Clarification of Catalytic Effect on Large Stretchable and Compressible Rubber Dye-Sensitized Solar Cells
Abstract
1. Introduction
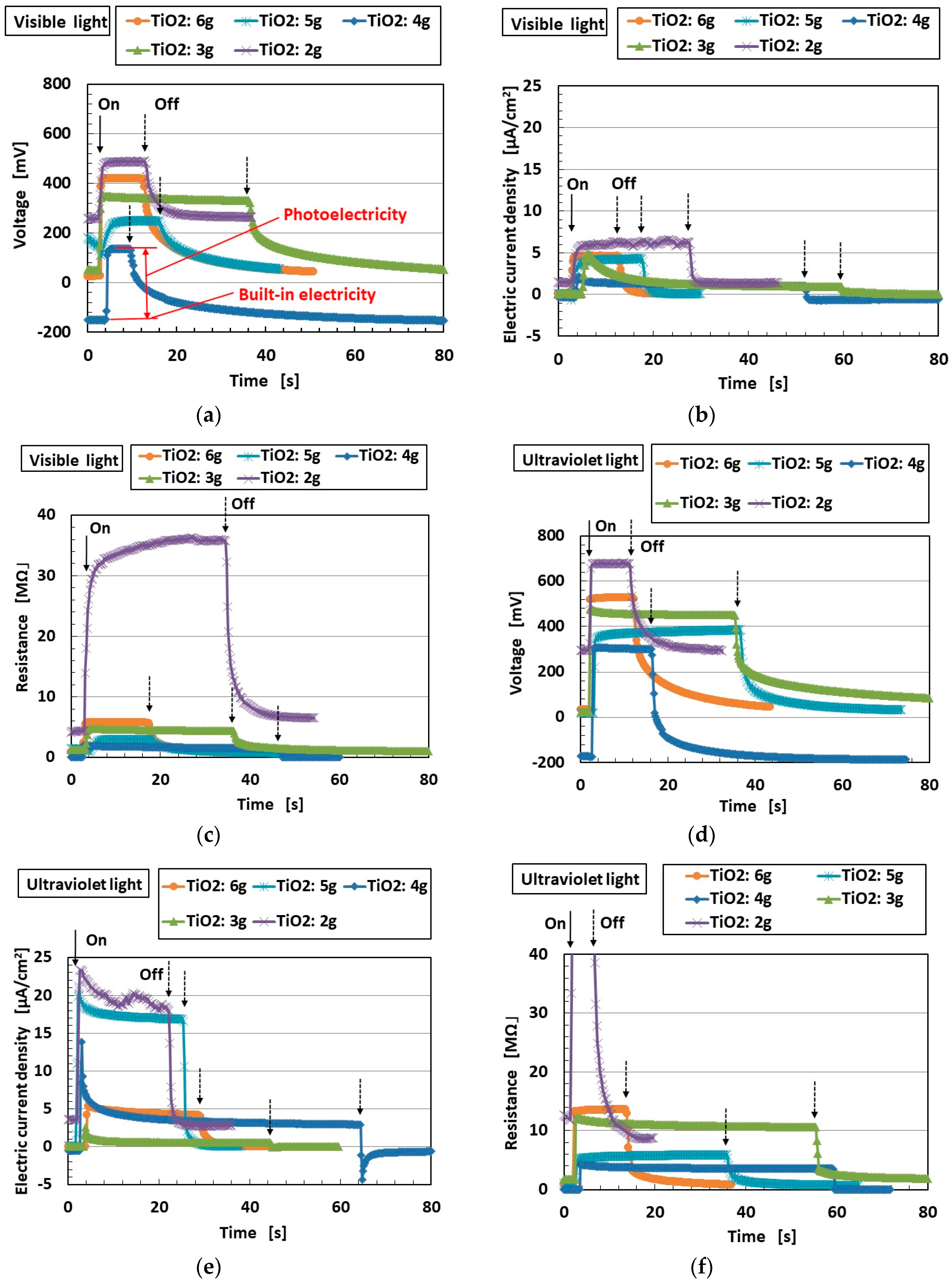
2. Effect of TiO2 on Photovoltaics
2.1. Experimental Procedure
2.2. Experimental Photovolatics
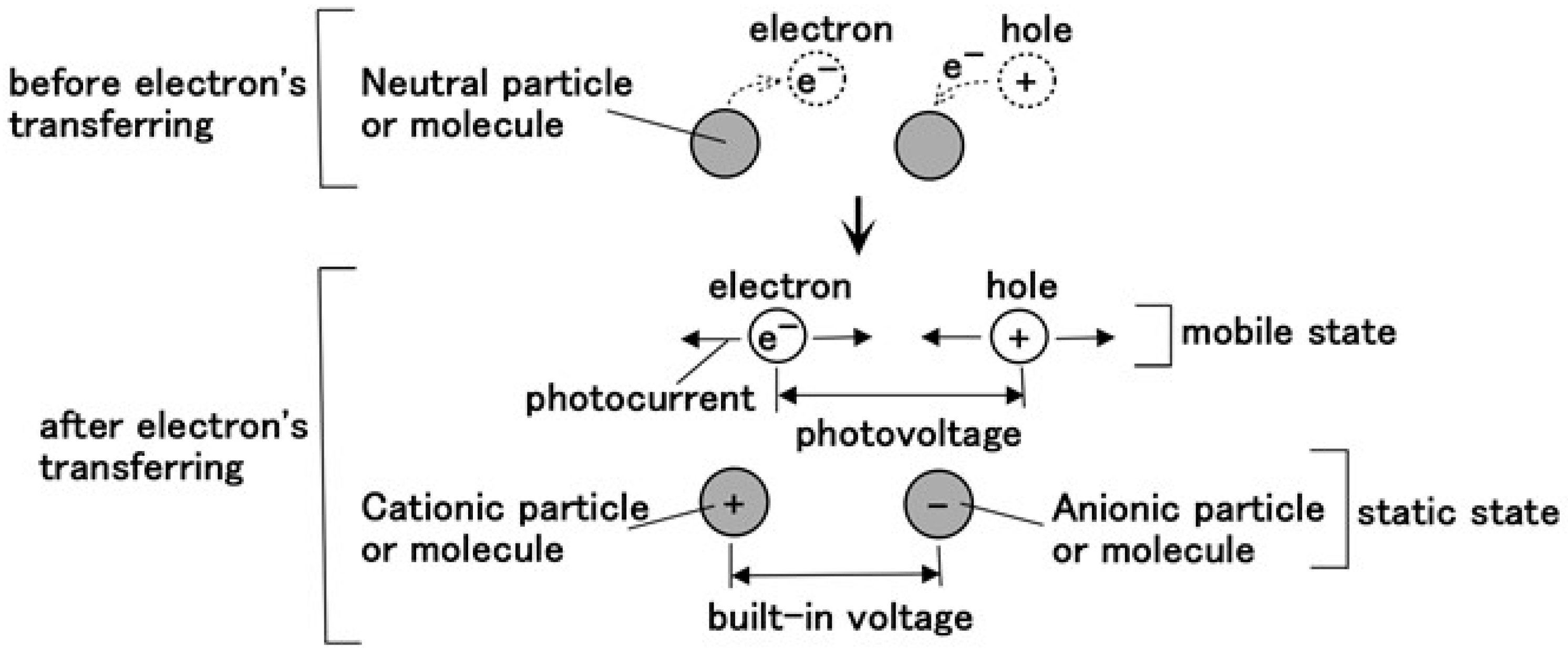
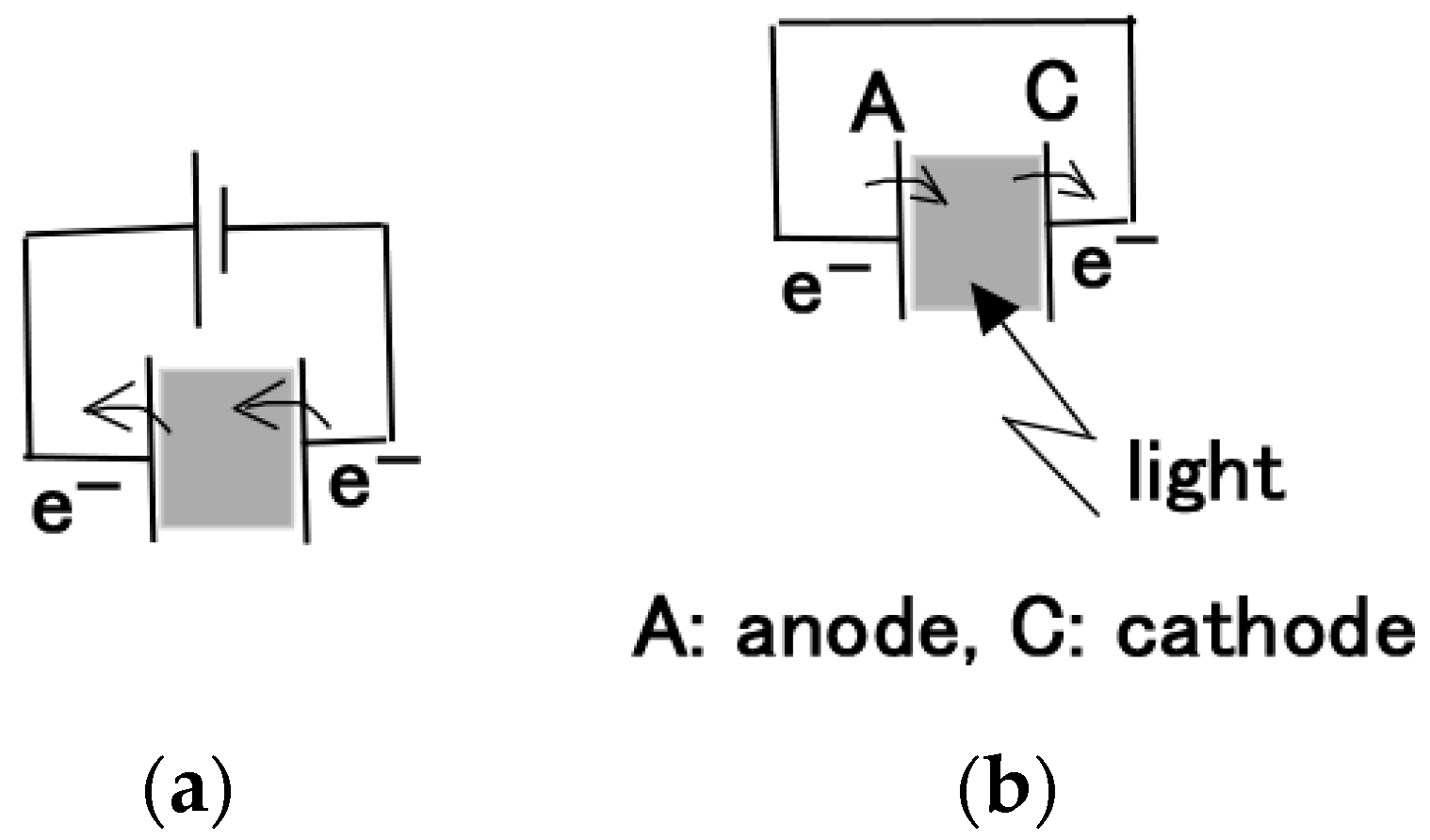
2.3. Chemical–Photovoltaic Mechanism
2.3.1. Case of Photovoltaic Mechanism by Dye and Electrolyte
2.3.2. Case of Electrical–Chemical Mechanism before Electrolytic Polymerization and without Light Scattering
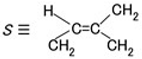 .
.
2.3.3. Case of Electrical–Chemical Mechanism by Electrolytic Polymerization and without Light Scattering
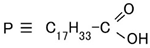
2.3.4. Case of Photovoltaic Mechanism after Electrolytic Polymerization and with Light Scattering
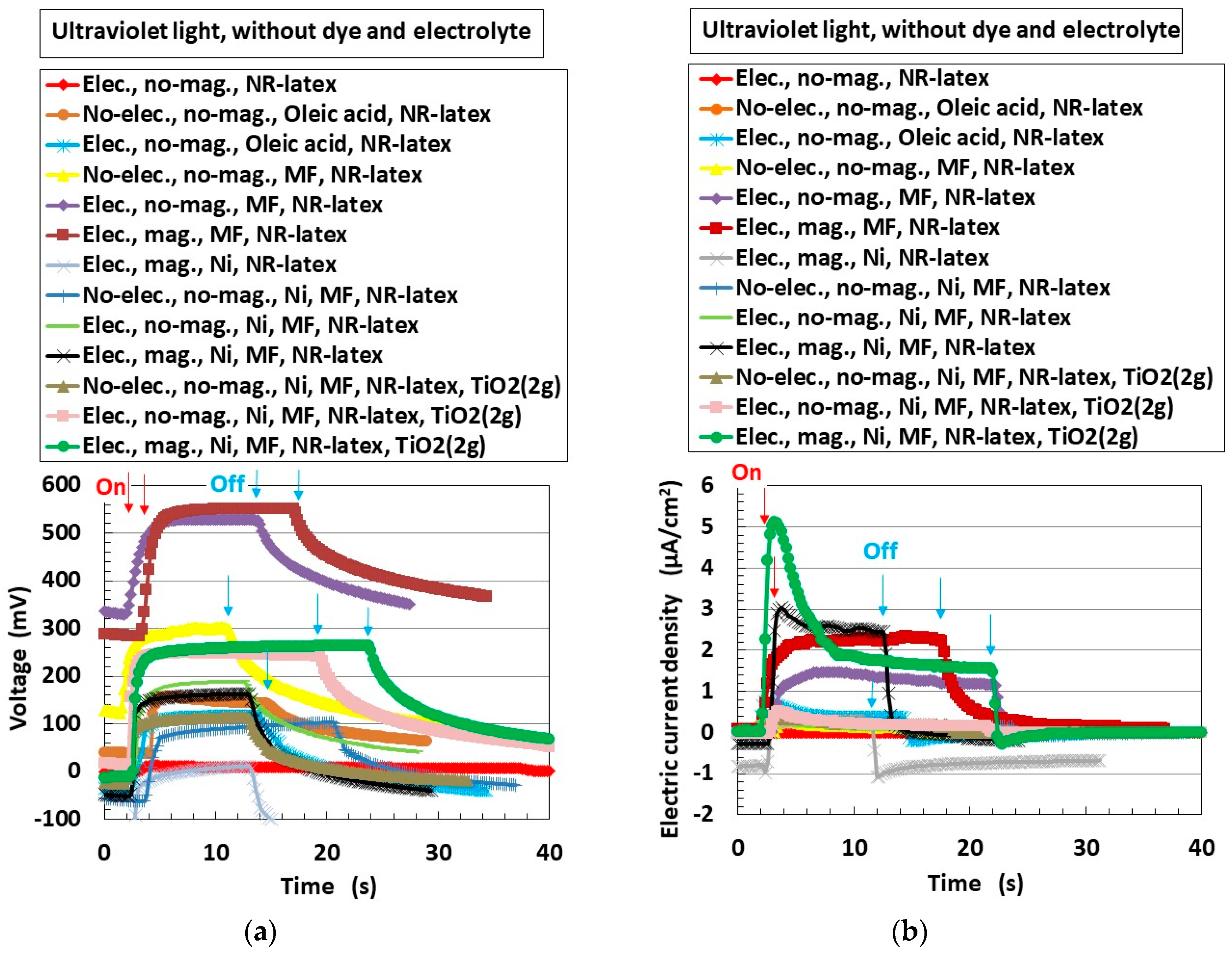
2.3.5. Case of Photovoltaic Mechanism by Catalyst Effect of TiO2, Ni, and Fe3O4.
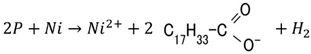 .
.
 .
.
- (a)
- As shown in Equations (15) and (16), electrons transfer. Electrons are generated not only as shown in Equation (8) through S− in Equations (15) and (16) but also as shown in Equation (19) through H+ in Equation (16). This chemical–photovoltaic mechanism is irrelevant to the behavior of the rubber molecule.
- (b)
- The operations expressed in Equations (19) and (21) are facilitated by the catalyst effect of TiO2, Ni, and Fe3O4 through the operations shown in Equations (17), (20), and (22). The ones expressed in Equation (10) are also facilitated through the one shown in Equation (18). This chemical–photovoltaic mechanism is irrelevant to the catalyst behavior of TiO2, Ni, and Fe3O4.
- (c)
- Equations (30) and (37) show that electrons are scavenged. This induces the reduction of photovoltaic current. In addition, not only Equations (17) and (18) but also Equations (9), (10), (14), and (19) show that the electrons are generated by water with an aiding catalyst. These summarized results provide the complicated changes in photovoltaic current. This chemical–photovoltaic mechanism is relevant to the behavior of correlation between TiO2 catalyst and H2O.
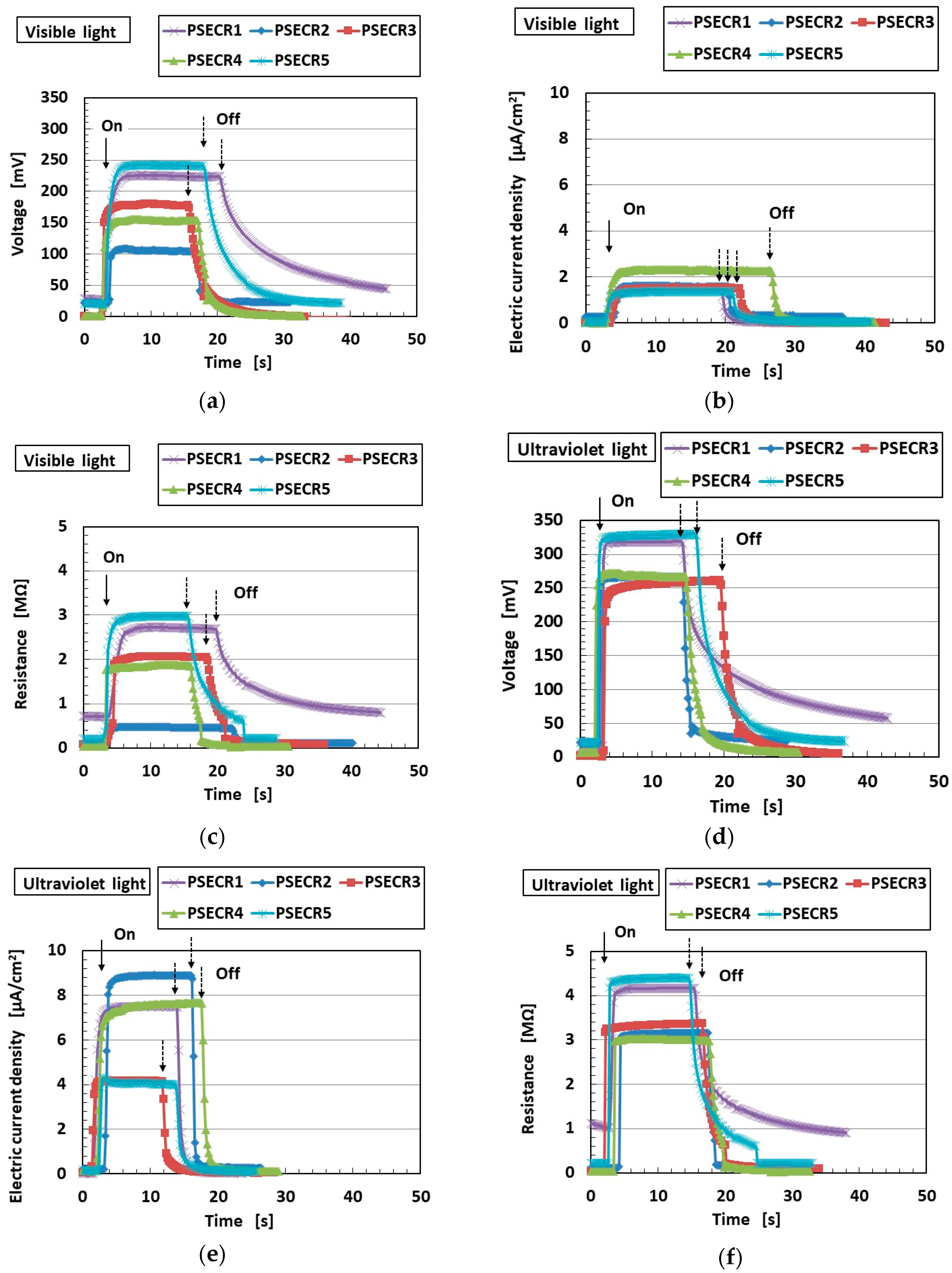
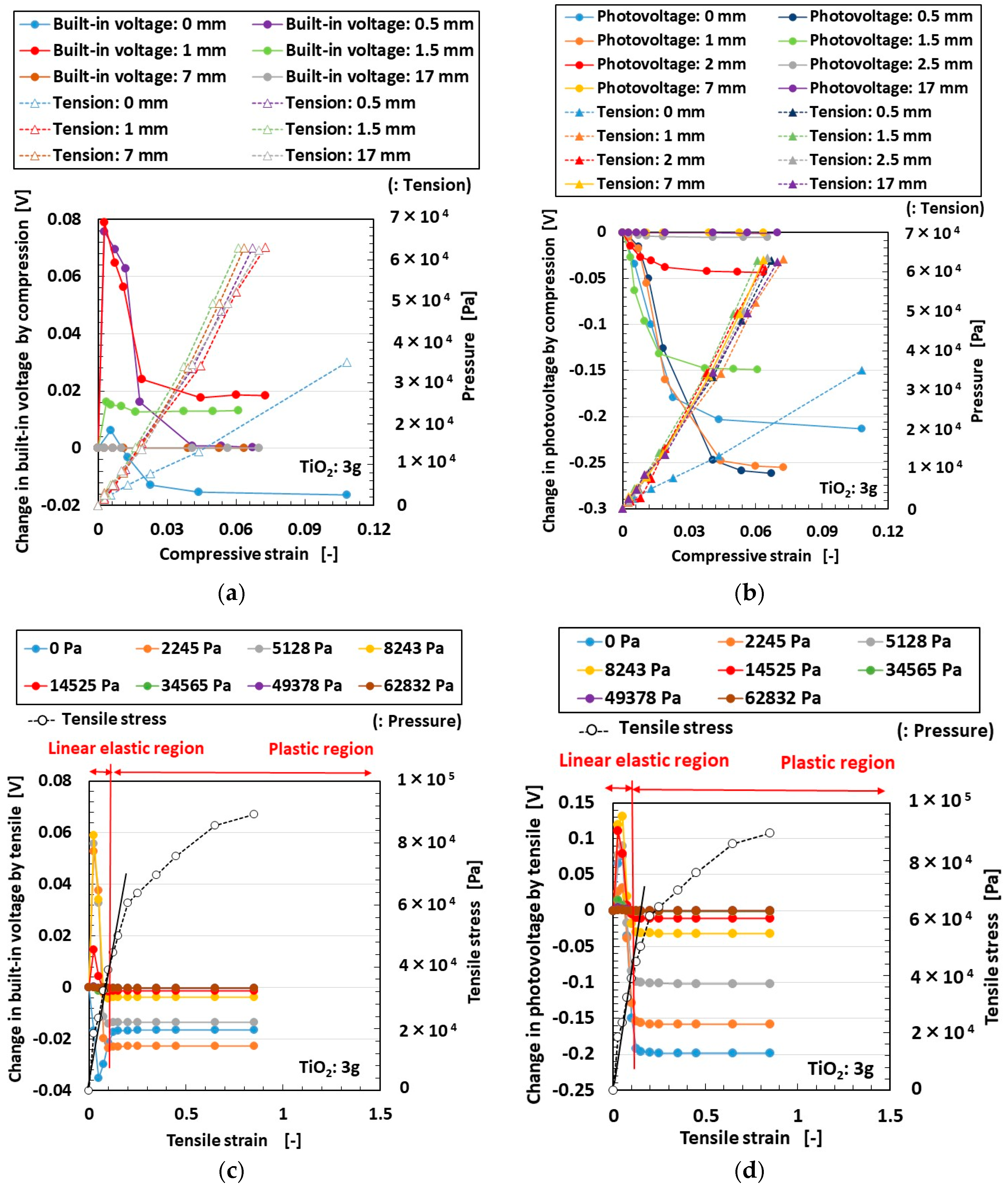
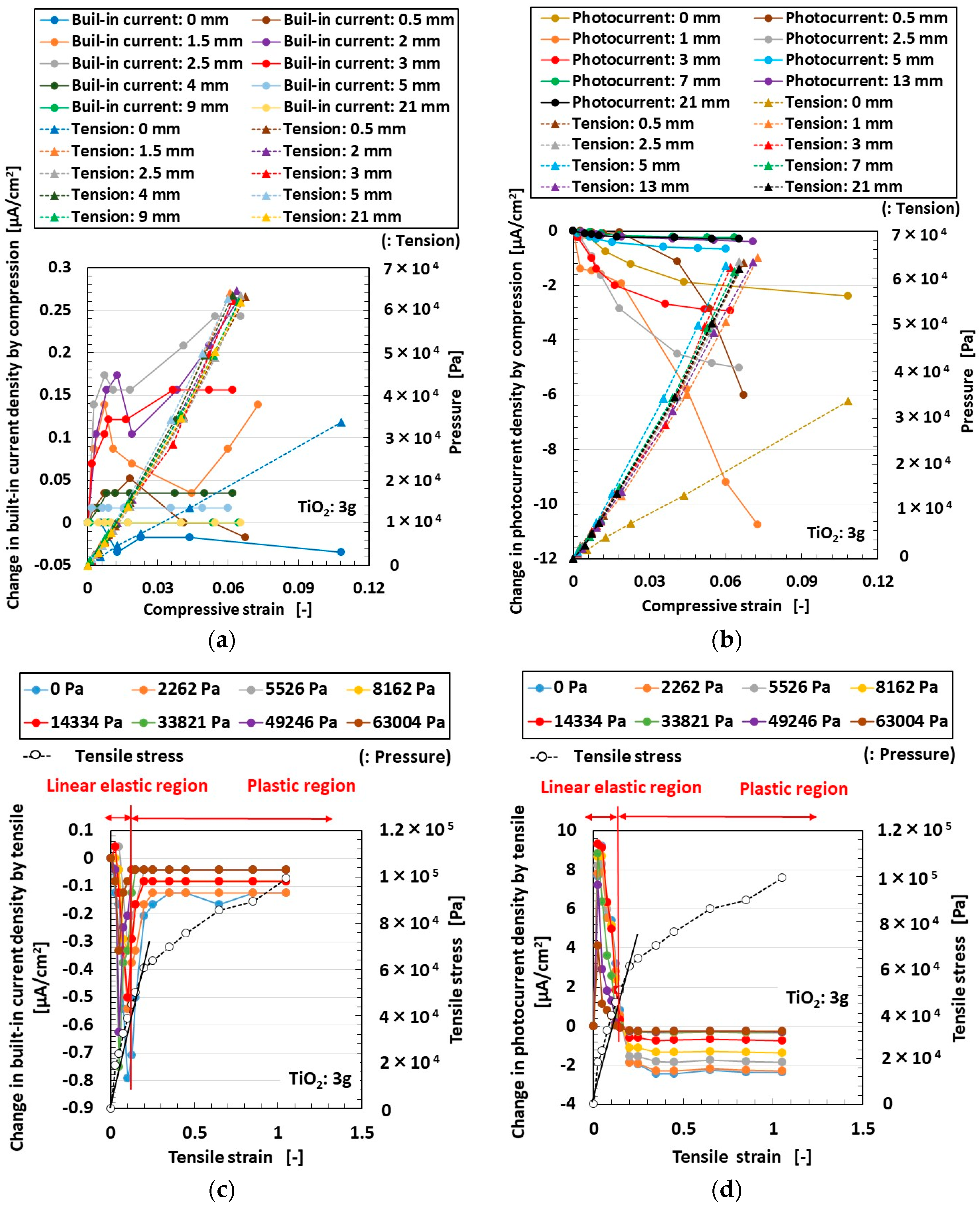
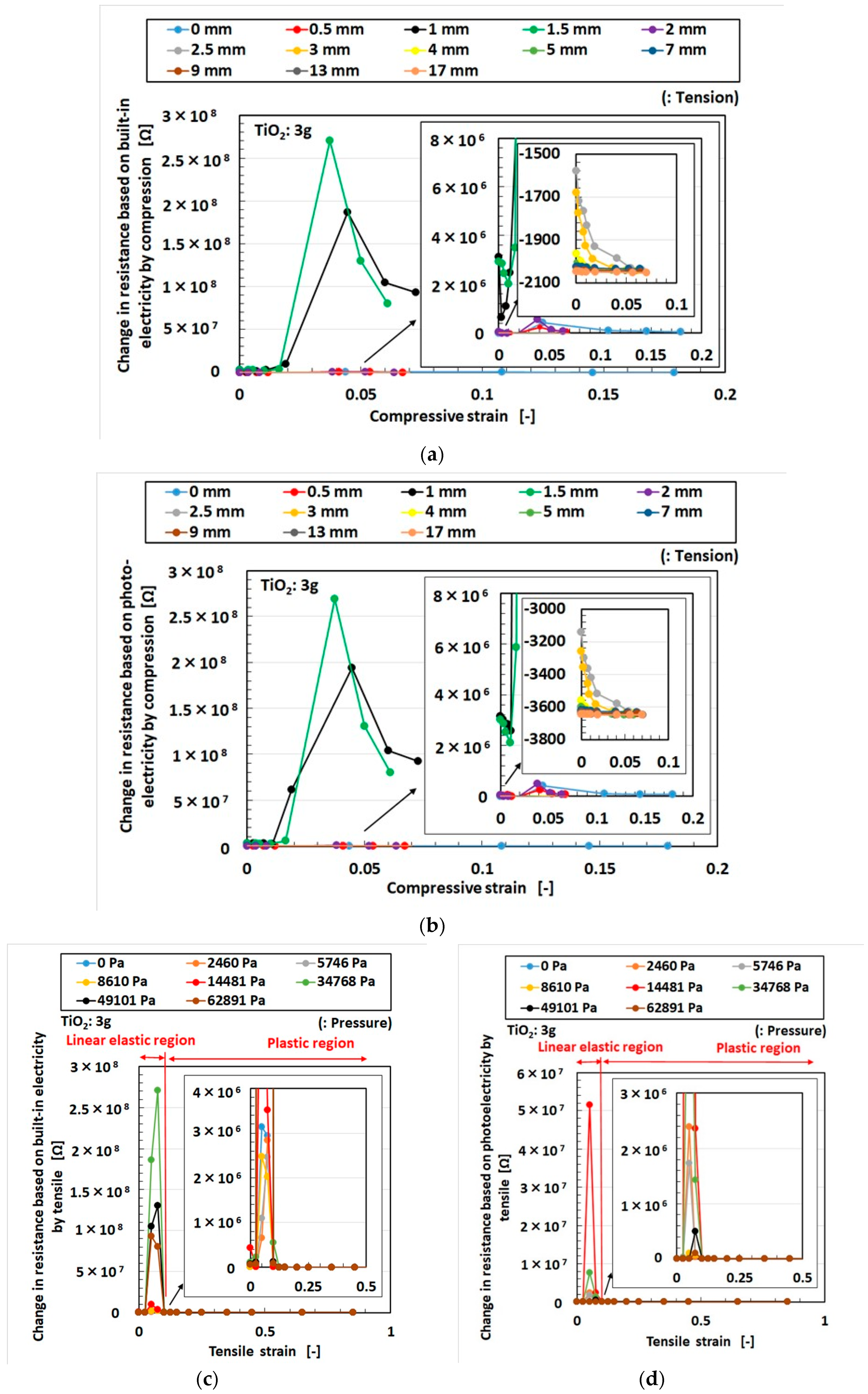
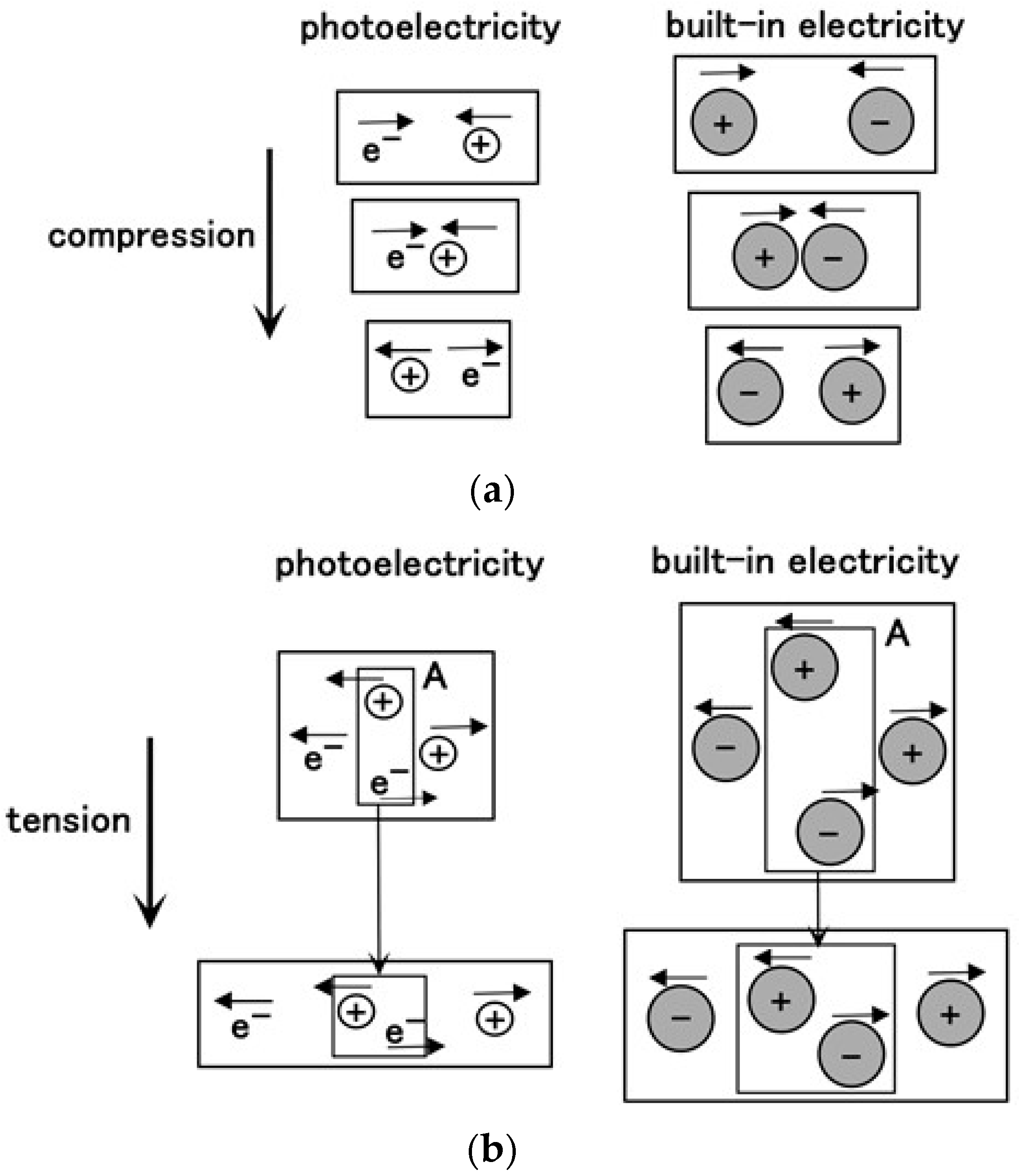
3. Effect of Tension and Compression
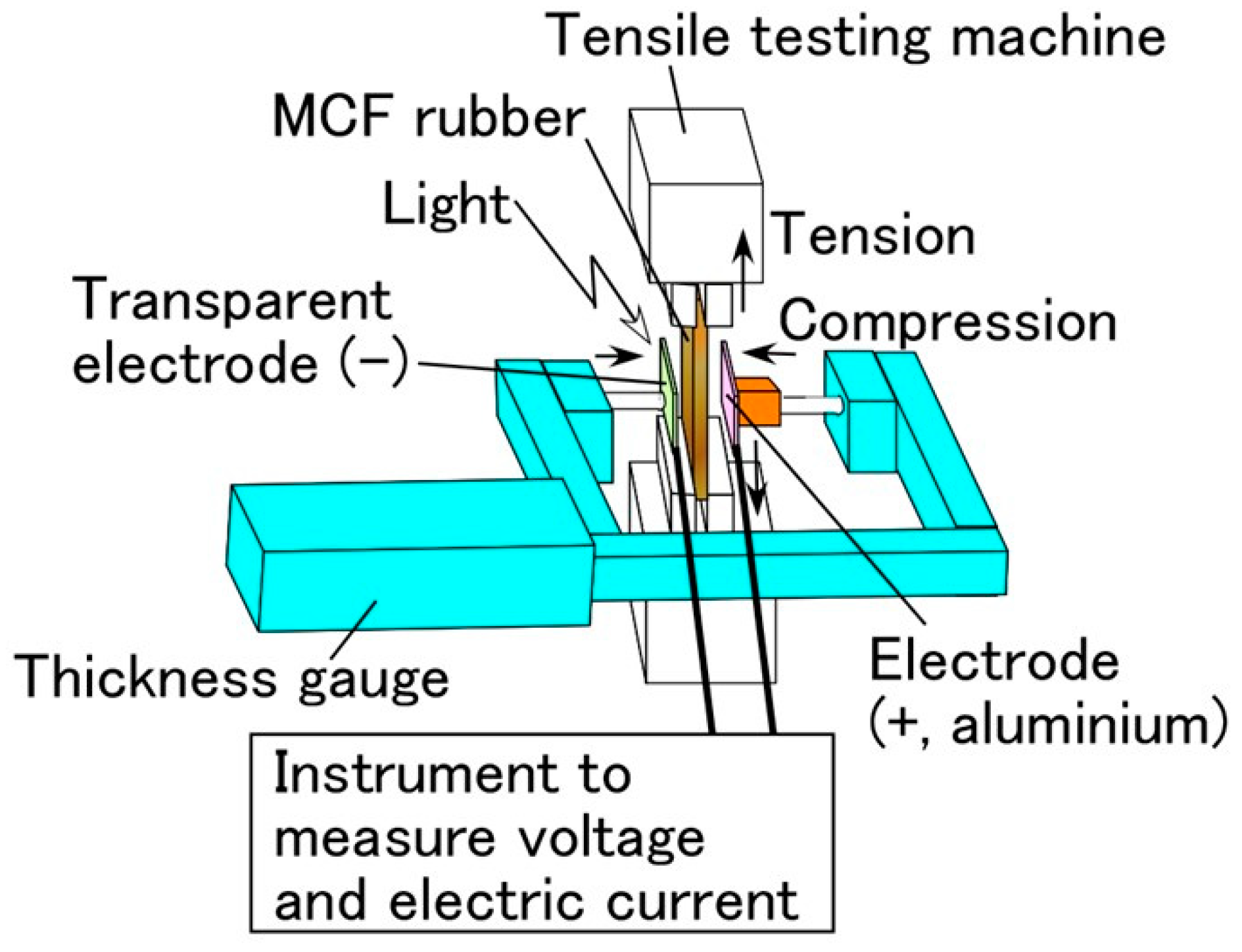
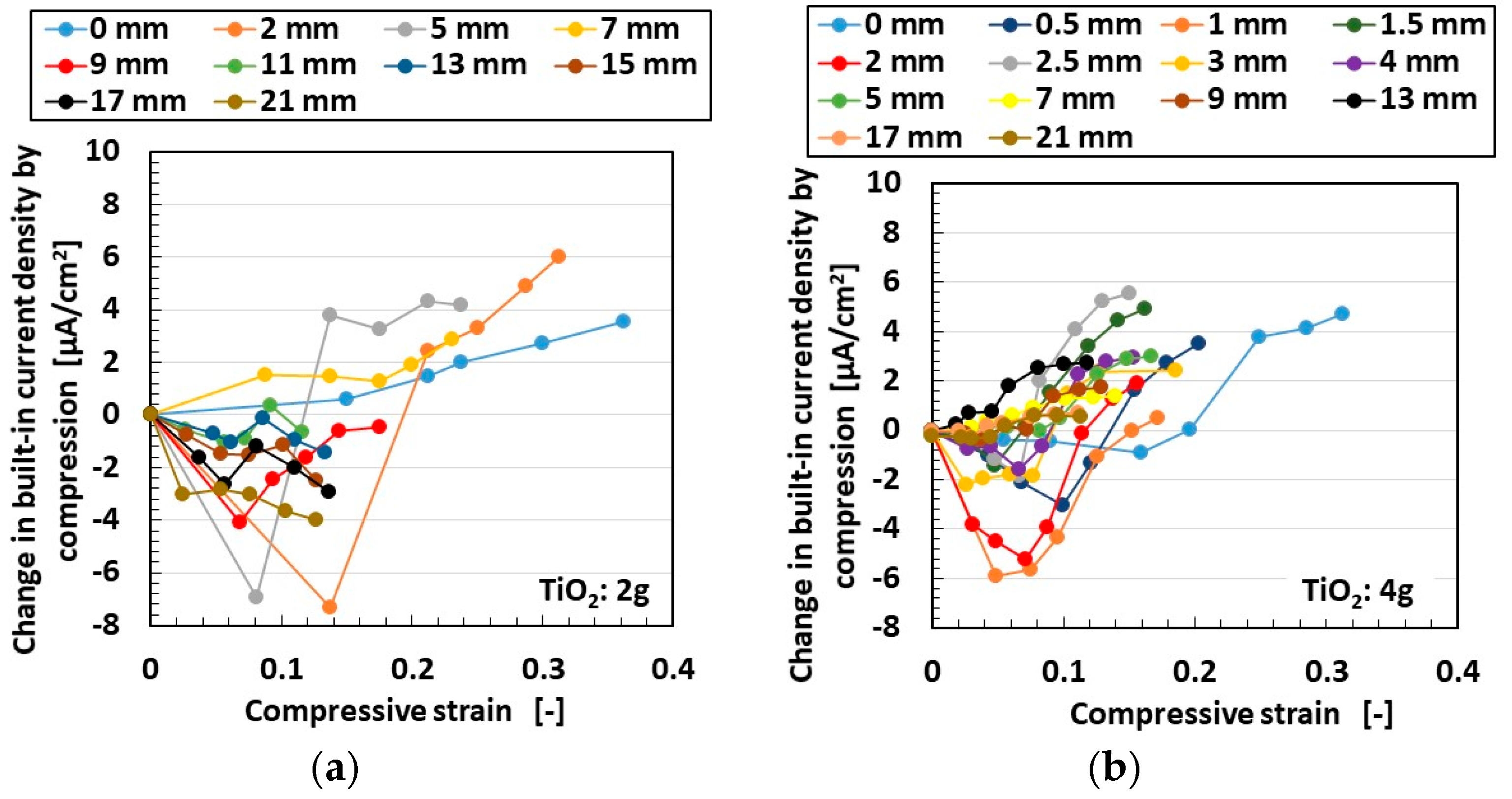
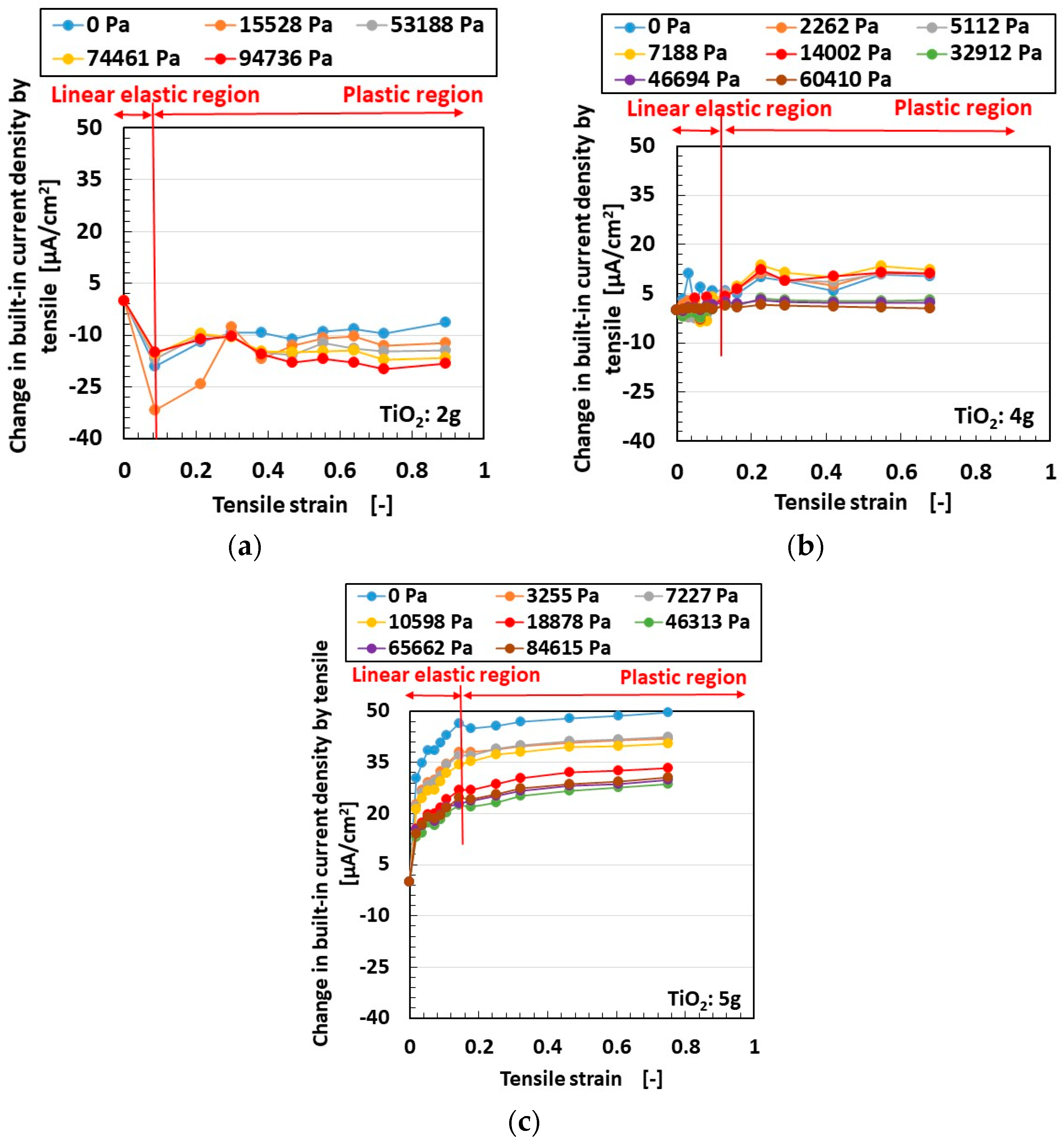
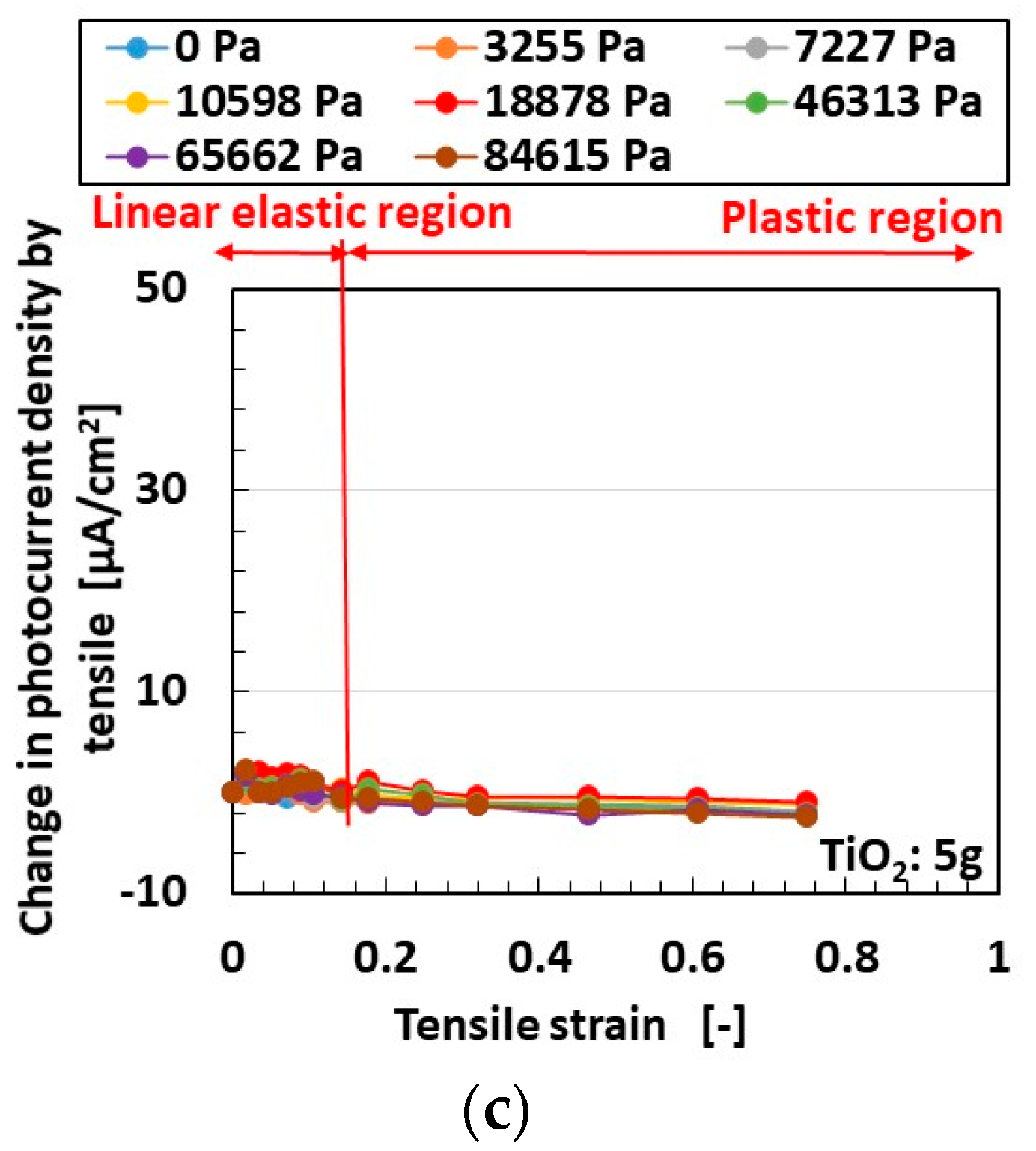
3.1. Experimental Procedure under Tension and Compression
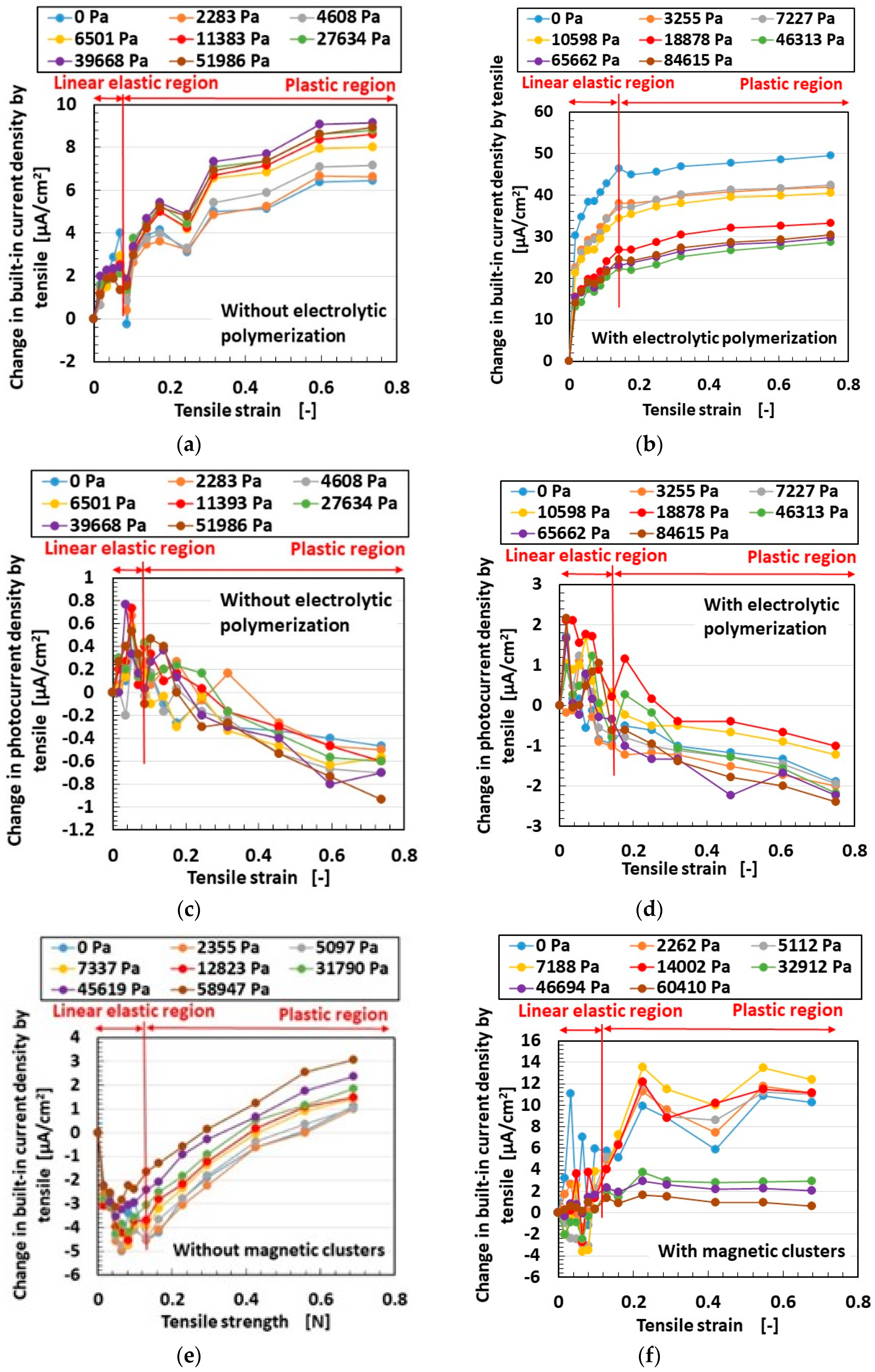
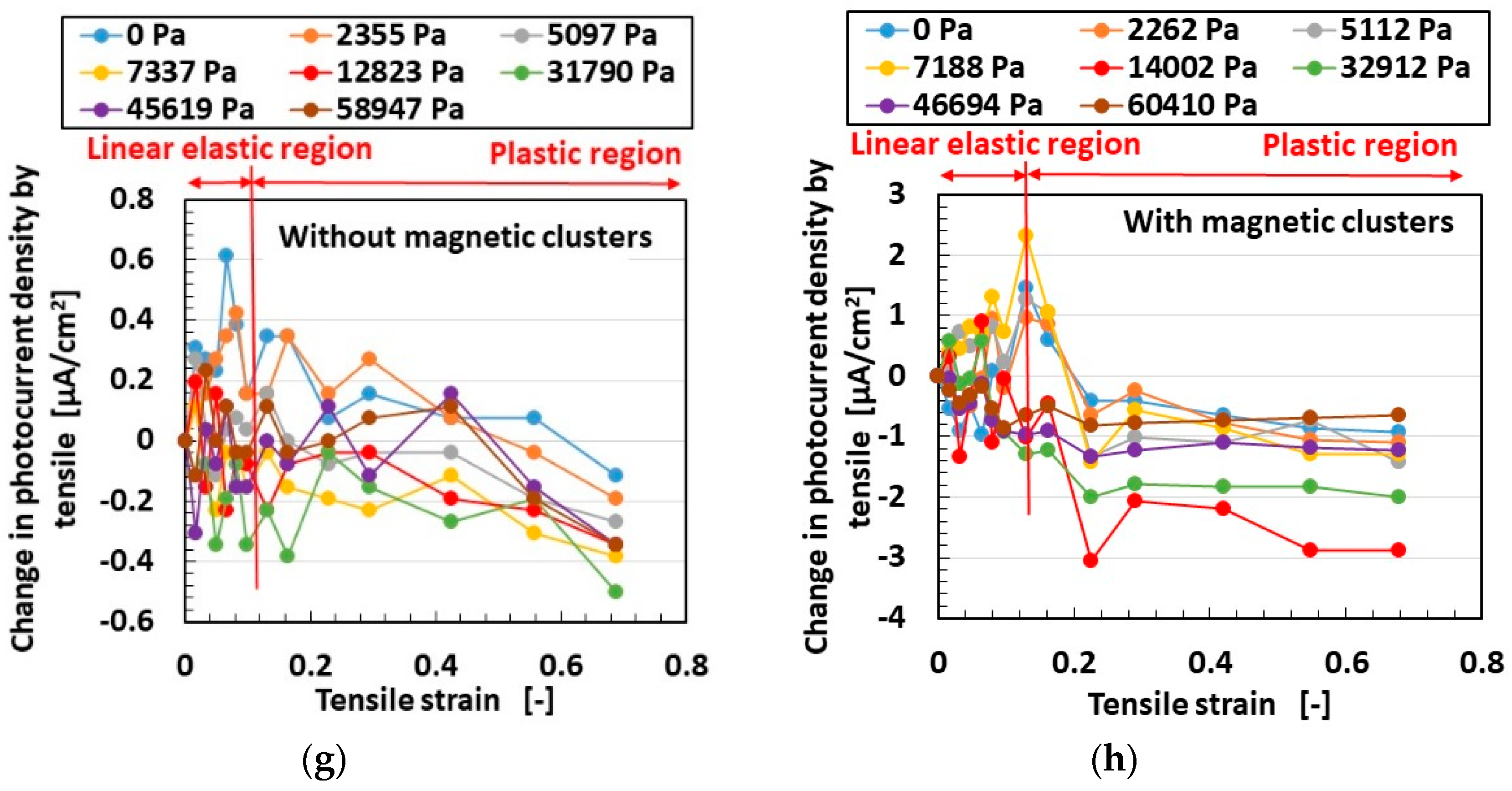
3.2. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Chen, C.P.; Chiang, C.Y.; Yu, Y.Y.; Hsiao, Y.S.; Chen, W.C. High-performance, robust, stretchable organic photovoltaics using commercially available tape as a deformable substrate. Sol. Energy Mat. Sol. Cells 2017, 165, 111–118. [Google Scholar] [CrossRef]
- Cong, H.N.; Dieng, M.; Sene, C.; Chartier, P. Hybrid organic-inorganic solar cells: Case of the all thin film PMeT(Y)/CdS(X) junctions. Sol. Energy Mat. Sol. Cells 2000, 63, 23–35. [Google Scholar] [CrossRef]
- Kudo, N.; Shimazaki, Y.; Ohkita, H.; Ohoka, M.; Ito, S. Organic–inorganic hybrid solar cells based on conducting polymer and SnO2 nanoparticles chemically modified with a fullerene derivative. Sol. Energy Mat. Sol. Cells 2007, 91, 1243–1247. [Google Scholar] [CrossRef]
- Maruthamuthu, P.; Fiechter, S.; Tributsch, H. Lipid (detergent)-based composite-dye solar cell. C. R. Chim. 2006, 9, 684–690. [Google Scholar] [CrossRef]
- Won, S.C.; Sung, H.A.; Harim, J.; Yong, G.S.; Jong, H.K. Rubbery copolymer electrolytes containing polymerized ionic liquid for dye-sensitized solar cells. J. Solid State Electrochem. 2012, 16, 3037–3043. [Google Scholar]
- Gamstedt, H.; Hagfeldt, A.; Kloo, L. Photoelectrochemical studies of ionic liquid-containing solar cells sensitized with different polypyridyl-ruthenium complexes. Polyhedron 2009, 28, 757–762. [Google Scholar] [CrossRef]
- Guo, L.; Pan, X.; Wang, M.; Zhang, C.; Fang, X.; Chen, S.; Dai, S. Novel hydrophobic ionic liquids electrolyte based on cyclic sulfonium used in dye-sensitized solar cells. Sol. Energy 2011, 85, 7–11. [Google Scholar] [CrossRef]
- Rastogi, A.C.; Janardhana, N.R. Properties of CuSbS2 thin films electrodeposited from ionic liquids as p-type absorber for photovoltaic solar cells. Thin Solid Film. 2014, 565, 285–292. [Google Scholar] [CrossRef]
- Zhai, P.; Lee, H.; Huang, Y.T.; Wei, T.C.; Feng, S.P. Study on the blocking effect of a quantum-dot TiO2 compact layer in dye-sensitized solar cells with ionic liquid electrolyte under low-intensity illumination. J. Power Sources 2016, 329, 502–509. [Google Scholar] [CrossRef]
- Gadilohar, B.; Shankarling, G.S. Choline based ionic liquids and their applications in organic transformation. J. Mol. Liq. 2017, 227, 234–261. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Zhu, L. The performance and long-term stability of silicon concentrator solar cells immersed in dielectric liquids. Energy Convers. Manag. 2013, 66, 189–198. [Google Scholar] [CrossRef]
- Tsubomura, H.; Matsumura, M.; Nomura, Y.; Amamiya, T. Dye sensitized zinc oxide: Aqueous electrolyte platinum photocell. Nature 1976, 261, 402–403. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- O’regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.A.; Ali, M.; Memon, A.A.; Sun, K.C.; Choi, B.J.; Jeong, S.H. An all carbon dye sensitized solar cell: A sustainable and low-cost design for metal free wearable solar cell devices. J. Colloid Int. Sci. 2020, 569, 386–401. [Google Scholar] [CrossRef]
- Jung, J.W.; Bae, J.H.; Ko, J.H.; Lee, W. Fully solution-processed indium tin oxide-free textile-based flexible solar cells made of an organic-inorganic perovskite absorber: Toward a wearable power source. J. Power Source 2018, 402, 327–332. [Google Scholar] [CrossRef]
- Brosseau, C. Emerging technologies of plastic carbon nanoelectronics: A review. Surf. Coat. Technol. 2011, 206, 753–758. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwodiauer, R.; Graz, I.; Gogonea, S.B.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458. [Google Scholar] [CrossRef]
- Savagatrup, S.; Printz, A.D.; Wu, H.; Rajan, K.M.; Sawyer, E.J.; Zaretski, A.V.; Bettinger, C.J.; Lipomi, D.J. Viability of stretchable poly (3-heptylthiophene)(P3HpT) for organic slar cells and field-effect transistors. Synth. Met. 2015, 203, 208–214. [Google Scholar] [CrossRef]
- Reinoso, J.; Paggi, M.; Areias, P. A finite element framework for the interplay between delamination and buckling of rubber-like bi-material systems and stretchable electronics. J. Eur. Ceram. Soc. 2016, 36, 2371–2382. [Google Scholar] [CrossRef]
- Ma, R.; Feng, J.; Yin, D.; Sun, H.B. Highly efficient and mechanically robust stretchable polymer solar cells with random buckling. Org. Electron. 2017, 43, 77–81. [Google Scholar] [CrossRef]
- Liu, K.; Wei, A.; Liu, J.; Liu, Z.; Xiao, Z.; Zhao, Y. NiCo2S4 nanosheet thin film counter electrodes prepared by a two-step approach for dye-sensitized solar cells. Mater. Lett. 2018, 217, 185–188. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Z.; Huang, J.; Zhang, Y.; Liu, X.; Guo, W. Photoelectrochromic smart windows powered by flexible dye-sensitized solar cell using CuS mesh as counter electrode. Mater. Lett. 2019, 244, 92–95. [Google Scholar] [CrossRef]
- Yun, S.; Lim, S. Improved conversion efficiency in dye-sensitized solar cells based on electrospun Al-doped ZnO nanofiber electrodes prepared by seed layer treatment. J. Solid State Chem. 2011, 184, 273–279. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Z.; Zhang, J.; Cao, J.; Wang, S.; Tian, Q.; Gao, M.; Xu, Q. Fabrication of PVA/graphene oxide/TiO2 composite nanofibers through electrospinning and interface sol-gel reaction: Effect of graphene oxide on PVA nanofibers and growth of TiO2. Colloids Sur. A Phys. Eng. Asp. 2014, 457, 318–325. [Google Scholar] [CrossRef]
- Yun, M.J.; Cha, S.I.; Seo, S.H.; Lee, D.Y. Highly flexible dye-sensitized solar cells produced by sewing textile electrodes on cloth. Sci. Rep. 2014, 4, 5322. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Chen, J.Y.; Shih, C.C.; Chueh, C.C.; Chen, W.C. Mechanically robust, stretchable organic solar cells via buckle-on-elastomer strategy. Org. Electron. 2018, 53, 339–345. [Google Scholar] [CrossRef]
- Muller, S.; Wieschollek, D.; Junger, I.J.; Helkamp, E.S.; Ehrmann, A. Back electrodes of dye-sensitized solar cells on textile fabrics. Optik 2019, 198, 163243. [Google Scholar] [CrossRef]
- Shimada, K. Elastic MCF rubber with photovoltaics and sensing for use as artificial or hybrid skin (H-Skin): 1st report on dry-type solar cell rubber with piezoelectricity for compressive sensing. Sensors 2018, 18, 1841. [Google Scholar] [CrossRef]
- Shimada, K. Elastic MCF rubber with photovoltaics and sensing on hybrid skin (H-Skin) for artificial skin by utilizing natural rubber: 2nd report on effect of tension and compression on properties of hybrid photo- and piezo-electricity in wet-type solar cell rubber. Sensors 2018, 18, 1848. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K. MCF rubber with photovoltaics and sensing for use as artificial or hybrid skin (H-Skin): Third report on electric charge and storage under tension and compression. Sensors 2018, 18, 1853. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Kato, R.; Ikeda, R.; Kikura, H.; Takahashi, H. γ-ray irradiation effect on MCF rubber solar cells with both photovoltaics and sensing involving semiconductors fabricated under magnetic and electric fields. World J. Mech. 2020, 10, 95–119. [Google Scholar]
- Shimada, K.; Saga, N. Mechanical enhancement of sensitivity in natural rubber using electrolytic polymerization aided by a magnetic field and MCF for application in haptic sensors. Sensors 2016, 16, 1521. [Google Scholar] [CrossRef]
- Shimada, K.; Saga, N. Detailed mechanism and engineering applicability of electrolytic polymerization aided by a magnetic field in natural rubber by mechanical approach for sensing (Part 1): The effect of experimental conditions on electrolytic polymerization. World J. Mech. 2016, 6, 357–378. [Google Scholar] [CrossRef]
- Shimada, K.; Saga, N. Detailed mechanism and engineering applicability of electrolytic polymerization aided by a magnetic field in natural rubber by mechanical approach for sensing (Part 2): Other and intrinsic effects on MCF rubber property. World J. Mech. 2016, 6, 379–395. [Google Scholar] [CrossRef]
- Shimada, K.; Saga, N. Development of a hybrid piezo natural rubber piezoelectricity and piezoresistivity sensor with magnetic clusters made by electric and magnetic field assistance and filling with magnetic compound fluid. Sensors 2017, 17, 1521. [Google Scholar] [CrossRef]
- Shimada, K. Enhancement of MCF rubber utilizing electric and magnetic fields, and clarification of electrolytic polymerization. Sensors 2017, 17, 767. [Google Scholar] [CrossRef]
- Shimada, K.; Michizuki, O.; Kubota, Y. The effect of particles on electrolytically polymerized thin natural MCF rubber for soft sensors installed in artificial skin. Sensors 2017, 17, 896. [Google Scholar] [CrossRef]
- Shimada, K.; Ikeda, R.; Kikura, H.; Takahashi, H. Development of a magnetic compound fluid rubber stability sensor and a novel production technique via combination of natural, chloroprene and silicone rubbers. Sensors 2019, 189, 3901. [Google Scholar] [CrossRef]
- Shimada, K.; Ikeda, R.; Kikura, H.; Takahashi, H. Enhancement of diversity in production and application utilizing electrolytically polymerized rubber sensors with MCF: 1st report on consummate fabrication combining varied kinds of constituents with porous permeant stocking-like rubber. Sensors 2020, 20, 4658. [Google Scholar] [CrossRef]
- Shimada, K.; Ikeda, R.; Kikura, H.; Takahashi, H. Enhancement of diversity in production and application utilizing electrolytically polymerized rubber sensors with MCF: The second report on various engineering applications. Sensors 2020, 20, 4674. [Google Scholar] [CrossRef]
- Miettunen, K.; Toivola, M.; Hashmi, G.; Salpakari, J.; Asghar, I.; Lund, P. A carbon gel catalyst layer for the roll-to-roll production of dye solar cells. Carbon 2011, 49, 528–532. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Meenakshamma, A.; Susmitha, K.; Charanadhar, N.; Srikanth, V.V.S.S.; Babu, S.N.; Subbaiah, Y.P.V.; Venkateswarlu, K.; Raghavender, M. A transparent and Pt-free all-carbon nanocomposite counter electrode catalyst for efficient dye sensitized solar cells. Sol. Energy 2019, 193, 568–575. [Google Scholar] [CrossRef]
- Qian, X.; Xu, C.; Jiang, Y.; Zhang, J.; Guan, G.; Huang, Y. Ni-Co-MoSx ball-in-ball hollow nanoshperes as Pt-free bifunctional catalysts for high-performance solar cells and hydrogen evolution reactions. Chem. Eng. J. 2019, 368, 202–211. [Google Scholar] [CrossRef]
- Li, J.; Yun, S.; Han, F.; Si, Y.; Arshad, A.; Zhang, Y.; Chidambaram, B.; Zafar, N.; Qiao, X. Biomass-derived carbon boosted catalytic properties of tungsten-based nanohybrids for accelerating the triiodide in dye-senstitized slar cells. J. Colloid Interfaces Sci. 2020, 578, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Makhdoom, A.R.; Bilal, M.K.; Badar, M.A. Comparative study of the photovoltaic behavior of ruthenium and the other organic and inorganic Dye-Sensitized Solar Cells (DSSC). Optik 2018, 157, 11–15. [Google Scholar] [CrossRef]
- Buffa, M.; Carturan, S.; Debije, M.G.; Quaranta, A.; Maggioni, G. Dye-doped polysiloxane rubbers for luminescent solar concentrator systems. Sol. Eng. Mater. Sol. Cells 2012, 103, 114–118. [Google Scholar] [CrossRef]
- Nyberg, T.; Zhang, F.; Inganas, O. Macromolecular nanoelectronics. Curr. Appl. Phys. 2002, 2, 27–31. [Google Scholar] [CrossRef]
- Vannucci, A.K.; Alibabaei, L.; Losego, M.D.; Concepcion, J.J.; Kalanyan, B.; Parsons, G.N.; Meyer, T.J. Crossing the divide between homogeneous and heterogeneous catalyst in water oxidation. Proc. Natl. Acad. Sci. USA 2013, 110, 20918–20922. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, Y. Tailored Metal Catalysts; D. Reidel Publishing Company: Dordrecht, The Neverlands, 1986; pp. 167–172. ISBN 90-277-1866-0. [Google Scholar]
- Duran, A.; Monteagudo, J.M.; Martin, I.S. Operation costs of the solar photo-catalytic degradation of pharmaceuticals in water: A mini-review. Chemosphere 2018, 211, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bai, Y.; Jin, W.; Xu, N. Visible-light-driven TiO2 catalysts doped with low-concentration nitrogen species. Sol. Energy Mater. Sol. Cells 2008, 92, 76–83. [Google Scholar] [CrossRef]
- Ito, S.; Nazeeruddin, K.M.; Zakeeruddin, S.M.; Pechy, P.; Comte, P.; Gratzel, M.; Mizuno, T.; Tanaka, A.; Koyanagi, T. Study of dye-sensitized solar cells by scanning electron micrograph observation and thickness optimization of porous TiO2 electrodes. Int. J. Photoenergy 2009, 2009, 517609. [Google Scholar] [CrossRef]
- Iyas, A.M.; Gondal, M.A.; Baig, U.; Akhtar, S.; Yamani, Z.H. Photovoltaic performance and photocatalytic activity of facile synthesized graphene decorated TiO2 monohybrid using nanosecond pulsed ablation in liquid technique. Solar Energy 2016, 137, 246–255. [Google Scholar]
- Lu, W.H.; Chou, C.S.; Chen, C.Y.; Wu, P. Preparation of Zr-doped mesoporous TiO2 particles and their applications in the novel working electrode of a dye-sensitized solar cell. Adv. Powder Technol. 2017, 28, 2186–2197. [Google Scholar] [CrossRef]
- Shamsudin, N.H.; Shafie, S.; Kadir, M.Z.A.A.; Ahmad, F.; Sadrolhosseini, A.R.; Sulaiman, Y.; Chachuli, S.A.M. Power conversion efficiency (PCE) performance of back-illuminated DSSCs with different Pt catalyst contents at the optimized TiO2 thickness. Optik 2020, 203, 163567. [Google Scholar] [CrossRef]
- Haimeur, A.E.; Makha, M.; Bakkali, H.; Leal, J.M.G.; Blanco, E.; Dominguez, M.; Voitenko, Z.V. Enhanced performance of planar perovskite solar cells using dip-coated TiO2 as electron transporting layer. Sol. Energy 2020, 195, 475–482. [Google Scholar] [CrossRef]
- Maksoud, Y.K.A.; Imam, E.; Ramada, A.R. TiO2 water-bell photoreactor for wastewater treatment. Sol. Energy 2018, 170, 323–335. [Google Scholar] [CrossRef]
- Qin, G.; Wu, Q.; Sun, Z.; Wang, Y.; Luo, J.; Xue, S. Enhanced photoelectrocatalytic degradation of phenols with bifunctionalized dye-sensitized TiO2 film. J. Hazard. Mater. 2012, 199–200, 226–232. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Duran, A.; Chazisymeon, E.; Martin, I.S.; Naranjo, S. Solar activation of TiO2 intensified with graphene for degradation of Bisphenol-A in water. Sol. Energy 2018, 174, 1035–1043. [Google Scholar] [CrossRef]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, K.; Kikura, H.; Ikeda, R.; Takahashi, H. Clarification of Catalytic Effect on Large Stretchable and Compressible Rubber Dye-Sensitized Solar Cells. Energies 2020, 13, 6658. https://doi.org/10.3390/en13246658
Shimada K, Kikura H, Ikeda R, Takahashi H. Clarification of Catalytic Effect on Large Stretchable and Compressible Rubber Dye-Sensitized Solar Cells. Energies. 2020; 13(24):6658. https://doi.org/10.3390/en13246658
Chicago/Turabian StyleShimada, Kunio, Hiroshige Kikura, Ryo Ikeda, and Hideharu Takahashi. 2020. "Clarification of Catalytic Effect on Large Stretchable and Compressible Rubber Dye-Sensitized Solar Cells" Energies 13, no. 24: 6658. https://doi.org/10.3390/en13246658
APA StyleShimada, K., Kikura, H., Ikeda, R., & Takahashi, H. (2020). Clarification of Catalytic Effect on Large Stretchable and Compressible Rubber Dye-Sensitized Solar Cells. Energies, 13(24), 6658. https://doi.org/10.3390/en13246658




