Abstract
We measured the spatial distribution and composition of ozone-forming hydrocarbons, alcohols, and carbonyls in Utah’s Uinta Basin during the winter months of 2019 and 2020. The Uinta Basin contains about 10,000 producing oil and gas wells. Snow cover and the region’s unique topography (i.e., a large basin entirely surrounded by mountains) promote strong, multi-day temperature inversion episodes that concentrate pollution and lead to wintertime ozone production. Indeed, organic compound concentrations were about eight times higher during inversion episodes than during snow-free springtime conditions. We examined spatial associations between wintertime concentrations of organics and oil and gas sources in the region, and we found that concentrations of highly reactive alkenes were higher in areas with dense oil production than in areas with dense gas production. Total alkene+acetylene concentrations were 267 (42, 1146; lower and upper 95% confidence limits) µg m−3 at locations with 340 or more producing oil wells within 10 km (i.e., 75th percentile) versus 12 (9, 23) µg m−3 at locations with 15 or fewer oil wells (i.e., 25th percentile). Twenty-eight percent of the potential for organic compounds to produce ozone was due to alkenes in areas with dense oil production. Spatial correlations and organic compound ratios indicated that the most likely source of excess alkenes in oil-producing areas was natural gas-fueled engines, especially lean-burning (i.e., high air:fuel ratio) artificial lift engines.
1. Introduction
Utah’s Uinta Basin suffers periodically during winter months from high concentrations of ozone [1,2,3,4], an air pollutant regulated by the Environmental Protection Agency (EPA). The number of exceedance days and concentrations of ozone that occur in a given year are directly related to meteorology, especially persistent snow cover and extended periods of high barometric pressure, which lead to persistent, multi-day thermal inversions that trap pollution and allow ozone to form [5,6]. Ozone tends to be higher at lower elevations in the Uinta Basin mostly because inversion conditions start earlier and last longer at low elevations, allowing more time for ozone and its precursors to accumulate [7].
The majority of ozone precursor pollutants in the Uinta Basin are emitted from the oil and gas industry [8,9,10], but emissions inventories for the industry contain significant uncertainty. Three-dimensional photochemical models are generally not able to simulate high wintertime ozone when official emissions inventories are used. At least part of the reason for underprediction of wintertime ozone is that official inventories appear to underestimate the magnitude of organic compound emissions [1,3] and the percentage of total emissions comprised by reactive organics [11].
Emission factors and composition data used to develop emissions inventories sometimes rely on outdated datasets with few samples, and regionally representative data are rarely available. The U.S. Environmental Protection Agency’s SPECIATE database is a repository of thousands of emissions composition datasets (also known as speciation profiles) that photochemical modelers use with total emission rates of organics in inventories to allocate emissions of individual organic compounds or groups of compounds [12]. The U.S. EPA has worked to update many oil and gas-related emissions profiles, including some that are specific to the Uinta Basin [13]. Some emissions profiles are still in need of updating, however. For example, the composition information contained in SPECIATE for natural gas-fueled stationary internal combustion engines (profile 1001) is derived from a study of a handful of engines measured in the 1980s in California [14,15].
Many recent studies have focused on improving estimates of methane emissions from oil and gas facilities [16,17,18,19,20,21,22], including in the Uinta Basin [8,9,23,24], but methane is not an important ozone precursor [2]. A few recent studies have directly measured speciated organic compound emissions from oil and gas sources, including individual components at well pads [25,26], at the well pad-level [25,27], water storage tanks [28], from oil and gas waste streams [29,30,31,32], during drilling and well completion operations [33], and from subsurface well pad sources [34]. Measurements of speciated non-methane organics in air influenced by the oil and gas sector have been reported [35,36,37,38,39,40,41,42,43,44], and some of these studies have attempted to infer information about specific sources or source types from ambient air data [45,46,47,48]. These studies have provided critical information about how oil and gas development impacts local and regional air quality, but more work is needed to comprehensively characterize the composition of emissions from many oil and gas sources.
In this study, we built 17 portable air sample collection stations and deployed them across the Uinta Basin during the winter months of 2019 and 2020. We analyzed samples for a suite of 72 organic compounds, including alkanes, alkenes, acetylene, aromatics, light alcohols, and carbonyls. We used this dataset to assess spatial trends in organic compound composition and to understand how specific source types influenced organics in ambient air. In this paper, we focus on observed high concentrations of alkenes (especially ethylene and propylene), their possible sources, and implications for wintertime ozone.
2. Methods
2.1. Study Location
We conducted this study in the Uinta Basin in northeastern Utah, United States (Figure 1). The Uinta Basin is a rural, desert region with a population of about 50,000 people and 9600 producing oil and gas wells. The western Basin produces mostly oil, while the eastern Basin produces mostly natural gas. Wells were first drilled in the Uinta Basin in the 1940s, but only 15% of currently-producing oil and gas wells were completed before 2000 (UDOGM, 2018). An industry downturn that started in 2014 [49,50,51] led to a decrease in drilling, such that 87% of currently-producing wells were completed before 2014.
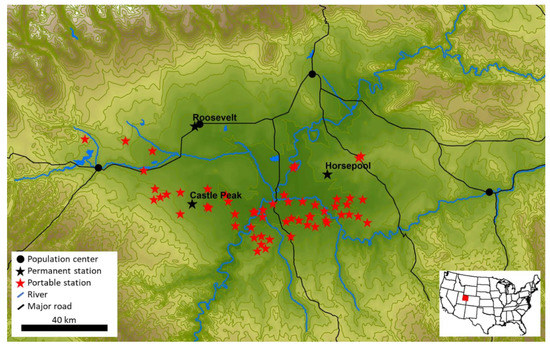
Figure 1.
Map of the Uinta Basin, with all sampling stations shown. Portable station locations shown are locations where at least one measurement occurred. Elevation contours are at 100 m intervals. For reference, the Horsepool station is at 1569 m.
2.2. Portable Sampling Stations
We deployed 14 portable sample collection stations during the winter months of 2019, and 17 stations during winter 2020 (see a diagram of the stations in Figure S1). Four of the stations were equipped with measurements of ambient temperature, relative humidity, wind speed and direction, and barometric pressure during 2019, and ten were equipped with these measurements during 2020. All stations incorporated a GPS (±5 m accuracy) in 2020, whereas we used cell phone GPS to record station locations in 2019. Figure 1 shows all unique portable station sampling locations from both winters.
For each station, a 0.5 μm PTFE filter pack was situated at the sampling inlet. The inlet protruded 3 cm from an insulated plastic box. PFA tubing (3 mm diameter) and PFA fittings led from the filter inlet to (1) an evacuated silonite-coated 6 L stainless steel canister (for hydrocarbons and light alcohols) and (2) a trans-1,2-bis(2-pyridyl)ethylene and 2,4-dinitrophenylhydrazine-coated silica (BPE-DNPH) cartridge (Sigma-Aldrich P/N 54278-U; for carbonyls). A critical orifice and miniature sampling pump downstream of the BPE-DNPH cartridge maintained flow at 1.25 L min−1. We measured the BPE-DNPH sampling flow rate for each station with a BIOS DryCal flow meter that had been factory-calibrated within less than a year. A high-purity solenoid valve (Clippard P/N O-ET-2-12) upstream of each silonite canister controlled flow into the canister and a stainless steel critical orifice regulated the flow rate. All sampling components, excluding the silonite-coated canisters, were located within the insulated box. We placed 48-h disposable hand warmers within the insulated plastic box and on the tubing that led from the box to the canisters. The hand warmers kept the box and tubing at 8.1 (6.6, 9.5) °C.
Clippard valves and BPE-DNPH sampling pumps were controlled by a relay board, and a Raspberry Pi with a custom Python program controlled the relay board. The Python program also allowed users to designate sampling times and dates for each deployment, so all stations sampled at exactly the same time, and it allowed the Pi to record all station parameters at 5-min intervals. Sampling stations collected air samples over 6 h intervals that always began at 10:00 or 22:00 local standard time. We positioned our measurement stations in several different spatial configurations over the two years to understand the distribution, magnitude, and composition of organic compounds in the oil and gas-producing areas of the Uinta Basin.
2.3. Permanent Monitoring Stations
In addition to the portable stations, our group operated permanent monitoring stations during the 2019 and 2020 winters at Roosevelt, Horsepool, and Castle Peak (Figure 1). We collected daily 3-h silonite-coated canister samples at each of these stations that began at either midnight or noon local standard time (except that only three samples per week were collected at Roosevelt in 2020). We also collected daily 3-h DNPH cartridge samples at Horsepool and Castle Peak during winter 2020. We used Waters Sep-Pak cartridges (P/N WAT037500) with Sep-Pak KI ozone scrubbers (P/N WAT054420), rather than the Supelco BPE-DNPH cartridges, for the permanent stations because an inadequate number of BPE-DNPH cartridges were available from the manufacturer. An intercomparison of the two types of cartridges showed no significant differences.
The sampling apparatuses at the permanent stations were similar to those at the portable stations. Samples were pulled through a PTFE filter, then through PFA tubing and into the canisters and cartridges. Stainless critical orifices regulated the flow rate of sample air into the canisters, and high-purity valves turned flow on and off. Sample air passed through a PTFE manifold and then into the DNPH cartridges. Mass flow meters recorded the flow through each cartridge, and valves and critical orifices downstream of the cartridges regulated the flow. A Campbell Scientific data logger controlled the valves and recorded sampling flow rates and valve positions.
Each of the permanent monitoring stations measured meteorological conditions, including wind speed and direction, temperature, relative humidity, barometric pressure, snow depth, and solar radiation. We checked all meteorological instruments against U.S. National Institutes of Standards and Technology (NIST)-traceable standards at least annually. The Horsepool station measured incoming and reflected UV-A and UV-B radiation with dual Kipp and Zonen UV radiometers. We calibrated the radiometers at the factory every three years. We measured ozone at the Horsepool station with an Ecotech 9810 ozone analyzer, and we calibrated the analyzer at three points weekly, and at 5 points at the beginning and end of each winter season, with an Ecotech GasCal calibrator. We checked the calibrator against a NIST-traceable ozone standard at the beginning and end of each winter season. We only used ozone data bracketed by calibration checks within 5% of expected non-zero values, and within ± 3 ppb when zero was expected.
2.4. Laboratory Analysis
We analyzed canister samples within 45 days of sampling for 54 hydrocarbons and three light alcohols using a derivation of the U.S. EPA Photochemical Assessment Monitoring Stations (PAMS) method [52]. Lyman, et al. [29] showed that all measured compounds, including alcohols, were stable in canisters over this period, and others have shown stability in canisters for hydrocarbons generally [53]. We used an Entech 7200 pre-concentrator in cold trap dehydration mode and a 7016D autosampler to cryogenically concentrate organic compounds from the air samples and introduce them to a gas chromatograph (GC) system, similar to Lyman, et al. [29]. The GC system included two Shimadzu GC-2010 GCs, one with a flame ionization detector (FID) and one with a Shimadzu QP2010 Mass Spectrometer (MS). Samples introduced to the GC system first passed through a Restek rtx1 ms column (60 m, 0.32 mm I.D.), and then entered a heated VICI four-port valve with a Valcon E rotor. For the first few minutes after injection, the sample then passed into a Restek AluminaBOND/Na2SO4 column (50 m, 0.32 mm I.D.) and into the FID. After the first few minutes, the valve position changed, and the sample was directed into another Restek rtx1 ms column (30 m, 0.25 mm I.D.), and then into the MS. Hydrocarbons with two and three carbon atoms were quantified by FID, whereas all other compounds were quantified by MS. We used a 5-point curve to calibrate the flame ionization detector and mass spectrometer before each analytical batch. We used an Entech 4600A to prepare calibration standards by diluting them in N2 from certified compressed gas standards. We also analyzed a duplicate sample, at least one analytical blank, and a calibration standard at the beginning and end of each batch.
We analyzed the DNPH cartridges for 13 carbonyl compounds, following Uchiyama, et al. [54] (also see Lyman, et al. [29]). We eluted cartridge samples into 5 mL volumetric flasks using a mixture of 75% acetonitrile and 25% dimethyl sulfoxide. We then used the same mixture to increase the total volume of the flasks to 5 mL. We transferred 1 mL of eluent from the flasks into an auto-sampler vial and analyzed samples using high-performance liquid chromatography (HPLC). We used a Hewlett Packard series 1050 HPLC with a Restek Ultra AQ C18 column and a guard column of the same material with a diode array detector. The eluent consisted of acetonitrile and ultra-pure water in gradient mode. We prepared calibration standards by diluting carbonyl-DNPH standards purchased from Sigma Aldrich (P/N 47650-U, CRM47651) and AccuStandard (P/N m-1004), and we calibrated the instrument regularly with a five-point calibration curve. We eluted samples within 14 days of collection, and we analyzed the eluent within 30 days of elution, following U.S. EPA protocols [55]. We analyzed several calibration standards at the beginning and the end of each analysis batch to check for retention time drift or other errors, and we also analyzed laboratory blanks and duplicate samples for each batch.
2.5. Oil and Gas Industry Data
We used oil and gas industry data available from the Utah Division of Oil, Gas, and Mining [56] and emissions data from the 2014 Utah Air Agencies Oil and Gas Emissions Inventory [10]. The 2014 Utah Air Agencies Inventory is a component of the 2014 U.S. EPA National Emissions Inventory [57,58]. We also calculated compound-specific emissions from the Utah Air Agencies Inventory. The Inventory reports total volatile organic compound emissions from oil and gas equipment and activities (e.g., engines, dehydrators, truck loadings, tanks, etc.), each of which is assigned a speciation profile by the EPA’s National Emissions Inventory [57] that is mapped to the SPECIATE database version 5.1 [14]. We converted total volatile organic compound emissions to total organic gas and then determined emissions of each specific compound based on its ratio to total organic gas as specified by SPECIATE. We also used raw and flash gas profiles that were derived from the Uinta Basin Composition Study [26].
2.6. Statistical Analysis
We present values as mean (lower 95% confidence limit, upper 95% confidence limit) when presentation of confidence limits is appropriate. We calculated confidence limits in Python with the scikits.bootstrap package, following Lyman, et al. [59]. Because the datasets collected in this study are heavy-tailed (as are most oil and gas-related datasets [60]), most of the statistical techniques we used are non-parametric. For convenience, most of the discussion below focuses on compound groups (alkanes, alkenes+acetylene, aromatics, alcohols, and carbonyls), rather than individual compounds. We present specific techniques as they are discussed below. We use units of µg m−3, rather than ppb, since this paper makes comparisons against emissions data and inventories, which are traditionally presented in mass units. Table S1 provides a conversion for each compound we measured.
We used ArcGIS version 10.2 for spatial analyses. We followed the methods presented by Lyman and Tran [7] to determine the proximity of sampling locations to possible emission sources. Similar to Lyman and Tran, we found that correlations of organic compounds with proximity to oil and gas facilities and related parameters were strongest when summed within a 10 km radius. The spatially-relevant results and discussion below are thus based on relationships within 10 km radii of sampling locations. We calculated the following parameters within 10 km of each sampling location. Parameters b through l are from the 2014 Utah Air Agencies Oil and Gas Emissions Inventory [10], and parameters m and n are from the Utah Division of Oil, Gas, and Mining’s online database [56].
- (a)
- Average elevation
- (b)
- Number of engines
- (c)
- Total engine organic compound emissions
- (d)
- Number of gas-fueled engines
- (e)
- Number of artificial lift engines
- (f)
- Number of two-stroke engines
- (g)
- Number of produced water ponds
- (h)
- Total area of produced water ponds
- (i)
- Number of glycol dehydrators
- (j)
- Total dehydrator organic compound emissions
- (k)
- Number of liquid storage tanks
- (l)
- Number of controls on liquid storage tanks
- (m)
- Number of producing oil and gas wells
- (n)
- Total amount of oil, gas, and water production
2.7. Quality Assurance
We collected canister field blanks by sampling hydrocarbon-free air generated by a Teledyne T701H, and we collected DNPH cartridge field blanks by deploying cartridges in field collection systems and immediately collecting them without pulling sample air through them. Canister field blanks resulted in hydrocarbon concentrations of 0.2 (0.1, 0.4) µg m−3, and DNPH cartridge field blanks resulted in carbonyl concentrations of 0.5 (0.2, 1.0) µg m−3 (all statistics in this section are averages for individual organic compounds). We collected field spikes for canister samples by filling high-pressure cylinders with hydrocarbon and alcohol mixtures with concentrations that were similar to ambient levels. We filled canisters directly from these cylinders in the laboratory, and then we attached the cylinders to field sampling systems and filled additional canisters. Laboratory-filled canisters recovered 6 (4, 7)% less hydrocarbons and alcohols than field-filled canisters.
Replicate analyses of canister samples were 0 (0, 0)% different from each other, and replicate DNPH cartridge analyses were 0 (−3, 3)% different. Calibration checks yielded 95 (0, 0)% recovery for hydrocarbons and alcohols, and 101 (101, 102)% recovery for carbonyls. Detection limits (determined as 3 times the standard deviation of standards with concentrations near the detection limit) were 0.3 (0.2, 0.4) µg m−3 for hydrocarbons and alcohols and 0.7 (0.5, 0.9) µg m−3 for carbonyls.
3. Results and Discussion
3.1. Impact of Inversion Conditions on Organic Compound Concentrations
Five of our sampling periods occurred during winter inversion episodes, seven were outside of inversion episodes but within the winter season, and one occurred during spring (Figure 2). Most inversion episodes in 2019 were associated with ozone in excess of the U.S. EPA 70 ppb ozone standard, but episodes in 2020 were not. Ozone and precursor concentrations have decreased over time in the Uinta Basin [61], and this could have influenced the year-on-year change in ozone. Meteorological conditions in 2020 were also different, however. Inversion episodes in winter 2019 were mostly sunny, while winter 2020 was consistently cloudy, so less sunlight was available to produce ozone (Figure 2). Inversion episodes in 2020 were also shorter, except for one episode in early January, but low solar elevation in January leads to inefficient ozone production.
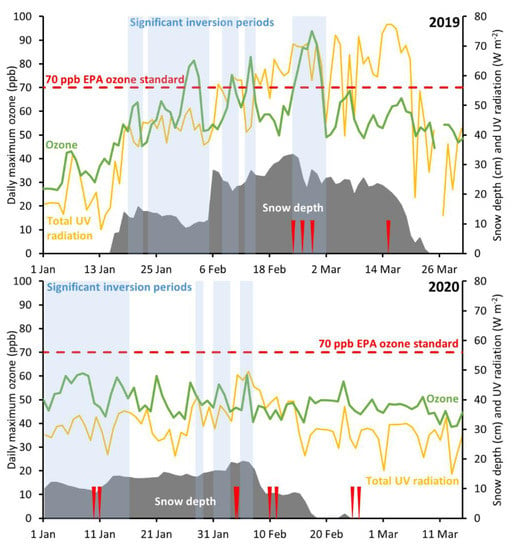
Figure 2.
Time series of ozone, snow depth, significant inversion periods, and total daytime UV radiation (UV-A + UV-B, incoming + outgoing) at the Horsepool monitoring station during winters 2019 (top) and 2020 (bottom). Periods during which samples were collected at portable stations are indicated by red ticks. A collection on 17 April 2019 is not shown.
Several deployments of the sample collection stations coincided with a temperature inversion episode that occurred from 23 February through 28 February 2019. This episode led to ozone concentrations exceeding 100 ppb at some area monitoring stations. During this episode, organic compound concentrations were elevated when compared to samples taken outside of inversion periods. Samples collected during an inversion on 27 February had organic compound concentrations that were 7.9 times higher, on average, than at the same locations during the 17 April 2019 deployment, which did not have snow cover or inversion conditions (Figure 3). We observed a similar trend during winter 2020, and Edwards, et al. [2] and Helmig, et al. [36] observed a similar trend in 2013.
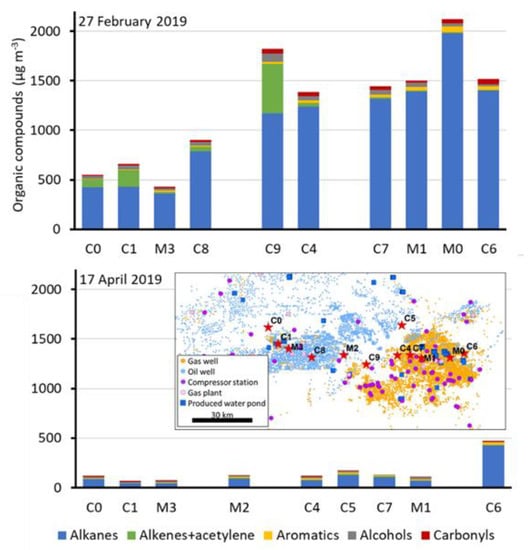
Figure 3.
Organic compound concentrations at all measurement stations on 27 February 2019 during a wintertime temperature inversion episode (top panel) and on 17 April 2019 when snow had melted and no inversion existed (bottom panel). Blank spaces exist because some samples failed to collect at some stations. The inset map shows the locations of the monitoring stations as red stars.
Helmig et al. found that alkane concentrations during inversion episodes were at least two times higher than periods without inversions, leading to higher ozone production rates. Non-inversion conditions studied by Helmig et al. sustained widespread snow cover, which may have inhibited vertical mixing and kept concentrations relatively high, leading to a less profound difference between periods with and without temperature inversion. Average values measured at the Horsepool station from 23 to 27 February 2019 in this study were only 39 (32, 46)% of values reported by Helmig et al., consistent with the finding that ozone precursor concentrations in the Uinta Basin have decreased since 2013, as discussed by Mansfield and Lyman [61].
Increases in pollutant concentrations during inversion episodes occur because emitted pollutants are trapped under the inverted layer, allowing their concentrations to build day-upon-day [7]. The concentration of organics was negatively correlated with elevation during inversion periods (Spearman r from −0.31 to −0.49 for total non-methane hydrocarbons; NMHC), partly because oil and gas sources are negatively correlated with elevation, and also because inversion conditions are more pronounced at lower elevation [7]. Ambient organics were not correlated with elevation outside of inversion periods.
3.2. Spatial Distribution
Figure 4 and Figure 5, Figures S2–S12 show the magnitude and composition of organic compounds at each measurement station for each deployment period. In general, alkanes comprised the dominant portion (by mass) of total organics at the stations (average of 85 (82, 86)%). Alkenes+acetylene, aromatics, alcohols, and carbonyls comprised averages of 8 (6, 10)%, 7 (6, 9)%, 12 (10, 15)%, and 7 (6, 8)%, respectively. Occasionally, each of these groups comprised a large portion of total organics. The 90th percentiles of composition for alkenes+acetylene, aromatics, alcohols, and carbonyls were 18%, 13%, 28%, and 16%, respectively, and maxima were 84%, 64%, 88%, and 45%, respectively.
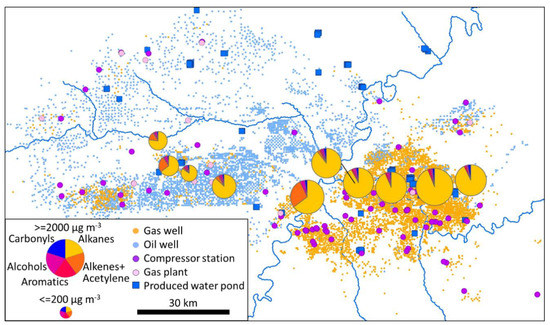
Figure 4.
Composition (weight percent) and total concentration of organic compounds measured on 27 February 2019 from 10:00 to 16:00.
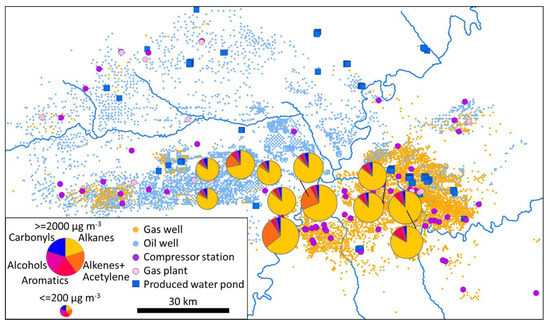
Figure 5.
Composition (weight percent) and total concentration of organic compounds measured on 11 January 2020 from 10:00 to 16:00.
Tables S2–S4 show Spearman rank correlations for concentrations of organic compound types with various spatial parameters for the entire dataset, the average of three deployments at the same locations in February 2019, and the average of four deployments at the same locations in early February 2020, respectively. The tables show consistent negative correlations of total NMHC, alkanes, and aromatics with average elevation within a 10 km radius, as discussed above. Moreover, proximity to producing gas wells correlated somewhat consistently with total NMHC, alkanes, and aromatics. Alkenes+acetylene, in contrast, correlated most strongly with proximity to producing oil wells, proximity to two-stroke engines, and (somewhat consistently) proximity to engines generally.
To further understand these relationships, we extracted organic compound measurements for all sampling locations at which each oil and gas-related spatial parameter listed in Section 2.6 was less than or equal to the 25th percentile or greater than or equal to the 75th percentile. Table 1 shows average concentrations of organic compound types for the 25th percentile and 75th percentile bins for each parameter and whether the two bins are significantly different from each other. Table 1 shows that alkane and aromatic concentrations were significantly higher in areas with more producing gas wells, while alkenes + acetylene were lower in those areas. Oil-producing and gas-producing wells are spatially anti-correlated in the Uinta Basin (Pearson r = −0.62), so a correlation with one well type leads to an anti-correlation with the other. Alkanes and total NMHC were significantly higher where more produced water pond area existed, perhaps because produced water pond area and gas well count were spatially correlated (Pearson r = 0.87) and because alkanes and total NMHC were correlated (Pearson r = 0.99). Alkenes+acetylene were significantly higher in areas with more oil wells and more engines (engines and oil wells were spatially correlated; Pearson r = 0.94). Alcohols and carbonyls showed the same pattern as alkenes+acetylene, but for engines only, not oil wells. Alkenes+acetylene were also significantly lower in areas with more glycol dehydrators, probably because dehydrators were spatially correlated with gas wells (Pearson r = 0.74).

Table 1.
Average concentrations of organic compound types (µg m−3) for all sampling locations at which the sum of each listed oil and gas-related parameter within a 10 km radius was less than or equal to the 25th percentile or greater than or equal to the 75th percentile. Bold values are significantly different (Mann–Whitney test).
Emissions from oil and gas production exhibit a heavy-tailed distribution, which means a few sources emit a high percentage of total pollution [60,62]. Similarly, the maps in Figure 4 and Figure 5, Figures S2–S12 show that the percent of total organics comprised of alkenes+acetylene is much higher for a few samples than for the dataset as a whole. In spite of high variability, Figure 6 shows that alkenes+acetylene comprised a larger portion of total organics in samples collected in areas with more producing oil wells. Alkenes+acetylene comprised 3.5 (2.4, 5.3)% of total organics at sampling locations with 0–20 wells in a 10 km radius, compared to 8.1 (6.4, 11.2)% for locations with greater than 20 wells. The results for permanent monitoring stations showed the same trend (Figure S13), with alkenes + acetylene comprising 2.0 (1.8, 3.2)% of total organics at the gas production-dominated Horsepool station, compared to 3.6 (3.0, 3.9)% at the oil production-dominated Castle Peak station. This trend was much weaker for carbonyls (5.3 (4.1, 7.0)% for <= 20 wells in 10 km; 5.4 (4.6, 6.3)% for >20) and alcohols (8.1 (5.8, 11.4)% for <= 20 wells in 10 km; 8.8 (7.4, 10.6)% for >20).
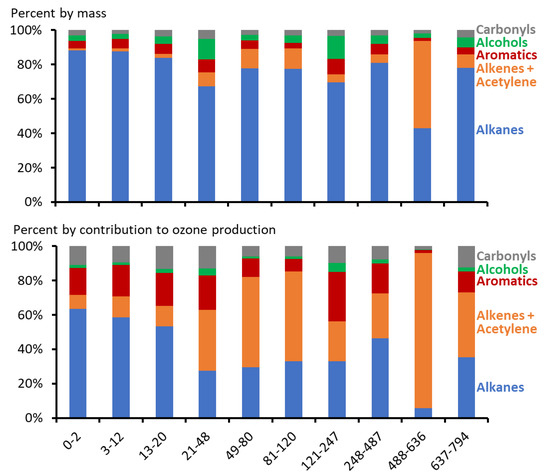
Figure 6.
Average contribution of organic compound types to total organic compound mass (top) and ability of organics to produce ozone (bottom; calculated from maximum incremental reactivity [63]). Data are 10-percentile bins of the number of producing oil wells within a 10 km radius.
Compared to most other organic compounds, alkenes are extremely reactive, so a given amount of these alkenes is able to produce more ozone than a given amount of most other organics. The impact of an organic compound on ozone formation can be estimated in computer models by changing the mass of that compound emitted in the model and recording the modeled change in ozone mass in the atmosphere. The amount of ozone produced per amount of organic compound emitted when other modeled conditions are optimized for ozone production is called maximum incremental reactivity (MIR) [63]. MIR can be used to compare the ability of different compounds to produce ozone. For example, the MIR of ethane derived by Carter [63] is 0.28 (meaning that a given mass of ethane can produce 28% of that mass of ozone in ideal conditions). In contrast, the MIR of ethylene for the same conditions is 9.00, 32 times higher than ethane.
In Figure 6 (bottom panel), we applied MIR values for concentrations of individual compounds measured in ambient air samples to obtain MIR-weighted composition information. We used MIR values from Carter [63], which were developed for urban, summertime ozone, not wintertime ozone in oil and gas-producing areas. Thus, they are only an approximation of actual MIR for winter ozone episodes in the Uinta Basin. Carter and Seinfeld [64] calculated incremental reactivities for winter ozone episodes in Wyoming in 2008, but the conditions they modeled were not chemically similar to conditions in the Uinta Basin, and thus may not be more accurate for the Uinta Basin case. The bottom panel of Figure 6 shows that alkenes are very important contributors to ozone production, particularly in areas dominated by oil production. On average, alkenes+acetylene constituted 28 (24, 33)% of MIR-adjusted organic compound composition for measurement locations with more than 20 oil wells in a 10 km radius, compared with 14 (10, 20)% at locations with 20 or fewer oil wells.
Several previous ambient air studies have found little impact from upstream oil and gas development on alkene concentrations and little influence from alkenes on the total ozone formation potential of organics [33,35,42,65], but these studies have occurred in natural gas-producing areas (Marcellus Basin in Pennsylvania, Piceance Basin in Colorado) or oil-producing areas without artificial lift engines (Denver-Julesberg Basin in Colorado [66]). Schade and Roest [67] found elevated concentrations of alkenes associated with chemical markers of oil and gas development in the Eagle Ford Basin in Texas, which they attributed to emissions from flaring and compressor engines. Koss, et al. [38] and Edwards, et al. [2] found that alkenes+acetylene were a relatively small component of OH reactivity at the Horsepool station in the Uinta Basin in 2013, which is consistent with our finding in this study of low alkene concentrations at the Horsepool station. The difference between our finding for the Uinta Basin overall and the findings of Koss et al. and Edwards et al. for the Horsepool station highlights the strong spatial variability that exists in the Basin and the need to understand spatial trends in emissions and ozone precursor concentrations.
3.3. Sources of Alkenes in Oil-Producing Areas
Combustion processes are the most important sources of alkenes+acetylene [68], and their emissions from non-combustion oil and gas sources like liquid storage tank venting and raw gas leaks are low [11,26]. Thus, most alkenes+acetylene emitted into the Uinta Basin atmosphere likely originate from combustion sources. Common combustion sources in the oil and gas industry include natural gas-fueled heaters, natural gas-fueled engines, diesel and gasoline engines (including vehicles), combustors, and flares [10]. Since alkenes+acetylene comprised a higher percentage of total organics in oil-producing areas, it is likely that (1) one or more combustion source types is more common in oil-producing areas than in gas-producing areas, and/or (2) one or more combustion source types that is common in both areas has a different emissions profile in oil-producing areas, leading to higher emissions of alkenes+acetylene.
Engines are more common in oil-producing areas because most oil wells have natural gas-fueled artificial lift engines (though some oil wells in the Uinta Basin have electric motors), while almost no gas wells have artificial lift engines [10]. Ninety-eight percent of all stationary engines in the Uinta Basin are natural gas-fueled, and 95% of natural gas engines in the Uinta Basin do not have emissions control equipment [10]. Oil wells in the Uinta Basin are also more likely to have emissions control devices, including combustors or flares, on tanks [69]. Oil and gas wells both have natural gas-fueled heaters. Natural gas-fueled compressor engines are more common in gas-producing areas (Figure 4), but there are relatively few compressor engines in the Uinta Basin. Truck traffic is likely to be more frequent in the oil-producing Uinta Basin because trucks are used to transport liquids (oil, condensate, and water) from most wells, and oil wells produce 7.3 times more liquids than gas wells [56].
Figure 7 shows a plot of the relationship between ethylene and propylene in all measurements collected from the portable sampling stations. The two compounds were strongly correlated (Spearman r = 0.91), and the average of the propylene:ethylene ratio in each ambient air sample was 0.89 (0.78, 1.06). Hesterberg, et al. [70] showed that, compared to uncontrolled diesel-fueled engines, uncontrolled natural gas-fueled engines have higher propylene:ethylene ratios (0.07 versus 0.28). The propylene:ethylene ratio in our measurements, however, was more than three times higher than that reported for natural gas engines by Hesterberg et al. Ethylene and propylene can be generated from the combustion of methane or from combustion of NMHC [71], and Kim and Bae [72] showed that propylene emissions (but not ethylene emissions) increased with the NMHC content of fuel (also see Drobot, et al. [73]). The raw gas available at Uinta Basin oil wells is richer in propane and other NMHC [26] than the purified natural gas used by Hesterberg et al., possibly explaining the difference between the two studies.
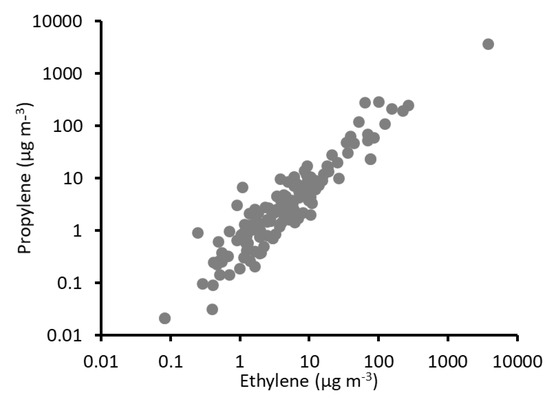
Figure 7.
Ethylene versus propylene in all portable station measurements. Both axes are shown in log scale.
Ethylene and propylene in this study were correlated with acetylene (Spearman r of 0.64 and 0.48, respectively), and the average ethylene:acetylene and propylene:acetylene ratios for measurements in this study were 6.05 (3.53, 11.45) and 10.64 (3.95, 37.94). Median ethylene:acetylene and propylene:acetylene ratios were 1.33 and 0.85, respectively. Samples with higher alkenes+acetylene tended also to have a higher percentage of ethylene+propylene. For samples with total alkenes+acetylene greater than 50 µg m−3 (14% of all samples), 84 (81, 87)% of total alkenes+acetylene was comprised of ethylene and propylene, compared to 52 (49, 56)% for samples with total alkenes+acetylene less than 50 µg m−3. Furthermore, having high total alkenes+acetylene and having a higher percentage of total alkenes + acetylene comprised of ethylene and propylene were both properties associated with proximity to oil wells. Natural gas engines produce much more ethylene and propylene than diesel engines (17 times more ethylene and 69 times more propylene [70]). Further, Nine, et al. [74] showed that natural gas engines under a light load emit more total alkenes+acetylene than those under a heavy load.
Ethylene:acetylene and propylene:acetylene ratios from gasoline and diesel-fueled vehicle emissions have been reported in the range of 1–3 and 0.5–1.5, respectively [75,76,77], much lower than the ratios observed in the Uinta Basin in ambient air. Emissions profiles from EPA for stationary natural gas engines also have a relatively low ethylene:acetylene ratio (2.0) [14,15]. Ratios from open flames are typically even lower; Allen and Torres [78] found an ethylene:acetylene ratio of only 0.5.
Nine, et al. [74], in contrast, found ethylene:acetylene and propylene:acetylene ratios from a four-cylinder natural gas engine to be 9.8 and 1.7, respectively. They also showed that these ratios depended strongly on the engines’ oxygen/fuel ratio. At an oxygen/fuel ratio 1.1 times the stoichiometric value (“rich-burn” conditions), the ethylene:acetylene ratio was only 4.8, while at 1.55 times the stoichiometric value (“lean-burn” conditions), the ratio was 12.94. Kim and Bae [72] found a similar trend for both the ethylene:acetylene ratio and the propylene:acetylene ratio. Russ, et al. [79] also showed that the ethylene:acetylene ratio increases with decreasing engine temperature (which is associated with lean-burn conditions). Seventy-three percent of natural gas-fueled engines in the Uinta Basin are categorized as two-stroke lean-burning [10], and most of these are artificial lift engines at oil wells.
Thus, the magnitude of ethylene and propylene contained in samples with high alkenes+acetylene, along with the ratios of individual species, point to lean-burning artificial lift engines as the probable source of excess alkenes+acetylene in the oil-producing part of the Uinta Basin. Other sources (e.g., truck traffic, combustors, and flares) do not have emissions profiles that match observed ratios of alkenes and acetylene. We acknowledge, however, that few studies of the detailed organic compound speciation of combustors and flares exist. It is possible that, like natural gas engines, emissions from combustors, and flares that burn fuel with a high hydrocarbon content are enriched in ethylene and propylene. Strosher [80] reported a higher percentage of total unburned hydrocarbons in emissions from flares burning fuel with higher NMHC content but did not include information about emissions of alkenes and acetylene.
3.4. Comparison with Inventoried Emissions
Consistent with measurements, the 2014 Utah Air Agencies Emissions Inventory shows a higher ratio of alkenes+acetylene to alkanes in areas with more oil wells (compare Figure 8 to locations of oil wells in Figure 4). The magnitude of the ratio was much higher in the measurement data, however. The average alkenes+acetylene:alkanes ratio was 13 (9, 29)% for all measurements, compared to 0.4 (0.3, 0.5)% for all sources in the emissions inventory. Locations with more than 20 oil wells within 10km had an alkenes+acetylene:alkanes ratio in ambient air of 17 (10, 40)%, compared with only 0.5 (0.4, 0.6)% for inventoried sources. This inventory analysis only includes stationary sources, but the analysis of compound ratios above shows that vehicles are likely not the most important sources of alkenes+acetylene in the wintertime Uinta Basin atmosphere. The emissions inventory also overpredicts the acetylene:alkenes ratio relative to measured values. The inventoried acetylene:alkenes ratio was 50 (48, 51)%, compared to 26 (24, 29)% for measurements.
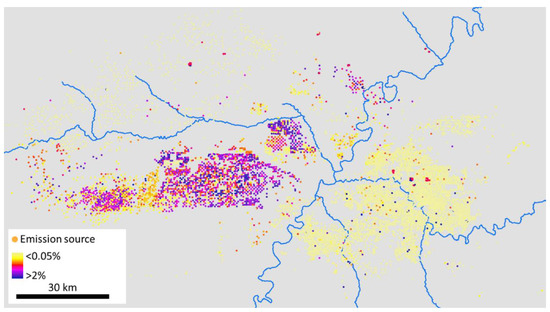
Figure 8.
Oil and gas-related emission sources listed in the 2014 Utah Air Agencies Inventory, colored by the alkenes+acetylene:alkane ratio.
4. Conclusions
Concentrations of all measured organic compounds increased under the strong temperature inversion conditions that commonly occur during Uinta Basin winters. The composition of organics varied in different parts of the Basin, however. We observed spatial trends for alkanes, aromatics, carbonyls, and alcohols. The most consistent trends we observed, however, were for alkenes+acetylene. Alkenes+acetylene were consistently more abundant in the oil-producing parts of the Uinta Basin, comprising more than 25% of total ozone formation potential from organics in areas with greater than 20 oil wells within 10 km. While these unsaturated hydrocarbons derive from a variety of (mostly combustion) sources, compound ratios and other evidence indicate that natural gas-fueled engines, especially lean-burn artificial lift engines, are likely their main source. More work, including direct measurements of emissions from various combustion sources in the Uinta Basin, is needed to confirm this finding.
NOx emissions from engines reach a maximum, and total hydrocarbon emissions (as well as the reactivity of hydrocarbon emissions) reach a minimum, at stoichiometric or near-stoichiometric oxygen:fuel ratios (i.e., rich-burn conditions). The converse is true at higher (i.e., lean-burn) oxygen:fuel ratios because of cooler temperatures and incomplete combustion [72,74,79,81]. Whether engines should be operated to minimize NOx emissions or to minimize organics emissions will depend on the chemical properties of the airshed those engines inhabit. Data from one location in the Uinta Basin during winter 2013 indicated that reductions in NOx emissions would reduce ozone more effectively than reductions in organics emissions [2,82]. If this is true currently and for the entire Basin, continuing to operate engines under lean-burn conditions to reduce NOx may be the most appropriate tactic to keep winter ozone concentrations low, even though it appears to result in excess emissions of reactive organics.
The 2014 Utah Air Agencies Oil and Gas Emissions Inventory contains a much lower percentage of alkenes+acetylene in organic compound emissions than our measurements suggest. Adjusting the inventory to include a higher percentage of alkenes+acetylene would result in much higher inventoried reactivity. Application of official emissions inventories in photochemical models have resulted in much less simulated wintertime ozone than reality, and some studies have shown that increasing total organic compound emissions above inventoried values results in more realistic ozone concentrations [1,3]. The current study is inadequate to evaluate the magnitude of inventoried organics emissions, but it does suggest that the total reactivity of inventoried emissions is too low. Future photochemical modeling studies should consider how inaccuracies in emissions composition, and not just emissions magnitude, may contribute to underprediction of both organic compound reactivity and winter ozone production.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4433/12/1/1/s1. Composition (weight percent) and total concentration of organic compounds Figures S1–S11, Composition (weight percent) and total concentration of organic compounds Figures S1–S11, Spearman rank correlation of concentrations of organic compound types measured with the listed parameters Tables S1–S3.
Author Contributions
Conceptualization, T.T., H.N.Q.T., and S.N.L.; methodology, H.N.Q.T., T.O., and S.N.L.; formal analysis, S.N.L., M.L.H., and H.N.Q.T.; investigation, S.N.L., M.L.H., and H.N.Q.T.; data curation, S.N.L. and H.N.Q.T.; writing—original draft preparation, S.N.L. and M.L.H.; writing—review and editing, all authors; project administration, T.T.; funding acquisition, T.T. and H.N.Q.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Utah Division of Air Quality, the Utah Legislature, the Uintah Impact Mitigation Special Service District, and an endowment for student research internships provided by Anadarko Petroleum Corporation.
Data Availability Statement
Ambient air measurement data used in this study are available here: https://usu.box.com/s/iivgyp7v0m2kka4l6l3401h9y9sm9vr6.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmadov, R.; McKeen, S.; Trainer, M.; Banta, R.; Brewer, A.; Brown, S.; Edwards, P.M.; De Gouw, J.; Frost, G.J.; Gilman, J.; et al. Understanding high wintertime ozone pollution events in an oil- and natural gas-producing region of the western US. Atmos. Chem. Phys. Discuss. 2015, 15, 411–429. [Google Scholar] [CrossRef]
- Edwards, P.M.; Brown, S.S.; Roberts, J.M.; Ahmadov, R.; Banta, R.M.; Degouw, J.A.; Dubé, W.P.; Field, R.A.; Flynn, J.H.; Gilman, J.B.; et al. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature 2014, 514, 351–354. [Google Scholar] [CrossRef]
- Matichuk, R.; Tonnesen, G.; Luecken, D.; Gilliam, R.; Napelenok, S.L.; Baker, K.R.; Schwede, D.; Murphy, B.; Helmig, D.; Lyman, S.N.; et al. Evaluation of the Community Multiscale Air Quality Model for Simulating Winter Ozone Formation in the Uinta Basin. J. Geophys. Res. Atmos. 2017, 122, 13545–13572. [Google Scholar] [CrossRef]
- Schnell, R.; Johnson, B.J.; Oltmans, S.J.; Cullis, P.; Sterling, C.; Hall, E.; Jordan, A.F.; Helmig, D.; Pétron, G.; Ahmadov, R.; et al. Quantifying wintertime boundary layer ozone production from frequent profile measurements in the Uinta Basin, UT, oil and gas region. J. Geophys. Res. Atmos. 2016, 121. [Google Scholar] [CrossRef]
- Mansfield, M.L.; Hall, C.F. A survey of valleys and basins of the western United States for the capacity to produce winter ozone. J. Air Waste Manag. Assoc. 2018, 68, 909–919. [Google Scholar] [CrossRef]
- Oltmans, S.J.; Schnell, R.C.; Johnson, B.; Pétron, G.; Mefford, T.; Neely, R. Anatomy of wintertime ozone associated with oil and natural gas extraction activity in Wyoming and Utah. Lementa Sci. Anthr. 2014, 2, 000024. [Google Scholar] [CrossRef]
- Lyman, S.N.; Tran, T. Inversion structure and winter ozone distribution in the Uintah Basin, Utah, U.S.A. Atmos. Environ. 2015, 123, 156–165. [Google Scholar] [CrossRef]
- Foster, C.S.; Crosman, E.T.; Holland, L.; Mallia, D.V.; Fasoli, B.; Bares, R.; Horel, J.; Lin, J.C. Confirmation of Elevated Methane Emissions in Utah’s Uintah Basin with Ground-Based Observations and a High-Resolution Transport Model. J. Geophys. Res. Atmos. 2017, 122, 13026–13044. [Google Scholar] [CrossRef]
- Foster, C.S.; Crosman, E.T.; Horel, J.D.; Lyman, S.N.; Fasoli, B.; Bares, R.; Lin, J.C. Quantifying methane emissions in the Uintah Basin during wintertime stagnation episodes. Elem. Sci. Anthr. 2019, 7, 24. [Google Scholar] [CrossRef]
- UDAQ. Uinta Basin: 2014 Air Agencies Oil and Gas Emissions Inventory. Available online: https://deq.utah.gov/legacy/destinations/u/uintah-basin/air-agencies-emissions-inventory/index.htm (accessed on 1 July 2020).
- Lyman, S.; Tran, T. Measurement of Carbonyl Emissions from Oil and Gas Sources in the Uintah Basin; Utah State University: Vernal, UT, USA, 2015; Available online: http://binghamresearch.usu.edu/files/CarbonylEmiss_FnlRprt_31jul2015.pdf (accessed on 1 July 2020).
- Simon, H.; Beck, L.; Bhave, P.V.; Divita, F.; Hsu, Y.; Luecken, D.; Mobley, J.D.; Pouliot, G.A.; Reff, A.; Sarwar, G.; et al. The development and uses of EPA’s SPECIATE database. Atmos. Pollut. Res. 2010, 1, 196–206. [Google Scholar] [CrossRef]
- Matichuk, R.; Tonnesen, G.; Eisele, A.; Thoma, E.; Kosusko, M.; Strum, M.; Beeler, C. Advancing Understanding of Emissions from Oil and Natural Gas Production Operations to Support Epa’s Air Quality Modeling of Ozone Non-Attainment Areas; Environmental Protection Agency: Washington, DC, USA, 2016. Available online: https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=NRMRL&dirEntryId=335190 (accessed on 1 July 2020).
- EPA. Addendum Speciate Version 5.1 Database Development Documentation; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2020. Available online: https://www.epa.gov/sites/production/files/2020-07/documents/speciate_5.1.pdf (accessed on 1 July 2020).
- Oliver, W.R.; Scott, H.P. Improvement of the Emission Inventory for Reactive Organic Gases and Oxides of Nitrogen in the South Coast Air Basin; Main Report; Final Report; Systems Applications, Inc.: San Rafael, CA USA, 1985; Volume 1. Available online: https://ww3.arb.ca.gov/ei/speciate/r21t40/rf25doc/refnum25.htm (accessed on 1 July 2020).
- Allen, D.T.; Torres, V.M.; Thomas, J.; Sullivan, D.W.; Harrison, M.; Hendler, A.; Herndon, S.C.; Kolb, C.E.; Fraser, M.P.; Hill, A.D.; et al. Measurements of methane emissions at natural gas production sites in the United States. Proc. Natl. Acad. Sci. USA 2013, 110, 17768–17773. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.A.; Zavala-Araiza, D.; Lyon, D.R.; Allen, D.T.; Barkley, Z.R.; Brandt, A.R.; Davis, K.J.; Herndon, S.C.; Jacob, D.J.; Karion, A.; et al. Assessment of methane emissions from the U.S. oil and gas supply chain. Science 2018, 361, eaar7204. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Covington, A.N.; Clark, N.N. Methane Emissions from Leak and Loss Audits of Natural Gas Compressor Stations and Storage Facilities. Environ. Sci. Technol. 2015, 49, 8132–8138. [Google Scholar] [CrossRef] [PubMed]
- Lamb, B.K.; Edburg, S.L.; Ferrara, T.W.; Howard, T.; Harrison, M.R.; Kolb, C.E.; Townsend-Small, A.; Dyck, W.; Possolo, A.; Whetstone, J.R. Direct Measurements Show Decreasing Methane Emissions from Natural Gas Local Distribution Systems in the United States. Environ. Sci. Technol. 2015, 49, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Omara, M.; Zimmerman, N.; Sullivan, M.R.; Li, X.; Ellis, A.; Cesa, R.; Subramanian, R.; Presto, A.A.; Robinson, A.L. Methane Emissions from Natural Gas Production Sites in the United States: Data Synthesis and National Estimate. Environ. Sci. Technol. 2018, 52, 12915–12925. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.M.; Edie, R.; Snare, D.; Soltis, J.; Field, R.A.; Burkhart, M.D.; Bell, C.S.; Zimmerle, D.; Murphy, S.M. Variation in Methane Emission Rates from Well Pads in Four Oil and Gas Basins with Contrasting Production Volumes and Compositions. Environ. Sci. Technol. 2017, 51, 8832–8840. [Google Scholar] [CrossRef]
- Schwietzke, S.; Petron, G.; Conley, S.; Pickering, C.; Mielke-Maday, I.; Dlugokencky, E.J.; Tans, P.P.; Vaughn, T.; Bell, C.; Zimmerle, D.; et al. Improved Mechanistic Understanding of Natural Gas Methane Emissions from Spatially Resolved Aircraft Measurements. Environ. Sci. Technol. 2017, 51, 7286–7294. [Google Scholar] [CrossRef] [PubMed]
- Karion, A.; Sweeney, C.; Pétron, G.; Frost, G.; Hardesty, R.M.; Kofler, J.; Miller, B.R.; Newberger, T.; Wolter, S.; Banta, R.M.; et al. Methane emissions estimate from airborne measurements over a western United States natural gas field. Geophys. Res. Lett. 2013, 40, 4393–4397. [Google Scholar] [CrossRef]
- Thoma, E.D.; Deshmukh, P.; Logan, R.; Stovern, M.; Dresser, C.; Brantley, H.L. Assessment of Uinta Basin Oil and Natural Gas Well Pad Pneumatic Controller Emissions. J. Environ. Prot. 2017, 8, 394–415. [Google Scholar] [CrossRef][Green Version]
- Brantley, H.L.; Thoma, E.D.; Eisele, A.P. Assessment of volatile organic compound and hazardous air pollutant emissions from oil and natural gas well pads using mobile remote and on-site direct measurements. J. Air Waste Manag. Assoc. 2015, 65, 1072–1082. [Google Scholar] [CrossRef]
- Wilson, L.; Tran, T.; Lyman, S.; Pearson, M.; McGrath, T.; Cubrich, B. Uinta Basin Composition Study; Utah Department of Environmental Quality: Salt Lake City, UT, USA, 2020. Available online: https://documents.deq.utah.gov/air-quality/planning/technical-analysis/DAQ-2020-004826.pdf (accessed on 1 July 2020).
- Goetz, J.D.; Floerchinger, C.; Fortner, E.C.; Wormhoudt, J.; Massoli, P.; Knighton, W.B.; Herndon, S.C.; Kolb, C.E.; Knipping, E.; Shaw, S.L.; et al. Atmospheric Emission Characterization of Marcellus Shale Natural Gas Development Sites. Environ. Sci. Technol. 2015, 49, 7012–7020. [Google Scholar] [CrossRef] [PubMed]
- Airtech. Report on the Upper Green River Basin Closed Top Produced Water Tank Emission Study; Wyoming Department of Environmental Quality: Cheyenne, WY, USA, 2014. Available online: http://deq.wyoming.gov/media/attachments/Air%20Quality/Winter%20Ozone/Technical%20Documents/2015-0331_AQD_Produced-Water-Tank-Study-Final-Report.pdf (accessed on 1 July 2020).
- Lyman, S.N.; Mansfield, M.L.; Tran, H.N.; Evans, J.D.; Jones, C.; O’Neil, T.; Bowers, R.; Smith, A.; Keslar, C. Emissions of organic compounds from produced water ponds I: Characteristics and speciation. Sci. Total Environ. 2018, 619, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.L.; Tran, H.N.; Lyman, S.N.; Bowers, R.L.; Smith, A.P.; Keslar, C. Emissions of organic compounds from produced water ponds III: Mass-transfer coefficients, composition-emission correlations, and contributions to regional emissions. Sci. Total Environ. 2018, 627, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Lyman, S.N.; Mansfield, M.L.; O’Neil, T.; Bowers, R.L.; Smith, A.P.; Keslar, C. Emissions of organic compounds from produced water ponds II: Evaluation of flux chamber measurements with inverse-modeling techniques. J. Air Waste Manag. Assoc. 2018, 68, 713–724. [Google Scholar] [CrossRef]
- Lyman, S.N.; Mansfield, M.L. Organic compound emissions from a landfarm used for oil and gas solid waste disposal. J. Air Waste Manag. Assoc. 2018, 68, 637–642. [Google Scholar] [CrossRef]
- Hecobian, A.; Clements, A.L.; Shonkwiler, K.B.; Zhou, Y.; Macdonald, L.P.; Hilliard, N.; Wells, B.L.; Bibeau, B.; Ham, J.M.; Pierce, J.R.; et al. Air Toxics and Other Volatile Organic Compound Emissions from Unconventional Oil and Gas Development. Environ. Sci. Technol. Lett. 2019, 6, 720–726. [Google Scholar] [CrossRef]
- Lyman, S.N.; Watkins, C.; Jones, C.P.; Mansfield, M.L.; McKinley, M.; Kenney, D.; Evans, J. Hydrocarbon and Carbon Dioxide Fluxes from Natural Gas Well Pad Soils and Surrounding Soils in Eastern Utah. Environ. Sci. Technol. 2017, 51, 11625–11633. [Google Scholar] [CrossRef]
- Gilman, J.B.; Lerner, B.M.; Kuster, W.C.; De Gouw, J.A. Source signature of volatile organic compounds from oil and natural gas operations in northeastern Colorado. Environ. Sci. Technol. 2013, 47, 1297–1305. [Google Scholar] [CrossRef]
- Helmig, D.; Thompson, C.R.; Evans, J.; Boylan, P.; Hueber, J.; Park, J.-H. Highly Elevated Atmospheric Levels of Volatile Organic Compounds in the Uintah Basin, Utah. Environ. Sci. Technol. 2014, 48, 4707–4715. [Google Scholar] [CrossRef]
- Koss, A.; Yuan, B.; Warneke, C.; Gilman, J.B.; Lerner, B.M.; Veres, P.R.; Peischl, J.; Eilerman, S.; Wild, R.; Brown, S.S.; et al. Observations of Voc Emissions and Photochemical Products over Us Oil-and Gas-Producing Regions Using High-Resolution H 3 O+ Cims (Ptr-Tof-Ms). Atmos. Meas. Technol. 2017, 10, 2941. [Google Scholar] [CrossRef]
- Koss, A.R.; De Gouw, J.; Warneke, C.; Gilman, J.B.; Lerner, B.M.; Graus, M.; Yuan, B.; Edwards, P.; Brown, S.S.; Wild, R.; et al. Photochemical Aging of Volatile Organic Compounds Associated with Oil and Natural Gas Extraction in the Uintah Basin, Ut, During a Wintertime Ozone Formation Event. Atmos. Chem. Phys. 2015, 15, 5727–5741. [Google Scholar] [CrossRef]
- Pétron, G.; Karion, A.; Sweeney, C.; Miller, B.R.; Montzka, S.A.; Frost, G.J.; Trainer, M.; Tans, P.P.; Andrews, A.; Kofler, J.; et al. A new look at methane and nonmethane hydrocarbon emissions from oil and natural gas operations in the Colorado Denver-Julesburg Basin. J. Geophys. Res. Atmos. 2014, 119, 6836–6852. [Google Scholar] [CrossRef]
- Prenni, A.J.; Day, D.E.; Evanoski-Cole, A.R.; Sive, B.C.; Hecobian, A.; Zhou, Y.; Gebhart, K.A.; Hand, J.; Sullivan, A.P.; Li, Y.; et al. Oil and gas impacts on air quality in federal lands in the Bakken region: an overview of the Bakken Air Quality Study and first results. Atmos. Chem. Phys. Discuss. 2016, 16, 1401–1416. [Google Scholar] [CrossRef]
- Schade, G.W.; Roest, G. Analysis of non-methane hydrocarbon data from a monitoring station affected by oil and gas development in the Eagle Ford shale, Texas. Elem. Sci. Anthr. 2016, 4, 000096. [Google Scholar] [CrossRef]
- Swarthout, R.F.; Russo, R.S.; Zhou, Y.; Miller, B.M.; Mitchell, B.; Horsman, E.; Lipsky, E.; McCabe, D.C.; Baum, E.; Sive, B.C. Impact of Marcellus Shale Natural Gas Development in Southwest Pennsylvania on Volatile Organic Compound Emissions and Regional Air Quality. Environ. Sci. Technol. 2015, 49, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.R.; Hueber, J.; Helmig, D. Influence of oil and gas emissions on ambient atmospheric non-methane hydrocarbons in residential areas of Northeastern Colorado. Elem. Sci. Anthr. 2014, 3, 000035. [Google Scholar] [CrossRef]
- Zavala-Araiza, D.; Sullivan, D.W.; Allen, D.T. Atmospheric Hydrocarbon Emissions and Concentrations in the Barnett Shale Natural Gas Production Region. Environ. Sci. Technol. 2014, 48, 5314–5321. [Google Scholar] [CrossRef]
- Field, R.A.; Soltis, J.; McCarthy, M.C.; Murphy, S.; Montague, D.C. Influence of oil and gas field operations on spatial and temporal distributions of atmospheric non-methane hydrocarbons and their effect on ozone formation in winter. Atmos. Chem. Phys. Discuss. 2015, 15, 3527–3542. [Google Scholar] [CrossRef]
- Pétron, G.; Frost, G.; Miller, B.R.; Hirsch, A.I.; Montzka, S.A.; Karion, A.; Trainer, M.; Sweeney, C.; Andrews, A.E.; Miller, L.; et al. Hydrocarbon emissions characterization in the Colorado Front Range: A pilot study. J. Geophys. Res. Space Phys. 2012, 117. [Google Scholar] [CrossRef]
- Roest, G.; Schade, G. Quantifying alkane emissions in the Eagle Ford Shale using boundary layer enhancement. Atmos. Chem. Phys. Discuss. 2017, 17, 11163–11176. [Google Scholar] [CrossRef]
- Warneke, C.; Geiger, F.; Edwards, P.M.; Dubé, W.; Pétron, G.; Kofler, J.; Zahn, A.; Brown, S.S.; Graus, M.; Gilman, J.B.; et al. Volatile organic compound emissions from the oil and natural gas industry in the Uintah Basin, Utah: oil and gas well pad emissions compared to ambient air composition. Atmos. Chem. Phys. Discuss. 2014, 14, 10977–10988. [Google Scholar] [CrossRef]
- Negron, A.M.G.; McDonald, B.C.; McKeen, S.A.; Peischl, J.; Ahmadov, R.; De Gouw, J.; Frost, G.J.; Hastings, M.G.; Pollack, I.B.; Ryerson, T.B.; et al. Development of a Fuel-Based Oil and Gas Inventory of Nitrogen Oxides Emissions. Environ. Sci. Technol. 2018, 52, 10175–10185. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.; Nicot, J.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can we beneficially reuse produced water from oil and gas extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef] [PubMed]
- Wilkey, J.; Kelly, K.; Jaramillo, I.C.; Spinti, J.; Ring, T.; Hogue, M.; Pasqualini, D. Predicting emissions from oil and gas operations in the Uinta Basin, Utah. J. Air Waste Manag. Assoc. 2016, 66, 528–545. [Google Scholar] [CrossRef] [PubMed]
- EPA. Technical Assistance Document for Sampling and Analysis of Ozone Precursors; United States Environmental Protection Agency: Research Triangle Park, NC, USA, 1998. Available online: http://www.epa.gov/ttn/amtic/files/ambient/pams/newtad.pdf (accessed on 1 July 2020).
- Plass-Dülmer, C.; Schmidbauer, N.; Slemr, J.; Šlemr, F.; D’Souza, H. European hydrocarbon intercomparison experiment AMOHA part 4: Canister sampling of ambient air. J. Geophys. Res. Space Phys. 2006, 111. [Google Scholar] [CrossRef]
- Uchiyama, S.; Naito, S.; Matsumoto, M.; Inaba, Y.; Kunugita, N. Improved Measurement of Ozone and Carbonyls Using a Dual-Bed Sampling Cartridge Containing Trans-1,2-Bis (2-Pyridyl) Ethylene and 2,4-Dinitrophenylhydrazine-Impregnated Silica. Anal. Chem. 2009, 81, 6552–6557. [Google Scholar] [CrossRef] [PubMed]
- EPA. Compendium Method to-11a; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 1999. Available online: https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-11ar.pdf (accessed on 1 July 2020).
- UDOGM. Data Research Center. Utah Division of Oil, Gas and Mining. Available online: http://oilgas.ogm.utah.gov/Data_Center/DataCenter.cfm (accessed on 1 July 2020).
- EPA. 2014 National Emissions Inventory, Version 2: Technical Support Document; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2018. Available online: https://www.epa.gov/sites/production/files/2018-07/documents/nei2014v2_tsd_05jul2018.pdf (accessed on 1 July 2020).
- EPA. 2017 National Emissions Inventory Complete Release: Technical Support Document; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2020. Available online: https://www.epa.gov/sites/production/files/2020-04/documents/nei2017_tsd_full_30apr2020.pdf (accessed on 1 July 2020).
- Lyman, S.N.; Tran, H.N.; Mansfield, M.L.; Bowers, R.; Smith, A. Strong temporal variability in methane fluxes from natural gas well pad soils. Atmos. Pollut. Res. 2020, 11, 1386–1395. [Google Scholar] [CrossRef]
- Mansfield, M.L. Numerical tools for obtaining power-law representations of heavy-tailed datasets. Eur. Phys. J. B 2016, 89, 1–13. [Google Scholar] [CrossRef]
- Mansfield, M.L.; Lyman, S.N. Winter Ozone Pollution in Utah’s Uinta Basin Is Attenuating. Atmosphere 2020, submitted. [Google Scholar]
- Frankenberg, C.; Thorpe, A.K.; Thompson, D.R.; Hulley, G.; Kort, E.A.; Vance, N.; Borchardt, J.; Krings, T.; Gerilowski, K.; Sweeney, C.; et al. Airborne Methane Remote Measurements Reveal Heavy-Tail Flux Distribution in Four Corners Region. Proc. Natl. Acad. Sci. USA 2016, 9, 9734–9739. [Google Scholar] [CrossRef]
- Carter, W.P.L. Updated Maximum Incremental Reactivity Scale and Hydrocarbon Bin Reactivities for Regulatory Applications. In California Air Resources Board Contract, 07-339; 2009. Available online: https://ww3.arb.ca.gov/regact/2009/mir2009/mir10.pdf (accessed on 1 July 2020).
- Carter, W.P.; Seinfeld, J.H. Winter ozone formation and VOC incremental reactivities in the Upper Green River Basin of Wyoming. Atmos. Environ. 2012, 50, 255–266. [Google Scholar] [CrossRef]
- Swarthout, R.F.; Russo, R.S.; Zhou, Y.; Hart, A.H.; Sive, B.C. Volatile organic compound distributions during the NACHTT campaign at the Boulder Atmospheric Observatory: Influence of urban and natural gas sources. J. Geophys. Res. Atmos. 2013, 118, 10614–10637. [Google Scholar] [CrossRef]
- WRAP. Oil/Gas Emissions Workgroup: Phase Iii Inventory. Available online: http://www.wrapair.org/forums/ogwg/PhaseIII_Inventory.html (accessed on 1 July 2020).
- Schade, G.W.; Geoffrey, R. Source Apportionment of Non-Methane Hydrocarbons, No X and H 2 S Data from a Central Monitoring Station in the Eagle Ford Shale, Texas. Elem. Sci. Anthr. 2018, 6. [Google Scholar] [CrossRef]
- Aikin, A.C.; Herman, J.R.; Maier, E.J.; McQuillan, C.J. Atmospheric chemistry of ethane and ethylene. J. Geophys. Res. Ocean. 1982, 87, 3105–3118. [Google Scholar] [CrossRef]
- Lyman, S.N.; Tran, T.; Mansfield, M.L.; Ravikumar, A.P. Aerial and ground-based optical gas imaging survey of Uinta Basin oil and gas wells. Elem. Sci. Anthr. 2019, 7, 43. [Google Scholar] [CrossRef]
- Hesterberg, T.W.; Lapin, C.A.; Bunn, W.B. A Comparison of Emissions from Vehicles Fueled with Diesel or Compressed Natural Gas. Environ. Sci. Technol. 2008, 42, 6437–6445. [Google Scholar] [CrossRef]
- Ranzi, E.; Sogaro, A.; Gaffuri, P.; Pennati, G.; Westbrook, C.; Pitz, W. A new comprehensive reaction mechanism for combustion of hydrocarbon fuels. Combust. Flame 1993, 99, 201–211. [Google Scholar] [CrossRef]
- Kim, C.U.; Bae, C.S. Speciated hydrocarbon emissions from a gas-fuelled spark-ignition engine with various operating parameters. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2000, 214, 795–808. [Google Scholar] [CrossRef]
- Drobot, K.; Cheng, W.K.; Trinker, F.H.; Kaiser, E.; Siegl, W.O.; Cotton, D.F.; Underwood, J. Hydrocarbon oxidation in the exhaust port and runner of a spark ignition engine. Combust. Flame 1994, 99, 422–430. [Google Scholar] [CrossRef]
- Nine, R.D.; Clark, N.N.; Mace, B.E.; Elgazzar, L. Hydrocarbon Speciation of a Lean Burn Spark Ignited Engine. SAE Tech. Pap. Ser. 1997, 972971. [Google Scholar] [CrossRef]
- Lai, C.-H.; Peng, Y.-P. Volatile hydrocarbon emissions from vehicles and vertical ventilations in the Hsuehshan traffic tunnel, Taiwan. Environ. Monit. Assess. 2012, 184, 4015–4028. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.H.; Wallace, J.S. Operating Parameter Effects on the Speciated Hydrocarbon Emissions from a Natural Gas Fueled Engine. SAE Tech. Pap. Ser. 1994, 942007. [Google Scholar] [CrossRef]
- Ryerson, T.B.; Trainer, M.; Angevine, W.M.; Brock, C.A.; Dissly, R.W.; Fehsenfeld, F.C.; Frost, G.J.; Goldan, P.D.; Holloway, J.S.; Hübler, G.; et al. Effect of Petrochemical Industrial Emissions of Reactive Alkenes and Nox on Tropospheric Ozone Formation in Houston, Texas. J. Geophys. Res. Atmos. 2003, 108, 4249. [Google Scholar] [CrossRef]
- Allen, D.T.; Vincent, M.T. Tceq 2010 Flare Study Final Report; The University of Texas: Austin, TX, USA, 2011. Available online: https://www.tceq.texas.gov/assets/public/implementation/air/rules/Flare/2010flarestudy/2010-flare-study-final-report.pdf (accessed on 1 July 2020).
- Russ, S.G.; Kaiser, E.W.; Siegl, W.O.; Podsiadlik, D.H.; Barrett, K.M. Compression Ratio and Coolant Temperature Effects on HC Emissions from a Spark- Ignition Engine. SAE Tech. Pap. Ser. 1995, 139–151. [Google Scholar] [CrossRef]
- Strosher, M.T. Characterization of Emissions from Diffusion Flare Systems. J. Air Waste Manag. Assoc. 2000, 50, 1723–1733. [Google Scholar] [CrossRef]
- Saanum, I.; Bysveen, M.; Tunestål, P.; Johansson, B. Lean Burn versus Stoichiometric Operation with EGR and 3-Way Catalyst of an Engine Fueled with Natural Gas and Hydrogen Enriched Natural Gas. SAE Tech. Pap. Ser. 2007, 35–45. [Google Scholar] [CrossRef]
- Womack, C.C.; McDuffie, E.E.; Edwards, P.M.; Bares, R.; de Gouw, J.A.; Docherty, K.S.; Dubé, W.P.; Fibiger, D.L.; Franchin, A.; Gilman, J.B.; et al. An Odd Oxygen Framework for Wintertime Ammonium Nitrate Aerosol Pollution in Urban Areas: Nox and Voc Control as Mitigation Strategies. Geophys. Res. Lett. 2019, 46, 4971–4979. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).