Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds
2.1.1. Phytochemical Composition of Breynia Fruits, Leaves and Bark Extracts by NMR
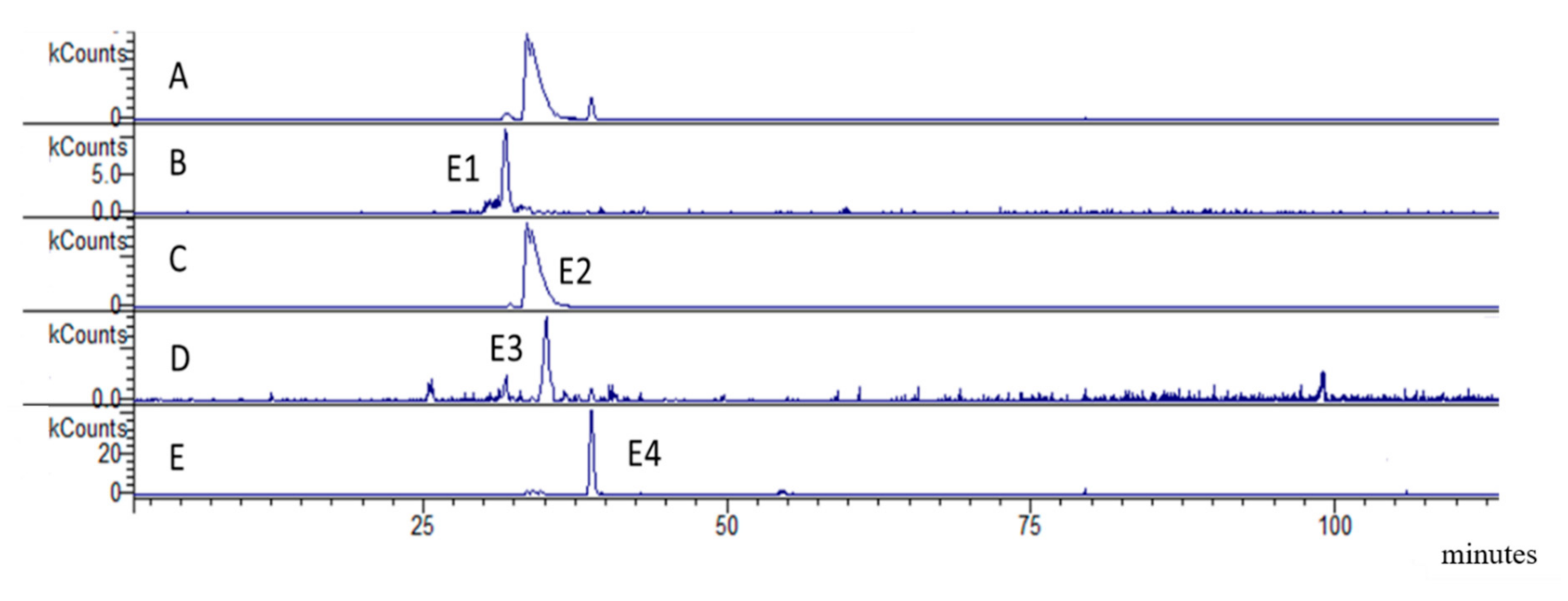
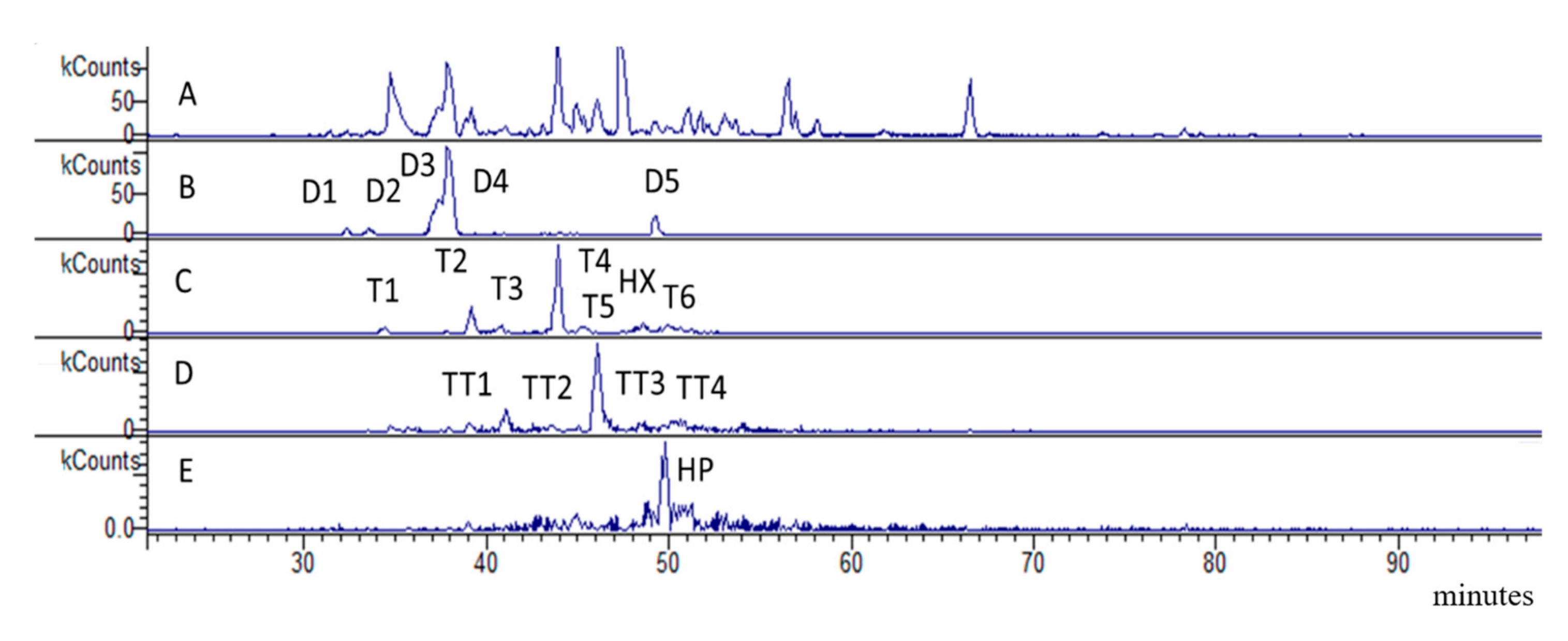
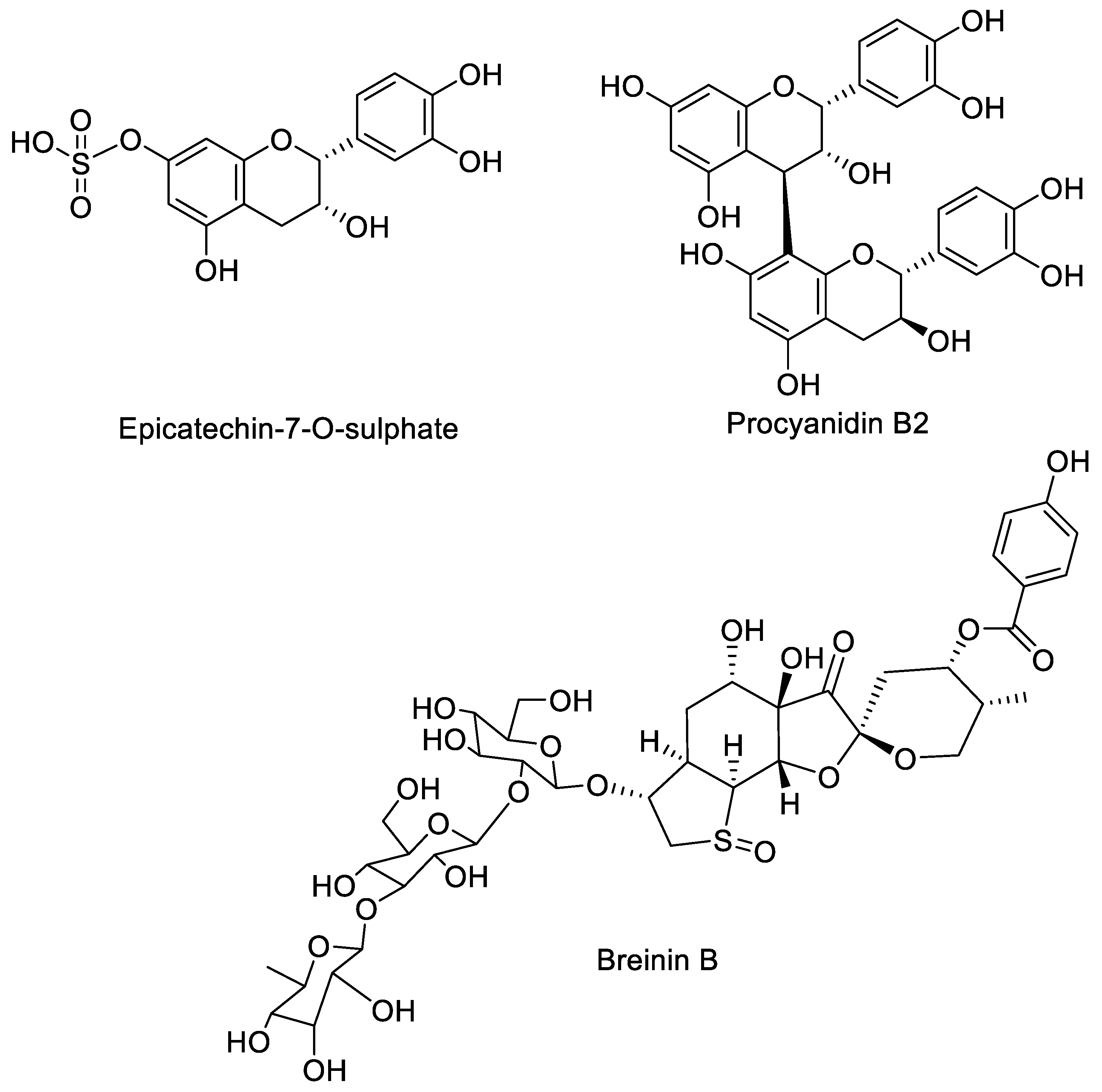
2.1.2. LC-DAD-MSn of Bark Extract
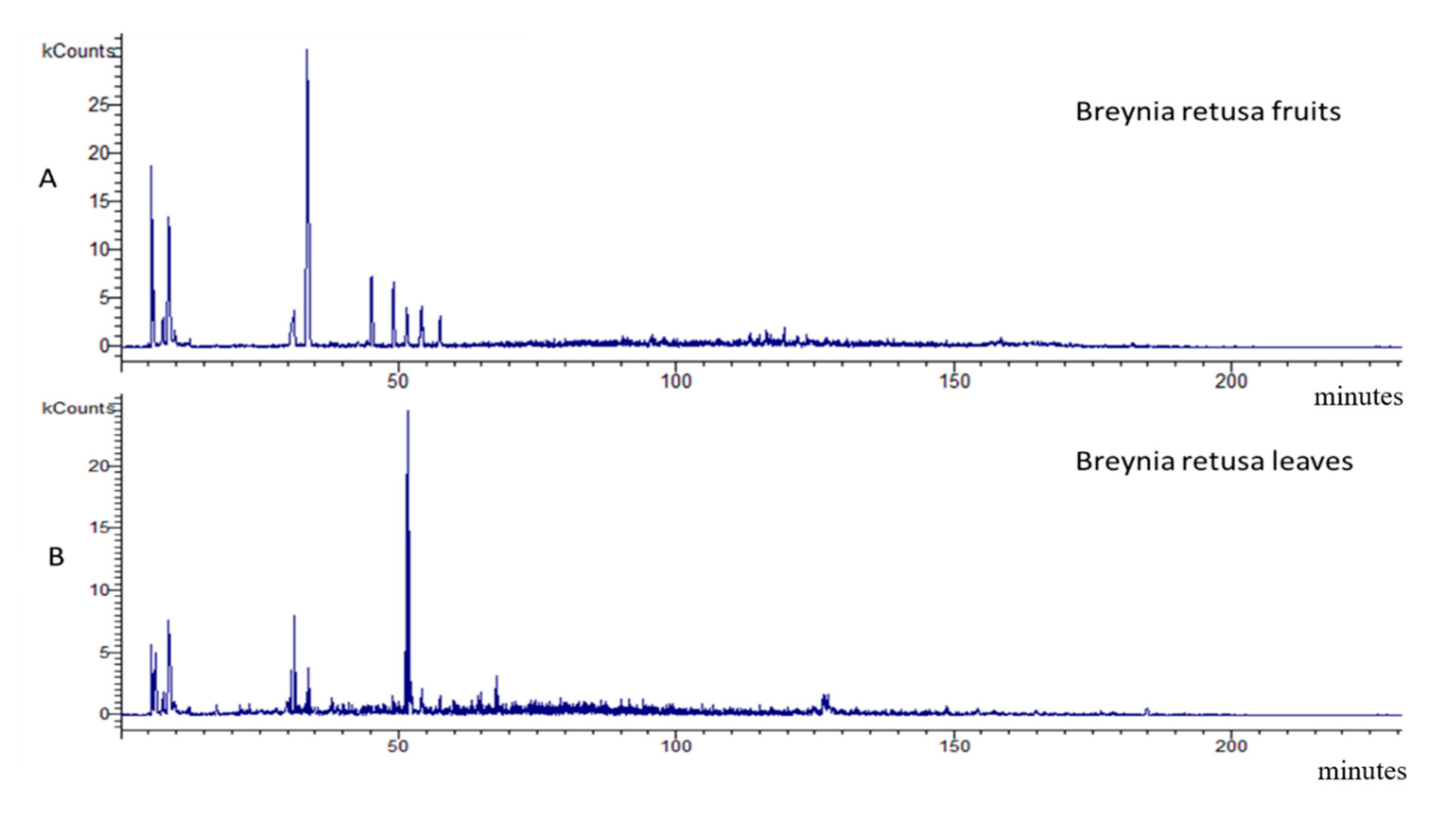
2.1.3. Breynia Fruits and Leaves
2.2. Antioxidant Activities
2.3. Enzyme Inhibitory Effects
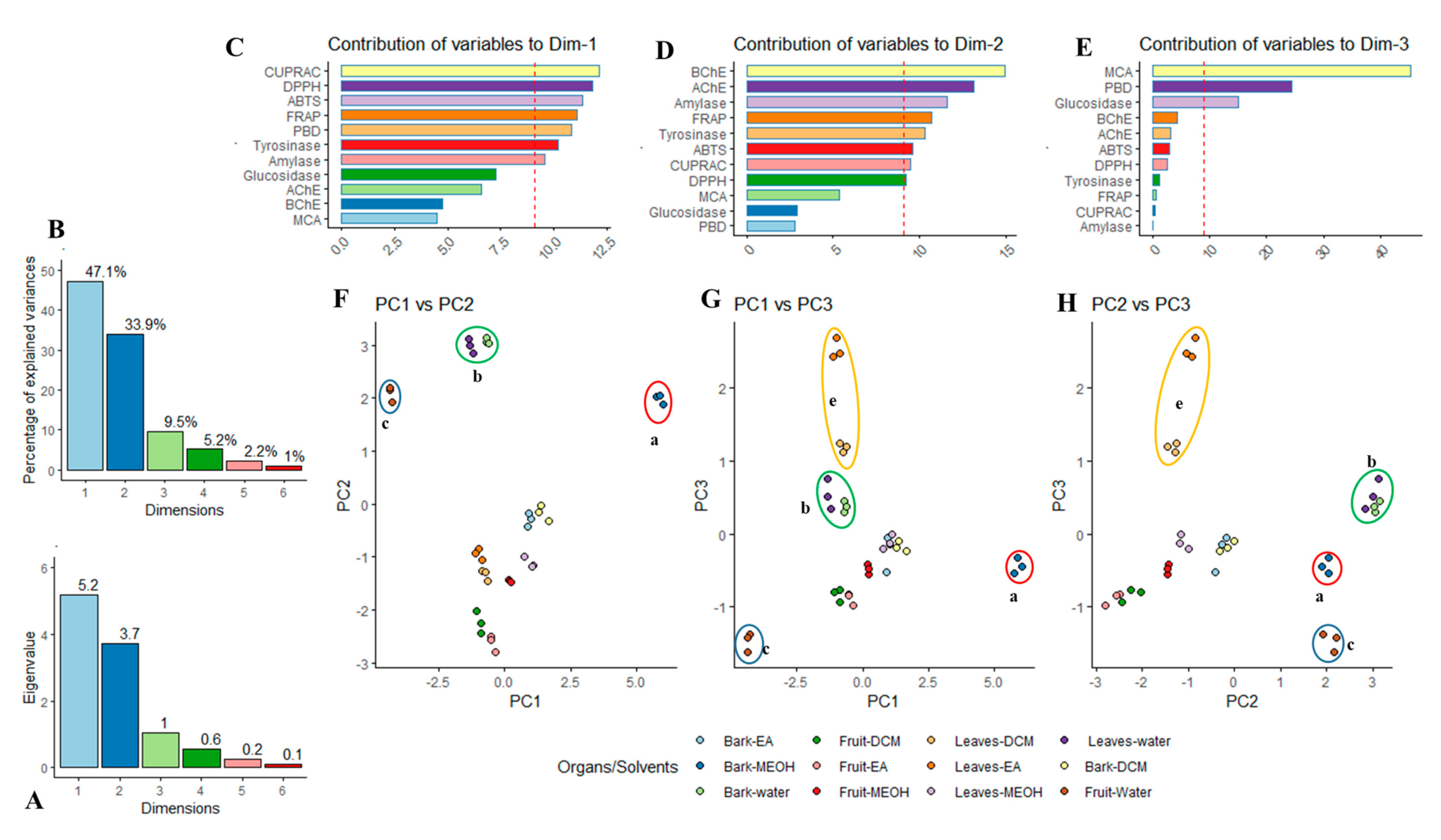
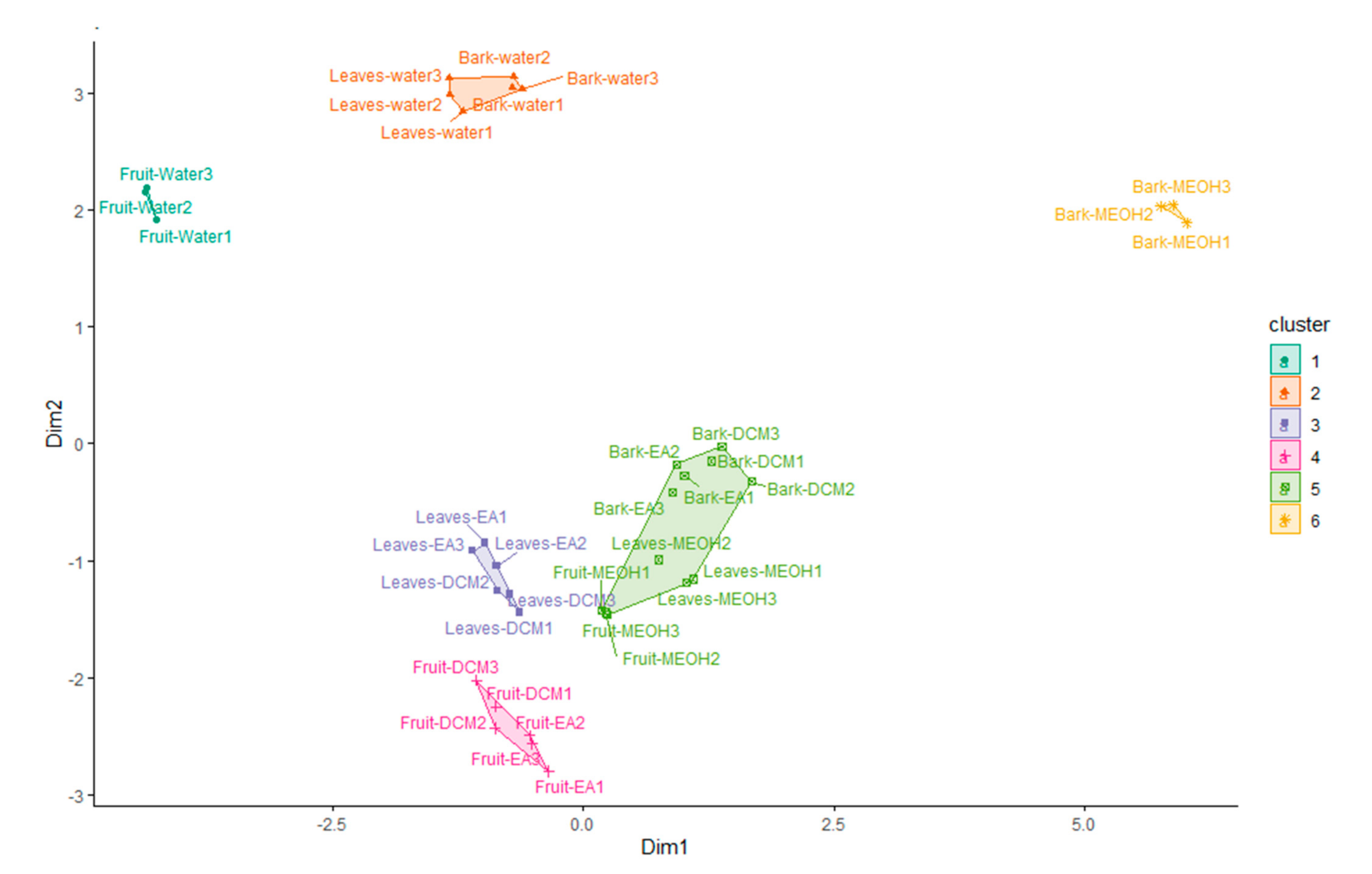
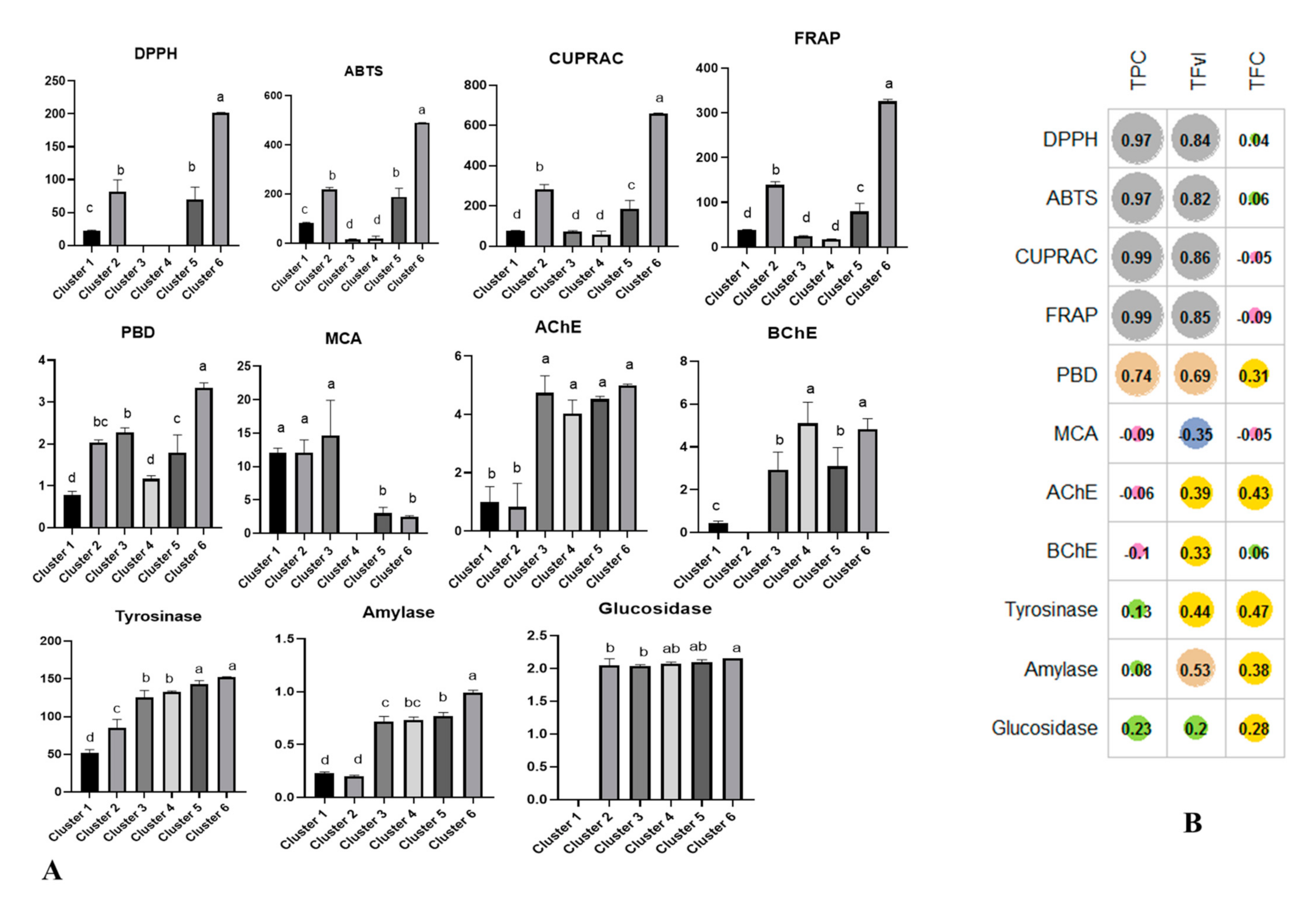
2.4. Multiple Statistical Data Mining
3. Materials and Methods
3.1. Plant Material and Preparation of Extracts
3.2. Profile of Bioactive Compounds
HPLC-DAD-MSn Analysis, Isolation of Epicatechin-7-O-Sulphate
3.3. Determination of Antioxidant and Enzyme Inhibitory Effects
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vickers, A.; Zollman, C.; Lee, R. Herbal medicine. West. J. Med. 2001, 175, 125. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Rammohan, A.; Bhaskar, B.V.; Camilo, A.; Gunasekar, D.; Gu, W.; Zyryanov, G.V. In silico, in vitro antioxidant and density functional theory based structure activity relationship studies of plant polyphenolics as prominent natural antioxidants. Arab. J. Chem. 2020, 13, 3690–3701. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, A.; Mantzioris, E.; Cross, G.; Deo, P.; Garg, S.; Hill, A.M. Nutraceuticals: Reviewing their Role in Chronic Disease Prevention and Management. Pharm. Med. 2019, 33, 291–309. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; DallAcqua, S. Nutraceuticals, a new challenge for medicinal chemistry. Curr. Med. Chem. 2016, 23, 3198–3223. [Google Scholar] [CrossRef]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 July 2020).
- Korczyn, A.D. Why have we failed to cure Alzheimer’s disease? J. Alzheimers Dis. 2012, 29, 275–282. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzyme Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes#:~:text=Diabetes%20is%20a%20major%20cause,high%20blood%20glucose%20in%202012 (accessed on 10 May 2020).
- Hostalek, U.; Gwilt, M.; Hildemann, S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs 2015, 75, 1071–1094. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Gupta, R. Alpha-amylase inhibition can treat diabetes mellitus. Res. Rev. J. Med. Health Sci. 2016, 5, 1–8. [Google Scholar]
- Orhan, N.; Gökbulut, A.; Orhan, D.D. Antioxidant potential and carbohydrate digestive enzyme inhibitory effects of five Inula species and their major compounds. S. Afr. J. Bot. 2017, 111, 86–92. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Bhagyasri, Y.; Subramanian, N.S.; Karthikeyan, R.; Jagadesh, K. A pharmacological review on Breynia retusa. J. Pharmacogn. Phytochem. 2017, 6, 511–513. [Google Scholar]
- Murugan, R.; Prabu, J.; Chandran, R.; Sajeesh, T.; Iniyavan, M.; Parimelazhagan, T. Nutritional composition, in vitro antioxidant and anti-diabetic potentials of Breynia retusa (Dennst.) Alston. Food Sci. Hum. Wellness 2016, 5, 30–38. [Google Scholar] [CrossRef]
- Nithiya, S.; Sangeetha, R. Amylase inhibitory potential of silver nanoparticles biosynthesized using Breynia retusa leaf extract. World J. Pharm. Res 2014, 3, 1055–1066. [Google Scholar]
- Koshiyama, H.; Hatori, M.; Ohkuma, H.; Sakai, F.; Imanishi, H.; Ohbayashi, M.; Kawaguchi, H. Breynins, new sulfur-containing glycosides with hypocholesterolemic activity. Chem. Pharm. Bull. 1976, 24, 169–172. [Google Scholar] [CrossRef]
- Ohkuma, H.; Tsuno, T.; Konishi, M.; Naito, T.; Kawaguchi, H. Complete structures of breynins A and B. Chem. Pharm. Bull. 1991, 39, 942–944. [Google Scholar] [CrossRef][Green Version]
- Smith, A.B., III; Gallagher, R.T.; Keenan, T.P.; Furst, G.T.; Dormer, P.G. Structures of breynins A and B, architecturally complex, hypocholesterolemic spiroketal glycosides. A revised formulation of breynin B. Tetrahedron Lett. 1991, 32, 6847–6850. [Google Scholar] [CrossRef]
- Smith, A.B., III; Keenan, T.P.; Gallagher, R.T.; Furst, G.T.; Dormer, P.G. Structures of breynins A and B, architecturally complex orally active, hypocholesterolemic spiroketal glycosides. J. Org. Chem. 1992, 57, 5115–5120. [Google Scholar] [CrossRef]
- Meng, D.; Chen, W.; Zhao, W. Sulfur-containing spiroketal glycosides from Breynia fruticosa. J. Nat. Prod. 2007, 70, 824–829. [Google Scholar] [CrossRef] [PubMed]
- He, X.-L.; Lv, J.-J.; Wang, X.; Zhang, Q.; Zhang, B.; Cao, K.; Liu, L.-L.; Xu, Y. The identification and isolation of anti-inflammatory ingredients of ethno medicine Breynia fruticosa. J. Ethnopharmacol. 2019, 239, 111894. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Gololo, S. Potential Adverse Effects of Alteration of Phytochemical Accumulation in Fruits and Vegetables. Phytochem. Source Antioxid. Role Dis. Prev. 2018. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef]
- Zhang, M.; Jagdmann, G.E., Jr.; Van Zandt, M.; Sheeler, R.; Beckett, P.; Schroeter, H. Chemical synthesis and characterization of epicatechin glucuronides and sulfates: Bioanalytical standards for epicatechin metabolite identification. J. Nat. Prod. 2013, 76, 157–169. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.R.; Fawzi Mahomoodally, M. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Lazarova, I.; Zengin, G.; Sinan, K.I.; Aneva, I.; Uysal, S.; Picot-Allain, M.C.N.; Aktumsek, A.; Bouyahya, A.; Mahomoodally, M.F. Metabolomics profiling and biological properties of root extracts from two Asphodelus species: A. albus and A. aestivus. Food Res. Int. 2020, 134, 109277. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Zengin, G.; Sinan, K.I.; Polat, R.; Canlı, D.; Picot-Allain, M.C.N.; Mahomoodally, M.F. Impact of different extraction solvents and techniques on the biological activities of Cirsium yildizianum (Asteraceae: Cynareae). Ind. Crops Prod. 2020, 144, 112033. [Google Scholar] [CrossRef]
- Sajeesh, T.; Arunachalam, K.; Parimelazhagan, T. Antioxidant and antipyretic studies on Pothos scandens L. Asian Pac. J. Trop. Med. 2011, 4, 889–899. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S. Antioxidant and free radical scavenging capacity of the underutilized legume, Vigna vexillata (L.) A. Rich. J. Food Compos. Anal. 2011, 24, 160–165. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and Anti-tyrosinase Activities of Phenolic Extracts from Rape Bee Pollen and Inhibitory Melanogenesis by cAMP/MITF/TYR Pathway in B16 Mouse Melanoma Cells. Front. Pharmacol. 2017, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.N.A.; Khatib, A.; Latip, J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. Biomed. Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef]
- Pauli, E.D.; Malta, G.B.; Sanchez, P.M.; Moreira, I.C.; Scarminio, I.S. Mixture design analysis of solvent extractor effects on epicatechin, epigallocatechin gallate, epigallocatechin and antioxidant activities of the Camellia sinensis L. leaves. Anal. Chem. Res. 2014, 2, 23–29. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]




| Parts | Solvents | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) | Total Flavanol Content (mg CE/g) | Phosphomolybdenum (mmol TE/g) |
|---|---|---|---|---|---|
| Bark | DCM | 63.54 ± 1.23 d | 24.13 ± 0.90 b | 11.59 ± 0.18 b | 2.19 ± 0.22 bc |
| EA | 49.43 ± 0.42 e | 24.27 ± 0.38 b | 9.26 ± 0.18 c | 1.89 ± 0.23 c | |
| MeOH | 145.79 ± 0.84 a | 4.79 ± 0.21 f | 35.24 ± 0.39 a | 3.33 ± 0.13 a | |
| Water | 75.28 ± 0.65 c | 1.19 ± 0.20 hi | 1.25 ± 0.02 g | 2.08 ± 0.06 bc | |
| Fruit | DCM | 18.23 ± 0.13 j | 2.80 ± 0.08 g | 2.30 ± 0.02 e | 1.15 ± 0.11 de |
| EA | 14.17 ± 0.33 k | 2.66 ± 0.10 g | 1.80 ± 0.03 f | 1.19 ± 0.04 d | |
| MeOH | 37.52 ± 0.27 g | 2.28 ± 0.03 gh | 2.94 ± 0.08 d | 1.17 ± 0.02 d | |
| Water | 26.00 ± 0.32 h | 0.64 ± 0.05 i | 0.70 ± 0.01 h | 0.79 ± 0.08 e | |
| Leaf | DCM | 24.51 ± 0.17 h | 11.79 ± 0.36 d | 2.01 ± 0.01 ef | 2.27 ± 0.09 b |
| EA | 21.63 ± 0.69 i | 19.18 ± 0.76 c | 1.74 ± 0.01 f | 2.29 ± 0.14 b | |
| MeOH | 47.59 ± 0.66 f | 34.49 ± 0.18 a | 1.84 ± 0.02 f | 1.93 ± 0.16 bc | |
| Water | 77.63 ± 0.63 b | 7.28 ± 0.51 e | 0.74 ± 0.01 h | 2.00 ± 0.02 bc |
| Retention Time | m/z | Fragments | Identification | mg/g | |
|---|---|---|---|---|---|
| Catechins | |||||
| E1 | 31.7 | 385 | 305 (100) 287 (15) 195 (3) 161 (3) | epigallocatechin sulphate | 1.61 ± 0.05 |
| E2 | 34.9 | 369.5 | 289 (100) 245 (24) 217 (52) | Epicatechin-7-O-sulphate | 60.53 ± 0.35 |
| E3 | 35.2 | 305 | 287 (15) 195 (3) 161 (3) | Epigallocatechin | 0.46 ± 0.05 |
| E4 | 38.7 | 289 | 245 (100) 205 (52) 175 (20) | Epicatechin | 4.89 ± 0.08 |
| total amounts | 67.48 ± 0.08 | ||||
| Proanthocyanidins | |||||
| D1 | 30.9 | 577.5 | 451 (28) 425 (100) 408 (15) 289 (30) 245 (6) | PAC-B dimer | 0.81 ± 0.02 |
| D2 | 31.9 | 577.5 | 451 (26) 425 (100) 408 (15) 289 (30) 245 (6) | PAC-B dimer | 0.97 ± 0.04 |
| D3 | 35.8 | 577.5 | 451 (29) 425 (100) 408 (17) 289 (35) 245 (9) | PAC-B dimer * | 6.68 ± 0.21 |
| D4 | 37.9 | 577.5 | 451 (28) 425 (100) 408 (15) 289 (27) 245 (8) | PAC-B2 | 13.07 ± 0.51 |
| D5 | 47.8 | 577.5 | 451 (31) 425 (100) 408 (18) 289 (26) 245 (10) | PAC-B dimer * | 2.21 ± 0.10 |
| T1 | 34.4 | 865 | 739 (14) 713 (24) 695 (100) 577 (28) 575 (21) 543 (9) 425 (8) 407(8) | PAC-B trimer | 1.17 ± 0.07 |
| T2 | 39.3 | 865 | 739 (14) 713 (24) 695 (100) 577 (28) 575 (21) 543 (9) 425 (8) 407(8) | PAC-B trimer | 4.06 ± 0.55 |
| T3 | 40.7 | 865 | 739 (17) 713 (29) 695 (100) 577 (33) 575 (16) 543 (9) 425 (10) 407(8) | PAC-B trimer | 1.39 ± 0.05 |
| T4 | 43.6 | 865 | 739 (11) 713 (30) 695 (100) 577 (22) 575 (23) 543 (11) 425 (8) 407(11) | PAC-B trimer * | 14.57 ± 0.57 |
| T5 | 45.4 | 865 | 739 (14) 713 (24) 695 (100) 577 (28) 575 (21) 543 (9) 425 (8) 407(8) | PAC-B trimer | 1.61 ± 0.05 |
| T6 | 49.7 | 865 | 739 (16) 713 (21) 695 (100) 577 (25) 575 (22) 543 (12) 425 (11) 407(8) | PAC-B trimer | 1.32 ± 0.07 |
| TT1 | 41.0 | 1154.5 | 1028 (78) 1002 (40) 984 (100) 865 (58) 739 (51) 577 (39) | PAC-B tetramer | 1.76 ± 0.06 |
| TT2 | 46.1 | 1154.5 | 1028 (68) 1002 (45) 984 (100) 865 (63) 739 (53) 577 (39) | PAC-B tetramer | 6.09 ± 0.15 |
| TT3 | 46.6 | 1154.5 | 1028 (66) 1002 (43) 984 (100) 865 (60) 739 (50) 577 (39) | PAC-B tetramer | 1.43 ± 0.05 |
| TT4 | 50.5 | 1154.5 | 1028 (70) 1002 (44) 984 (100) 865 (62) 739 (53) 577 (39) | PAC-B tetramer | 1.47 ± 0.05 |
| HX | 48.6 | 865 * | 1441 (25) 1151 (100) 989 (20) 713 (24) 695 (100) 577 (5) | PAC-B hexamer | 1.28 ± 0.07 |
| HP | 49.8 | 1009 * | 1441 (20) 1151 (100) 989 (22) 713 (26) 695 (100) 577 (3) | PAC-B heptamer | 3.73 ± 0.15 |
| total amounts | 63.63 ± 0.11 | ||||
| Small Phenolics | |||||
| 13.01 | 331 | 271 169 151 | galloylglucose | 0.21 ± 0.’02 | |
| 20.1 | 331 | 271 169 151 | galloylglucose | 0.23 ± 0.01 | |
| 20.9 | 315 | 153 | Protocatechuic acid-O-hexoside | 0.95 ± 0.03 | |
| 31.3 | 401 | 293 269 161 | benzoyl alchol hexose pentose | 0.60 ± 0.02 | |
| total amounts | 1.99 ± 0.04 | ||||
| Spiroketal Glycosides | |||||
| 44.8 | 952 | 813 463 368 | Breinin B | ||
| 47.6 | 936 | 798 472 453 | Breinin D | ||
| 51.9 | 952 | 813 463 368 | Epibreinin B | ||
| 56.9 | 922 | 790 464 353 | Breinin F derivative no sulfoxide | ||
| Leaves | Fruits | |||||
|---|---|---|---|---|---|---|
| rt | [M − H]− | Fragments | Identification | mg/g | mg/g | |
| 1 | 5.6 | 377 | 342 202 | Disaccharide (chlorine adduct) | Not quantified | Not quantified |
| 2 | 5.78 | 376 | 341 202 | Disaccharide (chlorine adduct) | Not quantified | Not quantified |
| 3 | 5.97 | 195 | 177 159 129 | Gluconic acid | Not quantified | Not quantified |
| 4 | 6.2 | 341 | 179 | caffeoyl hexose | 0.84 ± 0.01 | 2.37 ± 0.01 |
| 5 | 8.55 | 290 | 272 254 200 128 | Unknown | nd | nd |
| 6 | 31.0 | 401 | 293 269 161 | benzoyl alchol hexose pentose | 3.88 ± 0.01 | 1.99 ± 0.01 |
| 7 | 33.4 | 423,5 | 313 169 125 | Galloyl hexose derivative | 1.01 ± 0.01 | 11.18 ± 0.01 |
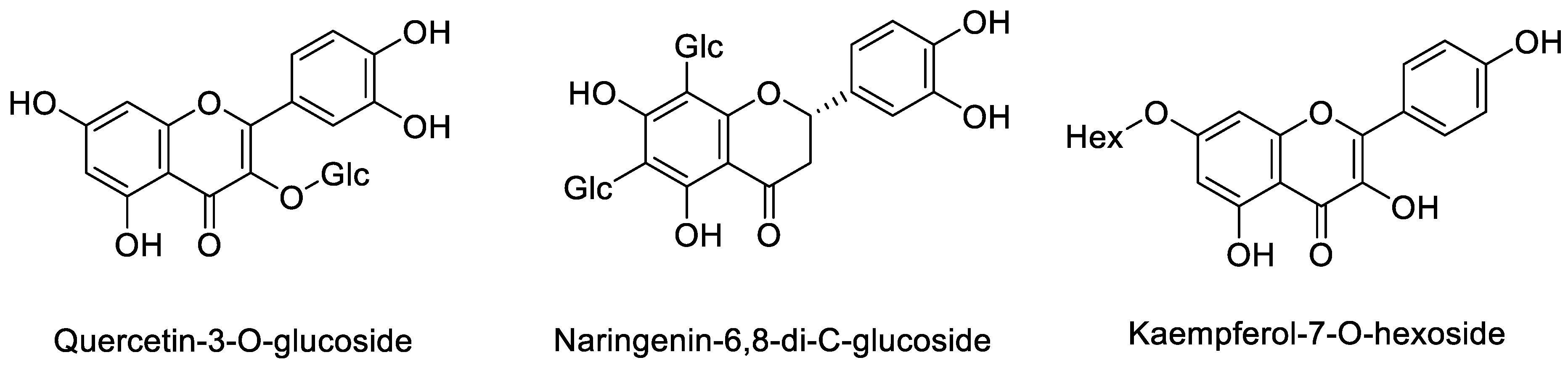
| 8 | 45.2 | 625 | 301 272 255 | quercetin di hexoside | 16.37 ± 0.01 | 1.89 ± 0.01 |
| 9 | 49.1 | 609 | 448 285 | Kaempferol-O-hexoside-O-hexoside | 5.43 ± 0.01 | 1.59 ± 0.01 |
| 10 | 51.5 | 463 | 301 255 179 | Quercetin-3-O-glucoside | 11.92 ± 0.01 | 11.16 ± 0.01 |
| 11 | 54.1 | 187 | 129 97 | Gallic acid monohydrate | 0.30 ± 0.01 | 1.37 ± 0.01 |
| 12 | 39.0 | 449 | 311 287 269 259 | Dihydrokaempferol hexoside | 1.37 ± 0.01 | nd |
| 13 | 34.8 | 595 | 505 475 415 385 355 | Naringenin-6,8-di-C-glucoside | 3.35 ± 0.01 | nd |
| 14 | 50.0 | 421 | 405 289 251 221 178 | Epicatechin/catechin pentoside | 2.47 ± 0.01 | nd |
| 15 | 55.9 | 447 | 284 255 227 | Kaempferol-7-O-hexoside | 4.31 ± 0.01 | nd |
| Total amount | 51.27 ± 0.02 | 31.55 ± 0.02 | ||||
| Parts | Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Metal Chelating Activity (mg EDTAE/g) |
|---|---|---|---|---|---|---|
| Bark | DCM | 93.53 ± 0.17 c | 231.93 ± 1.38 b | 227.75 ± 5.92 d | 103.77 ± 2.29 d | 2.40 ± 0.87 de |
| EA | 80.43 ± 0.69 d | 204.34 ± 1.82 c | 215.33 ± 1.15 e | 87.40 ± 1.35 e | 2.49 ± 0.73 de | |
| MeOH | 202.09 ± 0.15 a | 490.12 ± 0.18 a | 661.82 ± 0.40 a | 325.86 ± 4.36 a | 2.50 ± 0.13 de | |
| Water | 98.09 ± 0.36 b | 225.38 ± 3.91 b | 304.88 ± 1.16 b | 145.36 ± 0.82 b | 10.84 ± 0.73 bc | |
| Fruit | DCM | Na | 7.23 ± 1.33 i | 43.32 ± 0.26 i | 16.44 ± 0.11 j | Na |
| EA | Na | 27.53 ± 1.32 g | 73.16 ± 4.20 h | 18.06 ± 0.51 j | Na | |
| MeOH | 48.43 ± 0.82 g | 142.74 ± 2.53 e | 127.35 ± 3.25 g | 59.90 ± 0.73 g | 3.82 ± 0.67 d | |
| Water | 21.72 ± 1.44 h | 83.25 ± 1.98 f | 77.66 ± 0.69 h | 38.45 ± 0.61 h | 12.03 ± 0.72 bc | |
| Leaf | DCM | na | 14.95 ± 1.15 h | 72.98 ± 1.69 h | 24.07 ± 0.63 i | 9.88 ± 1.75 c |
| EA | na | 17.02 ± 0.99 h | 76.27 ± 1.40 h | 25.29 ± 0.42 i | 19.34 ± 0.31 a | |
| MeOH | 56.94 ± 0.74 f | 176.18 ± 3.71 d | 174.75 ± 1.85 f | 71.77 ± 1.92 f | 3.41 ± 0.56 d | |
| Water | 66.15 ± 3.51 e | 209.79 ± 4.50 c | 259.48 ± 2.61 c | 132.85 ± 1.31 c | 13.35 ± 1.94 b |
| Parts | Solvents | AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | α-Amylase Inhibition (mmol ACAE/g) | α-Glucosidase Inhibition (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| Bark | DCM | 4.55 ± 0.08 ab | 2.69 ± 0.86 cde | 143.23 ± 0.60 b | 0.74 ± 0.03 cd | 2.06 ± 0.01 c |
| EA | 4.57 ± 0.14 ab | 2.13 ± 0.16 e | 135.76 ± 0.55 c | 0.76 ± 0.02 bcd | 2.07 ± 0.01 c | |
| MeOH | 5.00 ± 0.03 ab | 4.82 ± 0.50 ab | 152.79 ± 0.27 a | 0.99 ± 0.01 a | 2.16 ± 0.01 a | |
| Water | 1.55 ± 0.17 d | na | 75.51 ± 0.56 f | 0.21 ± 0.01 e | 2.14 ± 0.01 a | |
| Fruit | DCM | 3.64 ± 0.32 c | 4.43 ± 0.47 ab | 132.04 ± 2.57 c | 0.72 ± 0.01 cd | 2.06 ± 0.01 c |
| EA | 4.40 ± 0.08 abc | 5.77 ± 0.94 a | 132.79 ± 0.72 c | 0.75 ± 0.03 bcd | 2.10 ± 0.01 b | |
| MeOH | 4.55 ± 0.05 ab | 3.65 ± 0.03 bcd | 145.34 ± 0.13 b | 0.77 ± 0.01 bc | 2.14 ± 0.01 a | |
| Water | 0.98 ± 0.54 d | 0.46 ± 0.09 f | 52.44 ± 3.87 g | 0.23 ± 0.01 e | na | |
| Leaf | DCM | 4.33 ± 0.61 bc | 3.58 ± 0.24 bcde | 117.92 ± 4.12 d | 0.75 ± 0.04 cd | 2.06 ± 0.01 cd |
| EA | 5.13 ± 0.11 a | 2.25 ± 0.62 de | 133.75 ± 0.54 c | 0.69 ± 0.03 d | 2.03 ± 0.01 d | |
| MeOH | 4.41 ± 0.05 abc | 3.89 ± 0.67 bc | 147.68 ± 0.53 ab | 0.82 ± 0.02 b | 2.12 ± 0.01 b | |
| Water | 0.09 ± 0.01 e | na | 95.50 ± 3.24 e | 0.19 ± 0.01 e | 1.97 ± 0.02 e |
| Position | δ H | δ C |
|---|---|---|
| 2 | 4.90 (under methanol) | 78.0 |
| 3 | 4.25 br s (J = 1.6) | 65.9 |
| 4 | 2.97–3.03 | 27.9 |
| 5 | - | 157.8 |
| 6 | 6.23 (J = 1.6) | 98.9 |
| 7 | - | 156.4 |
| 8 | 6.64 (J = 1.6) | 101.8 |
| 9 | - | 151.4 |
| 10 | - | 104.0 |
Sample Availability: Samples of the compound are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Acqua, S.; Sinan, K.I.; Ferrarese, I.; Sut, S.; Bene, K.; Mahomoodally, M.F.; Bibi Sadeer, N.; Ak, G.; Zengin, G. Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts. Molecules 2020, 25, 5537. https://doi.org/10.3390/molecules25235537
Dall’Acqua S, Sinan KI, Ferrarese I, Sut S, Bene K, Mahomoodally MF, Bibi Sadeer N, Ak G, Zengin G. Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts. Molecules. 2020; 25(23):5537. https://doi.org/10.3390/molecules25235537
Chicago/Turabian StyleDall’Acqua, Stefano, Kouadio Ibrahime Sinan, Irene Ferrarese, Stefania Sut, Kouadio Bene, Mohamad Fawzi Mahomoodally, Nabeelah Bibi Sadeer, Gunes Ak, and Gokhan Zengin. 2020. "Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts" Molecules 25, no. 23: 5537. https://doi.org/10.3390/molecules25235537
APA StyleDall’Acqua, S., Sinan, K. I., Ferrarese, I., Sut, S., Bene, K., Mahomoodally, M. F., Bibi Sadeer, N., Ak, G., & Zengin, G. (2020). Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts. Molecules, 25(23), 5537. https://doi.org/10.3390/molecules25235537








