; Jambrak, A.R.; Herceg, Z.; Brnčić, S.R. Influence of Novel Food Processing Technologies on the Rheological and Thermophysical Properties of Whey Proteins. J. Food Eng. 2008, 87, 64–73. [Google Scholar] [CrossRef]
- Pathania, S.; Ho, Q.T.; Hogan, S.A.; McCarthy, N.; Tobin, J.T. Applications of Hydrodynamic Cavitation for Instant Rehydration of High Protein Milk Powders. J. Food Eng. 2018, 225, 18–25. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kelly, P.M.; Maher, P.G.; Fenelon, M.A. Dissolution of Milk Protein Concentrate (MPC) Powders by Ultrasonication. J. Food Eng. 2014, 126, 142–148. [Google Scholar] [CrossRef]
- Zisu, B.; Schleyer, M.; Chandrapala, J. Application of Ultrasound to Reduce Viscosity and Control the Rate of Age Thickening of Concentrated Skim Milk. Int. Dairy J. 2013, 31, 41–43. [Google Scholar] [CrossRef]
- Almanza-Rubio, J.L.; Gutiérrez-Méndez, N.; Leal-Ramos, M.Y.; Sepúlveda, D.; Salmerón, I. Modification of the Textural and Rheological Properties of Cream Cheese Using Thermosonicated Milk. J. Food Eng. 2016, 168, 223–230. [Google Scholar] [CrossRef]
- Jeličić, I.; Božanić, R.; Brnčić, M.; Tripalo, B. Influence and Comparison of Thermal, Ultrasonic and Thermo-Sonic Treatments on Microbiological Quality and Sensory Properties of Rennet Cheese Whey. Mljekarstvo Časopis Unaprjeđenje Proizv. Prerade Mlijeka 2012, 62, 165–178. [Google Scholar]
- Bermúdez-Aguirre, D.; Barbosacanovas, G.V. Processing of Soft Hispanic Cheese (“Queso Fresco”) Using Thermo-Sonicated Milk: A Study of Physicochemical Characteristics and Storage Life. J. Food Sci. 2010, 75, S548–S558. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; AshokKumar, M. Ultrasonics in Food Processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Balzan, S.; Montemurro, F.; Fasolato, L.; Andrighetto, I.; Segato, S.; Novelli, E. Effect of Ultrasound Alone or Ultrasound Coupled with CO2 on the Chemical Composition, Cheese-Making Properties and Sensory Traits of Raw Milk. Innov. Food Sci. Emerg. Technol. 2012, 16, 391–397. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, S.; Uluko, H.; Liu, L.; Lu, J.; Xue, H.; Kong, F.; Lv, J. Effect of Ultrasound Pretreatment on Rennet-Induced Coagulation Properties of Goat’s Milk. Food Chem. 2014, 165, 167–174. [Google Scholar] [CrossRef] [PubMed]








| Name | Range, (Hz) |
|---|---|
| Infrasound | IS < 16 |
| Audible Sound | 16 ≤ AS ≤ 17.8 |
| Ultrasound | 17.8 < US < 1 G |
| Hypersound | HS ≥ 1 G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Martínez, A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Bolivar-Jacobo, N.A. Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality. Foods 2020, 9, 1688. https://doi.org/10.3390/foods9111688
Chávez-Martínez A, Reyes-Villagrana RA, Rentería-Monterrubio AL, Sánchez-Vega R, Tirado-Gallegos JM, Bolivar-Jacobo NA. Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality. Foods. 2020; 9(11):1688. https://doi.org/10.3390/foods9111688
Chicago/Turabian StyleChávez-Martínez, América, Raúl Alberto Reyes-Villagrana, Ana Luisa Rentería-Monterrubio, Rogelio Sánchez-Vega, Juan Manuel Tirado-Gallegos, and Norma Angélica Bolivar-Jacobo. 2020. "Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality" Foods 9, no. 11: 1688. https://doi.org/10.3390/foods9111688
APA StyleChávez-Martínez, A., Reyes-Villagrana, R. A., Rentería-Monterrubio, A. L., Sánchez-Vega, R., Tirado-Gallegos, J. M., & Bolivar-Jacobo, N. A. (2020). Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality. Foods, 9(11), 1688. https://doi.org/10.3390/foods9111688





