Non-Classical Gluconeogenesis-Dependent Glucose Metabolism in Rhipicephalus microplus Embryonic Cell Line BME26
Abstract
:1. Introduction
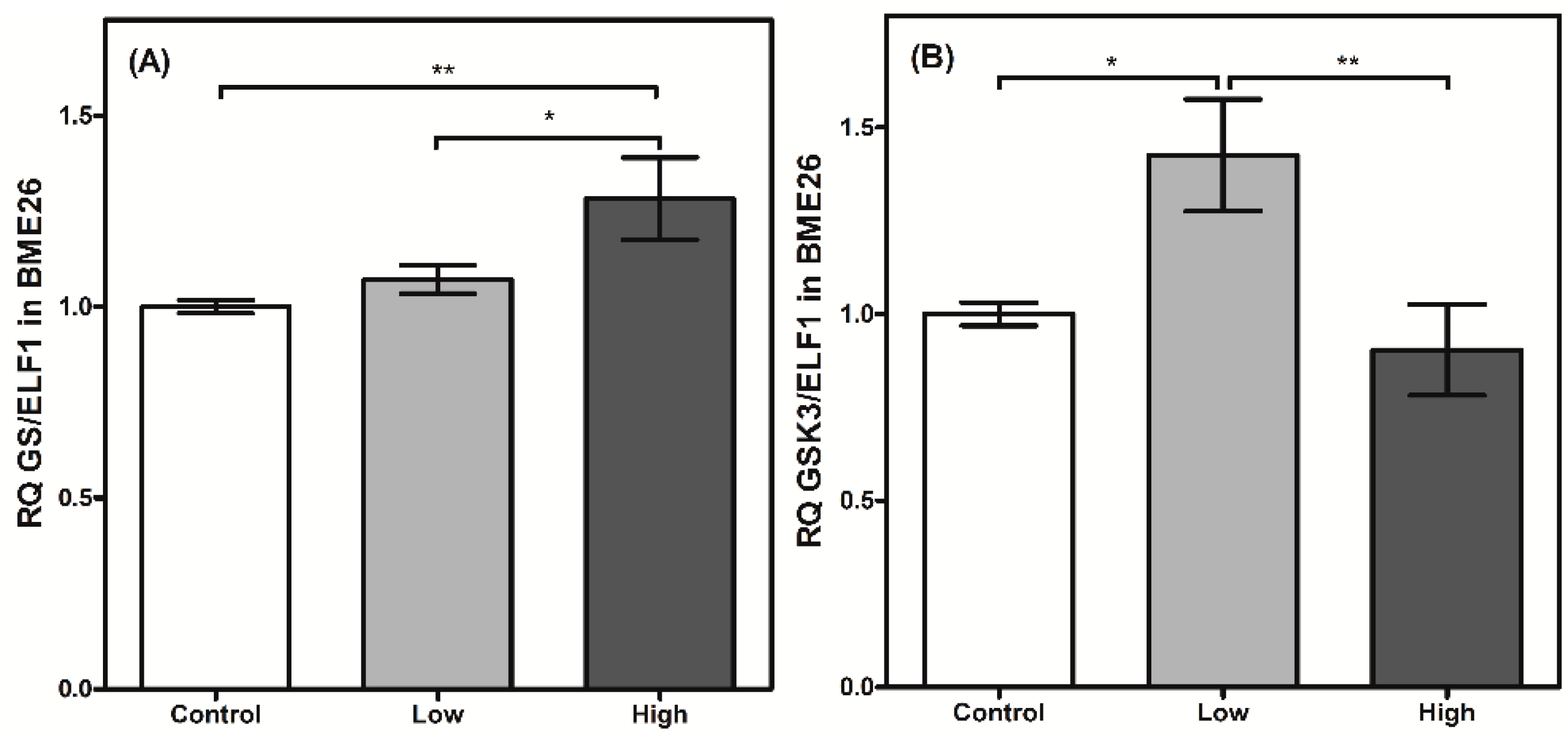
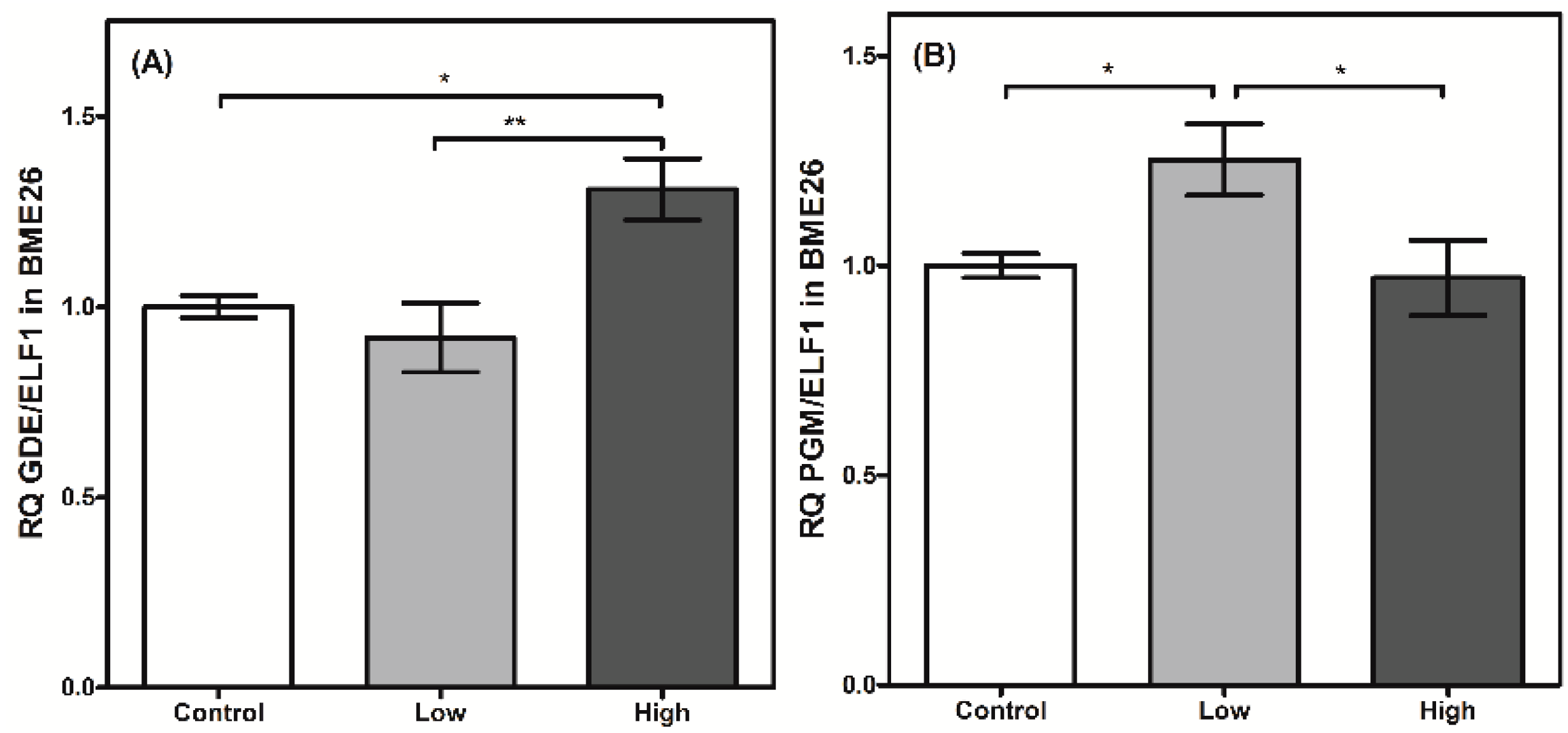
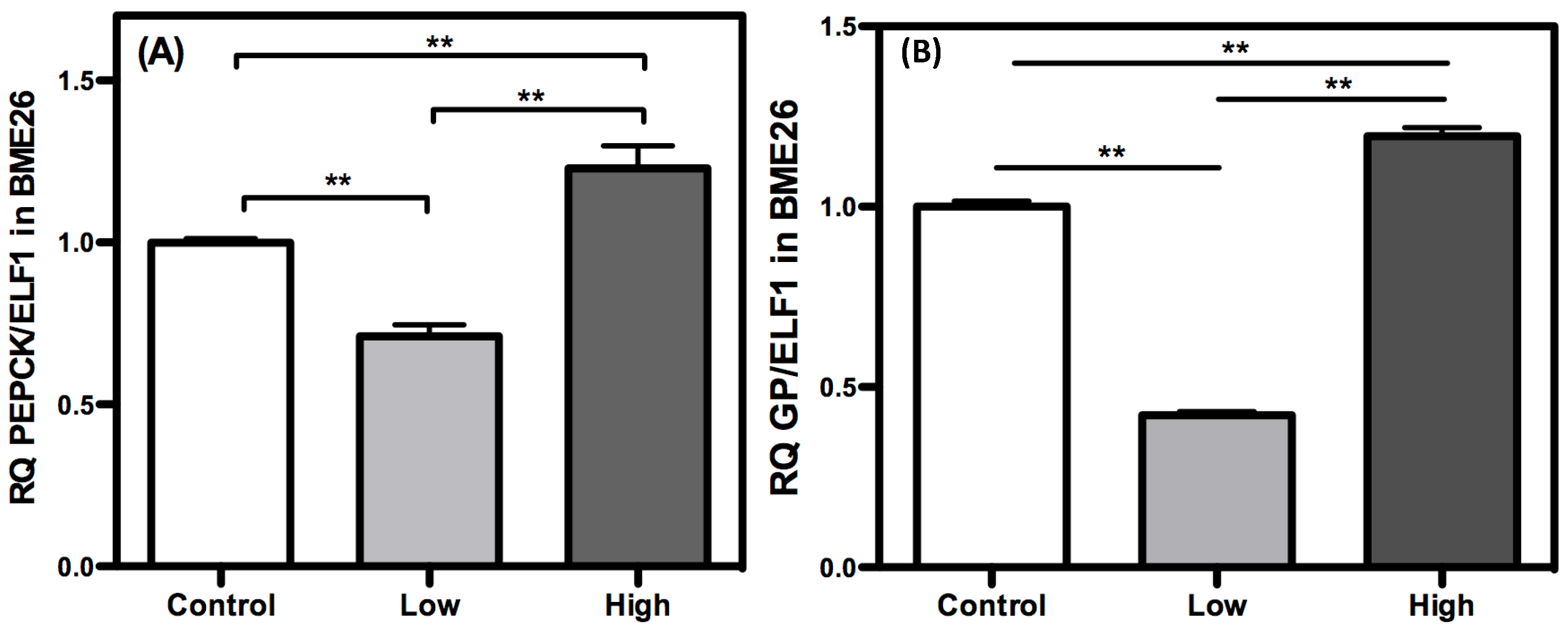
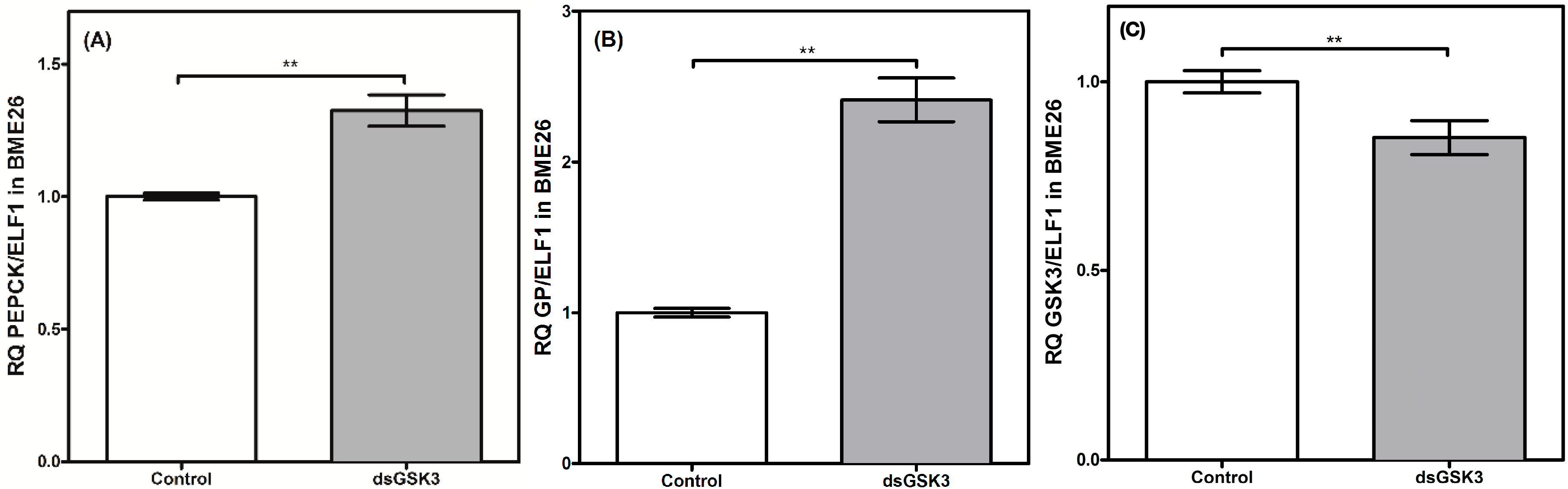
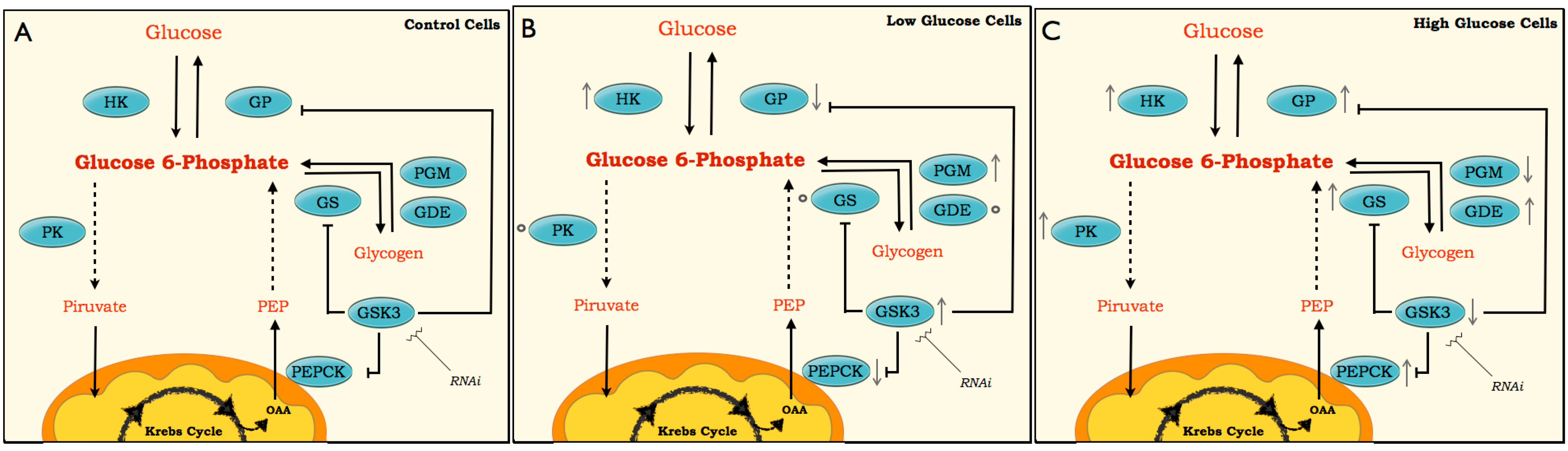
2. Results and Discussion
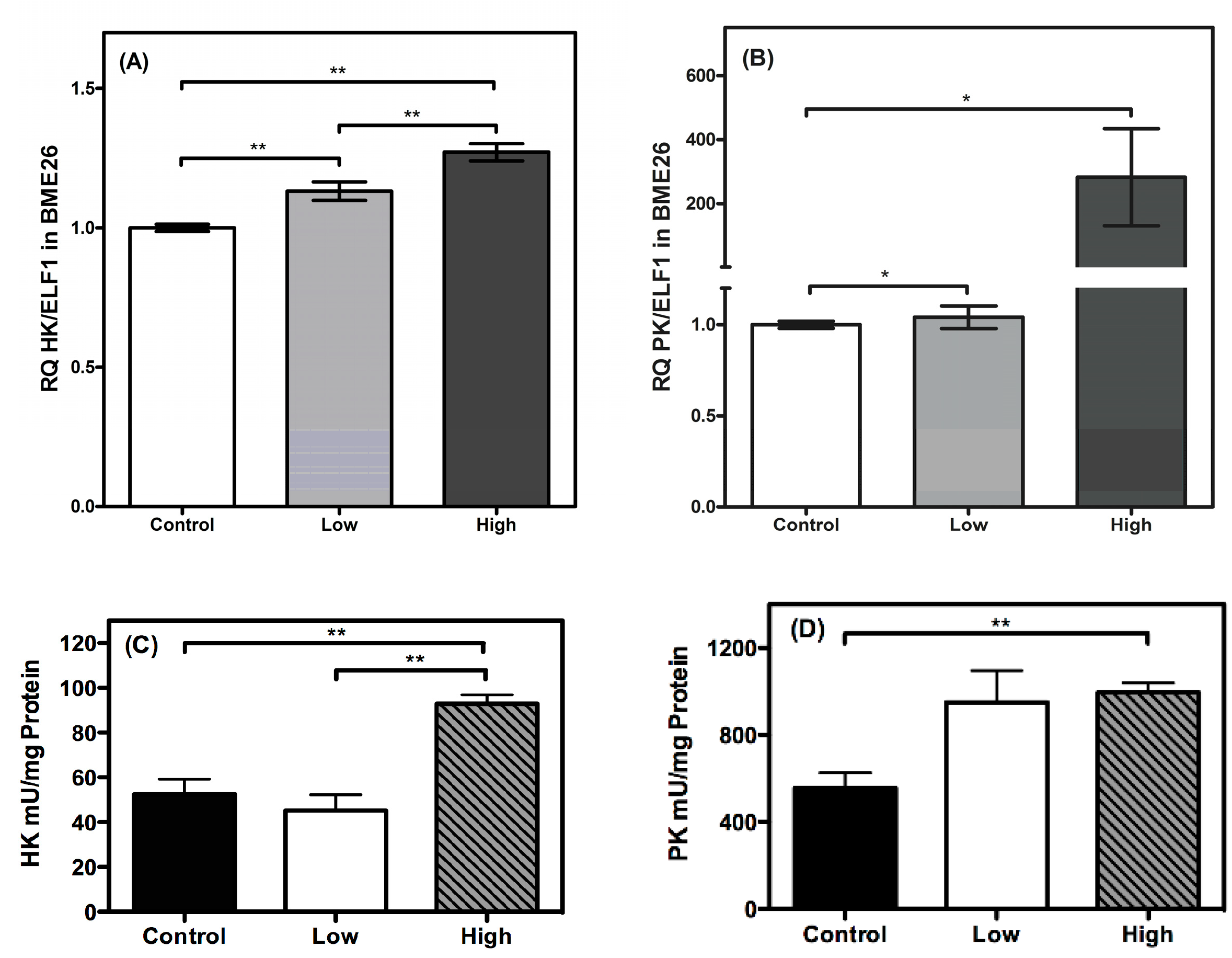
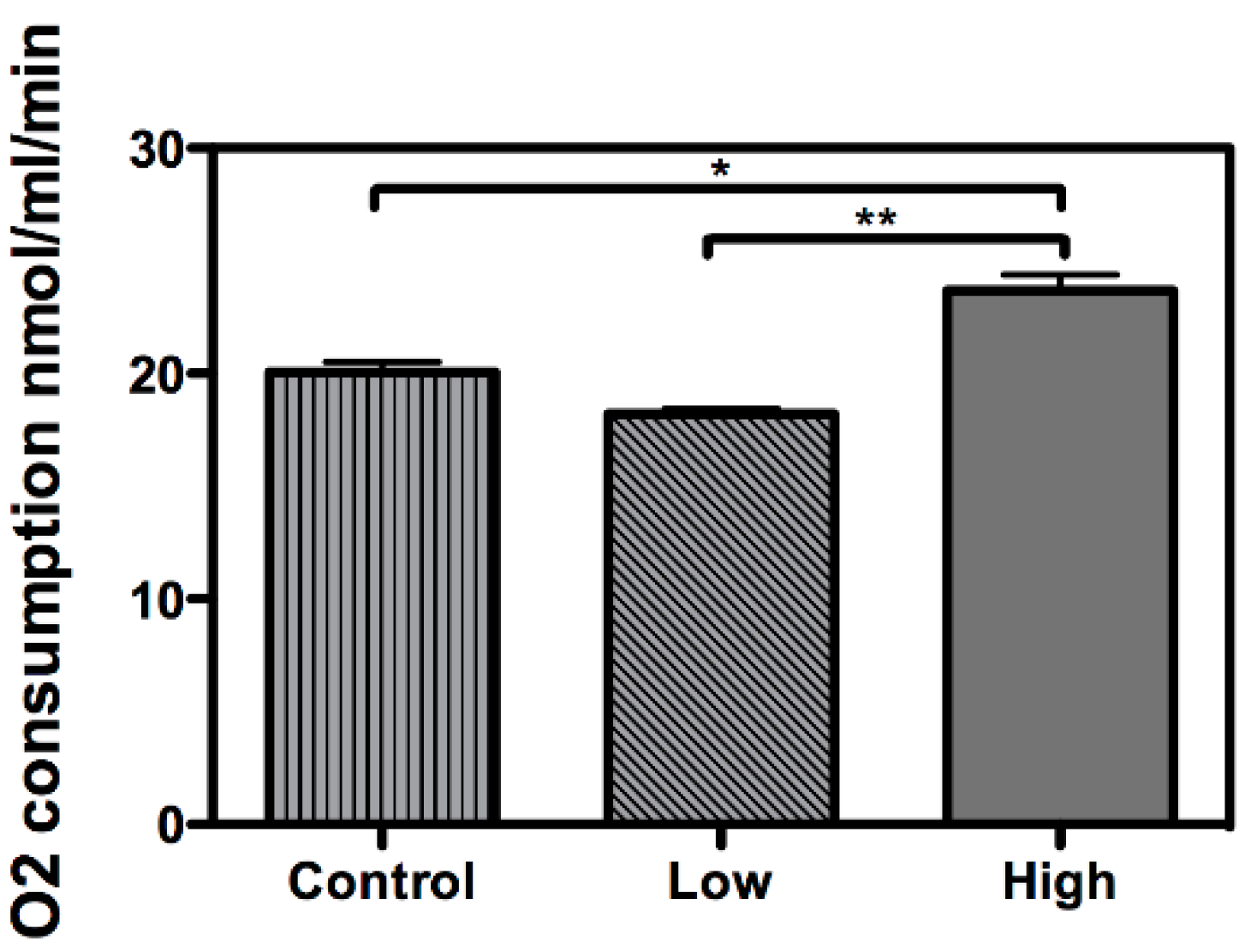
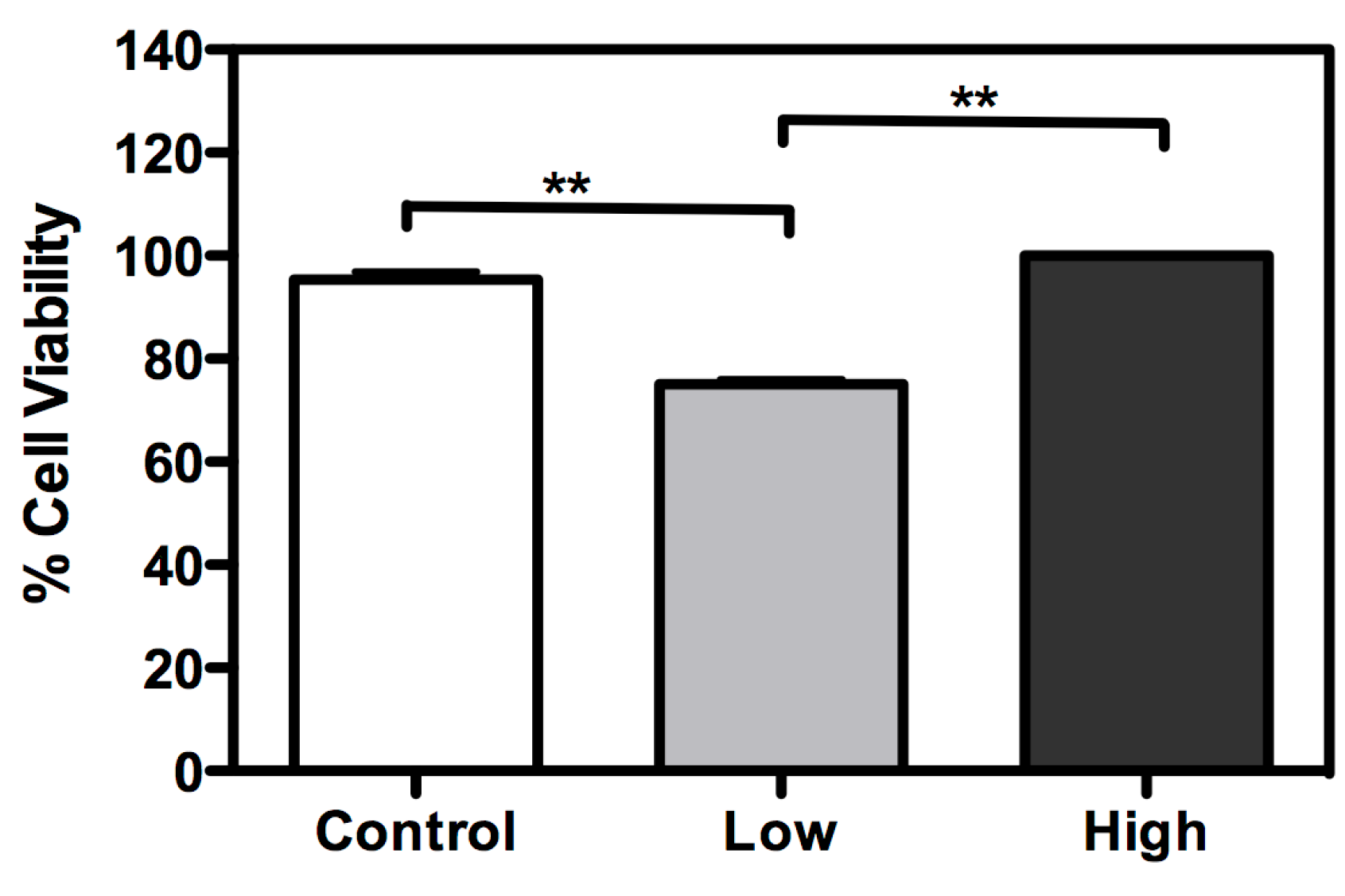
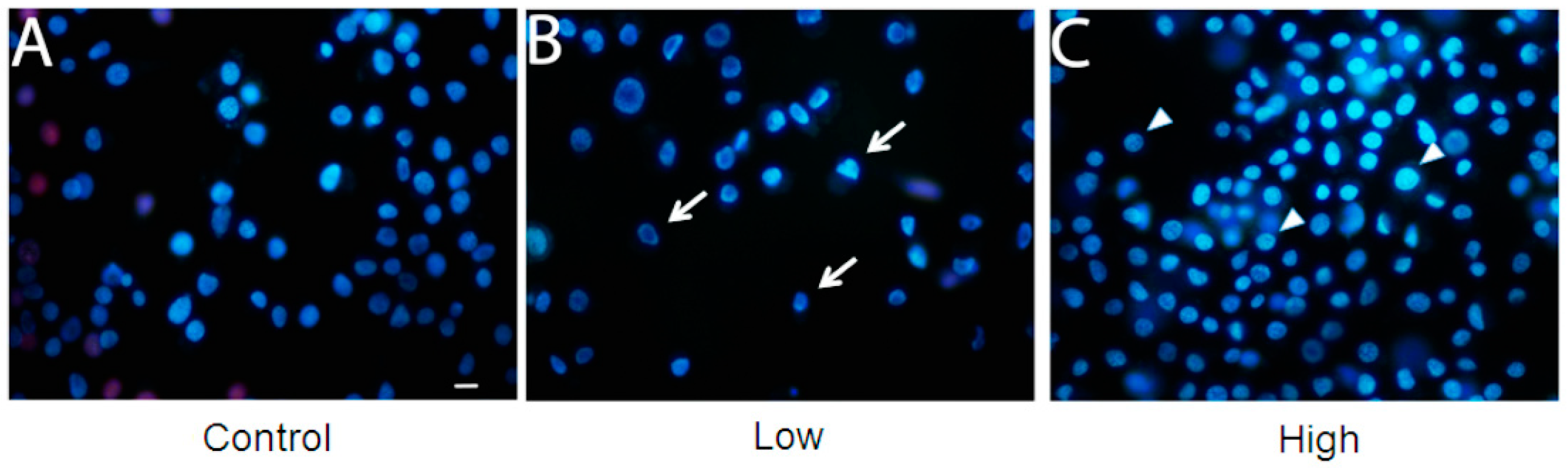
3. Methods
3.1. BME26 Cell Line
3.2. Viability Assay
3.3. Membrane Integrity Analysis
3.4. Double-Stranded RNA (dsRNA) Synthesis
3.5. dsRNA Delivery into BME26 Cells
3.6. RNA Extraction
3.7. Relative Quantification of Metabolism Genes by Real-Time PCR
| Gene | Primers Used for R. microplus Genes | Amplicon Size (bp) | Gene Used as Reference * | GenBank Access Number of R. microplus Gene |
|---|---|---|---|---|
| Hexokinase | CATGGACAAAGAGCTTCAACTGCTC GGAAAGCTCCCTTGACCAGGGTA | 150 | XM_002412706.1 Ixodes scapularis | KF951259 |
| Pyruvate kinase | GGGCAAGAGGGCAAGACAACTG CACGTTGAGCACCTTGGTGATG | 141 | XM_002407420.1 Ixodes scapularis | KF951260 |
| Phophoenolpyruvate carboxykinase | CAAGCAATGAGTGCCTGCCAC ACAGTCTTCCGTTTTCATCTTG | 147 | XM_002413329.1 Ixodes scapularis | KF951261 |
| Glucose-6-phosphatase | GGCAGCCATTTGGTACATCATCC CGACAGGCTGACAATGCACAGG | 133 | XM_002407091.1 Ixodes scapularis | KF951262 |
| Glycogen synthase-6 | GCTGGTATCGGGCTGATCCTG GATGCCTCTGTCTCCAGCCTCC | 165 | XM_002435718.1 Ixodes scapularis | KF951264 |
| Phosphoglucomutase | CGGATCTGGGCAAGCTGGG CCGTCGTGACCCTTGATGAGG | 151 | XM_003695907.1 Apis florea | KF951265 |
| Triose phosphate isomerizes | CCTCGCTGCACAAAATTGCTAC TCCGAATGACCCAGTATGACCC | 128 | EF014474 | |
| Protein kinase B | GGCCAAAGCCATTCACCTTCA CCTCCTCACTCGCCAACTTCTC | 151 | JX648548 | |
| Glycogen synthase kinase 3 | CCCACACCCGCTATTTATTG TGTGCAGGAGAGCCAGTTTA | 113 | EF142066 | |
| Glycogen-debranching enzyme | ATGCTCAGGATCACGCAGAAGC GTACGTCGGTTGGGAAGGACAAGG | 173 | XM_002401176.1 Ixodes scapularis |
3.8. Hexokinase (HK) Activity
3.9. Pyruvate Kinase (PK) Activity
3.10. Oxygen Consumption
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Abbreviation
| HK | hexokinase |
| PK | pyruvate kinase |
| GS | glycogen synthase |
| TIM | triose phosphate isomerase |
| AKT | protein kinase B |
| GSK3 | glycogen synthase kinase 3 |
| PGM | phosphoglucomutase |
| GDE | glycogen-debranching enzyme |
| PEPCK | phosphoenolpyruvate carboxykinase |
| GP | glucose-6 phosphatase |
| GK | glucokinase |
Conflicts of Interest
References
- Parizi, L.F.; Githaka, N.W.; Logullo, C.; Konnai, S.; Masuda, A.; Ohashi, K.; Silva, V.I. The quest for a universal vaccine against ticks: Cross-immunity insights. Vet. J. 2012, 194, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Grisi, L.; Leite, R.C.; Martins, J.R.S.; Barros, A.T.M.; Andreotti, R.; Cançado, P.H.D.; León, A.A.P.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.; Fabres, A.; Esteves, E.; Masuda, A.; Vaz, I.S., Jr.; Daffre, S.; Logullo, C. Exogenous insulin stimulates glycogen accumulation in Rhipicephalus (Boophilus) microplus embryo cell line BME26 via PI3K/AKT pathway. Comp. Biochem. Physiol. B 2009, 153, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.; Moraes, J.; Façanha, A.; Moreira, E.; Valle, D.; Abreu, L.; Manso, P.; Lenzi, H.; Masuda, A.; Vaz, I.S., Jr.; et al. Kinetics of energy source utilization in Boophilus microplus (Canestrini, 1887) (Acari:Ixodidae) embryonic development. Vet. Parasitol. 2006, 138, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Galina, A.; Alvarenga, P.; Rezende, G.; Masuda, A.; Vaz, I.S., Jr.; Logullo, C. Glucose metabolism during embryogenesis of the hard tick Boophilus microplus. Comp. Biochem. Physiol. A 2007, 146, 528–533. [Google Scholar] [CrossRef]
- Saramago, L.; Franceschi, M.; Logullo, C.; Masuda, A.; Vaz, I.S., Jr.; Estrazulas, S.; Moraes, J. Inhibition of enzyme activity of Rhipicephalus (Boophilus) microplus Triosephosphate Isomerase and BME26 cell growth by monoclonal antibodies. Int. J. Mol. Sci. 2012, 13, 13118–13133. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Stewart, R. Embryonic metabolism and growth in lizards of the genus Eumeces. Comp. Biochem. Physiol. 1997, 118A, 647–654. [Google Scholar] [CrossRef]
- Vleck, C.; Hoyt, D. Metabolism and energetic of reptilian and avian embryos. In Egg Incubation: Its Effects On Embryonic Development in Birds and Reptiles; Cambridge Univ. Press: Cambridge, UK, 1991; pp. 285–306. [Google Scholar]
- Wallace, R. Vitellogenesis and oocyte growth in nonmammalian vertebrates. In Developmental Biology; Browder, L.W., Ed.; Plenum Press: New York, NY, USA, 1985; Volume 1, pp. 127–177. [Google Scholar]
- Vital, W.; Rezende, G.; Abreu, L.; Moraes, J.; Lemos, F.; Vaz, I.S., Jr.; Logullo, C. Germ band retraction as a landmark in glucose metabolism during Aedes aegypti embryogenesis. BMC Dev. Biol. 2010, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Fabres, A.; de Andrade, C.; Guizzo, M.; Sorgine, M.; Paiva-Silva, G.; Masuda, A.; Vaz, I.S., Jr.; Logullo, C. Effect of GSK-3 activity, enzymatic inhibition and gene silencing by RNAi on tick oviposition and egg hatching. Parasitology 2010, 137, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Esteves, E.; Lara, F.; Lorenzini, D.; Costa, G.; Fukuzawa, A.; Pressinotti, L.; Silva, J.; Ferro, J.; Kurtti, T.; Munderloh, U.; et al. Cellular and molecular characterization of an embryonic cell line (BME26) from the tick Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol. 2008, 38, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Lohmeyer, K.; Pound, J.; George, J. Effects of photoperiod on reproduction, nymphal developmental timing, and diapauses in Amblyomma maculatum (Acari: Ixodidae). J. Med. Entomol. 2009, 46, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.; Green, R.; Hoodless, A.; Peacey, M. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 2002, 32, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Devlin, T. Textbook of Biochemistry with Clinical Correlations, 4th ed.; Wiley-Liss: New York, NY, USA, 1997. [Google Scholar]
- Dupriex, V.; Rousseau, G. Glucose response elements in a gene that codes for 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. DNA Cell. Biol. 1997, 9, 1075–1085. [Google Scholar] [CrossRef]
- Vaulont, S.; Kahn, A. Transcriptional control of metabolic regulation genes by carbohydrates. FASEB J. 1994, 8, 28–35. [Google Scholar] [PubMed]
- Scott, D.; O’Doherty, R.; Stafford, J.; Newgard, C.; Granner, D. The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J. Biol. Chem. 1998, 273, 24145–24151. [Google Scholar] [CrossRef] [PubMed]
- Towle, H. Metabolic regulation of gene transcription in mammals. J. Biol. Chem. 1995, 270, 23235–23238. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.; Albery, W. Perfection in enzyme catalysis: The energetics of triosephosphate isomerase. Acc. Chem. Res. 1977, 10, 105–111. [Google Scholar] [CrossRef]
- Bell, G.; Burant, C.; Takeda, J.; Gould, G. Structure and function of mammalian facilitative sugar transporters. J. Biol. Chem. 1993, 268, 19161–19164. [Google Scholar] [PubMed]
- Seixas, W.; Gomes-Puyou, A.; Tuena, M.; Moreno-Sanchez, R.; de Felice, F.; de Meis, L. Mitochondrial bound hexokinase activity as a preventive antioxidant defense. J. Biol. Chem. 2004, 279, 39846–39855. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Shukair, S.; Naik, T.; Moazed, F.; Ardehali, H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol. Cell. Biol. 2008, 28, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.; Hoek, J. Hexokinase II: The integration of energy metabolism and control of apoptosis. Curr. Med. Chem. 2003, 10, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Antonio, F.; Nimmo, G.; Yeaman, S.; Cohen, P. Comparison of the substrate specificities of protein phosphatases involved in the regulation of glycogen metabolism in rabbit skeletal muscle. Biochem. J. 1977, 162, 423–433. [Google Scholar] [PubMed]
- Nimmo, G.; Cohen, P. Hormonal control of protein phosphorylation. Adv. Cycl. Nucl. Res. 1977, 8, 124–266. [Google Scholar]
- Embi, N.; Rylatt, D.; Cohen, P. Glycogen synthase kinase-3 from rabbit skeletal muscle, separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur. J. Biochem. 1980, 107, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Plyte, S.; Hughes, K.; Nikolakaki, E.; Pulverer, B.; Woodgett, J. Glycogen synthase kinase-3: Functions in oncogenesis and development. Biochem. Biophys. Acta 1992, 1114, 147–162. [Google Scholar] [PubMed]
- Zhang, W.; Barry, J.; Thompson, J.; Hietakangas, V.; Cohen, S. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS Genet. 2011, 7, e1002429. [Google Scholar] [CrossRef] [PubMed]
- Lynedjian, P.; Roth, R.; Fleischmann, M.; Gjinovci, A. Activation of protein kinase B/cAkt in hepatocytes is sufficient for the induction of expression of the gene encoding glucokinase. Biochem. J. 2000, 351, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Chaodong, W.; Okar, D.; Stoeckman, A.; Peng, L.; Herrera, A.; Herrera, J.; Towle, H.; Lange, A. A potential role for fructose-2,6-bisphosphate in the stimulation of hepatic glucokinase gene expression. Endocrinology 2004, 145, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.; Calixto, C.; Waltero, C.; della Noce, B.; Wang’ombe, N.; Seixas, A.; Parizi, L.; Konnai, S.; Vaz, I.S., Jr.; Ohashi, K.; et al. The conserved role of the AKT/GSK3 axis in cell survival and glycogen metabolism in Rhipicephalus (Boophilus) microplus embryo tick cell line BME26. Biochim. Biophys. Acta 2013, 1830, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Burgering, B.; Coffer, P. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.; Yang, S.; Chan, T.; Datta, K.; Kazlauskas, A.; Morrison, D.; Kaplan, D.; Tsichlis, P. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995, 81, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Passonneau, J. Phosphoglucomutase Kinetics with the Phosphates of Fructose, Glucose, Mannose, Ribose, and Galactose. J. Biol. Chem. 1969, 244, 910–916. [Google Scholar] [PubMed]
- White, R.; Nelson, T. Re-evaluation of the subunit structure and molecular weight of rabbit muscle amylo-1,6-glucosidase-4-alpha-glucanotransferase. Biochim. Biophys. Acta 1974, 365, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ballard, F.; Oliver, I. Glycogen metabolism in embryonic chick and neonatal rat liver. Biochim. Biophys. Acta 1963, 71, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Ballard, F.; Hanson, R.; Leveille, G. Phosphoenolpyruvate carboxykinase and the synthesis of glyceride-glycerol from pyruvate in adipose tissue. J. Biol. Chem. 1967, 242, 2746–2750. [Google Scholar] [PubMed]
- Kreisberg, R.; Pennington, L.; Boshell, B. Lactate turnover and gluconeogenesis in normal and obese humans, E effect of starvation. Diabetes 1970, 19, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, S.; Hanson, R.; Reshef, L.; Hopgood, M.; Ballard, F. Rapid loss of translatable messenger RNA of phosphoenolpyruvate carboxykinase during glucose repression in liver. Proc. Natl. Acad. Sci. USA 1974, 71, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Massillon, D. Regulation of the glucose-6-phosphatase gene by glucose occurs by transcriptional and post-transcriptional mechanisms, differential effect of glucose and xylitol. J. Biol. Chem. 2001, 276, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Hanson, R.; Ballard, F. A possible physiological role for glyceroneogenesis in rat adipose tissue. Biol. Chem. 1970, 245, 5779–5785. [Google Scholar]
- Magnusson, I.; Rothman, D.L.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J. Clin. Investig. 1992, 90, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Martins, R.; Costa, E.; Casagrande, E.; Abreu, L.; Vaz, I.S., Jr.; Moreira, L.; Nunes, R.; Logullo, C. The modulation of the symbiont/host interaction between Wolbachia pipientis and Aedes fluviatilis embryos by glycogen metabolism. PLoS One 2014, 9, e98966. [Google Scholar] [CrossRef] [PubMed]
- Munderloh, U.; Kurtti, T. Formulation of medium for tick cell culture. Exp. Appl. Acarol. 1989, 7, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.; Balk, J.; Postigo, M.; Jongejan, F. Selection of reference genes for quantitative RT-PCR studies in Rhipicephalus (Boophilus) microplus and Rhipicephalus appendiculatus ticks and determination of the expression profile of Bm86. BMC Mol. Biol. 2009, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Galina, A.; da Silva, W.S. Hexokinase activity alters sugar-nucleotide formation in maize root homogenates. Phytochemistry 2000, 53, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Worthington, C. Worthington Enzyme Manual: Enzymes and Related Biochemicals; Worthington Biochemical Corporation: Freehold, NJ, USA, 1988. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, R.M.; Della Noce, B.; Waltero, C.F.; Costa, E.P.; De Abreu, L.A.; Githaka, N.W.; Moraes, J.; Gomes, H.F.; Konnai, S.; Da Silva Vaz, I., Jr.; et al. Non-Classical Gluconeogenesis-Dependent Glucose Metabolism in Rhipicephalus microplus Embryonic Cell Line BME26. Int. J. Mol. Sci. 2015, 16, 1821-1839. https://doi.org/10.3390/ijms16011821
Da Silva RM, Della Noce B, Waltero CF, Costa EP, De Abreu LA, Githaka NW, Moraes J, Gomes HF, Konnai S, Da Silva Vaz I Jr., et al. Non-Classical Gluconeogenesis-Dependent Glucose Metabolism in Rhipicephalus microplus Embryonic Cell Line BME26. International Journal of Molecular Sciences. 2015; 16(1):1821-1839. https://doi.org/10.3390/ijms16011821
Chicago/Turabian StyleDa Silva, Renato Martins, Bárbara Della Noce, Camila Fernanda Waltero, Evenilton Pessoa Costa, Leonardo Araujo De Abreu, Naftaly Wang'ombe Githaka, Jorge Moraes, Helga Fernandes Gomes, Satoru Konnai, Itabajara Da Silva Vaz, Jr., and et al. 2015. "Non-Classical Gluconeogenesis-Dependent Glucose Metabolism in Rhipicephalus microplus Embryonic Cell Line BME26" International Journal of Molecular Sciences 16, no. 1: 1821-1839. https://doi.org/10.3390/ijms16011821
APA StyleDa Silva, R. M., Della Noce, B., Waltero, C. F., Costa, E. P., De Abreu, L. A., Githaka, N. W., Moraes, J., Gomes, H. F., Konnai, S., Da Silva Vaz, I., Jr., Ohashi, K., & Logullo, C. (2015). Non-Classical Gluconeogenesis-Dependent Glucose Metabolism in Rhipicephalus microplus Embryonic Cell Line BME26. International Journal of Molecular Sciences, 16(1), 1821-1839. https://doi.org/10.3390/ijms16011821







