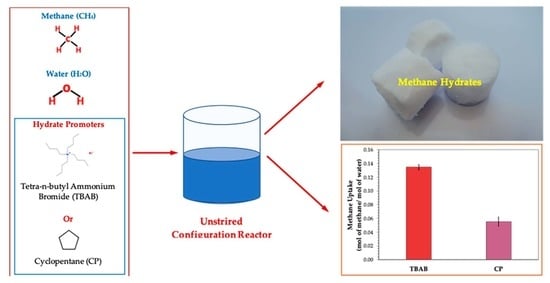
Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
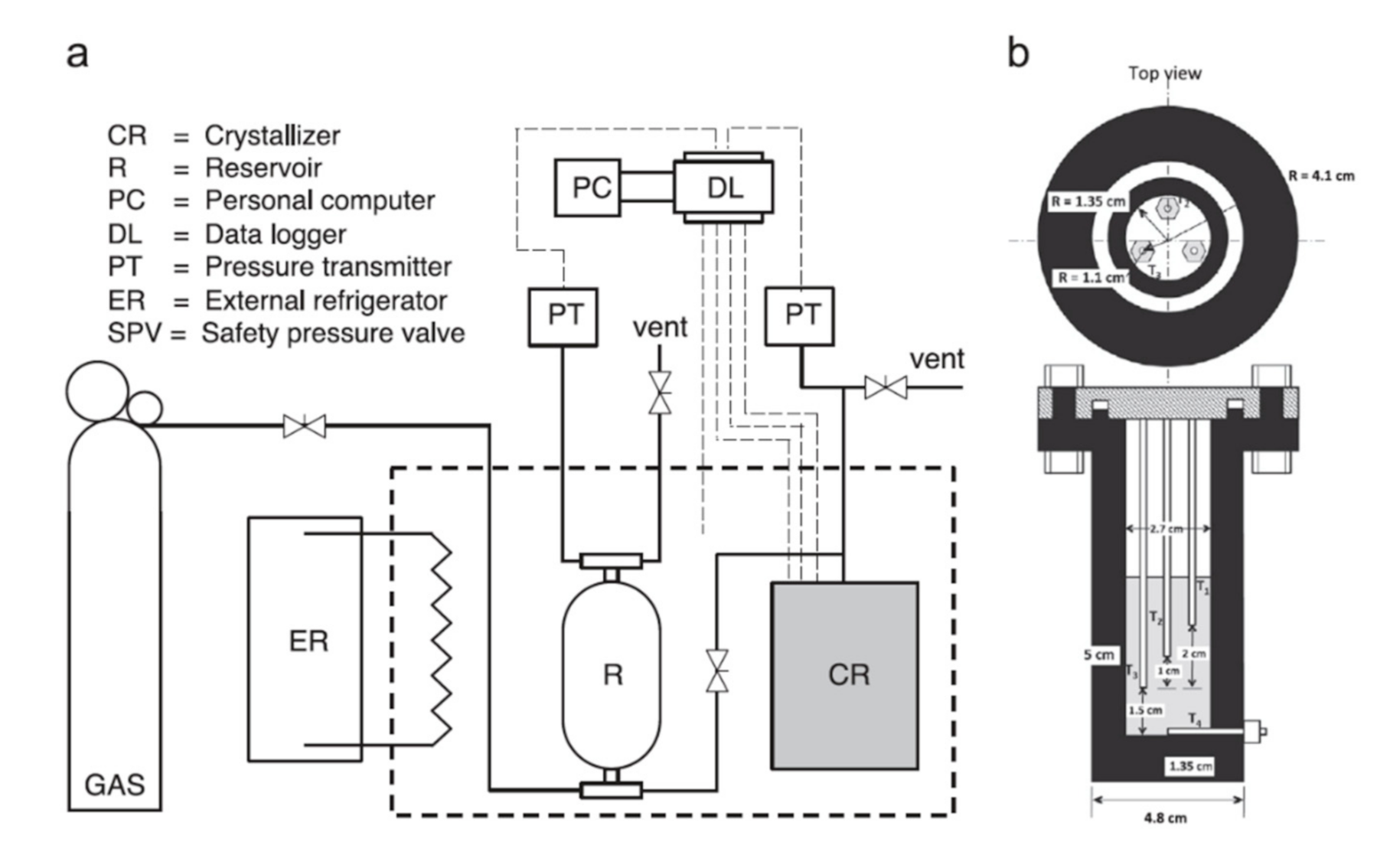
2.2. Apparatus for the Hydrate Formation and Dissociation Experiments
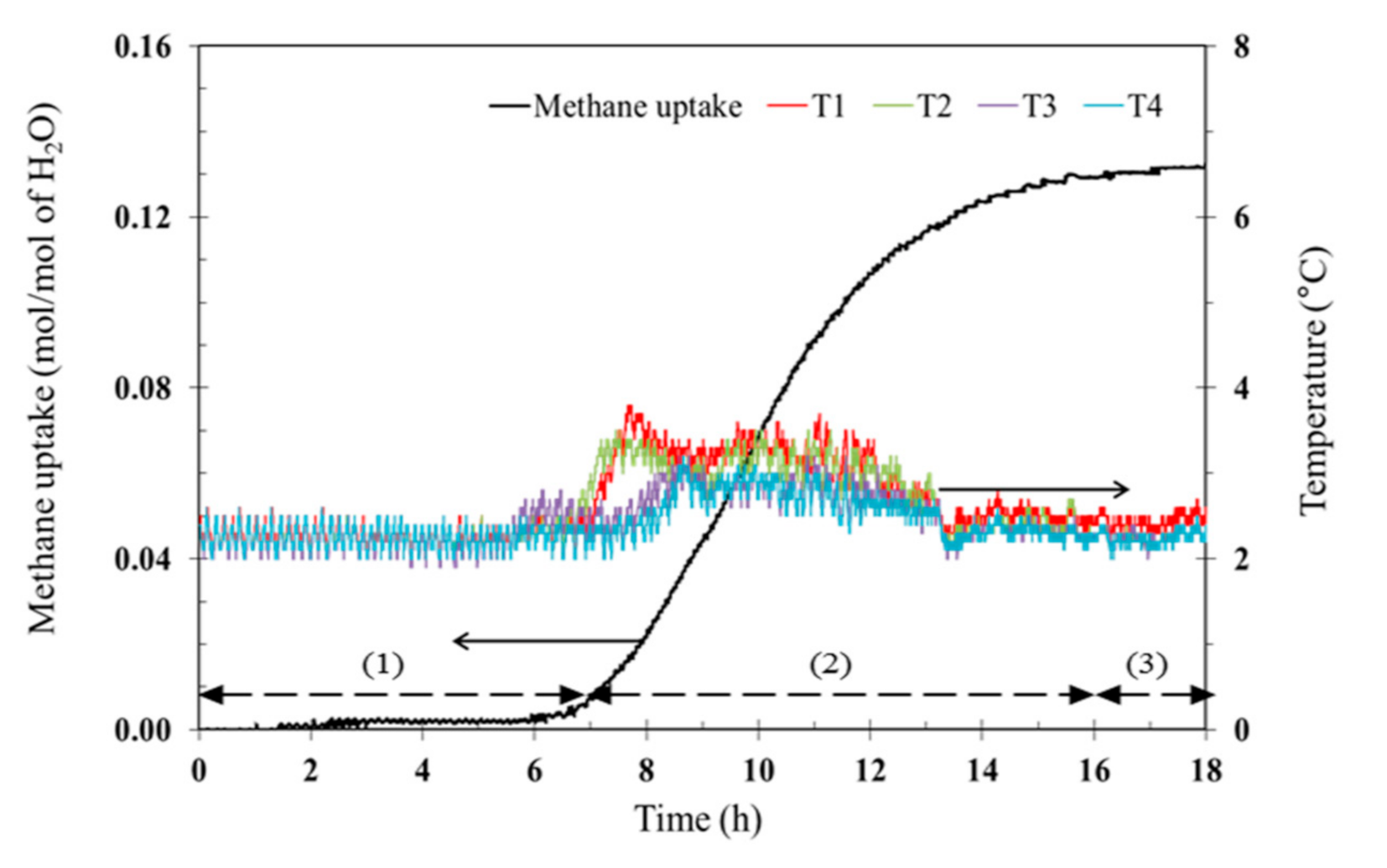
2.3. Hydrate Formation
2.4. Hydrate Dissociation
3. Results and Discussion
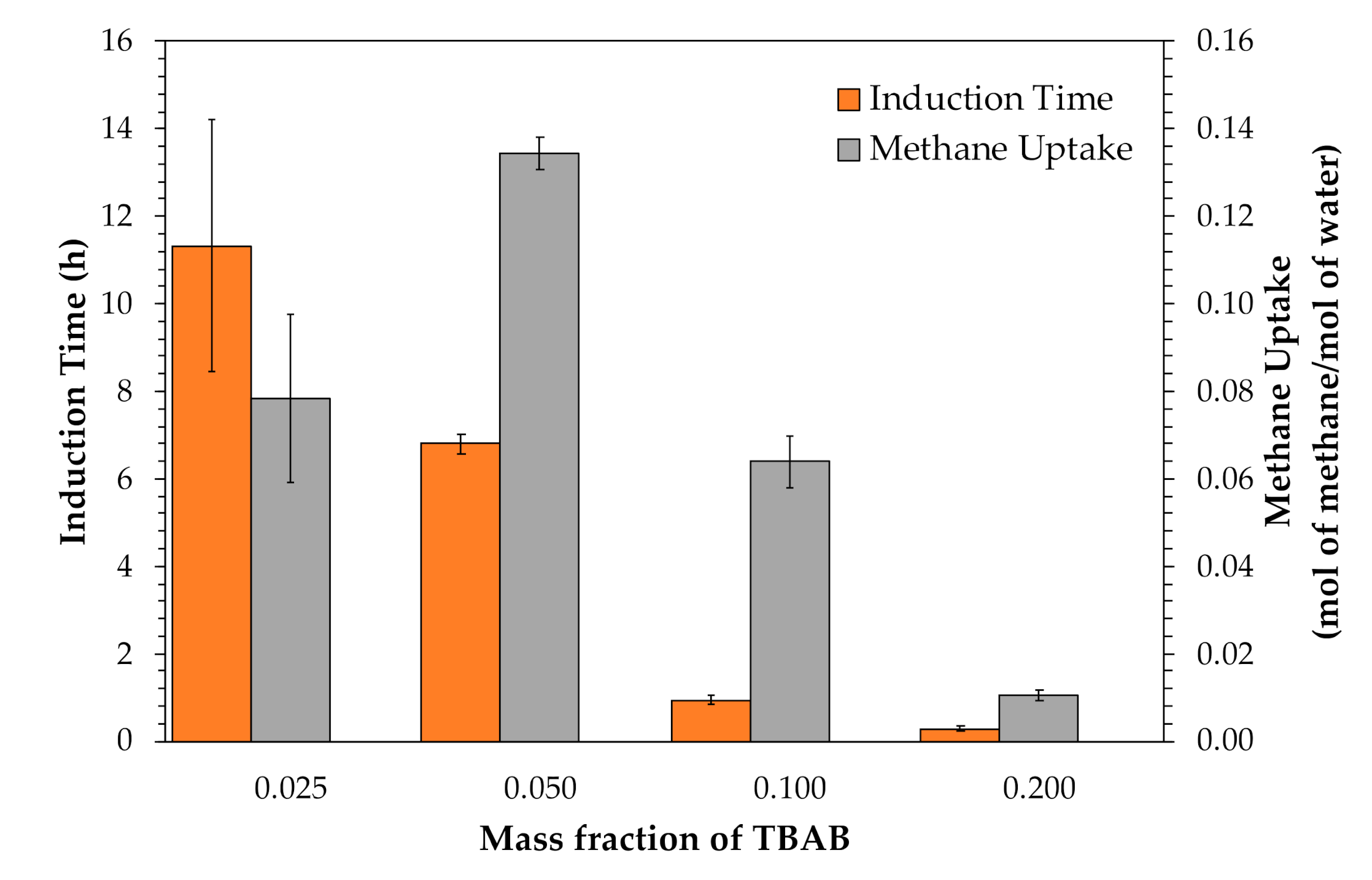
3.1. Effects of Tetra-N-Butyl Ammonium Bromide (TBAB)
3.2. Effects of Cyclopentane (CP)
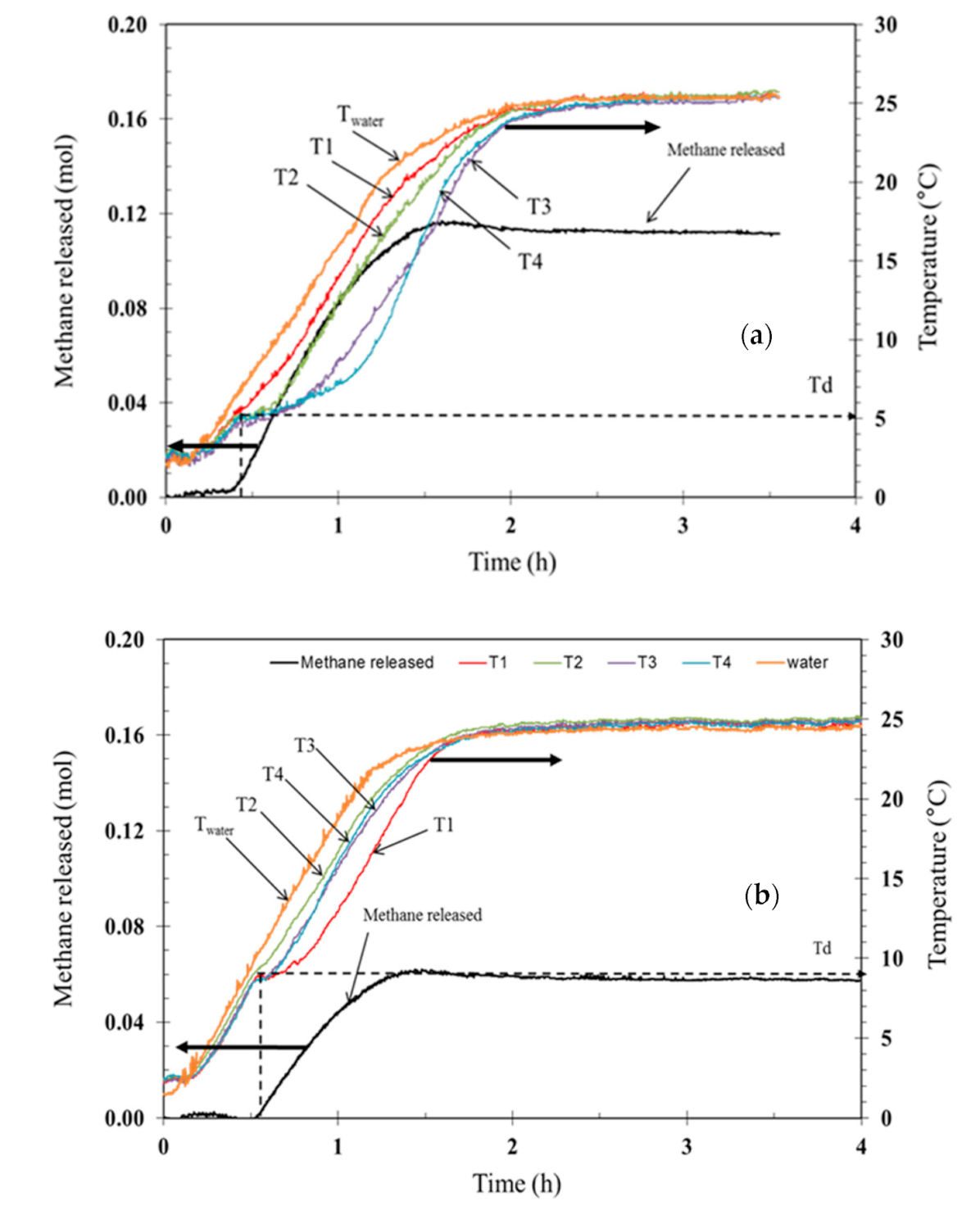
3.3. Hydrate Dissociation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IGU. Global Gas Report 2018; International Gas Union: Washington, DC, USA, 2018; p. 60. [Google Scholar]
- EIA. International Energy Outlook 2019 with Projections to 2050; U.S. Energy Information Administration, U.S. Department of Energy: Washington, DC, USA, 2019; p. 170.
- Demirbas, A. Methane hydrates as potential energy resource: Part 1–Importance, resource and recovery facilities. Energy Convers. Manag. 2010, 51, 1547–1561. [Google Scholar] [CrossRef]
- Englezos, P.; Lee, J.D. Gas hydrates: A cleaner source of energy and opportunity for innovative technologies. Korean J. Chem. Eng. 2005, 22, 671–681. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates–A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Zarenezhad, B.; Varaminian, F. A unified approach for description of gas hydrate formation kinetics in the presence of kinetic promoters in gas hydrate converters. Energy Convers. Manag. 2013, 73, 144–149. [Google Scholar] [CrossRef]
- Mech, D.; Gupta, P.; Sangwai, J.S. Kinetics of methane hydrate formation in an aqueous solution of thermodynamic promoters (THF and TBAB) with and without kinetic promoter (SDS). J. Nat. Gas Sci. Eng. 2016, 35, 1519–1534. [Google Scholar] [CrossRef]
- Verrett, J.; Posteraro, D.; Servio, P. Surfactant effects on methane solubility and mole fraction during hydrate growth. Chem. Eng. Sci. 2012, 84, 80–84. [Google Scholar] [CrossRef]
- Hao, W.; Wang, J.; Fan, S.; Hao, W. Evaluation and analysis method for natural gas hydrate storage and transportation processes. Energy Convers. Manag. 2008, 49, 2546–2553. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Kumar, A. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Sangwai, J.S.; Oellrich, L. Phase equilibrium of semiclathrate hydrates of methane in aqueous solutions of tetra-n-butyl ammonium bromide (TBAB) and TBAB–NaCl. Fluid Phase Equilibria 2014, 367, 95–102. [Google Scholar] [CrossRef]
- Li, D.; Du, J.-W.; Fan, S.-S.; Liang, D.-Q.; Li, X.-S.; Huang, N.-S. Clathrate Dissociation Conditions for Methane + Tetra-n-butyl Ammonium Bromide (TBAB) + Water. J. Chem. Eng. Data 2007, 52, 1916–1918. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Eslamimanesh, A.; Belandria, V.; Richon, D. Phase Equilibria of Semiclathrate Hydrates of CO2, N2, CH4, or H2 + Tetra-n-butylammonium Bromide Aqueous Solution. J. Chem. Eng. Data 2011, 56, 3855–3865. [Google Scholar] [CrossRef]
- Li, X.-S.; Xia, Z.-M.; Chen, Z.; Yan, K.-F.; Li, G.; Wu, H.-J. Gas Hydrate Formation Process for Capture of Carbon Dioxide from Fuel Gas Mixture. Ind. Eng. Chem. Res. 2010, 49, 11614–11619. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.-M. CO2 capture by hydrate crystallization–A potential solution for gas emission of steelmaking industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Fan, S.; Li, S.; Wang, J.; Lang, X.; Wang, Y. Efficient Capture of CO2 from Simulated Flue Gas by Formation of TBAB or TBAF Semiclathrate Hydrates. Energy Fuels 2009, 23, 4202–4208. [Google Scholar] [CrossRef]
- Zhong, D.-L.; Englezos, P. Methane Separation from Coal Mine Methane Gas by Tetra-n-butyl Ammonium Bromide Semiclathrate Hydrate Formation. Energy Fuels 2012, 26, 2098–2106. [Google Scholar] [CrossRef]
- Zheng, J.-N.; Yang, M.; Liu, Y.; Wang, D.; Song, Y.-C. Effects of cyclopentane on CO2 hydrate formation and dissociation as a co-guest molecule for desalination. J. Chem. Thermodyn. 2017, 104, 9–15. [Google Scholar] [CrossRef]
- Lim, Y.-A.; Babu, P.; Kumar, R.; Linga, P. Morphology of Carbon Dioxide–Hydrogen–Cyclopentane Hydrates with or without Sodium Dodecyl Sulfate. Cryst. Growth Des. 2013, 13, 2047–2059. [Google Scholar] [CrossRef]
- Li, X.-S.; Cai, J.; Chen, Z.; Xu, C.-G. Hydrate-Based Methane Separation from the Drainage Coal-Bed Methane with Tetrahydrofuran Solution in the Presence of Sodium Dodecyl Sulfate. Energy Fuels 2012, 26, 1144–1151. [Google Scholar] [CrossRef]
- Siangsai, A.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S.; Linga, P. Investigation on the roles of activated carbon particle sizes on methane hydrate formation and dissociation. Chem. Eng. Sci. 2015, 126, 383–389. [Google Scholar] [CrossRef]
- Siangsai, A.; Inkong, K.; Kulprathipanja, S.; Kitiyanan, B.; Rangsunvigit, P. Roles of Sodium Dodecyl Sulfate on Tetrahydrofuran-Assisted Methane Hydrate Formation. J. Oleo Sci. 2018, 67, 707–717. [Google Scholar] [CrossRef]
- Inkong, K.; Rangsunvigit, P.; Kulprathipanja, S.; Linga, P. Effects of temperature and pressure on the methane hydrate formation with the presence of tetrahydrofuran (THF) as a promoter in an unstirred tank reactor. Fuel 2019, 255, 115705. [Google Scholar] [CrossRef]
- Inkong, K.; Veluswamy, H.P.; Rangsunvigit, P.; Kulprathipanja, S.; Linga, P. Investigation on the kinetics of methane hydrate formation in the presence of methyl ester sulfonate. J. Nat. Gas Sci. Eng. 2019, 71, 102999. [Google Scholar] [CrossRef]
- Fandiño, O.; Ruffine, L. Methane hydrate nucleation and growth from the bulk phase: Further insights into their mechanisms. Fuel 2014, 117, 442–449. [Google Scholar] [CrossRef][Green Version]
- Ohmura, R.; Matsuda, S.; Uchida, T.; Ebinuma, T.; Narita, H. Clathrate Hydrate Crystal Growth in Liquid Water Saturated with a Guest Substance: Observations in a Methane + Water System. Cryst. Growth Des. 2005, 5, 953–957. [Google Scholar] [CrossRef]
- Arjmandi, M.; Chapoy, A.A.; Tohidi, B. Equilibrium Data of Hydrogen, Methane, Nitrogen, Carbon Dioxide, and Natural Gas in Semi-Clathrate Hydrates of Tetrabutyl Ammonium Bromide. J. Chem. Eng. Data 2007, 52, 2153–2158. [Google Scholar] [CrossRef]
- Linga, P.; Haligva, C.; Nam, S.C.; Ripmeester, J.A.; Englezos, P. Recovery of Methane from Hydrate Formed in a Variable Volume Bed of Silica Sand Particles. Energy Fuels 2009, 23, 5508–5516. [Google Scholar] [CrossRef]
- Du, J.; Li, H.; Wang, L. Effects of ionic surfactants on methane hydrate formation kinetics in a static system. Adv. Powder Technol. 2014, 25, 1227–1233. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, S.; Lee, J.W. Does SDS micellize under methane hydrate-forming conditions below the normal Krafft point? J. Colloid Interface Sci. 2007, 315, 313–318. [Google Scholar] [CrossRef]
- Ho, L.C.; Babu, P.; Kumar, R.; Linga, P. HBGS (hydrate based gas separation) process for carbon dioxide capture employing an unstirred reactor with cyclopentane. Energy 2013, 63, 252–259. [Google Scholar] [CrossRef]
- Lv, Q.; Li, L.; Li, X.-S.; Chen, Z. Formation Kinetics of Cyclopentane + Methane Hydrates in Brine Water Systems and Raman Spectroscopic Analysis. Energy Fuels 2015, 29, 6104–6110. [Google Scholar] [CrossRef]
- Yaws, C.L.; Richmond, P.C. Chapter 21-Surface tension—Organic compounds. In Thermophysical Properties of Chemicals and Hydrocarbons; Yaws, C.L., Ed.; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 686–781. [Google Scholar]
- Veluswamy, H.P.; Chen, J.Y.; Linga, P. Surfactant effect on the kinetics of mixed hydrogen/propane hydrate formation for hydrogen storage as clathrates. Chem. Eng. Sci. 2015, 126, 488–499. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Chin, W.I.; Linga, P. Clathrate hydrates for hydrogen storage: The impact of tetrahydrofuran, tetra-n-butylammonium bromide and cyclopentane as promoters on the macroscopic kinetics. Int. J. Hydrogen Energy 2014, 39, 16234–16243. [Google Scholar] [CrossRef]
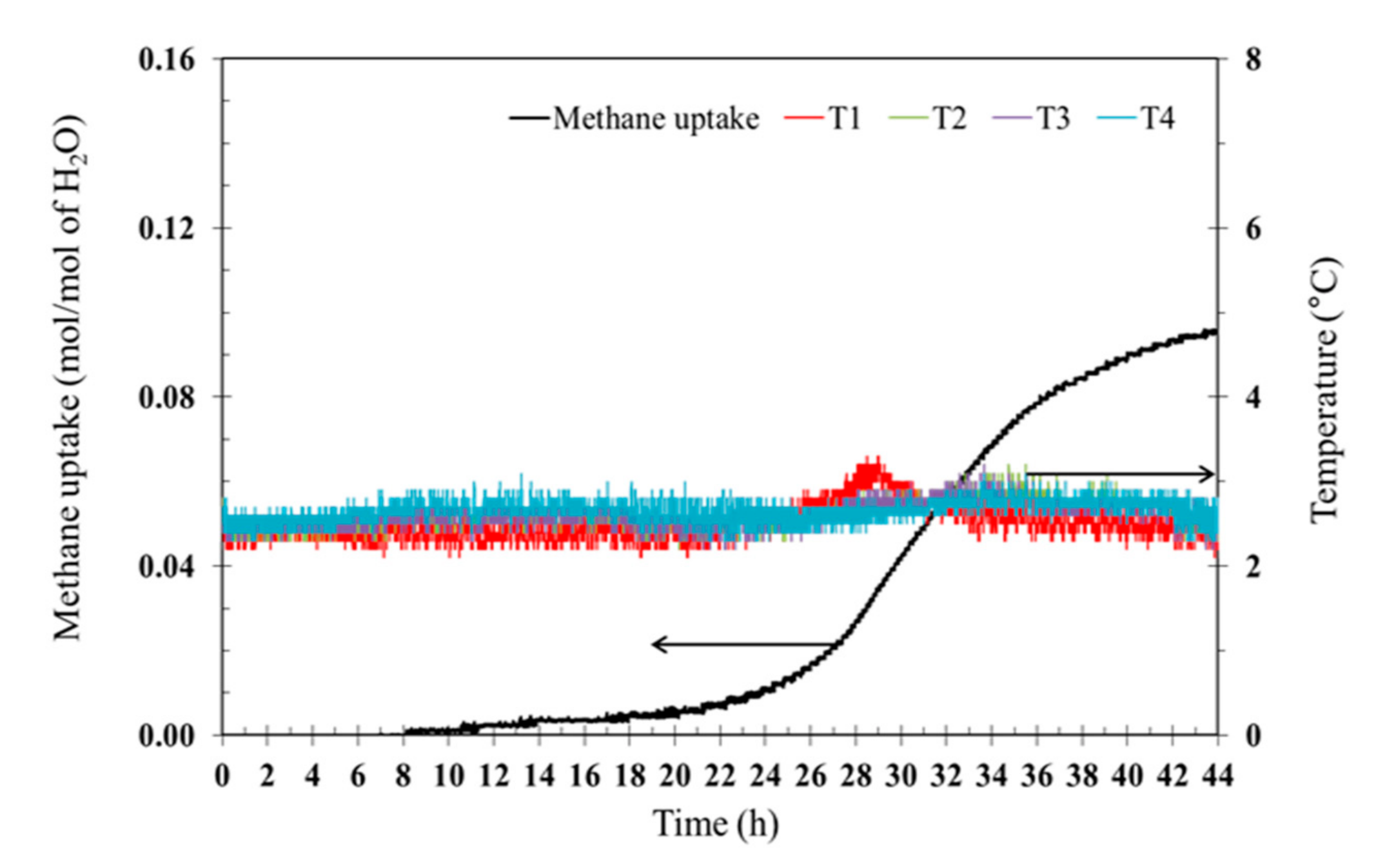
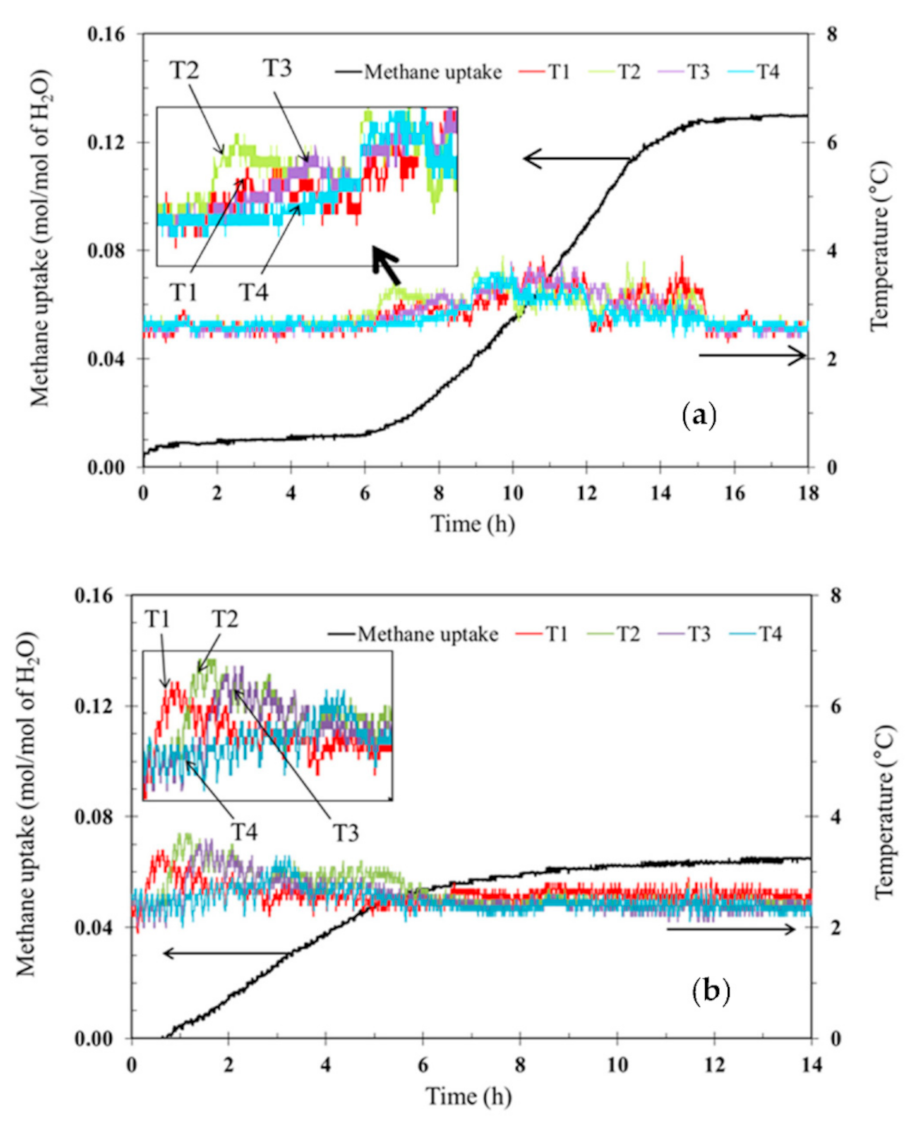
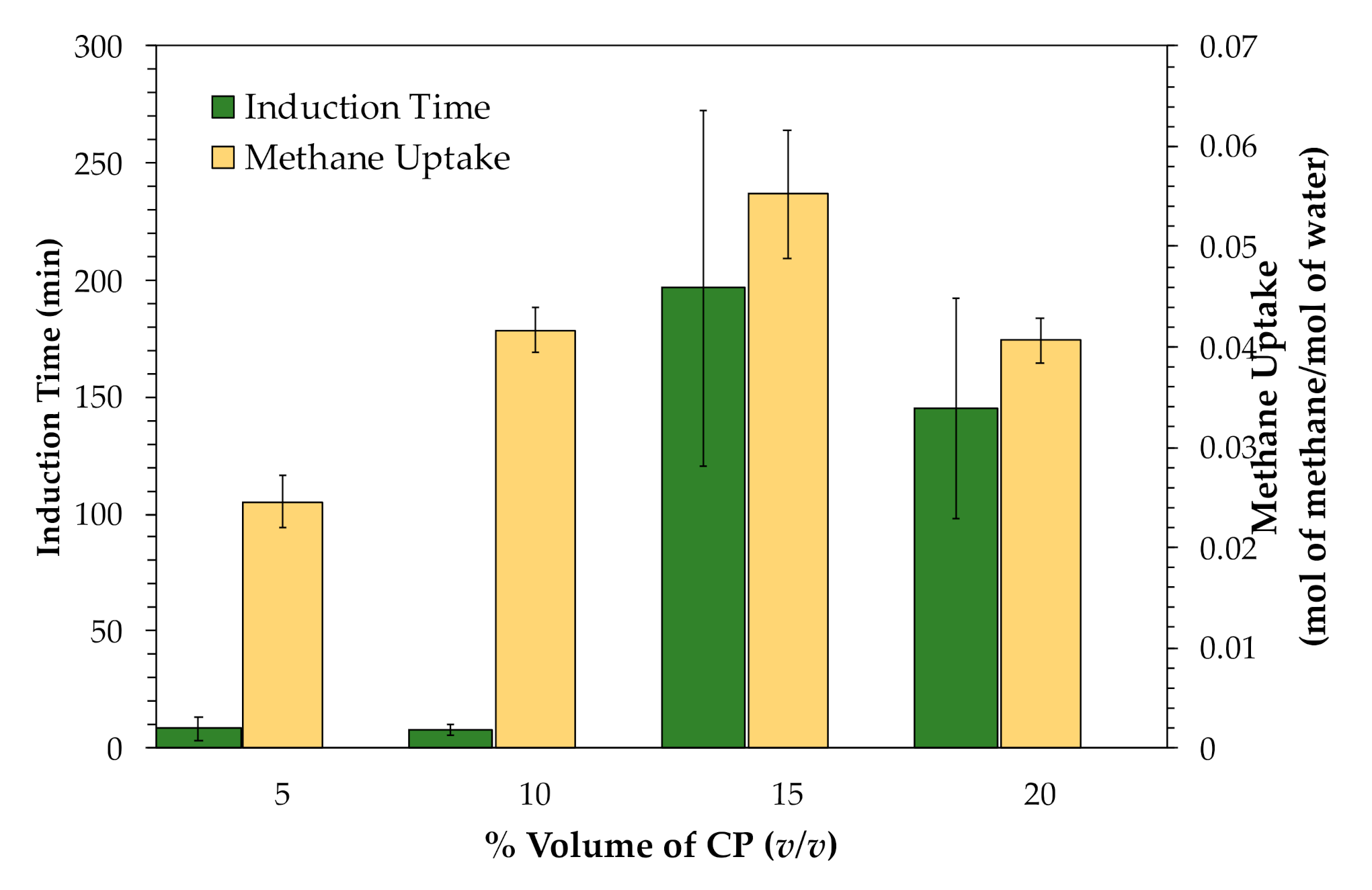
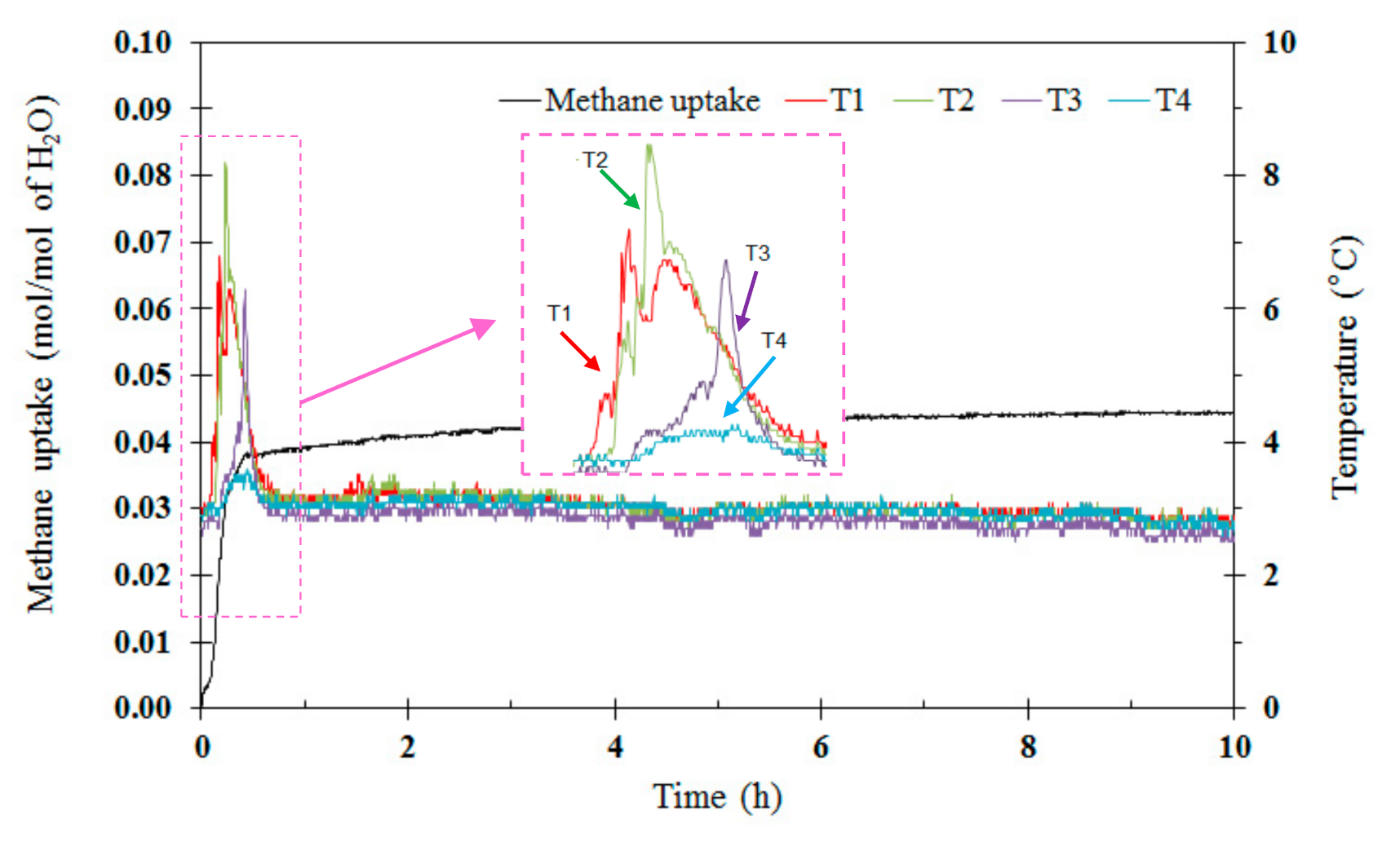

| Exp. No. | TBAB(Mass Fraction) | Induction Time *(h) | Methane Uptake(mol of Methane/mol of H2O) | %Recovery |
|---|---|---|---|---|
| 1 | 11 | 0.0514 | 90.69 | |
| 2 | w = 0.025 | 15 | 0.0956 | 92.89 |
| 3 | 8 | 0.0882 | 91.28 | |
| Avg | 11.33 ± 2.87 | 0.0784 ± 0.0193 | 91.62 ± 0.93 | |
| 4 | 6.5 | 0.1298 | 90.17 | |
| 5 | w = 0.05 | 7 | 0.1352 | 88.25 |
| 6 | 6.9 | 0.1386 | 90.53 | |
| Avg | 6.80 ± 0.22 | 0.1345 ± 0.0036 | 89.65 ± 1.00 | |
| 7 | 0.8 | 0.0559 | 89.38 | |
| 8 | w = 0.10 | 1 | 0.0702 | 94.39 |
| 9 | 1 | 0.0658 | 94.55 | |
| Avg | 0.93 ± 0.09 | 0.0639 ± 0.0060 | 92.77 ± 2.40 | |
| 10 | 0.2 | 0.0095 | 90.67 | |
| 11 | w = 0.20 | 0.3 | 0.0120 | 88.95 |
| 12 | 0.36 | 0.0097 | 90.93 | |
| Avg | 0.29 ± 0.06 | 0.0104 ± 0.0011 | 90.18 ± 0.88 | |
| Exp. No. | CP (%v/v) | Induction Time * (min) | Methane Uptake (mol of Methane/mol of H2O) | %Recovery |
|---|---|---|---|---|
| 13 | 5.00 | 0.0216 | 80.59 | |
| 14 | 5 | 15.00 | 0.0242 | 82.67 |
| 15 | 4.80 | 0.0279 | 81.98 | |
| Avg | 8.27 ± 4.76 | 0.0246 ± 0.0025 | 89.65 ± 1.00 | |
| 16 | 4.80 | 0.0446 | 80.35 | |
| 17 | 10 | 9.60 | 0.0413 | 78.58 |
| 18 | 9.00 | 0.0392 | 80.90 | |
| Avg | 7.13 ± 2.13 | 0.0417 ± 0.0022 | 79.94 ± 0.99 | |
| 19 | 170.00 | 0.0628 | 79.61 | |
| 20 | 15 | 300.00 | 0.0472 | 81.41 |
| 21 | 120.00 | 0.0557 | 80.45 | |
| Avg | 196.68 ± 75.86 | 0.0552 ± 0.0063 | 80.49 ± 0.73 | |
| 22 | 126.00 | 0.0438 | 80.67 | |
| 23 | 20 | 100.02 | 0.0395 | 78.75 |
| 24 | 210.00 | 0.0386 | 80.93 | |
| Avg | 145.34 ± 46.93 | 0.0406 ± 0.0023 | 80.12 ± 0.97 | |
| Exp. No. | Experimental Temperature (°C) | Induction Time (min) | Methane Uptake (mol of Methane/mol of H2O) |
|---|---|---|---|
| 16 | 170.00 | 0.0628 | |
| 17 | 2.5 | 300.00 | 0.0472 |
| 18 | 120.00 | 0.0557 | |
| Avg. | 196.67 ± 75.86 | 0.0417 ± 0.0027 | |
| 25 | 110.20 | 0.0367 | |
| 26 | 4 | 109.00 | 0.0336 |
| 27 | 110.80 | 0.0344 | |
| Avg. | 110.00 ± 0.92 | 0.0349 ± 0.0016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanakro, W.; Jaikwang, C.; Inkong, K.; Kulprathipanja, S.; Rangsunvigit, P. Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies 2020, 13, 6518. https://doi.org/10.3390/en13246518
Chanakro W, Jaikwang C, Inkong K, Kulprathipanja S, Rangsunvigit P. Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies. 2020; 13(24):6518. https://doi.org/10.3390/en13246518
Chicago/Turabian StyleChanakro, Warintip, Chutikan Jaikwang, Katipot Inkong, Santi Kulprathipanja, and Pramoch Rangsunvigit. 2020. "Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation" Energies 13, no. 24: 6518. https://doi.org/10.3390/en13246518
APA StyleChanakro, W., Jaikwang, C., Inkong, K., Kulprathipanja, S., & Rangsunvigit, P. (2020). Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies, 13(24), 6518. https://doi.org/10.3390/en13246518