Expression of Hybrid Peptide EF-1 in Pichia pastoris, Its Purification, and Antimicrobial Characterization
Abstract
1. Introduction
2. Results
2.1. Construction of Expression Plasmids
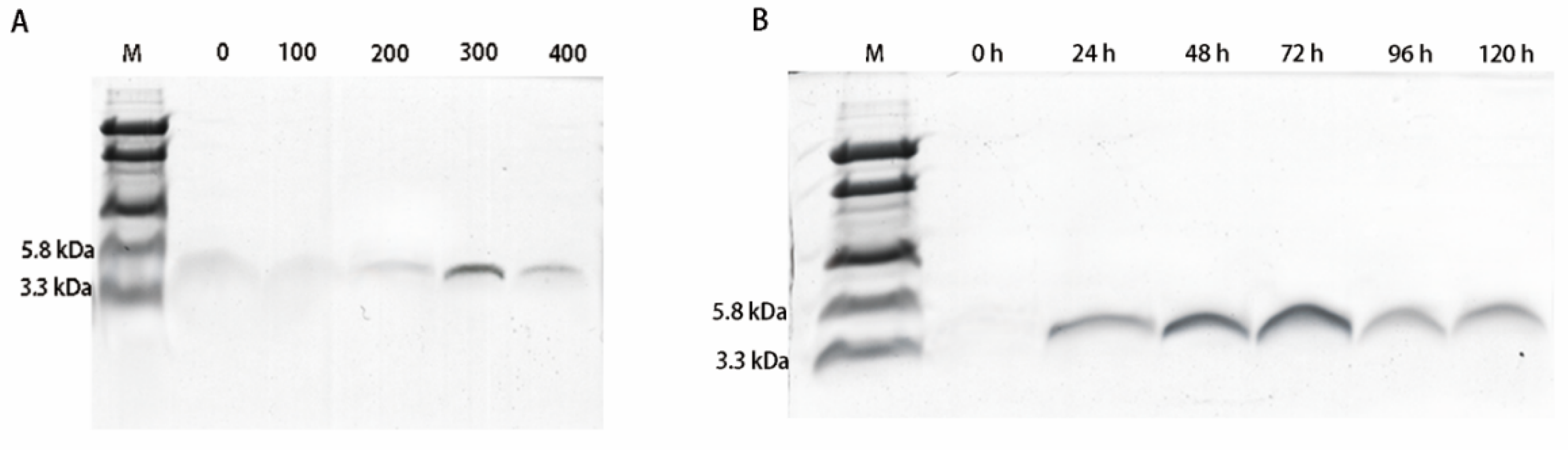
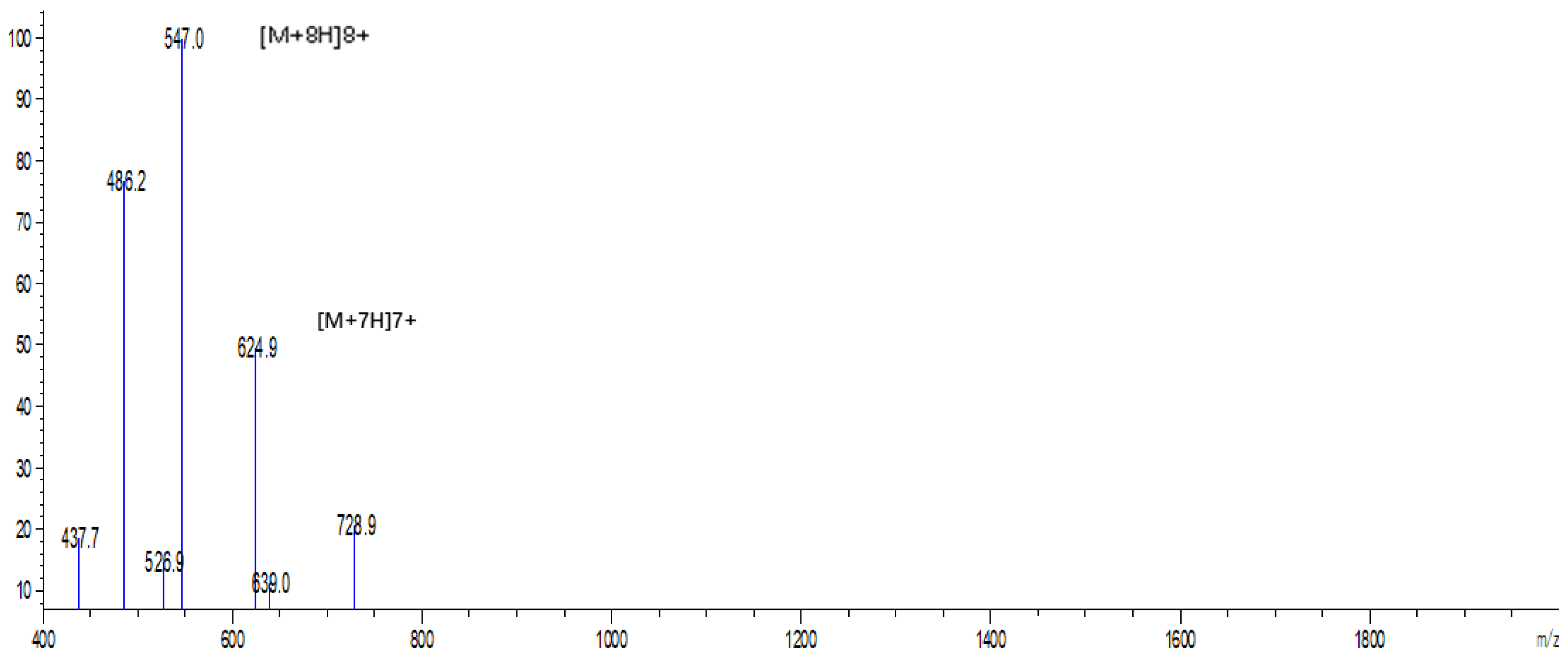
2.2. Expression and Purification of Hybrid EF-1 Peptide
2.3. Antimicrobial Activities of Synthesized PlnEF and Recombinant EF-1
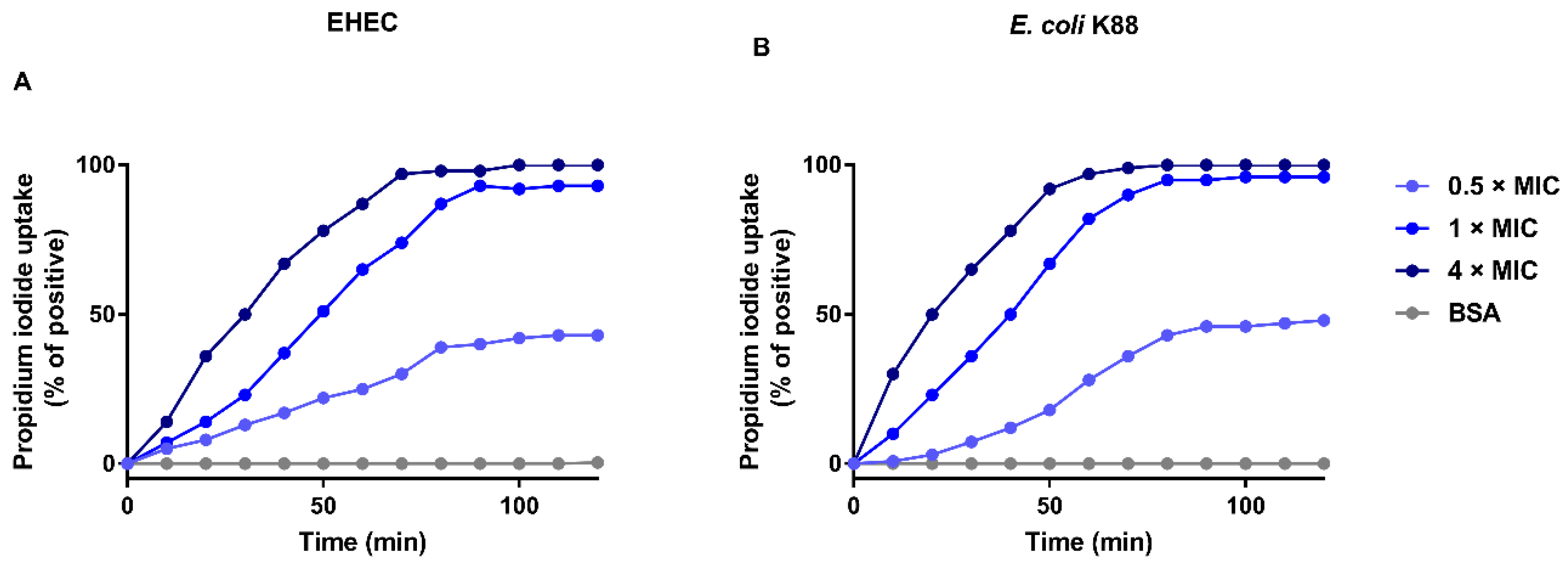
2.4. EF-1 Induces Bacterial Membrane Permeabilization
2.5. Hemolytic Activity of Purified Hybrid EF-1
3. Discussion
4. Materials and Methods
4.1. Regents and Enzymes
4.2. Bacterial Strains, Plasmid
4.3. Chemical Synthesis of Plantaricin EF
4.4. Construction of Expression Vectors
4.5. Expression of EF-1 Peptide in P. pastoris
4.6. Purification of Recombinant EF-1 Peptide
4.7. Analysis of EF-1 by Electrospray Ionization Mass
4.8. Antimicrobial Activity
4.8.1. Inhibition Zone
4.8.2. Minimal Inhibitory Concentrations and Minimum Bactericidal Concentrations
4.8.3. Membrane Integrity Evaluation
4.8.4. Scanning Electron Microscopy
4.9. Hemolytic Assay
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Hwang, P.M.; Vogel, H.J. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 1998, 76, 235. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Sehgal, D.; Valadi, J.K. Recent trends in antimicrobial peptide prediction using machine learning techniques. Bioinformation 2017, 13, 415–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [PubMed]
- Sengupta, D.; Leontiadou, H.; Mark, A.E.; Marrink, S.-J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta 2008, 1778, 2308–2317. [Google Scholar] [CrossRef] [PubMed]
- Oren, Z.; Shai, Y. Selective Lysis of Bacteria but Not Mammalian Cells by Diastereomers of Melittin: Structure—Function Study. Biochemistry 1997, 36, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.I.; Breukink, E. Expanding role of lipid II as a target for lantibiotics. Future Microbiol. 2007, 2, 513–525. [Google Scholar] [CrossRef]
- El Jastimi, R.; Edwards, K.; Lafleur, M. Characterization of Permeability and Morphological Perturbations Induced by Nisin on Phosphatidylcholine Membranes. Biophys. J. 1999, 77, 842–852. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.D.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [PubMed]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086–2092. [Google Scholar]
- Fimland, N.; Rogne, P.; Fimland, G.; Nissen-Meyer, J.; Kristiansen, P.E. Three-dimensional structure of the two peptides that constitute the two-peptide bacteriocin plantaricin EF. BBA Proteins Proteom. 2008, 1784, 1711–1719. [Google Scholar]
- Garneau, S.; Martin, N.I.; Vederas, J.C. Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 2002, 84, 577–592. [Google Scholar] [PubMed]
- Sharma, A.; Srivastava, S. Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/K) and their mode of action. Fungal Biol. 2014, 118, 264–275. [Google Scholar]
- Pal, G.; Srivastava, S. Inhibitory effect of plantaricin peptides (Pln E/F and J/K) against Escherichia coli. World J. Microbiol. Biotechnol. 2014, 30, 2829–2837. [Google Scholar] [PubMed]
- Hauge, H.H.; Mantzilas, D.; Eijsink, V.G.H.; Nissen-Meyer, J. Membrane-Mimicking Entities Induce Structuring of the Two-Peptide Bacteriocins Plantaricin E/F and Plantaricin J/K. J. Bacteriol. 1999, 181, 740. [Google Scholar] [PubMed]
- Nissen-Meyer, J.; Oppegård, C.; Rogne, P.; Haugen, H.S.; Kristiansen, P.E. Structure and Mode-of-Action of the Two-Peptide (Class-IIb) Bacteriocins. Probiotics Antimicrob. Proteins 2010, 2, 52–60. [Google Scholar]
- Anderssen, E.; Diep, D.B.; Nes, I.F.; Eijsink, V.G.H.; Nissen-Meyer, J. Antagonistic Activity of Lactobacillus plantarum C11: Two New Two-Peptide Bacteriocins, Plantaricins EF and JK, and the Induction Factor Plantaricin A. Appl. Environ. Microbiol. 1998, 64, 2269. [Google Scholar]
- Tiwari, S.K.; Srivastava, S. Purification and characterization of plantaricin LR14: A novel bacteriocin produced by Lactobacillus plantarum LR/14. Appl. Microbiol. Biotechnol. 2008, 79, 759–767. [Google Scholar]
- Cao, J.; De La Fuente-Nunez, C.; Ou, R.W.; Torres, M.D.T.; Pande, S.G.; Sinskey, A.J.; Lu, T.K. Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synth. Biol. 2018, 7, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pinera, P.; Han, H.; Cleto, S.; Cao, J.; Purcell, O.; Shah, K.; Lee, K.; Ram, R.; Lu, T.K. Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care. Nat. Commun. 2016, 7, 12211. [Google Scholar] [CrossRef] [PubMed]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [PubMed]
- Sreekrishna, K.; Brankamp, R.G.; Kropp, K.E.; Blankenship, D.T.; Tsay, J.-T.; Smith, P.L.; Wierschke, J.D.; Subramaniam, A.; Birkenberger, L.A. Strategies for optimal synthesis and secretion of heterologous proteins in the methylotrophic yeast Pichia pastoris. Gene 1997, 190, 55–62. [Google Scholar] [CrossRef]
- Jin, F.L.; Xu, X.-X.; Yu, X.-Q.; Ren, S.-X. Expression and characterization of antimicrobial peptide CecropinAD in the methylotrophic yeast Pichia pastoris. Process Biochem. 2009, 44, 11–16. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, S.; Li, B.C.; Qiu, W.; Jiao, B.; Zhang, J.; Diao, Z.Y. Expression of a cytotoxic cationic antibacterial peptide in Escherichia coli using two fusion partners. Protein Expr. Purif. 2008, 57, 303–311. [Google Scholar] [CrossRef]
- Li, Y. Recombinant production of antimicrobial peptides in Escherichia coli: A review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef]
- Ishida, H.; Nguyen, L.T.; Gopal, R.; Aizawa, T.; Vogel, H.J. Overexpression of Antimicrobial, Anticancer, and Transmembrane Peptides in Escherichia coli through a Calmodulin-Peptide Fusion System. J. Am. Chem. Soc. 2016, 138, 11318. [Google Scholar]
- Yang, Y.H.; Zheng, G.; Li, G.; Zhang, X.-J.; Cao, Z.-Y.; Rao, Q.; Wu, K.-F. Expression of bioactive recombinant GSLL-39, a variant of human antimicrobial peptide LL-37, in Escherichia coli. Protein Expr. Purif. 2004, 37, 229–235. [Google Scholar] [CrossRef]
- Moon, J.Y.; Henzler-Wildman, K.A.; Ramamoorthy, A. Expression and purification of a recombinant LL-37 from Escherichia coli. Biochim. Biophys. Acta 2006, 1758, 1351–1358. [Google Scholar] [CrossRef]
- Xu, X.; Jin, F.; Yu, X.; Ren, S.; Hu, J.; Zhang, W. High-level expression of the recombinant hybrid peptide cecropinA(1-8)-magainin2(1-12) with an ubiquitin fusion partner in Escherichia coli. Protein Expr. Purif. 2007, 55, 175–182. [Google Scholar] [CrossRef]
- Tingting, T.; Wu, D.; Li, W.; Zheng, X.; Fu, A.; Shan, A. High Specific Selectivity and Membrane-Active Mechanism of Synthetic Cationic Hybrid Antimicrobial Peptides Based on the Peptide FV7. Int. J. Mol. Sci. 2017, 18, 339. [Google Scholar]
- Moll, G.N.; Akker, E.V.D.; Hauge, H.H.; Nissen-Meyer, J.; Nes, I.F.; Konings, W.N.; Driessen, A.J.M. Complementary and Overlapping Selectivity of the Two-Peptide Bacteriocins Plantaricin EF and JK. J. Bacteriol. 1999, 181, 4848–4852. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, L.; Wei, Y.; Shang, N.; Zhang, X.; Li, P. Two-peptide bacteriocin PlnEF causes cell membrane damage to Lactobacillus plantarum. Biochim. Biophys. Acta 2016, 1858, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, B.; Kyriakou, P.K.; Oppegård, C.; Nissen-Meyer, J.; Kaznessis, Y.N.; Kristiansen, P.E. Structure-Function Analysis of the Two-Peptide Bacteriocin Plantaricin EF. Biochemistry 2016, 55, 5106–5116. [Google Scholar] [CrossRef]
- Cytryńska, M.G.; Zdybicka-Barabas, A. Defense peptides: Recent developments. Biomol. Concepts 2015, 6, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Schagger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Lee, J.-K.; Seo, C.H.; Luchian, T.; Park, Y. Antimicrobial Peptide CMA3 Derived from the CA-MA Hybrid Peptide: Antibacterial and Anti-inflammatory Activities with Low Cytotoxicity and Mechanism of Action in Escherichia coli. Antimicrob. Agents Chemother. 2015, 60, 495–506. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of antimicrobial peptides are available from the authors. |



| Indicator Strains | MIC (μM) | MBC (μM) | ||
|---|---|---|---|---|
| Synthesized PlnEF | Recombinant EF-1 | Synthesized PlnEF | Recombinant EF-1 | |
| S. aureus CVCC 1882 | 25 | 12.5 | 50 | 12.5 |
| M. luteusCMCC 28001 | 12.5 | 6.25 | 12.5 | 12.5 |
| S. Typhimurium ATCC 14028 | >100 | 25 | >100 | 25 |
| S. flexneriCMCC 51572 | >100 | 25 | >100 | 25 |
| EHEC | >100 | 6.25 | >100 | 6.25 |
| E. coli K88 | >100 | 3.125 | >100 | 3.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Cheng, Q.; Guo, H.; Zhang, R.; Si, D. Expression of Hybrid Peptide EF-1 in Pichia pastoris, Its Purification, and Antimicrobial Characterization. Molecules 2020, 25, 5538. https://doi.org/10.3390/molecules25235538
Li Z, Cheng Q, Guo H, Zhang R, Si D. Expression of Hybrid Peptide EF-1 in Pichia pastoris, Its Purification, and Antimicrobial Characterization. Molecules. 2020; 25(23):5538. https://doi.org/10.3390/molecules25235538
Chicago/Turabian StyleLi, Zhongxuan, Qiang Cheng, Henan Guo, Rijun Zhang, and Dayong Si. 2020. "Expression of Hybrid Peptide EF-1 in Pichia pastoris, Its Purification, and Antimicrobial Characterization" Molecules 25, no. 23: 5538. https://doi.org/10.3390/molecules25235538
APA StyleLi, Z., Cheng, Q., Guo, H., Zhang, R., & Si, D. (2020). Expression of Hybrid Peptide EF-1 in Pichia pastoris, Its Purification, and Antimicrobial Characterization. Molecules, 25(23), 5538. https://doi.org/10.3390/molecules25235538




