Surfactant-Polymer Interactions in a Combined Enhanced Oil Recovery Flooding
Abstract
1. Introduction
Polymer-Surfactant Flooding and Interaction
2. Aim of This Work
3. Model Description
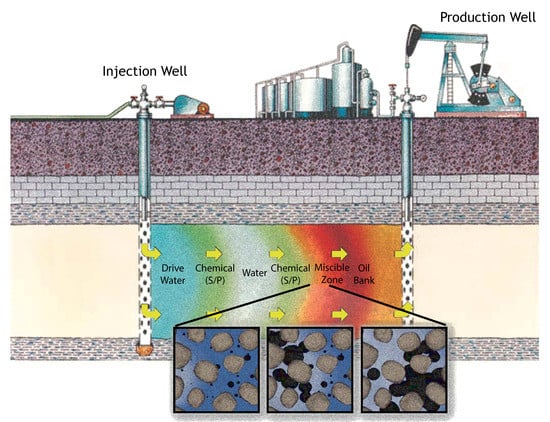
3.1. Physical Model
3.2. Mathematical Model
4. Physical Properties
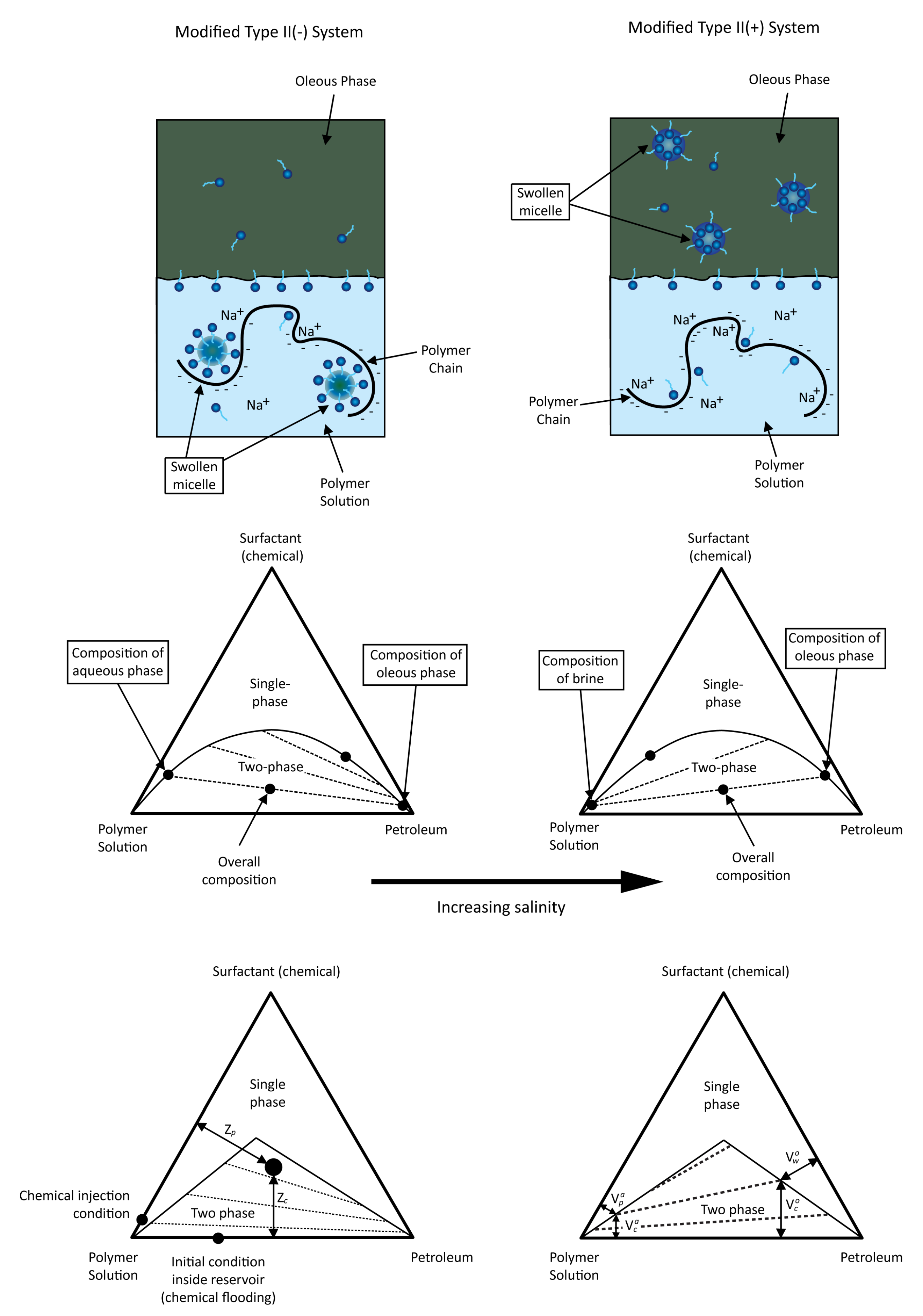
4.1. Chemical Component Partitioning
4.2. Interfacial Tension
4.3. Adsorption
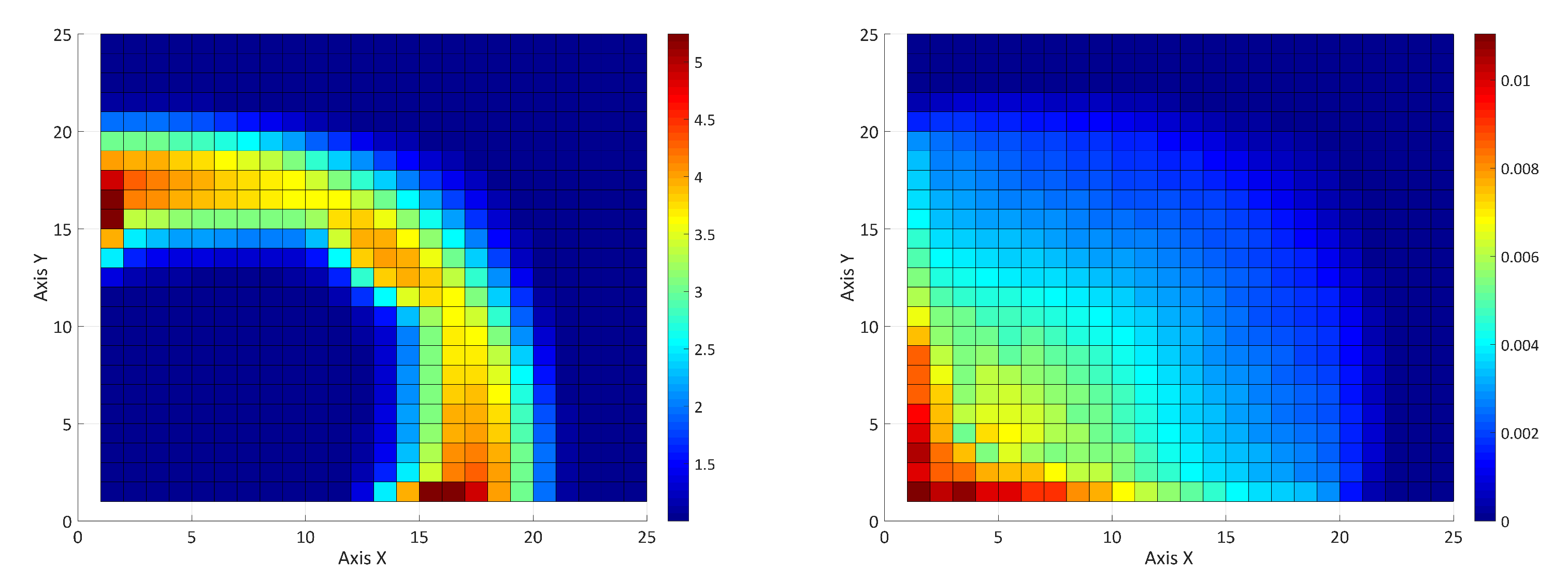
5. Results and Discussion
5.1. Introduction
Data
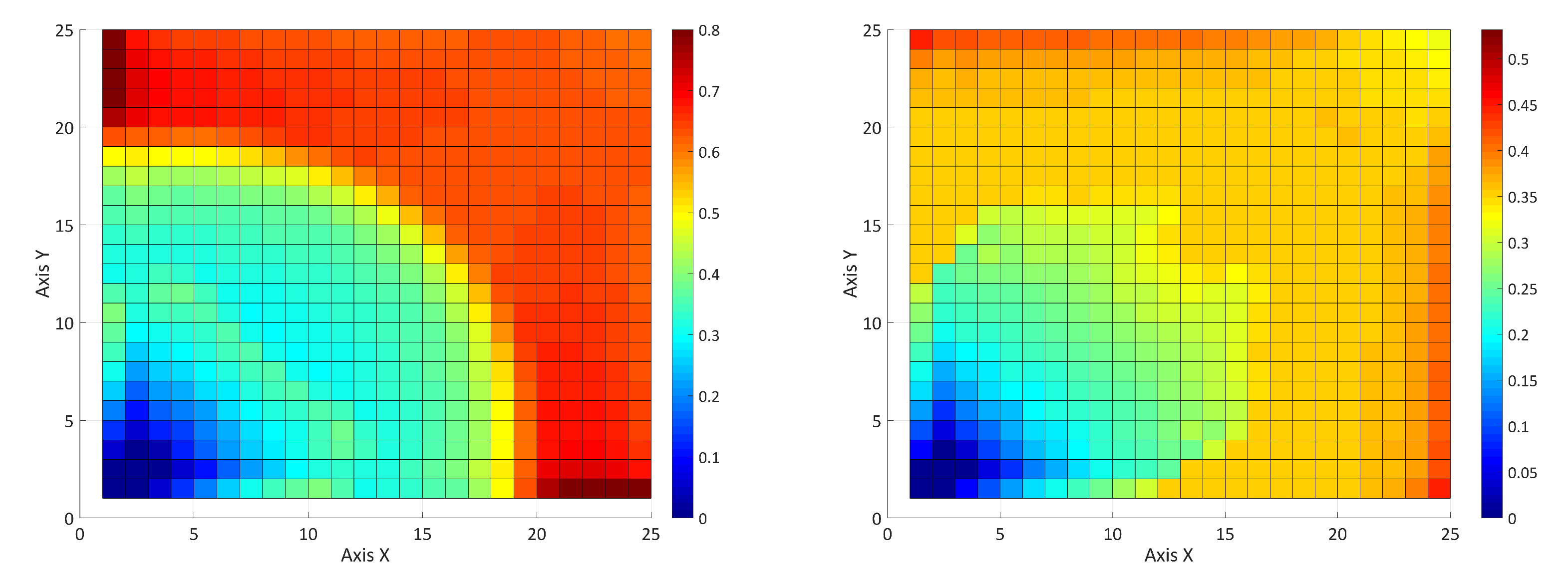
5.2. Influence of the Adsorption and the Surfactant-Polymer Interaction
5.3. Adsorption in a Five-Component System
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EOR | Enhanced Oil Recovery |
| TDS | Total Dissolved Solids |
| IAPV | Inaccessible Pore Volume |
| TVD | Total Variation Diminishing |
| PDE | Partial Differential Equation |
| IFT | Interfacial Tension |
| IMPEC | Implicit in Pressure, Explicit in Concentration |
| OOIP | Original Oil in Place |
Nomenclature
| Dispersion Tensor | Reservoir Domain | |||
| Molecular Diffusion | ||||
| Longitudinal Dispersion | Superscripts | |||
| Transversal Dispersion | a | Aqueous Phase | ||
| Relative Permeability | H | Water-Oil System (no Chemical) | ||
| p | Reservoir Pressure | j | Phase | |
| Bottomhole Pressure | Iteration Number | |||
| r | Volumetric Concentration Gradient | Time-Step | ||
| Well Radius | o | Oleous Phase | ||
| S | Phase Saturation | r | Residual | |
| s | Well Skin Factor | |||
| V | Volumetric Concentration | Subscripts | ||
| z | Overall Concentration | c | Chemical (Surfactant) Component | |
| i | Component | |||
| Greek Letters | Injection | |||
| Domain Boundary | Spatial Grid Blocks | |||
| Kronecker Delta | p | Petroleum Component | ||
| Phase Mobility | Polymer Component | |||
| Absolute Viscosity | Salt Component | |||
| Interfacial Tension | w | Water Component | ||
References
- Al-Mjeni, R.; Arora, S.; Cherukupalli, P.; Wunnik, J.V.; Edwards, J.; Felber, B.J.; Gurpinar, O.; Hirasaki, G.J.; Miller, C.A.; Jackson, C. Has the time come for EOR? Oilfield Rev. 2010, 2011, 4. [Google Scholar]
- Dake, L.P. Fundamentals of Reservoir Engineering; Elsevier: Amsterdam, The Netherlands, 1978; ISBN 0-444-41830-X. [Google Scholar]
- Lake, L.W. Enhanced Oil Recovery; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1989; ISBN 0-13-281601-6. [Google Scholar]
- Satter, A.; Iqbal, G.M.; Buchwalter, J.L. Practical Enhanced Reservoir Engineering; PennWell Books: Tulsa, OK, USA, 2008; ISBN 978-1-59370-056-0. [Google Scholar]
- Schmidt, R.L.; Venuto, P.B.; Lake, L.W. A Niche for Enhanced Oil Recovery in the 1990’s. Oilfield Rev. 1992, 4, 55–61. [Google Scholar]
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent Advances in Surfactant EOR. SPE J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Khan, M.Y.; Samanta, A.; Ojha, K.; Mandal, A. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac. J. Chem. Eng. 2008, 3, 579–585. [Google Scholar] [CrossRef]
- Ye, Z.; Guo, G.; Chen, H.; Shu, Z. Interaction between aqueous solutions of hydrophobically associating polyacrylamide and dodecyl dimethyl betaine. J. Chem. 2014, 932082. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Surfactant-Polymer flooding: Influence of the injection scheme. Energy Fuels 2018, 32, 12231–12246. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, M.; Zahrani, B.H.; Guo, L. Effect of surfactant-polymer interaction on the interfacial properties for chemical EOR. In SPE Middle East Oil & Gas Show and Conference; Society of Petroleum Engineers: Manama, Bahrain, 2015. [Google Scholar] [CrossRef]
- Ma, B.D.; Gao, B.Y.; Zhang, L.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhao, S. Influence of polymer on dynamic interfacial tensions of EOR surfactant solutions. J. Appl. Polym. Sci. 2014, 131, 40562. [Google Scholar] [CrossRef]
- Druetta, P.; Raffa, P.; Picchioni, F. Chemical enhanced oil recovery and the role of chemical product design. Appl. Energy 2019, 252, 113480. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.-C.; Yan, F.; Luo, L.; Zhang, L.; Zhao, S.; Yu, J.-Y. Interfacial dilational properties of partly hydrolyzed polyacrylamide and gemini surfactant at the decane-water interface. Colloid Polym. Sci. 2008, 286, 1291–1297. [Google Scholar] [CrossRef]
- Fathaddin, M.T.; Nugrahanti, A.; Buang, P.N.; Elraies, K.A. Surfactant-polymer flooding performance in heterogeneous two-layered porous media. IIUM Eng. J. 2011, 12, 31–38. [Google Scholar] [CrossRef]
- Bekbauov, B.; Berdyshev, A.; Baishemirov, Z. Numerical simulation of chemical enhanced oil recovery processes. In DOOR (suplement); CEUR-WS.org: Vladivostok, Russia, 2016; pp. 664–676. [Google Scholar]
- Abadli, F. Simulation Study of Enhanced Oil Recovery by ASP (Alkaline, Surfactant and Polymer) Flooding for Norne Field C-segment. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2012. [Google Scholar]
- Atthawutthisin, N. Numerical Simulation of Low Salinity Water Flooding Assisted with Chemical Flooding for Enhanced Oil Recovery. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2012. [Google Scholar]
- Roshanfekr, M.; Johns, R.T.; Pope, G. Effect of pressure, temperature, and solution gas on oil recovery from surfactant polymer floods. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: New Orleans, LA, USA, 2009. [Google Scholar] [CrossRef]
- Changli, Y. Commercial Scale Simulations of Surfactant/Polymer Flooding. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2012. [Google Scholar]
- Rai, S.; Bera, A.; Mandal, A. Modeling of surfactant and surfactant-polymer flooding for enhanced oil recovery using STARS (CMG) software. J. Pet. Explor. Prod. 2014, 1–11. [Google Scholar] [CrossRef]
- Rai, K.; Johns, R.T.; Lake, L.W.; Delshad, M. Oil-recovery predictions for surfactant polymer flooding. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: New Orleans, LA, USA, 2009. [Google Scholar] [CrossRef]
- Thirawarapan, C.; Thiele, M.R.; Kovscek, A.; Batycky, R.; Clemens, T. A simplified model for field-scale surfactant-polymer flooding. In SPE Heavy Oil Conference-Canada; Society of Petroleum Engineers: Calgary, AB, Canada, 2014. [Google Scholar] [CrossRef]
- Jin, B.; Jiang, H.; Zhang, X.; Wang, J.; Yang, J.; Zheng, W. Numerical simulation of surfactant-polymer flooding. Chem. Technol. Fuels Oils 2014, 50, 55–70. [Google Scholar] [CrossRef]
- Garrepally, S.; Jouenne, S.; Olmsted, P.; Lequeux, F. Scission of flexible polymers in contraction flow: Predicting the effects of multiple passages. J. Rheol. 2020, 64, 601–614. [Google Scholar] [CrossRef]
- Hosseini-Nasab, S.M.; Padalkar, C.; Battistutta, E.; Zitha, P.L.J. Mechanistic modeling of the alkaline/surfactant/polymer flooding process under sub-optimum salinity conditions for enhanced oil recovery. Ind. Eng. Chem. Res. 2016, 55, 6875–6888. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bera, A.; Raipuria, V.; Kumar, A.; Mandal, A.; Kumar, T. Numerical simulation of enhanced oil recovery by alkali-surfactant-polymer floodings. Pet. Sci. Technol. 2015, 33, 1229–1237. [Google Scholar] [CrossRef]
- Fadili, A.; Kristensen, M.R.; Moreno, J. Smart integrated chemical EOR simulation. In International Petroleum Technology Conference; Society of Petroleum Engineers: Doha, Qatar, 2009. [Google Scholar] [CrossRef]
- Druetta, P.; Yue, J.; Tesi, P.; Persis, C.D.; Picchioni, F. Numerical modeling of a compositional flow for chemical EOR and its stability analysis. Appl. Math. Model. 2017, 47, 141–159. [Google Scholar] [CrossRef]
- Druetta, P. Numerical Simulation of Chemical EOR Processes. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2018. [Google Scholar]
- Azad, M.; Trivedi, J. Quantification of the Viscoelastic Effects During Polymer Flooding: A Critical Review. SPE J. 2019, 24, 2731–2757. [Google Scholar] [CrossRef]
- Azad, M.; Trivedi, J. Quantification of Sor Reduction during Polymer Flooding Using Extensional Capillary Number. SPE J. 2020, 204212. [Google Scholar] [CrossRef]
- Sheng, J. Modern Chemical Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-1-85617-745-0. [Google Scholar]
- Bidner, M.S.; Savioli, G.B. On the numerical modeling for surfactant flooding of oil reservoirs. Mec. Comput. 2002, 21, 566–585. [Google Scholar]
- Chen, Z.; Huan, G.; Ma, Y. Computational Methods for Multiphase Flows in Porous Media; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2006; ISBN 978-0-89871-606-1. [Google Scholar] [CrossRef]
- Druetta, P.; Tesi, P.; Persis, C.D.; Picchioni, F. Methods in Oil Recovery Processes and Reservoir Simulation. Adv. Chem. Eng. Sci. 2016, 6, 39. [Google Scholar] [CrossRef]
- Barrett, R.; Berry, M.; Chan, T.; Demmel, J.; Donato, J.; Dongarra, J.; Eijkhout, V.; Pozo, R.; Romine, C.; van der Vorst, C. Templates for the Solution of Linear Systems: Building Blocks for Iterative Methods; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1994; ISBN 978-0-89871-328-2. [Google Scholar] [CrossRef]
- Kamalyar, K.; Kharrat, R.; Nikbakht, M. Numerical Aspects of the Convection-Dispersion Equation. Pet. Sci. Technol. 2014, 32, 1729–1762. [Google Scholar] [CrossRef]
- Bear, J. Dynamics of Fluids In Porous Media; American Elsevier Publishing Company: New York, NY, USA, 1972; ISBN 978-0-44400-114-6. [Google Scholar]
- Kuzmin, D. A Guide to Numerical Methods for Transport Equations; University Erlangen-Nuremberg: Nuremberg, Germany, 2010. [Google Scholar]
- Larson, R.G. The Influence of Phase Behavior on Surfactant Flooding. Soc. Pet. Eng. J. 1979, 19, 411–422. [Google Scholar] [CrossRef]
- Porcelli, P.; Bidner, M. Simulation and transport phenomena of a ternary 2-phase flow. Transp. Porous Media 1994, 14, 101–122. [Google Scholar] [CrossRef]
- Attwood, D.; Florence, A.T. Surfactant Systems—Their Chemistry, Pharmacy and Biology; Chapman and Hall: London, UK, 1983; ISBN 978-9-40095-775-6. [Google Scholar]
- Delshad, M.; Pope, G.; Sepehrnoori, K. UTCHEM Version 9.0 Technical Documentation; Center for Petroleum and Geosystems Engineering, The University of Texas at Austin: Austin, TX, USA, 2000; Volume 78751. [Google Scholar]
- Hirasaki, G.J. Application of the Theory of Multicomponent, Multiphase Displacement to 3-Component, 2-Phase Surfactant Flooding. Soc. Pet. Eng. J. 1981, 21, 191–204. [Google Scholar] [CrossRef]
- Bidner, M.; Porcelli, P. Influence of phase behavior on chemical flood transport phenomena. Transp. Porous Media 1996, 24, 247–273. [Google Scholar] [CrossRef]
- Bidner, M.; Porcelli, P. Influence of capillary pressure, adsorption and dispersion on chemical flood transport phenomena. Transp. Porous Media 1996, 24, 275–296. [Google Scholar] [CrossRef]
- Kumar, B. Effect of Salinity on the Interfacial Tension of Model and Crude Oil Systems. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2012. [Google Scholar]
- Goddard, E. Polymer/surfactant interaction: Interfacial aspects. J. Colloid Interface Sci. 2002, 256, 228–235. [Google Scholar] [CrossRef]
- Panmai, S.; Prud’homme, R.; Peiffer, D.; Jockusch, S.; Turro, N. Interactions between hydrophobically modified polymers and surfactants: A fluorescence study. Langmuir 2002, 18, 3860–3864. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, D.; Cao, X.; Guo, L.; Zhu, Y. Both-branch amphiphilic polymer oil displacing system: Molecular weight, surfactant interactions and enhanced oil recovery performance. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 509, 440–448. [Google Scholar] [CrossRef]
- Xu-Long, C.; Jing, L.; Yong, Y.; Ji-Chao, Z.; Lei, Z.; Lu, Z.; Sui, Z. Effects of Surfactants on Interfacial Shear Rheological Properties of Polymers for Enhanced Oil Recovery. Acta Phys. Chim. Sin. 2014, 30, 908–916. [Google Scholar] [CrossRef]
- Yan, L.; Cui, Z.; Song, B.; Pei, X.; Jiang, J. Glyceryl Ether Ethoxylates as Surfactants for Surfactant-Polymer Flooding. Energy Fuels 2016, 30, 5425–5431. [Google Scholar] [CrossRef]
- Al-Wahaibi, Y.; Al-Hashmi, A.; Joshi, S.; Mosavat, N.; Rudyk, S.; Al-Khamisi, S.; Al-Kharusi, T.; Al-Sulaimani, H. Mechanistic study of surfactant/polymer adsorption and its effect on surface morphology and wettability. In SPE Oil and Gas India Conference and Exhibition; Society of Petroleum Engineers: Mumbai, India, 2017. [Google Scholar] [CrossRef]
- Darvas, M.; Gilanyi, T.; Jedlovszky, P. Competitive adsorption of surfactants and polymers at the free water surface. a computer simulation study of the sodium dodecyl sulfate- poly(ethylene oxide) system. J. Phys. Chem. B 2011, 115, 933–944. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Influence of the polymer properties and numerical schemes on tertiary oil recovery processes. Comput. Math. Appl. 2020, 79, 1094–1110. [Google Scholar] [CrossRef]
- Curbelo, F.D.S.; Santanna, V.C.; Neto, E.L.B.; Dutra, T.V.; Dantas, T.N.C.; Neto, A.A.D.; Garnica, A.I.C. Adsorption of nonionic surfactants in sandstones. Colloids Surfaces -Physicochem. Eng. Asp. 2007, 293, 1–4. [Google Scholar] [CrossRef]
- Thomas, C.; Fleming, P.; Winter, W. A Ternary, 2-Phase, Mathematical-Model of Oil-Recovery with Surfactant Systems. Soc. Pet. Eng. J. 1984, 24, 606–616. [Google Scholar] [CrossRef]
- Nambam, J.S.; Philip, J. Competitive adsorption of polymer and surfactant at a liquid droplet interface and its effect on flocculation of emulsion. J. Colloid Interface Sci. 2012, 366, 88–95. [Google Scholar] [CrossRef]
- Postmus, B.R. Polymer vs. Surfactant—Competitive Adsorption at the Solid-Liquid Interface. Ph.D. Thesis, University of Wageningen, Wageningen, The Netherlands, 2008. [Google Scholar]
- Wang, J.; Han, M.; Fuseni, A.B.; Cao, D. Surfactant adsorption in surfactant-polymer flooding for carbonate reservoirs. In SPE Middle East Oil & Gas Show and Conference; Society of Petroleum Engineers: Manama, Bahrain, 2015. [Google Scholar] [CrossRef]

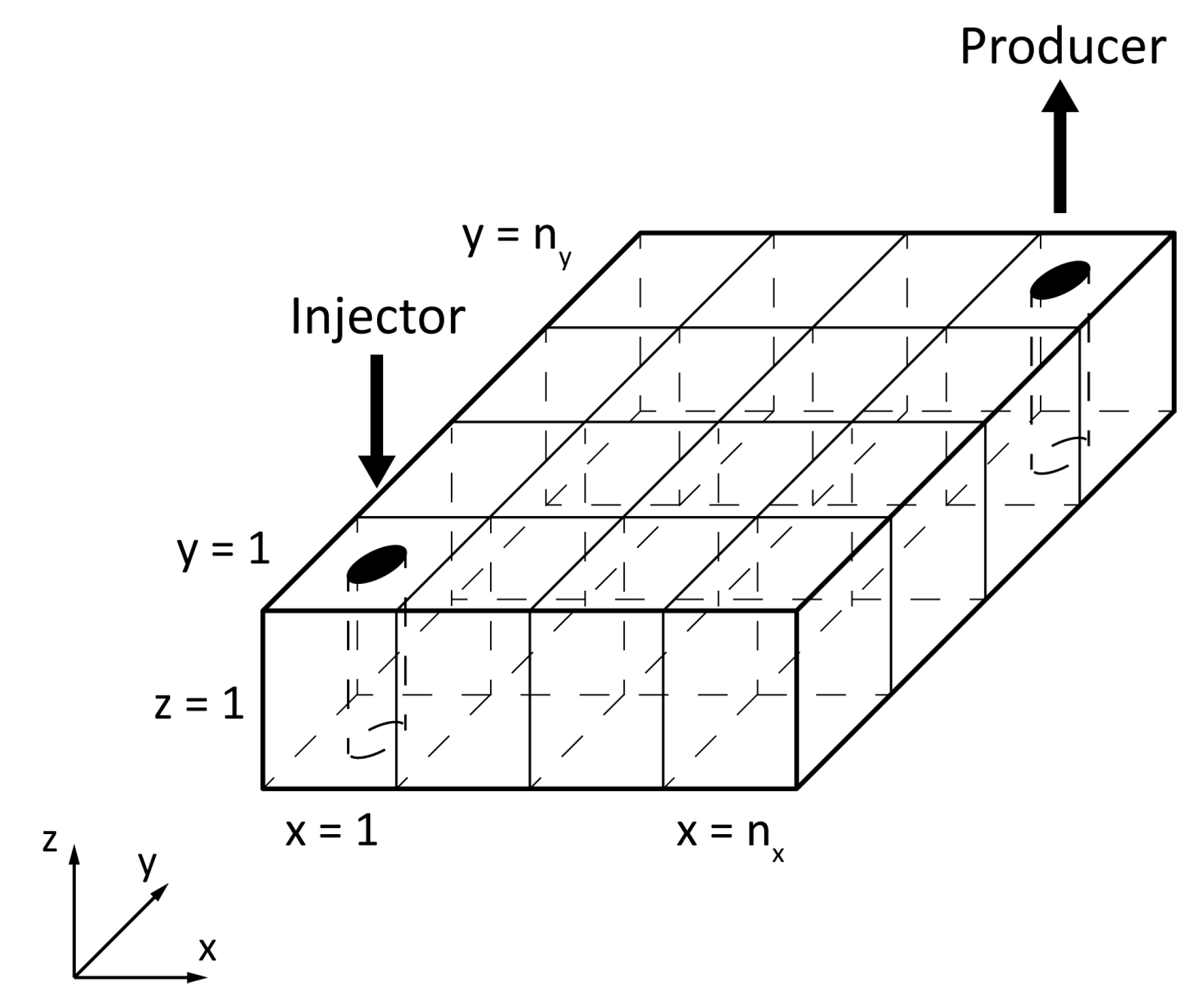










| Geometrical Data of the Reservoir | |||||
| Length in axis X | 500 m | Length in axis Y | 500 m | Layer thickness | 5 m |
| 25 blocks | 25 blocks | ||||
| Rock Properties | |||||
| Porosity | 0.25 | 200 mD | 200 mD | ||
| Initial Conditions | |||||
| 0.70 | (EOR) | 0.35 | 0.15 | ||
| Simulation Data | |||||
| Total time | 3000 days | Surf. inj. time | 100 days | 0.1 | |
| Pol. inj. time | 100 days | 0.025 | |||
| Physical Data of the Phases | |||||
| 1 cP | 10 cP | Oil density | 850 kg/m | ||
| Water density | 1020 kg/m | IFT | 50 mN/m | ||
| Physical Information | |||||
| Number of wells | 2 | Well radius | 0.25 m | Skin factor | 0 |
| Operating Conditions | |||||
| Total flowrate | 222.58 m/day | Bottomhole pressure | 55,160 kPa | ||
| Case | Oil Recovered | Case | Oil Recovered | ||
|---|---|---|---|---|---|
| m | %OOIP | m | %OOIP | ||
| Reference Polymer | 81,540 | 43.5 | Polymer + Surfactant (separated) | 87,530 | 46.7 |
| Reference Surfactant | 75,450 | 40.2 | Surfactant + Polymer (overlapped) | 90,036 | 48.0 |
| Polymer + Surfactant (overlapped) | 90,928 | 48.5 | Surfactant + Polymer (separated) | 87,740 | 46.9 |
| Competitive Adsorption Factor | Oil Recovered | Competitive Adsorption Factor | Oil Recovered | ||
|---|---|---|---|---|---|
| m | %OOIP | m | %OOIP | ||
| 0.1 | 86,750 | 46.3 | 2 | 87,767 | 46.8 |
| 1 | 86,930 | 46.4 | 5 | 89,516 | 47.7 |
| 1.5 | 87,530 | 46.7 | 10 | 91,594 | 48.9 |
| Case | Oil Recovered | Case | Oil Recovered | ||
|---|---|---|---|---|---|
| m | %OOIP | m | %OOIP | ||
| Reference Polymer | 80,300 | 42.8 | Polymer + Surfactant (separated) | 84,010 | 44.8 |
| Reference Surfactant | 74,860 | 39.9 | Surfactant + Polymer (overlapped) | 84,860 | 45.3 |
| Polymer + Surfactant (overlapped) | 85,780 | 45.8 | Surfactant + Polymer (separated) | 84,350 | 45.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druetta, P.; Picchioni, F. Surfactant-Polymer Interactions in a Combined Enhanced Oil Recovery Flooding. Energies 2020, 13, 6520. https://doi.org/10.3390/en13246520
Druetta P, Picchioni F. Surfactant-Polymer Interactions in a Combined Enhanced Oil Recovery Flooding. Energies. 2020; 13(24):6520. https://doi.org/10.3390/en13246520
Chicago/Turabian StyleDruetta, Pablo, and Francesco Picchioni. 2020. "Surfactant-Polymer Interactions in a Combined Enhanced Oil Recovery Flooding" Energies 13, no. 24: 6520. https://doi.org/10.3390/en13246520
APA StyleDruetta, P., & Picchioni, F. (2020). Surfactant-Polymer Interactions in a Combined Enhanced Oil Recovery Flooding. Energies, 13(24), 6520. https://doi.org/10.3390/en13246520