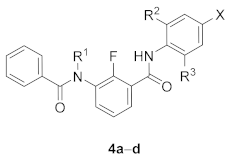
Abstract
Herein, we describe novel pentafluorosulfanyl (SF5) group-containing meta-diamide insecticides. For the facile preparation of the SF5-based compounds 4a–d, practical synthetic methods were applied. Among newly synthesized compounds, 3-benzamido-N-(2,6-dimethyl-4-(pentafluoro-λ6-sulfanyl)phenyl)-2-fluorobenzamide 4d showed (i) a high insecticidal activity, (ii) an excellent selectivity to insects, and (iii) good levels of water solubility and log P values. In this study, we demonstrated that the pentafluorosulfanyl moiety could serve as an attractive functionality for the discovery of a new scope of crop-protecting agents.
1. Introduction
The introduction of a fluorine atom into a biologically active compound can have a significant influence on its properties. One or more incorporated fluorine atoms can alter the electrostatic and hydrogen bonding parameters of the molecule as well as its physicochemical and pharmacokinetic properties [1,2]. Currently, fluorine-containing substituents, which are commonly encountered in commercial pharmaceuticals and agrochemicals, include fluoroaromatic, trifluoromethyl (CF3), trifluoromethoxy (OCF3), and trifluoromethlythio (SCF3) functionalities [3,4]. Another fluorinated substituent that reflects the continuing development of a relatively new fluorinated building block with distinct properties could be the pentafluorosulfanyl (SF5) group. The SF5 group is often called the “super-trifluoromethyl group”, and aryl sulfanyl pentafluorides display high thermal and chemical stability, electronegativity, and lipophilicity [5,6,7]. Due to its unique properties, the SF5 group has widely been applied in drug discovery and crop protection research (Figure 1) [8,9,10,11,12,13,14], since the first organic pentafluorosulfanyl compound was described in 1950 [15].
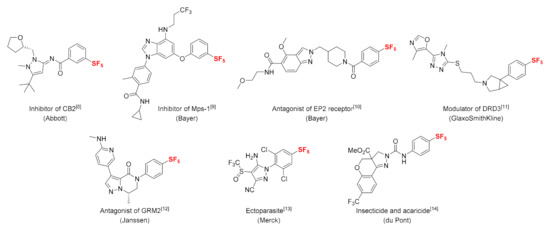
Figure 1.
SF5-containing biologically active compounds [8,9,10,11,12,13,14].
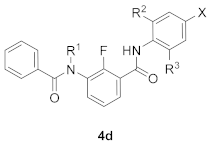
As a part of our ongoing efforts to discover new eco-friendly insecticides [16,17,18], we are particularly interested in small molecules containing the fluorine atom. One of the outstanding representatives of this category is broflanilide, which is known as an efficient broad-spectrum meta-diamide insecticide containing a high number of fluorine atoms [19,20]. Taking into account the suggested potential bioisosteric relationship between the SF5 and CF3 substituents [2,5], we proposed the novel design of the SF5-containing meta-diamide insecticide 4d (Figure 2). In order to examine the influence of SF5 moiety on the properties of the meta-diamide 4d (R1 = H, R2 and R3 = CH3) and investigate the significance of the effect caused by the replacement of the CF(CF3)2 group to SF5 functionality, the known meta-diamide insecticide BPB1 (R1 = H, R2 and R3 = CH3) was selected for this study (Figure 2). According to the previous studies on the development of meta-diamide-based insecticides, it was discovered that the insecticide BPB3 (R1 = CH3) can be metabolized to its active form, BPB1 (R1 = H), which in its turn demonstrates insecticidal activity by acting on the target RDL GABARs gene subunit and inhibiting its expression [19,20].
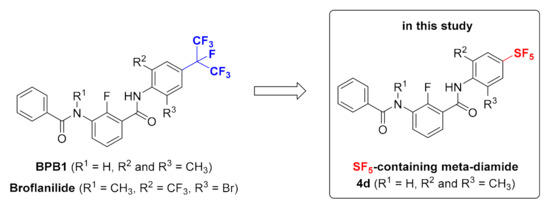
Figure 2.
SF5-containing meta-diamide insecticides in this study.
2. Results
The synthetic route shown in Scheme 1 was successfully applied for the preparation of the para-SF5 substituted aniline derivatives, 1b–d. The synthesis was initiated by the bromination of a commercially available aniline, 1a using N-bromosuccinimide (NBS), which led to the formation of mono- and di-bromo anilines 1a′ and 1a″, respectively. The molecules, 1a′ and 1a″ were subsequently methylated by the Pd-catalyzed cross-coupling to give the corresponding anilines, 1b and 1d in 64% and 75% yields, respectively. Finally, 2-methyl-aniline 1b was further reacted with NBS in DMF to produce 2-methyl-6-bromo-aniline 1c with excellent yield [21].
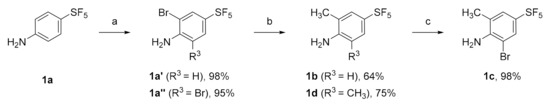
Scheme 1.
Reagents and conditions: (a) for 1a′: N-bromosuccinimide (1.1 eq), DMF, rt, 2 h; for 1a″: NBS (2.5 eq), DMF, rt, 2 h; (b) from 1a′ to 1b: CH3B(OH)2 (2.0 eq), Pd(dppf)2Cl2, Cs2CO3, 1,4-dioxane, reflux, 12 h; from 1a″ to 1d: CH3B(OH)2 (4.0 eq), Pd(dppf)2Cl2, Cs2CO3, 1,4-dioxane, reflux, 12 h; (c) from 1b to 1c: N-bromosuccinimide (1.5 eq), DMF, rt, 2 h.
The target compounds with the incorporated SF5 group were successfully prepared starting from 2-fluoro-3-nitrobenzoic acid, which is commercially available and can be readily converted into the corresponding aniline 2 [22]. Benzoylation of 2 provided 3-benzamido-2-fluorobenzoic acid 3 in excellent yield [23]. Then, SF5-containing compounds 4a–d were easily prepared by the condensation of benzoic acid 3 with 4-SF5-anilines 1a–d (Scheme 2) [24].
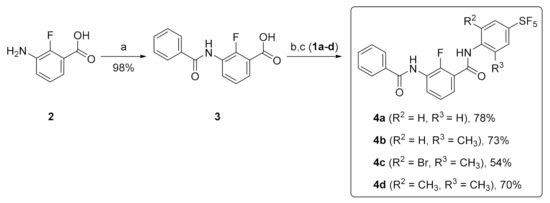
Scheme 2.
Reagents and conditions: (a) Benzoyl chloride, NaOH, H2O, rt, 6 h; (b) SOCl2, reflux, 1 h; (c) 4-SF5-anilines 1a–d, NaHCO3, Acetone/H2O, reflux, 2 h.
The synthesized SF5-containing derivatives 4a–d were examined for their insecticidal activities at 10 ppm concentration against the 3rd instar larvae of Plutella xylostella using the leaf-dip method [16,17,18,25]. Among them, the compounds 4c and 4d showed excellent activities with high inhibition of feeding behaviors (entry 3 and 4, Table 1). Interestingly, 2,6-dimethyl-substituted compound 4d, SF5-containing meta-diamide BPB1, displayed an excellent potency with eating area—0~5%. According to the data in Table 1, it is reasonable to believe that the SF5 group can be considered as an important part of the toxophore.

Table 1.
Insecticidal activities of SF5-containing meta-diamide insecticides 4a–d against the 3rd instar larvae of Plutella xylostella.
The target site specificities of newly prepared SF5-based meta-diamide insecticide 4d should differ in insect and mammalian GABA and glycine receptors. In this regard, the cell-based antagonist activities of the meta-diamide 4d against the human GABAAR and glycine receptor (GlyR) A1 were investigated. According to the results obtained from previous studies, the mammalian GABAAR α1β3γ2 and the human glycine receptor (GlyR) A1 were selected for this study [19,26,27]. As shown in Table 2, the estimated IC50 values of SF5-containing meta-diamide 4d and broflanilide against GABAAR and GlyR were more than 30 μM. This discovery implies that SF5-containing meta-diamide 4d has much higher selectivity toward targeted insects.

Table 2.
Summary of the antagonist activities of SF5-containing meta-diamide insecticide 4d according to ion channel cell-based ionflux assays of wild-type GABAARs and GlyRs.
There are a number of studies for confirming the existence of the strong relationship between insecticidal activity and bioavailability of the potential insecticides [18,28]. In this context, SF5-containing compound 4d, which showed the highest potency, was further investigated in its physicochemical properties, including LogP and solubility. As a reference, properties of broflanilide were also measured. For lipophilicity, most commonly referred to as LogP [29], replacing the heptafluoroisopropyl group with the SF5 moiety resulted in similar LogP values in broflanilide and the meta-diamide 4d (entry 1, Table 3). In addition, both the molecules meta-diamide 4d and broflanilide showed high levels of kinetic solubility [30].

Table 3.
Physical properties of SF5-containing meta-diamide insecticide 4d.
Generally, the presence of fluoroaromatics and perfluoroalkanes increases the lipophilicity values of the molecules in comparison to the parental hydrocarbon bonds [31,32,33,34,35]. In addition to that, regarding its structural differences, the SF5 group possesses superior properties over other available fluorine-containing functionalities. Taking into consideration that lipophilicity plays a key role in transport processes [36], this result could be an important finding to discover new functionalities for application in the development of crop protecting agents.
3. Material and Methods
3.1. General Information
Melting points: Barnstead/Electrothermal 9300—measurements were performed in open glass capillaries. NMR spectra: Bruker AV 300 MHz (Bruker corporation, Billerica, MA, USA)(1H-NMR: 300 MHz, 13C-NMR: 75 MHz), AV 400 MHz (1H-NMR: 400 MHz, 13C-NMR: 100 MHz), AV 500 MHz (1H-NMR: 500 MHz, 13C-NMR: 125 MHz), and AV2 500 MHz (19F-NMR: 470 MHz); the spectra were recorded in CDCl3 and DMSO-d6 using tetramethylsilane (TMS) as the internal standard and are reported in ppm. 1H-NMR data are reported as follows: (s—singlet; d—doublet; t—triplet; q—quartet; dd—doublet of doublet; m—multiplet; coupling constant(s) J are given in Hz; integration, proton assignment). High-resolution mass spectra (HRMS): JEOL JMS-700.
2-Methyl-4-(pentafluorothio)aniline (1b) [37,38]. A mixture of 2-bromo-4-(pentafluorothio)aniline (500 mg, 3.223 mmol), methylboronic acid (2.0 eq., 6.446 mmol), Pd(dppf)Cl2.DCM (0.1 eq., 0.322 mmol), and Cs2CO3 (3.0 eq., 9.669 mmol) in 1,4-dioxane (8.6 mL) was stirred at 105 °C for 5 h. The reaction mixture was diluted with EtOAc and washed with aq. NaHCO3. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by flash column chromatography on a silica gel (hexane:EtOAc = 15:1) to give the desired product 1b as a yellow solid (481 mg, 64%).
2-bromo-6-methyl-4-(pentafluorothio)aniline (1c). To a solution of 2-methyl-4-(pentafluorothio)aniline (350 mg, 1.5 mmol) in DMF (15 mL), NBS (1.03 eq., 1.545 mmol) was added. The reaction mixture was stirred at RT for 2 h, quenched with water, and extracted with EtOAc (10 mL). The organic layer was dried over NaSO4, filtered, and concentrated. The residue was purified by column chromatography on a silica gel (hexane:EtOAc = 20:1) to give the desired product 1c as a red solid (459 mg, 98%). mp 64~65 °C; 1H-NMR (500 MHz, CDCl3) δ 7.71 (d, J = 2.5 Hz, 1H), 7.40 (d, J = 2.5 Hz, 1H), 4.42 (s, 2H), 2.25 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 146.0, 144.7, 129.2, 127.9, 122.8, 107.8, 19.4; 19F-NMR (470 MHz, CDCl3) δ 86.46 (p, 1F, JSF–SF4 = 150.3 Hz, SF), 64.90 (d, 4F, JSF4–SF = 150.2 Hz, SF4); HRMS (EI) calcd. for C7H7BrF5NS 310.9403, found 310.9409 (see Supplementary Materials).
2,6-dimethyl-4-(pentafluorothio)aniline (1d). A mixture of 2-bromo-6-methyl-4-(pentafluorothio)aniline (200 mg, 0.64 mmol), methylboronic acid (2.0 eq., 1.28 mmol), Pd(dppf)Cl2.DCM (0.1 eq., 0.064 mmol), and Cs2CO3 (3.0 eq., 1.92 mmol) in 1,4-dioxane (1.7 mL) was stirred at 105 °C for 5 h. The mixture was diluted in EtOAc, washed with aq. NaHCO3, dried over Na2SO4, and concentrated. The residue was purified by flash column chromatography on a silica gel (hexane:EtOAc = 15:1) to give the desired product 1d as a brown solid (119 mg, 75%). mp 205~206 °C; 1H-NMR (300 MHz, CDCl3) δ 7.34 (s, 2H), 3.90 (s, 2H), 2.20 (s, 6H); 13C-NMR (100 MHz, CDCl3) δ 145.2, 144.1, 128.0, 126.5, 121.8, 120.7, 18.0; 19F-NMR (470 MHz, CDCl3) δ 87.32 (p, 1F, JSF–SF4 = 149.9 Hz, SF), 64.88 (d, 4F, JSF4–SF = 149.8 Hz, SF4); HRMS (EI) calcd. for C8H10F5NS 247.0454, found 247.0451 (see Supplementary Materials).
3-benzamido-2-fluorobenzoic acid (3). To 2-fluoro-3-nitro-benzoic acid (2 g, 10.8 mmol) in 44 mL of tetrahydrofuran, 20% palladium hydroxide on carbon (148 mg, 1.05 mmol) was added. The reaction was stirred under hydrogen for 2 h. The reaction mixture was filtered through a short pad of celite and the solution was evaporated (without a purification) to give the desired compound 3 as an ivory color solid (1.64 g, 98%). mp 257~258 °C; 1H-NMR (300 MHz, DMSO-d6) δ 13.32 (s, 1H), 10.22 (s, 1H), 8.02–7.95 (m, 2H), 7.86–7.79 (m, 1H), 7.76–7.69 (m, 1H), 7.66– 7.59 (m, 1H), 7.58–7.51 (m, 2H), 7.31 (t, J = 7.8 Hz, 1H); 13C-NMR (100 MHz, DMSO-d6) δ 165.5, 164.8, 133.7, 131.9, 131.1, 128.5, 128.5, 128.3, 127.8, 126.9, 123.8, 123.8; 19F-NMR (470 MHz, CDCl3) δ −119.6; HRMS (EI) calcd. for C14H10FNO3 259.0645, found 259.0638 (see Supplementary Materials).
3.2. General Method for the Synthesis of 4a–d
A mixture of 3-benzamido-2-fluorobenzoic acid 3 (50 mg, 0.193 mmol) and SOCl2 (3.0 eq., 0.579 mmol) was refluxed for 2 h. The solution of aniline (0.9 eq., 0.174 mmol) and NaHCO3 (2.7 eq., 0.52 mmol) in acetone/water (0.4 mL/0.04 mL) was added in the reaction mixture. The reaction mixture was refluxed for 1 h, quenched with water, and extracted with EtOAc (10 mL). The organic layer was dried over NaSO4, filtered, and concentrated. The residue was purified by column chromatography on a silica gel (hexane:EtOAc = 10:1) to give the desired product.
3-Benzamido-2-fluoro-N-(4-(pentafluorothio)phenyl)benzamide (4a). This follows the general method. The residue was purified by column chromatography on a silica gel (Hexane:EtOAc = 10:1) to give the desired diamide 4a as a white solid (55.3 mg, 78% yield). mp 193~194 °C; 1H-NMR (500 MHz, CDCl3) δ 8.63–8.57 (m, 1H), 8.44 (d, J = 12.4 Hz, 1H), 8.09 (s, 1H), 7.94–7.88 (m, 2H), 7.85–7.80 (m, 1H), 7.78 (s, 4H), 7.65–7.60 (m, 1H), 7.57–7.52 (m, 2H), 7.36 (t, J = 8.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 165.6, 161.3, 140.2, 133.9, 132.6, 129.1, 127.2, 127.1,127.0, 126.8, 126.4, 126.3, 125.5, 125.4, 121.3, 121.2, 119.6; 19F-NMR (470 MHz, CDCl3) δ 84.83 (quin, 1F, JSF–SF4, J = 150.3 Hz, SF), 63.41 (d, 4F, JSF4–SF, J = 149.8 Hz, SF4), −131.28–−131.40 (m, 1F); HRMS (EI) calcd. for C20H14F6N2O2S 460.0680, found 460.0680 (see Supplementary Materials).
3-benzamido-2-fluoro-N-(2-methyl-4-(pentafluorothio)phenyl)benzamide (4b). This follows the general method. The residue was purified by column chromatography on a silica gel (Hexane:EtOAc = 10:1) to give the desired diamide 4b as a white solid (54.3 mg, 73% yield). mp 189~190 °C; 1H-NMR (500 MHz, CDCl3) δ 8.64–8.59 (m, 1H), 8.42–8.34 (m, 2H), 8.11 (s, 1H), 7.94–7.87 (m, 3H), 7.67 (dd, J = 9.0, 2.7 Hz, 1H), 7.64–7.60 (m, 2H), 7.56–7.52 (m, 2H), 7.38 (t, J = 8.0 Hz, 1H), 2.42 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 165.6, 160.9, 152.2, 149.8, 138.6, 134.0, 132.6, 129.0, 127.9, 127.6, 127.1, 126.9, 126.8, 126.72, 126.70, 126.46, 126.44, 125.49, 125.46, 125.0, 121.3, 121.1, 121.0, 18.0; 19F-NMR (470 MHz, CDCl3) δ 85.10 (t, 1F, JSF–SF4, J = 150.2 Hz, SF), 63.42 (d, 4F, JSF4–SF, J = 149.9 Hz, SF4), −132.30 (s, 1F); HRMS (EI) calcd. for C21H16F6N2O2S 474.0837, found 474.0837 (see Supplementary Materials).
3-benzamido-N-(2-bromo-6-methyl-4-(pentafluorothio)phenyl)-2-fluorobenzamide (4c). This follows the general method. The residue was purified by column chromatography on a silica gel (Hexane:EtOAc = 10:1) to give the desired diamide 4c as a brown solid (51.2 mg, 54% yield). mp 209~210 °C; 1H-NMR (500 MHz, CDCl3) δ 8.69–8.63 (m, 1H), 8.17–8.07 (m, 2H), 7.94–7.89 (m, 3H), 7.89–7.83 (m, 1H), 7.67 (d, J = 2.5 Hz, 1H), 7.64–7.59 (m, 1H), 7.56–7.52 (m, 2H), 7.40–7.35 (m, 1H), 2.43 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 165.6, 161.0, 152.4, 149.9, 138.9, 137.0, 134.0, 132.6, 129.1, 128.0, 127.6, 127.2, 127.1, 127.0, 126.5, 126.3, 125.4, 125.3, 121.4, 120.5, 120.4, 20.0; 19F-NMR (470 MHz, CDCl3) δ 82.97 (quin, 1F, JSF–SF4, J = 150.5 Hz, SF), 63.35 (d, 4F, JSF4–SF, J = 150.0 Hz, SF4), −130.98 (s, 1F); HRMS (EI) calcd. for C21H15BrF6N2O2S 551.9942, found 551.9954 (see Supplementary Materials).
3-benzamido-N-(2,6-dimethyl-4-(pentafluorothio)phenyl)-2-fluorobenzamide (4d). This follows the general method. The residue was purified by column chromatography on a silica gel (Hexane:EtOAc = 10:1) to give the desired diamide 4d as a white solid (52.7 mg, 70% yield). mp 205~206 °C; 1H-NMR (500 MHz, CDCl3) δ 8.60 (t, J = 7.9 Hz, 1H), 8.13 (s, 1H), 7.93–7.90 (m, 2H), 7.85–7.80 (m, 2H), 7.64–7.59 (m, 1H), 7.56–7.51 (m, 4H), 7.36 (t, J = 8.0 Hz, 1H), 2.36 (s, 6H); 13C-NMR (100 MHz, CDCl3) δ 165.6, 161.4, 161.3, 136.5, 136.4, 133.9, 132.5, 129.0, 127.1, 126.9, 126.8, 126.4, 126.4, 126.1, 126.15, 125.8, 125.8, 125.7, 125.3, 125.2, 18.9; 19F-NMR (470 MHz, CDCl3) δ 84.69 (quin, 1F, JSF–SF4, J = 150.3, SF), 63.09 (d, 4F, JSF4–SF, J = 149.7 Hz, SF4), −131.55 (s, 1F); HRMS (EI) calcd. for C22H18F6N2O2S 488.0993, found 488.0988 (see Supplementary Materials).
4. Conclusions
In summary, starting from the known meta-diamide BPB1 containing a heptafluoroisopropyl group and its isosteric replacement with pentafluorosulfanyl moiety (-SF5) led to the meta-diamide insecticide 4d, a compound with good potency, high selectivity toward insects, and a similar level of lipophilicity with broflanilide. For the preparation of SF5-containing meta-diamide insecticides 4a–d, an efficient synthetic route was established. This study has demonstrated that the pentafluorosulfanyl group (-SF5) could be an appealing structural scaffold for the discovery of a new crop-protecting agent.
Supplementary Materials
The following are available online: 1H, 13C and 19F NMR spectroscopy of compound 1c.; 1H, 13C and 19F NMR spectroscopy of compound 1d.; 1H, 13C and 19F NMR spectroscopy of compound 3.; 1H, 13C and 19F NMR spectroscopy of compound 4a.; 1H, 13C and 19F NMR spectroscopy of compound 4b.; 1H, 13C and 19F NMR spectroscopy of compound 4c.; 1H, 13C and 19F NMR spectroscopy of compound 4d.; Table S1 and S2: Larvicidal activity against Plutella xylostella (4c and 4d); Figure S1: pH-metric Log P of compounds 4d (KI-03066); Figure S2: pH-metric Log P of Broflanilide and Figure S3. Ion Channels assay of 4d (KI-03066) and Broflanilide.
Author Contributions
Methodology, J.G.K., H.J.L. and S.J.P.; investigation, W.H.L., H.J.L. and S.J.P.; analysis, J.G.K., O.-Y.K., S.M.K., G.I., I.S.O., Y.L., W.H.L., H.J.L. and S.J.P.; writing—original draft preparation, H.J.L. and S.J.P.; writing—review and editing, O.-Y.K., S.M.K., G.I., I.S.O., Y.L., H.J.L. and S.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was fully supported by KRICT/Kyung Nong Co. Ltd. co-research project (TS191-06R, TS201-18R, and SI2031-40). This research was funded by the Ministry of Trade, Industry and Energy, Republic of Korea, grant number 10077494. This research was also supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2019M3E5D506617712).
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; Del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, G. Chapter 4 Fluorine-Containing Agrochemicals: An Overview of Recent Developments. In Advances in Fluorine Science; Tressaud, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 121–175. [Google Scholar] [CrossRef]
- Sowaileh, M.F.; Hazlitt, R.A.; Colby, D.A. Application of the Pentafluorosulfanyl Group as a Bioisosteric Replacement. ChemMedChem 2017, 12, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, S.; Zanda, M. Synthetic chemistry and biological activity of pentafluorosulfanyl (SF5) organic molecules. J. Fluorine Chem. 2012, 143, 57–93. [Google Scholar] [CrossRef]
- Savoie, P.R.; Welch, J.T. Preparation and Utility of Organic Pentafluorosulfanyl-Containing Compounds. Chem. Rev. 2015, 115, 1130–1190. [Google Scholar] [CrossRef] [PubMed]
- Carroll, W.A.; Dart, M.J.; Perez-Medrano, A.; Nelson, D.W.; Li, T.; Peddi, S.; Frost, J.; Kolasa, T.; Liu, B.; Latshaw, S.P.; et al. Novel compounds as cannabinoid receptor ligands. US Patent 2009/0105306 A1, 23 April 2009. [Google Scholar]
- Klar, U.; Koppitz, M.; Nguyen, D.; Kosemund, D.; Neuhaus, R.; Siemeister, G. Substituted benzimidazoles. WO Application 2012/130905 A1, 4 October 2012. [Google Scholar]
- Braeuer, N.; Mengel, A.; Roehn, U.; Rotgeri, A.; Buchmann, B.; Lindenthal, B.; Ter Laak, A. Preparation of novel 2H-indazoles as EP2 receptor antagonists. WO Application 2013/079425 A1, 6 June 2013. [Google Scholar]
- Andreotti, D.; Checchia, A.; Hamprecht, D.; Micheli, F. Preparation of 1-(pentafluorosulfanylphenyl)-3-(1,2,4-triazol-3-ylthioalkyl)-3-azabicyclo[3.1.0]hexanes as selective modulators of dopamine D3 receptors. WO Application 2006/108700 A1, 19 October 2006. [Google Scholar]
- Van Gool, M.L.M.; Andres-Gil, J.I.; Alcazar-Vaca, M.J.; Bormans, G.M.R.; Celen, S.J.L.; Joost, V. Radiolabelled mGluR2 PET ligands. WO Application 2016/087489 A1, 9 June 2016. [Google Scholar]
- Chern, R.T.; Zingerman, J.R.; Clark, J.N.; Drag, M.D. Sulfur pentafluorophenyl pyrazoles for controlling ectoparasitic infestations. WO Application 9947139 A1, 23 September 1999. [Google Scholar]
- Howard, M.H., Jr.; Stevenson, T.M. Preparation of arthropodicidal pentafluorothio-substituted anilides. WO Application 9516676 A1, 22 June 1995. [Google Scholar]
- Silvey, G.A.; Cady, G.H. Trifluoromethylsulfur Pentafluoride. J. Am. Chem. Soc. 1950, 72, 3624–3626. [Google Scholar] [CrossRef]
- Chang, S.Y.; Heo, J.N.; Lee, H.; Lim, H.J.; Kim, B.T.; Kim, J.K.; Kim, J. Diaminoaryl Derivatives Substituted by Carbamate and Pesticidal Composition Containing Same. WO Application 2013/168967 A1, 14 November 2013. [Google Scholar]
- Park, S.J.; Lim, H.J.; Kim, B.T. Pyrazole carboxamide compound containing organosulfur group and pesticide composition containing pyrazole carboxamide compound. WO Application 2019/156425 A1, 15 August 2019. [Google Scholar]
- Lim, H.J.; Lee, W.H.; Park, S.J. Synthesis, Physicochemical Properties, and Biological Activities of 4-(S-Methyl-N-(2,2,2-Trifluoroacetyl)Sulfilimidoyl) Anthranilic Diamide. Molecules 2019, 24, 3451. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, H.; Nomura, M.; Wakita, T.; Daido, H.; Kobayashi, Y.; Kawahara, A.; Banba, S. Discovery of broflanilide, a novel insecticide. J. Pestic. Sci. 2019, 44, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Menet, C.J.M.; Mammoliti, O.; Blanc, J.; Orsulic, M.; Roscic, M. Novel compounds and pharmaceutical compositions thereof for the treatment of inflammatory disorders. US Patent 2015/0203455 A1, 23 July 2015. [Google Scholar]
- Ibrahim, P.N.; Spevak, W.; Cho, H. Preparation of pyrrolo[2,3-b]pyrazine derivatives as Raf kinase modulators. US Patent 20090306087 A1, 10 December 2009. [Google Scholar]
- Nomura, M.; Tomura, N.; Kawahara, A.; Daido, H. Pesticide compositions containing amides. JP Patent 2010047478 A, 4 March 2010. [Google Scholar]
- Jingbo, X.; Hongfei, W.; Xueming, C.; Libao, X.; Hao, Y.; Ningning, S.; Haibo, Y. Method for preparing o-trifluoromethylaniline compound and intermediate thereof. CN Patent 109206335 A, 15 January 2019. [Google Scholar]
- According to the literature 16 method, the insecticidal assays were performed by Kyung Nong Co. Ltd., Korea. In detail, please see the supporting information.
- Nakao, T.; Hirase, K. Effects of novel meta-diamide insecticides on GABA type A receptors α1β2γ2 and α1β3γ2 and on glycine receptor α1β. J. Pestic. Sci. 2014, 39, 144–151. [Google Scholar] [CrossRef]
- Note that this ligand-gated ion channels assays were performed by eurofins (in details, please see the supplementary materials).
- Gnamm, C.; Jeanguenat, A.; Dutton, A.C.; Grimm, C.; Kloer, D.P.; Crossthwaite, A.J. Novel diamide insecticides: Sulfoximines, sulfonimidamides and other new sulfonimidoyl derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3800–3806. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Abed, S.N.; Al-Attraqchi, O.; Kuche, K.; Tekade, R.K. Computer-Aided Prediction of Pharmacokinetic (ADMET) Properties. In Dosage Form Design Parameters; Tekade, R.K., Ed.; Elsevier Inc.: London, UK, 2018; Volume 2, pp. 731–755. [Google Scholar]
- Kerns, E.H.; Di, L. Drug-like Properties: Concepts, Structure Design and Methods: From ADME to Toxicity Optimization; Elsevier Inc: San Diego, CA, USA, 2008; p. 65. [Google Scholar]
- Dykstra, K.D.; Ichiishi, N.; Krska, S.W.; Richardson, P.F. Chapter 1—Emerging fluorination methods in organic chemistry relevant for life science applications. In Fluorine in Life Sciences. Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe, G., Leroux, F., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 1–90. [Google Scholar]
- Müller, K. Chapter 2—Fluorination patterns in small alkyl groups: Their impact on properties relevant to drug discovery. In Fluorine in Life Sciences. Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe, G., Leroux, F., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 91–139. [Google Scholar]
- Pertusati, F.; Serpi, M.; Pileggi, E. Chapter 3—Polyfluorinated scaffolds in drug discovery In Fluorine in Life Sciences. Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe, G., Leroux, F., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 141–180. [Google Scholar]
- Xing, L.; Honda, T.; Fitz, L.; Ojima, I. Chapter 4—Case studies of fluorine in drug disvovery. In Fluorine in Life Sciences. Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe, G., Leroux, F., Eds.; Academic Press: San Diego, CA, USA , 2019; pp. 181–211. [Google Scholar]
- Tredwell, M.; Gouverneur, V. 1.5 Fluorine in Medicinal Chemistry: Importance of Chirality. Compr. Chirality 2012, 1, 70–85. [Google Scholar] [CrossRef]
- Van De Waterbeemd, H. In Silico Models to Predict Oral Absorption. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 669–697. [Google Scholar] [CrossRef]
- Arora, N.; Bacani, G.M.; Cai, M.; Barbay, J.K.; Bembenek, S.D.; Chen, W.; Deckhut, C.P.; Edwards, J.P.; Ghosh, B.; Hao, B.; et al. Inhibitors of bruton’s kinase and methods of their use. WO Application 2018/103058 A1, 14 June 2018. [Google Scholar]
- Kleemann, H.-W. Pentafluorosulfanylphenyl-substituted benzoylguanidines, processes for their preparation, their use as medicament or diagnostic aid, and medicament comprising them. US Patent 2005/0043401 A1, 24 February 2005. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).